Professional Documents
Culture Documents
Regeneration. Wound Healing: October 10, 2006
Uploaded by
Komang Tri Adi Suparwati0 ratings0% found this document useful (0 votes)
23 views47 pagesok
Original Title
Wound HealingPP2006
Copyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentok
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
23 views47 pagesRegeneration. Wound Healing: October 10, 2006
Uploaded by
Komang Tri Adi Suparwatiok
Copyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 47
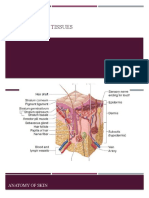
Regeneration.
Wound healing
October 10, 2006
Wound healing
is a natural restorative response to tissue injury.
Healing is the interaction of a complex cascade
of cellular events that generates resurfacing,
reconstitution, and restoration of the tensile
strength of injured skin.
Under the most ideal circumstances, healing is a
systematic process, traditionally explained in
terms of 3 classic phases: inflammation,
proliferation, and maturation.
Wound healing
The inflammatory phase: a clot forms
and cells of inflammation debride injured
tissue during
The proliferative phase: epithelialization,
fibroplasia, and angiogenesis occur;
additionally, granulation tissue forms and
the wound begins to contract.
The maturation phase: Collagen forms
tight cross-links to other collagen and with
protein molecules, increasing the tensile
strength of the scar.
Inflammatory phase
Endothelial cells retract to expose the
subendothelial collagen surfaces.
Platelets attach to these surfaces.
Adherence to exposed collagen surfaces
and to other platelets occurs through
adhesive glycoproteins: fibrinogen,
fibronectin, thrombospondin, and von
Willebrand factor.
Blood clot formation
Primary and secondary
hemostasis
Inflammatory phase
The aggregation of platelets results in the
formation of the primary platelet plug.
Aggregation and attachment to exposed
collagen surfaces activates the platelets.
Activation enables platelets to degranulate
and release chemotactic and growth
factors, such as platelet-derived growth
factor (PDGF), proteases, and vasoactive
agents (eg, serotonin, histamine).
Inflammatory phase
The coagulation cascade occurs by 2 different
pathways.
The intrinsic pathway begins with the activation
of factor XII (Hageman factor), when blood is
exposed to intravascular (subendothelial
surfaces.
The extrinsic coagulation pathway occurs
through the activation of tissue factor found in
extravascular cells in the presence of factors VII
and VIIa.
Coagulation
Inflammatory phase
The result of platelet aggregation and the coagulation
cascade is clot formation.
Clot formation is limited in duration and to the site of
injury.
Clot formation dissipates as its stimuli dissipate.
Plasminogen is converted to plasmin, a potent enzyme
aiding in cell lysis.
Clot formation is limited to the site of injury because
uninjured nearby endothelial cells produce prostacyclin,
an inhibitor of platelet aggregation. In the uninjured
nearby areas, antithrombin III binds vitamin K dependent
coagulation factors and protein C binds factors of the
coagulation cascade, namely, factors V and VII.
Fibrinolysis
Inflammatory phase
Both pathways proceed to the activation of
thrombin, which converts fibrinogen to fibrin.
The fibrin product is essential to wound
healing and is the primary component of the
wound matrix into which inflammatory cells,
platelets, and plasma proteins migrate.
Removal of the fibrin matrix impedes wound
healing.
Fibrinolysis
Inflammatory phase
In addition to activation of fibrin, thrombin
facilitates migration of inflammatory cells
to the site of injury by increasing vascular
permeability. By this mechanism, factors
and cells necessary to healing flow from
the intravascular space and into the
extravascular space.
Inflammatory Phase
Platelets also release factors that attract
other important cells to the injury.
Neutrophils enter the wound to fight
infection and to attract macrophages.
Macrophages break down necrotic debris
and activate the fibroblast response.
The inflammatory phase lasts about 24
hours and leads to the proliferation phase
of the healing process.
Proliferation Phase
On the surface of the wound, epidermal
cells burst into mitotic activity within 24 to
72 hours. These cells begin their migration
across the surface of the wound.
Fibroblasts proliferate in the deeper parts
of the wound. These fibroblasts begin to
synthesize small amounts of collagen
which acts as a scaffold for migration and
further fibroblast proliferation.
Proliferation Phase
Granulation tissue, which consists of
capillary loops supported in this
developing collagen matrix, also appears
in the deeper layers of the wound. The
proliferation phase lasts from 24 to 72
hours and leads to the fibroblastic phase
of wound healing.
Proliferation Phase
Four to five days after the injury occurs,
fibroblasts begin producing large amounts
of collagen and proteoglycans.
Collagen fibers are laid down randomly
and are cross-linked into large, closely
packed bundles.
Proliferation Phase
Proteoglycans appear to enhance the formation
of collagen fibers, but their exact role is not
completely understood.
Within two to three weeks, the wound can resist
normal stresses, but wound strength continues
to build for several months.
The proliferation phase lasts from 15 to 20 days
and then wound healing enters the maturation
phase.
Maturation Phase
During the maturation phase, fibroblasts
leave the wound and collagen is
remodeled into a more organized matrix.
Tensile strength increases for up to one
year following the injury. While healed
wounds never regain the full strength of
uninjured skin, they can regain up to 70 to
80% of its original strength.
Scar formation and time-dependent synthesis and release
of different forms of collagen
Keloid scars
are defined as an abnormal scar that grows
beyond the boundary of the original site of a skin
injury. It is a raised and ill defined growth of skin
in the area of damaged skin.
Although anyone can form a keloid scar some
ethnic groups are at more risk of developing
them. The African-American or Hispanic
populations are 16% more susceptable to form
the types of scars. Keloid scars are seen 15
times more in highly pigmented ethnic groups
rather than Caucasians.
Keloid scaring
Keloid formation
Skin and/or muscle tension seem to
contribute to keloid formation and this is
demonstrated by the most common sites
of their formation (the upper arm and
back).
Infection at a wound site, repeated trauma
to the same area, skin tension or a foreign
body in a wound can also be factors.
Genetic background
There does appears to be a genetic component
to keloid scarring (family history).
Other theories for the causes of keloid scarring
include a deficiency or an excess in melanocyte
hormone (MSH), decreased percentages of
mature collagen and increased soluble collagen,
or that very small blood vessels get blocked and
the resulting lack of oxygen contribute to keloid
formation.
Hypertrophic scar
looks similar to a keloid.
Hypertrophic scars are more common.
They don't get a big as keloids, and may
fade with time.
They occur in all racial groups.
You might also like
- Wound HealingDocument45 pagesWound HealingReda IsmaeelNo ratings yet
- Basic Pathology of Wound Healing and RepairDocument36 pagesBasic Pathology of Wound Healing and Repairaimi BatrisyiaNo ratings yet
- Tissue Repair and Wound Healing ProcessDocument70 pagesTissue Repair and Wound Healing ProcessRegi SeptianNo ratings yet
- Wound Healing Phases and ProcessesDocument24 pagesWound Healing Phases and ProcessesSrishti SrivastavaNo ratings yet
- 3 Phases Wound Healing ProcessDocument24 pages3 Phases Wound Healing ProcessJoher MendezNo ratings yet
- Wound Healing Normal and Abnormal Mechanisms and Closure TechniquesDocument40 pagesWound Healing Normal and Abnormal Mechanisms and Closure TechniquesAhmad Fakhrozi Helmi100% (1)
- Chapter 7: Wound HealingDocument12 pagesChapter 7: Wound HealingpoddataNo ratings yet
- Stages of Wound HealingDocument9 pagesStages of Wound HealingIrene Grace BalcuevaNo ratings yet
- The Four Phases of Wound HealingDocument4 pagesThe Four Phases of Wound HealingNadeeka GamageNo ratings yet
- Basic Mollecular Wound Healing of The SkinDocument22 pagesBasic Mollecular Wound Healing of The SkindavidNo ratings yet
- Skin Wound HealingDocument9 pagesSkin Wound HealingOEDIEN-kmbNo ratings yet
- Wound Healing and RepairDocument7 pagesWound Healing and Repairon miniNo ratings yet
- Wound Healing-1Document63 pagesWound Healing-1sunday danielNo ratings yet
- Wound Healing and Factors Affecting Wound Healing: Principles of SurgeryDocument57 pagesWound Healing and Factors Affecting Wound Healing: Principles of SurgeryAmit RamdinNo ratings yet
- Balsa 2015Document17 pagesBalsa 2015Pipe VodNo ratings yet
- Manejo de HeridasDocument17 pagesManejo de HeridassaortizpNo ratings yet
- Wound Healing Guide: Classification, Phases & Factors ImpactingDocument36 pagesWound Healing Guide: Classification, Phases & Factors ImpactingIbrahim AkinbolaNo ratings yet
- Biology of Periodontal Wound HealingDocument78 pagesBiology of Periodontal Wound HealingSudip SenNo ratings yet
- Healing & Repair Guide for DentistryDocument50 pagesHealing & Repair Guide for DentistryVrushali BhoirNo ratings yet
- Vulnus Wound HealingDocument20 pagesVulnus Wound HealingVicKy AmaliaNo ratings yet
- Wound HealingDocument36 pagesWound Healingkiran kcNo ratings yet
- Woundhealingseminar 150216070848 Conversion Gate02Document103 pagesWoundhealingseminar 150216070848 Conversion Gate02mohan100% (1)
- Wound Healing: Ziv Peled, M.DDocument8 pagesWound Healing: Ziv Peled, M.Dapi-26007957No ratings yet
- Soft Tissue Wound Healing GuideDocument59 pagesSoft Tissue Wound Healing GuideLavanya Kalapala50% (4)
- PBL 4 Wound Healing and Tissue AtrophyDocument3 pagesPBL 4 Wound Healing and Tissue AtrophyadaumedNo ratings yet
- Wound Phases 101Document2 pagesWound Phases 101TaraKyleUyNo ratings yet
- WoundDocument31 pagesWoundEnos ErastusNo ratings yet
- Hypertrophic Scar & KeloidDocument3 pagesHypertrophic Scar & KeloidVeroshini TeddyNo ratings yet
- Patho FullDocument633 pagesPatho FullAbir AlamNo ratings yet
- Tissue Repair, Wound Healing and FibrosisDocument48 pagesTissue Repair, Wound Healing and FibrosisIyanAsianaNo ratings yet
- Fecky F Ichsan - Wound HealingDocument60 pagesFecky F Ichsan - Wound HealingFecky Fihayatul IchsanNo ratings yet
- Keloids and Hypertrophic Scars: Pathophysiology, Classification, and TreatmentDocument16 pagesKeloids and Hypertrophic Scars: Pathophysiology, Classification, and TreatmentStella SunurNo ratings yet
- Unit 1msn Last PartDocument11 pagesUnit 1msn Last PartSusmita BeheraNo ratings yet
- Wound Healing & BurnsDocument232 pagesWound Healing & BurnsdillaNo ratings yet
- Surgery Assignment: Name: Venugopal Asbeliin IMD 14/15/16 TutorialDocument7 pagesSurgery Assignment: Name: Venugopal Asbeliin IMD 14/15/16 TutorialAsbelNo ratings yet
- Healing of Oral WoundsDocument73 pagesHealing of Oral WoundsMadhvendra Singh80% (5)
- Wound HealingDocument45 pagesWound HealingmaleehaNo ratings yet
- Wound Healing, or Wound Repair, Is An Intricate Process in Which The Skin (OrDocument20 pagesWound Healing, or Wound Repair, Is An Intricate Process in Which The Skin (OrDeepak AhujaNo ratings yet
- JCM 11 02744Document13 pagesJCM 11 02744Muhammad MujahidNo ratings yet
- Tissue RepairDocument9 pagesTissue RepairSakidu LegionNo ratings yet
- Chronic WoundsDocument7 pagesChronic WoundstaniaNo ratings yet
- Wound Healing & Care Guide: Stages, Factors & TreatmentDocument55 pagesWound Healing & Care Guide: Stages, Factors & TreatmentClaudio Luis VenturiniNo ratings yet
- Wound Healing PhasesDocument27 pagesWound Healing PhasesAnil BasnetNo ratings yet
- Wound RepairDocument100 pagesWound RepairVergel John ErciaNo ratings yet
- Lecture 20 SkinDocument9 pagesLecture 20 SkinOsama MalikNo ratings yet
- (SURGERY SGD) Wound HealingDocument8 pages(SURGERY SGD) Wound HealingPaulene RiveraNo ratings yet
- Skin and Soft TissuesDocument122 pagesSkin and Soft TissuesNagulan ChanemougameNo ratings yet
- Wound management stages healingDocument14 pagesWound management stages healingnjoomNo ratings yet
- Wound Healing: Santos, Patrick John S., MD First Year Resident Department of Surgery Manila MedDocument60 pagesWound Healing: Santos, Patrick John S., MD First Year Resident Department of Surgery Manila MedJhessie ChingNo ratings yet
- wound healing in surgeryDocument77 pageswound healing in surgerychguduruNo ratings yet
- 3a. Wound HealingDocument37 pages3a. Wound HealingOlivia Avriyanti HanafiahNo ratings yet
- Wound Healing and Tissue RepairDocument27 pagesWound Healing and Tissue RepairKELVIN MAYOMBONo ratings yet
- 2 Wound HealingDocument6 pages2 Wound HealingMohamad AlfarisNo ratings yet
- Wound Healing Wound Healing and Wound and Wound Care Care Care CareDocument7 pagesWound Healing Wound Healing and Wound and Wound Care Care Care Carejc_sibal13No ratings yet
- Wound Healing: Healing by First Intention (Primary Union)Document31 pagesWound Healing: Healing by First Intention (Primary Union)Muhammad Masoom AkhtarNo ratings yet
- Wound Healing and ScarDocument35 pagesWound Healing and ScarLayla CabduqaadirNo ratings yet
- L10 Wound HealingDocument26 pagesL10 Wound HealingAnggi HimranNo ratings yet
- The 4 Main Phases of Wound HealingDocument2 pagesThe 4 Main Phases of Wound HealingJacobMsangNo ratings yet
- Wound Healing Pathophysiology: Inflammatory PhaseDocument5 pagesWound Healing Pathophysiology: Inflammatory Phasechinta_prapoornaNo ratings yet
- Last Minute Embryology: Human embryology made easy and digestible for medical and nursing studentsFrom EverandLast Minute Embryology: Human embryology made easy and digestible for medical and nursing studentsNo ratings yet
- 13 14 1 PB PDFDocument7 pages13 14 1 PB PDFKomang Tri Adi SuparwatiNo ratings yet
- Standardized Balance Tests for Fall Risk AssessmentDocument8 pagesStandardized Balance Tests for Fall Risk AssessmentAlvin OngNo ratings yet
- Neurological PhysiotherapyDocument252 pagesNeurological PhysiotherapyZrakaSunca93% (14)
- Mandibular Movements Up L of DDocument79 pagesMandibular Movements Up L of DkrondanNo ratings yet
- Wound Healing LectureDocument80 pagesWound Healing LecturegogmasiraitNo ratings yet
- Temporamandibula JointDocument22 pagesTemporamandibula JointKomang Tri Adi SuparwatiNo ratings yet
- Wound Healing LectureDocument80 pagesWound Healing LecturegogmasiraitNo ratings yet
- Wound Healing Basic Concepts BhavsarDocument47 pagesWound Healing Basic Concepts BhavsarKomang Tri Adi SuparwatiNo ratings yet
- Journal of International Medical Research 2009 Velnar 1528 42Document15 pagesJournal of International Medical Research 2009 Velnar 1528 42NoorliyyantiNo ratings yet
- PrinciplesWoundHealing WCCSpring2011 PDFDocument5 pagesPrinciplesWoundHealing WCCSpring2011 PDFaajelNo ratings yet
- Temporamandibula JointDocument22 pagesTemporamandibula JointKomang Tri Adi SuparwatiNo ratings yet
- Neurological PhysiotherapyDocument252 pagesNeurological PhysiotherapyZrakaSunca93% (14)
- Temporamandibula JointDocument22 pagesTemporamandibula JointKomang Tri Adi SuparwatiNo ratings yet
- Joint Pain Leaflet PDFDocument12 pagesJoint Pain Leaflet PDFSuaeni Kurnia Wirda100% (1)
- Doping and Performance Enhancing Drug Use in SportDocument5 pagesDoping and Performance Enhancing Drug Use in SportKomang Tri Adi SuparwatiNo ratings yet
- Temporamandibula JointDocument22 pagesTemporamandibula JointKomang Tri Adi SuparwatiNo ratings yet
- CN - Jurnal Lansia 1Document5 pagesCN - Jurnal Lansia 1vidiaherdinaNo ratings yet
- TMJ DisordersDocument20 pagesTMJ DisordersErisa BllakajNo ratings yet
- CN - Jurnal Lansia 1Document5 pagesCN - Jurnal Lansia 1vidiaherdinaNo ratings yet
- Bdi Tdi PDFDocument52 pagesBdi Tdi PDFKomang Tri Adi SuparwatiNo ratings yet
- Ems UnitDocument28 pagesEms UnitKomang Tri Adi SuparwatiNo ratings yet
- Si PDFDocument7 pagesSi PDFKomang Tri Adi SuparwatiNo ratings yet
- Saraf PeriferDocument41 pagesSaraf PeriferLaurensia Erlina NataliaNo ratings yet
- Indonesian WhoqolDocument5 pagesIndonesian WhoqolSri Nindita RuslanNo ratings yet
- Icf CyDocument351 pagesIcf CyDyandraFA100% (1)
- Wound Healing LectureDocument80 pagesWound Healing LecturegogmasiraitNo ratings yet
- LaserDocument23 pagesLaserKomang Tri Adi SuparwatiNo ratings yet
- Head & Neck - Deep Cervical Fascia &cervical PlexusDocument9 pagesHead & Neck - Deep Cervical Fascia &cervical PlexusKomang Tri Adi SuparwatiNo ratings yet
- Head & Neck - Deep Cervical Fascia &cervical PlexusDocument9 pagesHead & Neck - Deep Cervical Fascia &cervical PlexusKomang Tri Adi SuparwatiNo ratings yet
- PhlebotomyDocument46 pagesPhlebotomyQaiser ZamanNo ratings yet
- Blood Work Case StudyDocument2 pagesBlood Work Case Studyapi-268478000No ratings yet
- Blood Plasma Fractionation ProcessDocument2 pagesBlood Plasma Fractionation ProcessAli Hussain100% (1)
- ANATOMY of The Musculoskeletal SystemDocument3 pagesANATOMY of The Musculoskeletal Systemsigfred02No ratings yet
- Information For Patients Needing Irradiated BloodDocument8 pagesInformation For Patients Needing Irradiated BloodCaddy MkaNo ratings yet
- Immuno-Serology & Blood Banking Case StudyDocument8 pagesImmuno-Serology & Blood Banking Case StudyRomie SolacitoNo ratings yet
- XN Cal TraceabilityDocument5 pagesXN Cal TraceabilityAnonymous brvvLxoIluNo ratings yet
- Function of RBCDocument9 pagesFunction of RBCAthea MelosantosNo ratings yet
- Muscle Tissue Lab AssignmentDocument5 pagesMuscle Tissue Lab AssignmentShreyasi DongreNo ratings yet
- Importance of Weak ABO SubgroupsDocument3 pagesImportance of Weak ABO SubgroupsCharmaine Corpuz GranilNo ratings yet
- Leukocytes or White Blood Cells (WBC)Document22 pagesLeukocytes or White Blood Cells (WBC)Md Atikur AminNo ratings yet
- Imuune Thrombocytopenia (Itp)Document34 pagesImuune Thrombocytopenia (Itp)Roshandiep GillNo ratings yet
- Blood TransfusionDocument6 pagesBlood TransfusionSophia Mae RoseteNo ratings yet
- ECL 760 Advanced Fully Automated Coag AnalyzerDocument2 pagesECL 760 Advanced Fully Automated Coag AnalyzerTrần Văn BìnhNo ratings yet
- Penerapan Massage Effluerage Terhadap Penurunan Tekanan DarahDocument7 pagesPenerapan Massage Effluerage Terhadap Penurunan Tekanan DarahindrinurmalasariNo ratings yet
- 5 Hemodynamic Disorders, Thromboembolism and ShockDocument162 pages5 Hemodynamic Disorders, Thromboembolism and Shocksinte beyuNo ratings yet
- Contemporary Management of Major Haemorrhage in Critical CareDocument13 pagesContemporary Management of Major Haemorrhage in Critical CareYo MeNo ratings yet
- Anatomical structures in Cells at Work animeDocument3 pagesAnatomical structures in Cells at Work animeRochele ForondaNo ratings yet
- Hema Primary DisordersDocument13 pagesHema Primary DisordersMiki NishiharaNo ratings yet
- BC3000 PDFDocument325 pagesBC3000 PDFhac huyNo ratings yet
- Unit-3rd, HAP-1st, 1st Sem, PCI, Carewell PharmaDocument23 pagesUnit-3rd, HAP-1st, 1st Sem, PCI, Carewell PharmaRithik ModiNo ratings yet
- Plant Tissues - Practice QuestionsDocument4 pagesPlant Tissues - Practice QuestionsAruna PatilNo ratings yet
- Epithelial Tissue PDFDocument20 pagesEpithelial Tissue PDFshafa cahyani100% (1)
- Ossification: The Process of Bone FormationDocument7 pagesOssification: The Process of Bone FormationAzmal FuadNo ratings yet
- Audit Criteria TransfusionDocument6 pagesAudit Criteria TransfusionDaniel ChristoNo ratings yet
- Training for Safe Blood Collection and TransfusionDocument82 pagesTraining for Safe Blood Collection and TransfusionMohamed Elmasry0% (1)
- Biology - Circulatory SystemDocument13 pagesBiology - Circulatory SystemEnkoujo KairiNo ratings yet
- HemostasisDocument23 pagesHemostasisSwathi BNo ratings yet
- BC-5390CRP Feature and SpecificationDocument3 pagesBC-5390CRP Feature and SpecificationNetfix DigitalNo ratings yet
- Animal TissuesDocument46 pagesAnimal TissuesTrisha ZamboangaNo ratings yet