Professional Documents
Culture Documents
1-Steady State Heat Conduction
Uploaded by
Jabir UnissaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1-Steady State Heat Conduction
Uploaded by
Jabir UnissaCopyright:
Available Formats
Steady state heat
conduction
Heat Transfer
A background in ODE
Important analogies between heat,
mass, and momentum transfer
Heat transfer is the science that seeks
to predict energy transfer that may
take place between material bodies as
a result of a temperature difference.
The science of heat transfer seeks
to predict the rate at which the heat
exchange will take place under
certain specified conditions.
Thus, heat transfer rate is the
desired objective
Difference between Heat Transfer
and Thermodynamics
Thermodynamics deals with the systems in
equilibrium.
Heat transfer predicts how fast change of a
system from one state to another will take
place.
Heat transfer supplements First and Second
Laws of Thermodynamics with additional
experimental rules.
Consider the cooling of a hot steel
bar placed in a jar full of water __
Thermodynamics may be used to predict
the final equilibrium temperature of the
steel bar water combination.
Thermodynamics will not tell us how long
it takes to reach this equilibrium
condition, or what the temperature of
the bar will e after a certain length of
time before equilibrium is achieved.
The three modes of heat transfer are:
Conduction,
convection,
and radiation.
x
T
A
q
c
c
x
T
kA q
c
c
=
Conduction heat transfer:
When a temperature gradient exists in a
body, there is an energy transfer from the
high-temperature region to the low-
temperature region.
The heat transfer rate per unit area is
proportional to the normal temperature
gradient,
Here, q is the heat transfer rate
and
is temperature gradient in the
direction of heat flow.
k is a positive constant, called
thermal conductivity of the
material, W/m/
O
C
x
T
c
c
Three-dimensional analysis:
Heat conduction in and out of a unit volume in all three
directions: Energy balance
Total heat conducted in to the system+ the heat
generated within the system= the total heat
conducted out of the system+ the change in
internal energy of the system
t c
c
+ + + = + + +
+ + +
E
q q q q q q q
dz z dy y dx x gen z y x
x
T
kdydz q
x
c
c
=
dydz dx
x
T
k
x x
T
k q
dx x
(
|
.
|
\
|
c
c
c
c
+
c
c
=
+
Cartesian Coordinates:
y
T
kdxdz q
y
c
c
=
dxdz dy
y
T
k
y y
T
k q
dy y (
|
|
.
|
\
|
c
c
c
c
+
c
c
=
+
z
T
kdxdy q
z
c
c
=
dxdy dz
z
T
k
z z
T
k q
dz z
(
|
.
|
\
|
c
c
c
c
+
c
c
=
+
dxdydz q q
gen
=
t
t c
c
=
T
Cdxdydz
d
dE
t
c
c
= +
|
.
|
\
|
c
c
c
c
+
|
|
.
|
\
|
c
c
c
c
+
|
.
|
\
|
c
c
c
c T
C q
z
T
k
z y
T
k
y x
T
k
x
Substituting the above all in the energy balance equation
t o c
c
= +
c
c
+
c
c
+
c
c T
k
q
z
T
y
T
x
T 1
2
2
2
2
2
2
C
k
o =
For constant thermal conductivity,
Here,
is thermal diffusivity.
Special Cases:
Steady state one-dimensional heat flow (no heat generation)
0
2
2
=
c
c
x
T
(1.4)
Two-dimensional steady state conduction without heat
sources
(1.7)
0
2
2
2
2
=
c
c
+
c
c
y
T
x
T
Steady state one-dimensional heat flow with heat sources
(1.6)
0
2
2
= +
c
c
k
q
x
T
Steady state one-dimensional heat flow in
cylindrical coordinates
0
1
2
2
= +
c
c
dr
dT
r r
T
(1.5)
Thermal conductivity
The Mechanism of Thermal Conductivity in a
Gas
We identify the kinetic energy of a molecule with
its temperature. Thus, in a high-temperature
region, the molecules have higher velocities than
in some lower temperature region. The
molecules are in continuous random motion,
colliding with one another and exchanging
energy and momentum.
The molecules have this random
motion whether or not a temperature
gradient exists in a gas. If a molecule
moves from a high-temperature region
to a low-temperature region, it
transports the kinetic energy to the
lower temperature part of the system
and gives up this energy thru
collisions with lower-energy molecules.
In general, thermal conductivity is
strongly temperature-dependent.
The numerical value of thermal
conductivity indicates how fast heat will
flow in a given material.
The faster the molecules move, the
faster they will transport energy.
Therefore, the thermal conductivity of a
gas depends on temperature.
Thermal conductivity, k, of a gas varies as
T k
where T is absolute temperature. For most
gases at moderate pressures, k is a function
of temperature alone.
Thermal energy may be conducted in
solids by two modes:
Lattice vibrations, and
Transport by free electrons.
In good electric conductors, a large number
of free electrons move about in the lattice of
material. These electrons also carry thermal
energy from a high-temperature region to a
low-temperature region. These electrons are
thus referred to as electron gas.
Energy may also be transported as
vibrational energy in the lattice
structure of a material. But this
component is usually smaller than
electron transport.
Thus, good electrical conductors are
almost always good heat conductors.
Thermal conductivity at 0
O
C
Copper (pure) 385 W/m
O
C
Diamond 2300
Sawdust 0.059
Glass wool 0.038
Window glass 0.78
Ice 2.22
Hg 8.21
Water 0.556
Air 0.024
Steady state one-dimensional heat flow (no heat generation)
0
2
2
=
c
c
x
T
Integrating once
dT/dx = C
1 >>>>>
eqn. 1
Integrating again
T = C
1
* x + C
2 >>>>>>>
eqn. 2
Applying Boundry condtions x=0 , T=T1 and x=L , T=T2
C
2
= T
1
and C
1
= (T
2
-T
1
)/L
Q = - KA dT/dx = K A ( T
1
-T
2
)/L
or Q = ( T
1
-T
2
)/ ( L/ KA)
1-Dimensional Heat Conduction
20
A flat wall is exposed to the environment
temperature of 27C. The wall is covered with two
layers of insulation of 2.5 mm thickness each
whose thermal conductivities are 1.4 and 1.7 W/m-
K respectively. The wall loses heat to the
environment by convection. Compute the value of
the convection heat transfer coefficient which must
be maintained on the outer surface of the insulation
to ensure that the outer surface temperature does
not exceed 41C. The innermost surface is
maintained at a temp of 70C.
You might also like
- Complete Resonance MathematicsDocument701 pagesComplete Resonance MathematicsRajendra Bisoi100% (6)
- Tidal CalculationsDocument6 pagesTidal CalculationsSandipanBiswasNo ratings yet
- Lifting Hook Calculation: 90° Standard Hook Development LengthDocument2 pagesLifting Hook Calculation: 90° Standard Hook Development LengthSi Chini100% (3)
- Boiling and Condensation 1Document48 pagesBoiling and Condensation 1hasan bish100% (1)
- Steel Road Plate DesignDocument1 pageSteel Road Plate DesignAshraf KhanNo ratings yet
- Car crushing hydraulic circuit analysisDocument22 pagesCar crushing hydraulic circuit analysisphankhoa83100% (1)
- IEEE-Std-C57-149-IEEE Guide For The Application and Interpretation of Frequency Response Analysis For Oil-Immersed Transformers PDFDocument72 pagesIEEE-Std-C57-149-IEEE Guide For The Application and Interpretation of Frequency Response Analysis For Oil-Immersed Transformers PDFJose Luis BarretoNo ratings yet
- Climbing Film Evaporation Data (2016) - All GroupsDocument12 pagesClimbing Film Evaporation Data (2016) - All GroupsJuwon Jeremiah MakuNo ratings yet
- Air Flow Velocity and Pressure Coefficient Around The 90o Rectangular Duct (Fluid Exp 5)Document9 pagesAir Flow Velocity and Pressure Coefficient Around The 90o Rectangular Duct (Fluid Exp 5)hayder alaliNo ratings yet
- Gating System Design GuideDocument11 pagesGating System Design GuideAshok Pradhan100% (1)
- ZKG IndiaDocument36 pagesZKG Indiajoe_kudo0% (1)
- E 881511 Om GSCTP C P RC GBR 6-A4Document72 pagesE 881511 Om GSCTP C P RC GBR 6-A4winarnobNo ratings yet
- 04-Binary Vapour Cycle and Co-GenerationDocument9 pages04-Binary Vapour Cycle and Co-GenerationMohtasin SheikhNo ratings yet
- Stefan Boltzmann Apparatus NewDocument4 pagesStefan Boltzmann Apparatus NewDhananjay KadamNo ratings yet
- Investigation of The Effect of Cooling Load On Cooling Tower Performance Thermodynamic Exp 6Document14 pagesInvestigation of The Effect of Cooling Load On Cooling Tower Performance Thermodynamic Exp 6hayder alaliNo ratings yet
- Lab 2 Throttling and Separating ExperimentDocument17 pagesLab 2 Throttling and Separating ExperimentYanganani SindeloNo ratings yet
- Radial Heat Conduction ExperimentDocument13 pagesRadial Heat Conduction ExperimentqwertyasdNo ratings yet
- Measuring Temp Distribution in a Linear Heat BarDocument52 pagesMeasuring Temp Distribution in a Linear Heat BarM Junaid tabassumNo ratings yet
- Full ReportDocument16 pagesFull ReportafiqahanuwarNo ratings yet
- Emissivity Measurement ProcedureDocument7 pagesEmissivity Measurement ProcedureNik SainiNo ratings yet
- EKC 291 9 Heat ConductionDocument11 pagesEKC 291 9 Heat ConductionLia HolmanNo ratings yet
- Solar Collectors: By: Dr. Amandeep Singh Oberoi Assistant Professor Mechanical Engineering DepartmentDocument24 pagesSolar Collectors: By: Dr. Amandeep Singh Oberoi Assistant Professor Mechanical Engineering DepartmentSachin MalhotraNo ratings yet
- Heat Exchanger Experiment AnalysisDocument14 pagesHeat Exchanger Experiment AnalysisKlinsmannJanujajJurgenNo ratings yet
- CHE 333 Chapter 6 Fundamentals of ConvectionDocument47 pagesCHE 333 Chapter 6 Fundamentals of ConvectionEnesEmreTaşNo ratings yet
- Losses in Fittings ReportDocument7 pagesLosses in Fittings ReportAmartya Mitra100% (1)
- Expt#3, FinalDocument3 pagesExpt#3, Finalakhtar140No ratings yet
- Boys Gas CalorimeterDocument6 pagesBoys Gas Calorimeterpolimerasriuma venkatacharishmaNo ratings yet
- Emissivity Guide for Common Materials Under 40 CharactersDocument1 pageEmissivity Guide for Common Materials Under 40 CharactersSinan YıldızNo ratings yet
- Loss of Head in BendsDocument6 pagesLoss of Head in BendsaikareiNo ratings yet
- Mechanical Workshop ReportDocument4 pagesMechanical Workshop ReportHumaid Al-'AmrieNo ratings yet
- Thermal Conductivity Metal Rod ExperimentDocument3 pagesThermal Conductivity Metal Rod ExperimentMuskan JainNo ratings yet
- Performance Test of A Vapor Compression Refrigeration CycleDocument11 pagesPerformance Test of A Vapor Compression Refrigeration CycleA-ar FebreNo ratings yet
- Exp.1 - Steady - State Thermal ConductionDocument5 pagesExp.1 - Steady - State Thermal Conductionنزار الدهاميNo ratings yet
- Lab2 Nozzle FlowDocument5 pagesLab2 Nozzle FlowpuhumightNo ratings yet
- Drying Sand in a Tray DryerDocument13 pagesDrying Sand in a Tray DryerHaziq AzliNo ratings yet
- Answer Scheme Set A /JULY 2012 Section A (Total Marks: 40) : X e T X TDocument11 pagesAnswer Scheme Set A /JULY 2012 Section A (Total Marks: 40) : X e T X TSaran JiNo ratings yet
- Supersonic Flow Over A Double Circular AirfoilDocument33 pagesSupersonic Flow Over A Double Circular AirfoilDragomirescu Andrei100% (1)
- Experiment 1Document9 pagesExperiment 1Mohsen MohammadNo ratings yet
- EGR365 Lab8 - Drag Coefficient of A SphereDocument4 pagesEGR365 Lab8 - Drag Coefficient of A Spherejoeblow4224No ratings yet
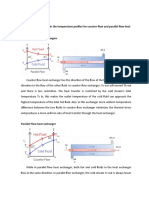
- Describe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat ExchangersDocument3 pagesDescribe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat Exchangersbyun baekNo ratings yet
- Thermal Conductivity of Liquids - MSTDocument11 pagesThermal Conductivity of Liquids - MSTsukhmaniNo ratings yet
- تجربه السيطرهDocument12 pagesتجربه السيطرهMOHAMMED HADINo ratings yet
- Fluidization Bed Column Lab ManualDocument10 pagesFluidization Bed Column Lab ManualAshish VermaNo ratings yet
- COMSATS UNIVERSITY LAHORE DEW POINT LAB REPORTDocument5 pagesCOMSATS UNIVERSITY LAHORE DEW POINT LAB REPORTTaqqi HaiderNo ratings yet
- Heat Engines ExplainedDocument78 pagesHeat Engines ExplainedKhushank MNo ratings yet
- Heat Exchangers Design: Effectiveness - NTU MethodDocument35 pagesHeat Exchangers Design: Effectiveness - NTU MethodSaurabh SengarNo ratings yet
- Compression Test Lab ReportDocument11 pagesCompression Test Lab ReportRobert K OtienoNo ratings yet
- Combined Convection and Radiation Mechanical Engineering ThermoDocument15 pagesCombined Convection and Radiation Mechanical Engineering ThermoBhaggyaLakshanVidanarachchiNo ratings yet
- Delhi Technological University: Heat and Mass Transfer (ME-302) Group: M4Document190 pagesDelhi Technological University: Heat and Mass Transfer (ME-302) Group: M4Subhash SharmaNo ratings yet
- Condensation in Drop and Film FormDocument5 pagesCondensation in Drop and Film FormAshish VermaNo ratings yet
- Melde's MethodDocument2 pagesMelde's MethodDR.P.V.Kanaka Rao0% (1)
- Meen 464 Lab 2 Linear Radial Heat Conduction 1-24-2020Document15 pagesMeen 464 Lab 2 Linear Radial Heat Conduction 1-24-2020Shoaib AhmedNo ratings yet
- Principle Heat TransferDocument34 pagesPrinciple Heat TransferZick HaziqNo ratings yet
- Measurement of Pressure Using McLeod GaugeDocument5 pagesMeasurement of Pressure Using McLeod GaugeSantosh SharmaNo ratings yet
- Important Dimensionless Numbers and Their SignificanceDocument5 pagesImportant Dimensionless Numbers and Their SignificanceMubarik AliNo ratings yet
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDocument68 pagesGas Power Cycles Study Guide in Powerpoint: To AccompanyexceptionalhighdeeNo ratings yet
- The Heat of Solution LabDocument4 pagesThe Heat of Solution Labapi-310957734No ratings yet
- Colling Tower: Mechanical Lab / Exp. NO.Document10 pagesColling Tower: Mechanical Lab / Exp. NO.Dalal Salih100% (1)
- Discussion Shell and TubeDocument3 pagesDiscussion Shell and TubeFarhah AinNo ratings yet
- Composite WallDocument6 pagesComposite WallRushabh PatelNo ratings yet
- Boiling and Condensation PDFDocument11 pagesBoiling and Condensation PDFYeditha Satyanarayana MurthyNo ratings yet
- 7405Document8 pages7405Ebby OnyekweNo ratings yet
- Test On Vapor Compression Refrigeration FINALDocument8 pagesTest On Vapor Compression Refrigeration FINALUdara ManawaduNo ratings yet
- Heat Pump Performance AnalysisDocument22 pagesHeat Pump Performance Analysisahmad pidotNo ratings yet
- Bactron Anaerobic ChamberDocument8 pagesBactron Anaerobic ChamberAsvene SharmaNo ratings yet
- TH35 Ejercicio ADocument3 pagesTH35 Ejercicio ALuis CuzcoNo ratings yet
- Heat Transfer Experiment 1Document16 pagesHeat Transfer Experiment 1atiqahNo ratings yet
- Thermal Expansion of Metals Measured by Dilatometer LabDocument7 pagesThermal Expansion of Metals Measured by Dilatometer LabTaqqi HaiderNo ratings yet
- Professional Etiquettes - CMPDocument1 pageProfessional Etiquettes - CMPJabir UnissaNo ratings yet
- Introduction To CADDocument22 pagesIntroduction To CADJabir UnissaNo ratings yet
- Sponsorship PackagesDocument1 pageSponsorship PackagesJabir UnissaNo ratings yet
- Optimization of Airflow Via An Air RestrictorDocument8 pagesOptimization of Airflow Via An Air RestrictorJabir UnissaNo ratings yet
- Co Pia de Garcia System Dynamics 1 Theory PDFDocument294 pagesCo Pia de Garcia System Dynamics 1 Theory PDFGuilherme AntunesNo ratings yet
- Measure Density & Test Hooke's LawDocument2 pagesMeasure Density & Test Hooke's LawArt Angel GingoNo ratings yet
- Aplicaciones Krohne PDFDocument58 pagesAplicaciones Krohne PDFcollegio101083No ratings yet
- Development of Xbloc Concrete Breakwater Armour Units Canada 2003Document12 pagesDevelopment of Xbloc Concrete Breakwater Armour Units Canada 2003r_anzarNo ratings yet
- Lecture TutorialDocument40 pagesLecture TutorialAhmed A. RadwanNo ratings yet
- Physics Duty Chapter SevenDocument23 pagesPhysics Duty Chapter SevenScribdTranslationsNo ratings yet
- Recursion: Fall 2002 CMSC 203 - Discrete Structures 1Document18 pagesRecursion: Fall 2002 CMSC 203 - Discrete Structures 1Kris BraNo ratings yet
- Structures: Muhammad Zain, Muhammad Usman, Syed Hassan FarooqDocument11 pagesStructures: Muhammad Zain, Muhammad Usman, Syed Hassan FarooqDilum VRNo ratings yet
- NS Meteorological Calculations GuideDocument3 pagesNS Meteorological Calculations GuidecagnashNo ratings yet
- Intensive and Extensive Properties Crowther-Robitaille 2019Document6 pagesIntensive and Extensive Properties Crowther-Robitaille 2019provocator74No ratings yet
- Mechanical EngineeringDocument19 pagesMechanical EngineeringhenoksolNo ratings yet
- Metals and AlloysDocument34 pagesMetals and AlloyszenrockNo ratings yet
- Assignment # 4 MomentsDocument9 pagesAssignment # 4 MomentsKalidNo ratings yet
- Metals 10 01121Document14 pagesMetals 10 01121dietersimaNo ratings yet
- 4709 Outline f15Document2 pages4709 Outline f15ricedragonNo ratings yet
- VTC 18MAT31 QP-Scheme-1Document55 pagesVTC 18MAT31 QP-Scheme-1Maithira HNo ratings yet
- Int - Ph.D. Math - SCDocument11 pagesInt - Ph.D. Math - SCapi-26401608No ratings yet
- SM04 Poster 82Document6 pagesSM04 Poster 82lkamalNo ratings yet
- Lesson 1.3: General Properties of Indefinite IntegralsDocument6 pagesLesson 1.3: General Properties of Indefinite IntegralsMarkNo ratings yet
- MS27069GDocument7 pagesMS27069Gawesome_600No ratings yet
- 6 W1 P 7 K FQ MlitDocument309 pages6 W1 P 7 K FQ MlitnicolaunmNo ratings yet