Professional Documents
Culture Documents
BS 3
Uploaded by
himanshu_agraOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BS 3
Uploaded by
himanshu_agraCopyright:
Available Formats
Unit 2
Chemical biology
Saumen Hajra
Department of Chemistry
Chemical biology is a scientific discipline spanning the fields of chemistry
and biology that involves the application of chemical techniques and tools,
often compounds produced through synthetic chemistry, to the study and
manipulation of biological systems.
Proteomics (study of proteins/enzymes)
Glycobiology
Molecular sensing
Enzymes (History)
First discovered by Eduard Buchner in 1897
who observed that yeast extracts can ferment
sugar to alcohol
This proved that fermentation was promoted
by molecules that continued to function when
removed from cells
The first enzyme to be purified and
crystallized was urease in 1926; these
crystals consisted entirely of protein
Later, pepsin, trypsin and other digestive
proteins were isolated and determined to be
purely protein as well
Enzymes are the catalysts of nature.
With the exception of catalytic RNA, all enzymes are
proteins.
Catalyst alter the rate of a chemical reaction without
undergoing a permanent change in structure.
Catalytic activity is dependant upon native
conformation; if it is lost, then catalytic activity is
lost as well
All levels of protein architecture must be intact and
correct for enzymes to perform their functions
They range in molecular weights from 12,000 to over
1 million
Enzymes
Simple Enzymes: composed of whole proteins
Complex Enzymes: composed of protein plus a relatively small
organic molecule
holoenzyme = apoenzyme + prosthetic group / coenzyme
A prosthetic group describes a small organic or metalloorganic molecule
bound to the apoenzyme by covalent bonds.
When the binding between the apoenzyme and non-protein
components is non-covalent, the small organic molecule is called a
coenzyme.
Coenzymes serve as transient carriers of specific functional groups.
They often come from vitamins (organic nutrients required in small amounts in the
diet)
Enzyme Classification
Oxidoreductases Act on many chemical groupings to add or remove
hydrogen atoms (transfer of electrons).
Transferases Transfer functional groups between donor and
acceptor molecules. Kinases are specialized
transferases that regulate metabolism by
transferring phosphate from ATP to other
molecules.
Hydrolases Add water across a bond, hydrolyzing it.
Lyases Add water, ammonia or carbon dioxide across
double bonds, or remove these elements to
produce double bonds.
Isomerases Carry out many kinds of isomerization: L to D
isomerizations, mutase reactions (shifts of
chemical groups) and others.
Ligases Catalyze reactions in which two chemical groups
are joined (or ligated) with the use of energy from
ATP.
International Classification of Enzyme
How enzymes work
Enzymes provide specific environments in which
chemical reactions that dont normally proceed under
neutral pH, mild temperature, and aqueous
environment conditions can occur
This region is a pocket on the enzyme known as the
active site
The molecule that is bound to the active site and
acted upon by the enzyme is called the substrate
The two together form what is known as the enzyme-
substrate complex
The function of an enzyme catalyst is to increase the
rate of a chemical reaction, not affect is equilibrium
Therefore, enzymes dont make more product, they
just make product faster
Active Site
The area of an enzyme that binds to the substrate
Structure has a unique geometric shape that is designed to
fit the molecular shape of the substrate
Each enzyme is substrate specific
Thus the active site that is complementary to the geometric
shape of a substrate molecule
Active site is lined with residues and sometimes contains a co-
factor
Active site residues have several important properties:
Charge [partial, dipoles, helix dipole]
pKa
Hydrophobicity
Flexibility
Reactivity
Substrate Binding specificity
Complementarity
Geometric
Electronic (electrostatic)
Stereospecificity (enzymes and
substrates are chiral)
1. Lock and Key model
2. Induced Fit model
Enzyme active site
Chymotrypsin (Cuts next to Hydrophobic Groups)
Trypsin (Cuts next to Arg & Lys)
Elastase (Cuts next to Val & Thr)
An enzyme binds a substrate in a region called the active site
Only certain substrates can fit the active site
Amino acid R groups in the active site help substrate bind
Lock and Key Model
Enzyme structure flexible, not rigid
Enzyme and active site adjust shape to bind substrate
Increases range of substrate specificity
Shape changes also improve catalysis during reaction
- transition-state like configuration
Induced Fit Model
Enzyme-Substrate Interaction
Hexokinase undergoes a conformational change upon
binding to a substrate
red: before substrate-binding
green: after substrate-binding
Carboxypeptidase A catalyzes the hydrolysis of the
C-terminal peptide
Effect of Temperature
Effect of pH
The pH-rate profile of an enzyme is a function of the
pK
a
values of the catalytic groups in the enzyme
a group is
catalytically
active in its basic
form
a group is
catalytically
active in its acidic
form
Dependence of lysozyme activity on the pH of the reaction
Asp 52
Glu 35
The pH at which
enzyme is 50% active
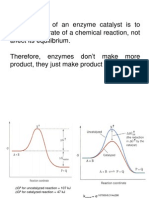
The function of an enzyme catalyst is to
increase the rate of a chemical reaction, not
affect is equilibrium.
Therefore, enzymes dont make more
product, they just make product faster.
AG
#
for uncatalyzed reaction = 107 kJ
AG
#
for catalyzed reaction = 47 kJ
Arrheneous Eqn.: k = Ae
-AG
#
/RT
k
uncat
= Ae
-107000/8.314x298
k
cat
= Ae
-47000/8.314x298
k
cat
/k
uncat
= ~5x10
10
How can an enzyme reduce the activation energy?
(1) Binding to the substrate can be done such that the formation
of the transition state is favored
(2) Orientation and positioning of substrate(s)
(3) Bonds in the substrate can be activated by functional groups
in the catalytic site
E = Enzyme S = Substrate P = Product
ES = Enzyme-Substrate complex
k
1
rate constant for the forward reaction
k
-1
= rate constant for the breakdown of the ES to
substrate
k
2
= rate constant for the formation of the products
Enzyme Kinetics
E+S
k
1
k
1
ES
k
2
E+ P
- the activation energies for the formation of the intermediate state,
and its conversion to the final product are each lower than the
activation energy for the uncatalyzed reaction
-intermediate state- resembles transition state but with lower energy,
(due to interaction with a catalyst)
- transition state defines free energy maximum state
uncatalyzed
reaction
E+S
k
1
k
1
ES
k
2
E+ P
When the substrate concentration becomes large enough to
force the equilibrium to form completely all ES the second
step in the reaction becomes rate limiting because no more ES
can be made and the enzyme-substrate complex is at its
maximum value.
| |
| | ES
P
2
k
dt
d
v = =
[ES] is the difference between the rates
of ES formation minus the rates of its
disappearance.
| |
| || | | | | | ES ES S E
ES
2 1 1
k k k
dt
d
=
1
E+S
k
1
k
1
ES
k
2
E+ P
Assumption of equilibrium
k
-1
>>k
2
the formation of product is so
much slower than the formation of the ES
complex. That we can assume:
| || |
| | ES
S E
1
1
= =
k
k
K
s
K
s
is the dissociation constant for the ES complex.
Assumption of steady state
Transient phase where in the course of a reaction the
concentration of ES does not change
| |
0
ES
=
dt
d
2
| | | | | | ES E E
T
+ =
3
Combining 1 + 2 + 3
| | | | ( )| | ( )| | ES k k S ES - E k
2 1 - T 1
+ =
| | | | ( ) | | | | S E k S k k k ES
T 1 1 2 1 -
= + +
| |
| | | |
| | S K
S E
ES
T
+
=
M
1
2 1 -
k
k k
K
+
=
M
rearranging
Divide by k
1
and solve for [ES] Where
| |
| |
| | | |
| | S K
S E
ES
P
T 2
2
0
+
= =
|
.
|
\
|
=
= M t
o
k
k
dt
d
v
v
o
is the initial velocity when the reaction is just starting out.
And is the maximum velocity | |
T 2 max
E k V =
| |
| | S K
S V
max
+
=
M
o
v
The Michaelis - Menten
equation
low [S], v is proportional to [S] - first order
high [S], v is independent of [S] - zero order
Michaelis Menten Kinetics
The K
m
is the substrate concentration where v
o
equals one-half V
max
The K
M
widely varies among different
enzymes
The K
M
can be expressed as:
1
2
1
2
1
1
K K
k
k
k
k
k
k
s M
+ = + =
As K
s
decreases, the affinity for the substrate
increases. The K
M
can be a measure for substrate
affinity if k
2
<k
-1
V
0
= V
max
[S]
K
m
+ [S]
- in order to change this equation to a form we can use in
our analysis of enzymatic rate constants, we invert both
sides of the equation:
1 = K
m
+ [S]
V
0
V
max
[S]
K
m
1 1
V
max
[S] V
max
=
+
1
V
0
0
1
/
V
0
1/[S]
Slope = K
m
/V
max
1/V
max
-1/K
m
Lineweaver-Burk Plot
The double reciprocal plot
Lineweaver-Burk plot: slope = K
M
/V
max
,
1/v
o
intercept is equal to 1/V
max
the extrapolated x intercept is equal to -1/K
M
For small errors in at low [S] leads to large errors in 1/v
o
| |
T
max
E
V
=
cat
k
k
cat
is how many reactions an
enzyme can catalyze per second
The turnover number
For Michaelis -Menton kinetics k
2
= k
cat
When [S] << K
M
very little ES is formed and [E] = [E]
T
and
| | | | | || | S E
K
k
S E
K
k
M
cat
T
M
2
~ ~
o
v
k
cat
/K
M
is a measure of catalytic efficiency
V
0
= V
max
[S]
K
M
+ [S]
| |
T
max
E
V
=
cat
k
K
m
Relates to how strongly an enzyme binds its substrate
k
cat
Relates to how rapid a catalyst the enzyme is
V
max
Related to k
cat
and [E] by: V
max
=k
cat
[E]
High K
m
means strength of binding is low
High k
cat
means high speed of catalysis
High V
max
means high rate of catalysis
A high k
cat
/K
M
ratio implies an efficient enzyme
This could result from: Large k
cat
Small K
M
k
cat
= turnover number; k
cat
= V
max
/[E]
T
k
cat
/K
m
is a measure of activity, catalytic efficiency
K
M
is a useful indicator of the affinity of an enzyme
for the substrate
A low K
M
indicates a high affinity for the substrate
Enzyme Inhibition
Inhibitors: compounds that decrease activity of the enzyme
Can decrease binding of substrate (affect K
M
), or turnover #
(affect k
cat
) or both
Most drugs are enzyme inhibitors
Inhibitors are also important for determining enzyme
mechanisms and the nature of the active site.
Important to know how inhibitors work facilitates drug design,
inhibitor design.
Antibiotics inhibit enzymes by affecting bacterial
metabolism
Nerve Gases cause irreversible enzyme inhibition
Insecticides choline esterase inhibitors
Many heavy metal poisons work by irreversibly
inhibiting enzymes, especially cysteine residues
Types of Enzyme Inhibition
Reversible inhibition
reversibly bind and dissociate from enzyme,
activity of enzyme recovered on removal of
inhibitor - usually non-covalent in nature
Competitive
Uncompetitive
Noncompetitive (Mixed)
Irreversible inhibition
inactivators that irreversibly associate with
enzyme
activity of enzyme not recovered on removal -
usually covalent in nature
Competitive Inhibition
Inhibitor competes for the substrate binding site
most look like substrate
substrate mimic / substrate analogue
Competitive Inhibition
Competitive Inhibition
Competitive Inhibition
No Reaction
Methanol poisoning is treated with ethanol; the formation of formaldehyde
is slowed and spread out over a longer period of time, lessening its effects
on the body
Uncompetitive Inhibition
Uncompetitive inhibitors bind at a site distinct from the substrate
active site and bind only to the ES complex
Active site distorted after binding of S ( usually occurs
in multisubstrate enzymes) Decreases both K
M
and k
cat
Vo = V
max
[S]/(K
M
+ o[S]) K
I
= [ES][I]/[ESI]
Cannot be reversed by increasing [S] available
enzyme decreases
Uncompetitive Inhibition
Uncompetitive Inhibition
Inhibitor can bind at a site distinct from the substrate
active site to either E or ES
Mixed (Noncompetitive) Inhibition
V
o
= Vmax[S]/(oK
M
+ o[S])
V
max
decreases; K
M
can go up or down.
Mixed Inhibition
Mixed inhibition refers to a combination of two different types of reversible enzyme inhibition
competitive inhibition and uncompetitive inhibition. The term 'mixed' is used when the
inhibitor can bind to either the free enzyme or the enzyme-substrate complex.
In mixed inhibition, the inhibitor binds to a site different from the active site where the substrate
binds. Mixed inhibition results in a decrease in the apparent affinity of the enzyme for the
substrate ( K
m
app
> K
m
, a decrease in apparent affinity means the K
m
value appears to
increase) and a decrease in the apparent maximum enzyme reaction rate (V
max
app
<
V
max
).
Mathematically, mixed inhibition occurs when the factors and (introduced into the
Michaelis-Menten equation to account for competitive and uncompetitive inhibition,
respectively) are both greater than 1.
In the special case where = , noncompetitive inhibition occurs, in which case V
max
app
is
reduced but K
m
is unaffected. This is very unusual in practice
Non-competitive inhibition models a system where the inhibitor and the substrate may
both be bound to the enzyme at any given time. When both the substrate and the inhibitor
are bound, the enzyme-substrate-inhibitor complex cannot form product and can only be
converted back to the enzyme-substrate complex or the enzyme-inhibitor complex. Non-
competitive inhibition is distinguished from general mixed inhibition in that the inhibitor has
an equal affinity for the enzyme and the enzyme-substrate complex.
Lineweaver-Burke plots
Irreversible inhibitors are those that combine with or
destroy a functional group on an enzyme that is
essential for activity
They usually form covalent linkages to the enzyme
Diisopropylfluorophosphate binds irreversibly with chymotrypsin at the
Ser195 residue; this gives info justifying this as the primary active site of the
enzyme
A special class of irreversible inhibitors is the suicide
inactivators
These are unreactive until bound to the active site
They are designed to carry out the first few steps of a
normal enzyme reaction, but instead of forming a
product, they form a highly reactive compound that
binds irreversibly to the enzyme
They are sometimes called mechanism-based
inactivators, because they use the normal enzyme
mechanism to lead to the inactivation
These are often used in drug design
Regulatory Enzymes
These are enzymes that set the rate of a metabolic pathway by
catalyzing the slowest or rate-limiting reaction
They experience increased or decreased catalytic activity in
response to certain external signals
There are two major classes of regulatory enzymes in metabolic
pathways
Allosteric enzymes bind regulatory compounds called allosteric
modulators reversible, noncovalent interactions
Others regulate by reversible covalent modification
In some pathways, the regulatory enzyme is inhibited by the end
product of the pathway whenever the end product concentration
exceeds the cells requirement
When the regulatory enzyme is slowed, all subsequent enzymes
operate at reduced rates
This is known as feedback inhibition
A B C D E Z
Threonine
o-ketobutyrate Isoleucine
Feedback Inhibition
CO
2
H
NH
2
H
3
CH
2
C
H
3
C
CO
2
H
NH
2
HO
H
3
C
Allosteric regulation
When a small molecule can act as an effector or
regulator to activate or inactivate an action of a
protein
- the protein is said to be under allosteric control. The
binding of the small ligand is distant from the
proteins active site and regulation is a result of a
conformational change in the protein when the ligand
is bound
Many types of proteins show allosteric control:
- haemoglobin (NOT myoglobin)
- various enzymes
- various gene-regulating proteins
Allosteric regulation
Example: Phosphofructokinase and
ATP
Substrate: Fructose-6-phosphate
Reaction
fructose-6-phosphate + ATP fructose-1,6-bisphosphate +
ADP
phosphofructokinase
2008 Paul Billiet ODWS
ATP is the end point
This reaction lies near the beginning of the
respiration pathway in cells
The end product of respiration is ATP
If there is a lot of ATP in the cell this
enzyme is inhibited
Respiration slows down and less ATP is
produced
As ATP is used up the inhibition stops and
the reaction speeds up again
2008 Paul Billiet ODWS
The switch: Allosteric
inhibition
Allosteric means other
site
E
Active site
Allosteric
site
2008 Paul Billiet ODWS
Switching off
These enzymes
have two
receptor sites
One site fits the
substrate like
other enzymes
The other site fits
an inhibitor
molecule
Inhibitor fits
into allosteric
site
Substrate
cannot fit
into the
active site
Inhibitor
molecule
2008 Paul Billiet ODWS
The allosteric site the enzyme
on-off switch
E
Active
site
Allosteric
site empty
Substrate
fits into
the active
site
The
inhibitor
molecule is
absent
Conformational
change
Inhibitor fits
into
allosteric
site
Substrate
cannot fit
into the
active
site
Inhibitor
molecule
is
present
E
2008 Paul Billiet ODWS
A change in shape
When the inhibitor is present it fits into its
site and there is a conformational
change in the enzyme molecule
The enzymes molecular shape changes
The active site of the substrate changes
The substrate cannot bind with the
substrate
2008 Paul Billiet ODWS
You might also like
- Lipids: CH403 Gayod, JiannaDocument3 pagesLipids: CH403 Gayod, JiannaCASTER JOSH RAMOSNo ratings yet
- Letter of TransmittalDocument9 pagesLetter of TransmittalTuhin KhanNo ratings yet
- Religion As GlobaizationDocument7 pagesReligion As Globaizationmae cadamusNo ratings yet
- Outline: Prepared by Andrea D. LeonardDocument57 pagesOutline: Prepared by Andrea D. LeonardGion LuisNo ratings yet
- Enzymes: OutlineDocument10 pagesEnzymes: OutlineManila MedNo ratings yet
- Lesson 1 Carboxylic AcidsDocument5 pagesLesson 1 Carboxylic AcidsMARY JANE ANGELICA SEVANo ratings yet
- Folic AcidDocument4 pagesFolic AcidBal Ri MekoleuNo ratings yet
- Bio Chemistry: Core Unit #1 - Review and IntroductionDocument28 pagesBio Chemistry: Core Unit #1 - Review and Introduction0921pyNo ratings yet
- Classification of Protein Based On CompositionDocument4 pagesClassification of Protein Based On CompositionHambaliNo ratings yet
- Terpenes and Terpenoids: Principles of Biochemistry BIOC-301 Dr. Gull-E-FaranDocument10 pagesTerpenes and Terpenoids: Principles of Biochemistry BIOC-301 Dr. Gull-E-FaranFahad NaeemNo ratings yet
- Glyoxylate CycleDocument14 pagesGlyoxylate CycleUtkarsh SharmaNo ratings yet
- Biology OxidationDocument50 pagesBiology Oxidationderhangker100% (2)
- Vitamin C: World's Healthiest Foods Rich in VitaminDocument12 pagesVitamin C: World's Healthiest Foods Rich in VitaminGusti Arya YunediNo ratings yet
- Lipid Chemistry: BiochemistryDocument14 pagesLipid Chemistry: BiochemistryManila Med0% (1)
- The role and importance of coenzymes in metabolismDocument14 pagesThe role and importance of coenzymes in metabolismsmollorenaNo ratings yet
- Denaturation of ProteinsDocument32 pagesDenaturation of ProteinsAhmad KhanNo ratings yet
- Intro To Metabo (IsmDocument8 pagesIntro To Metabo (IsmManila MedNo ratings yet
- Solution Brown Solution: Sample Used Time Oxidized Apple Banana Potato GuavaDocument7 pagesSolution Brown Solution: Sample Used Time Oxidized Apple Banana Potato GuavaLaelannie MagpayoNo ratings yet
- Absorption of LipidsDocument22 pagesAbsorption of LipidsEmm Noman100% (1)
- Amino Acids and Proteins ExplainedDocument17 pagesAmino Acids and Proteins ExplainedAbhijat Jha100% (1)
- Lipid Absorption and Transport UptakeDocument218 pagesLipid Absorption and Transport UptakeSimra Zahid100% (1)
- Chemistryofproteinswithclinicalapplications 190621192525 PDFDocument181 pagesChemistryofproteinswithclinicalapplications 190621192525 PDFAl-waleed Julkanain100% (1)
- Importance of proteins in living organisms (40 charactersDocument2 pagesImportance of proteins in living organisms (40 charactersChris Wills0% (1)
- Chapter17 Lipids Textbook PowerpointsDocument56 pagesChapter17 Lipids Textbook PowerpointsqaisarNo ratings yet
- Biomolecules: Biomolecules, Polymers, Chemistry in Everyday Life & Env. ChemistryDocument16 pagesBiomolecules: Biomolecules, Polymers, Chemistry in Everyday Life & Env. ChemistryIshanNo ratings yet
- SOM 201 Nutrients Notes 2021Document26 pagesSOM 201 Nutrients Notes 2021kxng crocked100% (1)
- Vitamin D: World's Healthiest Foods Rich in VitaminDocument13 pagesVitamin D: World's Healthiest Foods Rich in VitaminGusti Arya YunediNo ratings yet
- Protein Denaturation Factors and Nutritional PropertiesDocument4 pagesProtein Denaturation Factors and Nutritional PropertiesBOR KIPLANGAT ISAACNo ratings yet
- Fatty Acid Synthesis 11.12.19Document18 pagesFatty Acid Synthesis 11.12.19Sanreet Randhawa100% (1)
- Chapter 2 - Metabolism & Bioenergetics (Part 2) PDFDocument69 pagesChapter 2 - Metabolism & Bioenergetics (Part 2) PDFdarren100% (1)
- Glycogenolysis Basic PDFDocument25 pagesGlycogenolysis Basic PDFSamuel Morales Navarro100% (1)
- s15 Miller Chap 3b LectureDocument25 pagess15 Miller Chap 3b LectureDorice Clement100% (1)
- Human Skin Color - Evidence For Selection Activity Student HandoutDocument7 pagesHuman Skin Color - Evidence For Selection Activity Student HandoutKalina DimovNo ratings yet
- Carbohydrates: Definitions, Classification and PropertiesDocument6 pagesCarbohydrates: Definitions, Classification and PropertiesNarasimha MurthyNo ratings yet
- Biology Notes (Proteins)Document9 pagesBiology Notes (Proteins)Teo Jia Ming Nickolas100% (1)
- Haemoglobin: DR Nilesh Kate MBBS, MD Associate ProfDocument31 pagesHaemoglobin: DR Nilesh Kate MBBS, MD Associate ProfMarcellia100% (1)
- LEC18 MembraneLipids 08Document12 pagesLEC18 MembraneLipids 08Mây Chính ChủNo ratings yet
- Vitamin E: World's Healthiest Foods Rich in VitaminDocument12 pagesVitamin E: World's Healthiest Foods Rich in VitaminGusti Arya YunediNo ratings yet
- Lecture 25Document7 pagesLecture 25Vatshalla100% (1)
- 2114 Biochemistry and Clinical PathologyDocument32 pages2114 Biochemistry and Clinical PathologyMadhuri poulkar100% (1)
- Amino AcidsDocument32 pagesAmino AcidsStephen Leonel100% (1)
- 2.1 Molecules To Metabolism-STUDENTDocument54 pages2.1 Molecules To Metabolism-STUDENTAngel Alexandra SiregarNo ratings yet
- The Hardy-Weinberg Equation ExplainedDocument16 pagesThe Hardy-Weinberg Equation ExplainedLya MNo ratings yet
- HMP-shunt MEDDocument37 pagesHMP-shunt MEDAboubakar Moalim Mahad moh'dNo ratings yet
- Hexose Mono Phosphate (HMP) ShuntDocument28 pagesHexose Mono Phosphate (HMP) ShuntVishesh JainNo ratings yet
- Biochemistry - C4 Proteins Determination of Primary StructureDocument2 pagesBiochemistry - C4 Proteins Determination of Primary StructureKim LlamasNo ratings yet
- Qualitative Analysis of Amino Acids and ProteinsDocument21 pagesQualitative Analysis of Amino Acids and ProteinsJoshua AbelgasNo ratings yet
- Carbohydrate MetabolismDocument30 pagesCarbohydrate MetabolismAndraKamee100% (1)
- HtwoO and BufferDocument7 pagesHtwoO and BufferManila MedNo ratings yet
- General Protein MetabolismDocument72 pagesGeneral Protein MetabolismHafizie SyahmanNo ratings yet
- Vitamina D3Document10 pagesVitamina D3Adina ElenaNo ratings yet
- 3 LipidsDocument30 pages3 LipidsElena DalcaranNo ratings yet
- Lesson 10Document13 pagesLesson 10armin509No ratings yet
- Carbohydrates, Lipids & Nucleic Acids:: Forms Chain Like Molecules-PolymersDocument8 pagesCarbohydrates, Lipids & Nucleic Acids:: Forms Chain Like Molecules-PolymersJojo LouNo ratings yet
- Vitamin D (Calcitriol)Document4 pagesVitamin D (Calcitriol)goremicNo ratings yet
- 1.05 Biochemistry Trans - Coenzyme. Cofactors. Prosthetic Grps TRANS v2Document12 pages1.05 Biochemistry Trans - Coenzyme. Cofactors. Prosthetic Grps TRANS v2April AramNo ratings yet
- Sources and Applications of Carbohydrates.Document10 pagesSources and Applications of Carbohydrates.Narges Malik100% (1)
- Integration of Metabolism PathwaysDocument68 pagesIntegration of Metabolism PathwaysCahyani Tiara Safitri100% (1)
- Enzyme2 2012Document45 pagesEnzyme2 2012Surya Prakash KabiNo ratings yet
- IIPE Class 4 24.03Document41 pagesIIPE Class 4 24.03bharatNo ratings yet
- Micro ControllersDocument25 pagesMicro Controllershimanshu_agraNo ratings yet
- Novice's Guide To AVR DevelopmentDocument5 pagesNovice's Guide To AVR Developmentdeivs001100% (1)
- Max 232Document17 pagesMax 232Rohan KelkarNo ratings yet
- RF Module Quick Mannua v3Document3 pagesRF Module Quick Mannua v3himanshu_agraNo ratings yet
- Installing USBTINY Programmer in WindowsDocument3 pagesInstalling USBTINY Programmer in Windowshimanshu_agraNo ratings yet
- BS 4Document95 pagesBS 4himanshu_agraNo ratings yet
- Avr Aswarm v1.2 SchematicDocument1 pageAvr Aswarm v1.2 Schematichimanshu_agraNo ratings yet
- How To Start With AVR Series Micro ControllersDocument9 pagesHow To Start With AVR Series Micro ControllersAditya KamathNo ratings yet
- R-Sen Lecture 01bDocument57 pagesR-Sen Lecture 01bhimanshu_agraNo ratings yet
- L293DDocument7 pagesL293Dapi-370080950% (2)
- Science of LIVING SYSTEM AB (Part of Unit 2)Document46 pagesScience of LIVING SYSTEM AB (Part of Unit 2)himanshu_agraNo ratings yet
- Atmega 16Document357 pagesAtmega 16Edy 'nye' IrawanNo ratings yet
- R-Sen Lecture 01aDocument28 pagesR-Sen Lecture 01ahimanshu_agraNo ratings yet
- Science of Living Systems: Bio-Thermal-Fluid Sciences: Lecture by Prof. Soumen DasDocument36 pagesScience of Living Systems: Bio-Thermal-Fluid Sciences: Lecture by Prof. Soumen Dashimanshu_agraNo ratings yet
- Motifs of Protein StructureDocument41 pagesMotifs of Protein Structurehimanshu_agraNo ratings yet
- Bs18-1SC Kundu - Cell Division, Cell Cycle & Apoptosis (Two Lectures For Sci of Liv Sys-Autumn 2011)Document35 pagesBs18-1SC Kundu - Cell Division, Cell Cycle & Apoptosis (Two Lectures For Sci of Liv Sys-Autumn 2011)Akshat Kumar AgarwalNo ratings yet
- Evs - Soil PollutionDocument47 pagesEvs - Soil Pollutionhimanshu_agraNo ratings yet
- New 2012 04 Rabibrata Bio TransportDocument66 pagesNew 2012 04 Rabibrata Bio Transporthimanshu_agraNo ratings yet
- Bioscience: Protein Structure Function BS20001 Section 2Document27 pagesBioscience: Protein Structure Function BS20001 Section 2himanshu_agraNo ratings yet
- Waste Minimization and Cleaner ProductionDocument75 pagesWaste Minimization and Cleaner Productionhimanshu_agra100% (2)
- Science of Living SystemsDocument52 pagesScience of Living Systemshimanshu_agraNo ratings yet
- Water Treatment 25-01-2012Document57 pagesWater Treatment 25-01-2012himanshu_agra100% (1)
- Cell StructureDocument17 pagesCell StructureSoumyadeep MajumdarNo ratings yet
- Soild Waste AmangementDocument75 pagesSoild Waste Amangementhimanshu_agraNo ratings yet
- Photosynthesis: Prof. S. Dutta GuptaDocument41 pagesPhotosynthesis: Prof. S. Dutta Guptahimanshu_agraNo ratings yet
- Noise PolutionDocument48 pagesNoise Polutionhimanshu_agraNo ratings yet
- Eukariotic Cell StructureDocument26 pagesEukariotic Cell Structurehimanshu_agra100% (1)
- Water Pollution18!01!2012Document41 pagesWater Pollution18!01!2012himanshu_agraNo ratings yet
- Wastewater Treatment: Department of Civil Engineering Indian Institute of Technology KharagpurDocument26 pagesWastewater Treatment: Department of Civil Engineering Indian Institute of Technology Kharagpurhimanshu_agraNo ratings yet
- Under Water WeldingDocument23 pagesUnder Water WeldingNishanth GowdaNo ratings yet
- Structural Theory Eval Exam by SorianoDocument6 pagesStructural Theory Eval Exam by SorianoBenjie MorenoNo ratings yet
- Hot Bolting FPSO BrazilDocument1 pageHot Bolting FPSO BrazilKhan Arshi100% (1)
- AIR Modeller 75 2017-12-20 - 01Document68 pagesAIR Modeller 75 2017-12-20 - 01JoãoGilbertoAraújoPontes100% (4)
- Nitobond EP 0608Document2 pagesNitobond EP 0608James PittsNo ratings yet
- Next Gen Ford Ranger Digital BrochureDocument10 pagesNext Gen Ford Ranger Digital BrochureTri BureauNo ratings yet
- Fire Drencher System - Base-Engineer PDFDocument2 pagesFire Drencher System - Base-Engineer PDFpequenita34100% (1)
- PalindromeDocument7 pagesPalindromeZy AdrianneNo ratings yet
- Boiler MaintenanceDocument144 pagesBoiler Maintenanceaziz100% (2)
- Implementing A Maintenance Strategic Plan Using TPM MethodologyDocument13 pagesImplementing A Maintenance Strategic Plan Using TPM MethodologyJeyson Lendro ParedesNo ratings yet
- Vernier, Dial, and Electronic Digital Calipers: Session 3Document40 pagesVernier, Dial, and Electronic Digital Calipers: Session 3Emman Bosito100% (1)
- Elective-II: Pavement Analysis & Design: B.E. (Civil Engineering) Eighth Semester (C.B.S.)Document6 pagesElective-II: Pavement Analysis & Design: B.E. (Civil Engineering) Eighth Semester (C.B.S.)Adesh DeshbhratarNo ratings yet
- Strahlenfolter Stalking - TI - Baker - UK Targeted Individuals Activism & Safety Watch - February 2013 - UktargetedindividualsDocument3 pagesStrahlenfolter Stalking - TI - Baker - UK Targeted Individuals Activism & Safety Watch - February 2013 - UktargetedindividualsKarl-Hans-RohnNo ratings yet
- Isolation of Caffeine from TeaDocument6 pagesIsolation of Caffeine from TeaDaisy Joyce Seroje BuslonNo ratings yet
- A Practical Introductory Guide On Using Satellite Technology For CommunicationsDocument15 pagesA Practical Introductory Guide On Using Satellite Technology For CommunicationsJohan PrinslooNo ratings yet
- EA 4b ArchiMate Views and Viewpoints PDFDocument41 pagesEA 4b ArchiMate Views and Viewpoints PDFanon_834023132No ratings yet
- 4.failure Theories and Stress ConcentrationsDocument21 pages4.failure Theories and Stress ConcentrationsAmr El SaeedNo ratings yet
- Gas Sensors: Jiturvi Chokshi ENPM-808BDocument27 pagesGas Sensors: Jiturvi Chokshi ENPM-808Banon_44955929No ratings yet
- 1.1 Testing of PPE For Eye and Face Protection FPDocument6 pages1.1 Testing of PPE For Eye and Face Protection FPWalter PossoNo ratings yet
- Canusa GTS - 3LPEDocument2 pagesCanusa GTS - 3LPEarifin rizalNo ratings yet
- Solidworks SyllabusDocument7 pagesSolidworks SyllabusArun SubramanianNo ratings yet
- Gilding Manual PDFDocument14 pagesGilding Manual PDFIva VazNo ratings yet
- (1)Document119 pages(1)Virginia Rosales OlmosNo ratings yet
- Fall Protection Marking GuidelinesDocument2 pagesFall Protection Marking GuidelinescuervohijoguachoNo ratings yet
- Section11 Proportional ValvesDocument52 pagesSection11 Proportional ValvesyogitatanavadeNo ratings yet
- High Build Epoxy Coating for Hulls and Ballast TanksDocument3 pagesHigh Build Epoxy Coating for Hulls and Ballast Tankskasosei0% (1)
- ASTM E92-17 Standard Test Methods For Vickers Hardness and Knoop Hardness of Metallic MaterialsDocument27 pagesASTM E92-17 Standard Test Methods For Vickers Hardness and Knoop Hardness of Metallic MaterialsCarlos Pinto Pradilla88% (8)
- Twice As Sharp Operators ManualDocument34 pagesTwice As Sharp Operators ManualLeonardo CHTZNo ratings yet
- d-Copia3500MF 4500MF 5500MFsmY113351-4Document1,051 pagesd-Copia3500MF 4500MF 5500MFsmY113351-4ctecisbNo ratings yet
- Vacuum Chill BlockDocument2 pagesVacuum Chill BlockAditheya Varthan MNo ratings yet