Professional Documents
Culture Documents
ICS - 1st Le
Uploaded by
Abigail Mayled LausOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ICS - 1st Le
Uploaded by
Abigail Mayled LausCopyright:
Available Formats
ICS Abnormal signs and symptoms: Progressive pain right calf; about 5 day, as vague, intermittent pain aggravated
by movement and walking; 2 days PTC: cellulitis, erythema and tenderness, fever and chills Abnormal elements of Patient history: 30 years of DM, HPN and ischemic heart disease Abnormal PE findings: BP 160/100 high (120/80) HR 105/min high (60-100) RR 22/min high (12-18) Temp 38.9 high general: Obese, no ARDS HEENT diabetic retinopathy chronic compli of Dm Heart sounds bounding ( increased stroke volume aortic insufficiency and arterial stiffness, occur with aging) Apex beat left 5th ICS anterior axillary line (normal left 5th ICS MCL): LV and RV enlargement, CHF, Valvular dse. Abnormal Lab results: HB 165 (N: 120-160) HCT 48% (36 48%) WBC 16500 ( 4000-11000) PLT 350000 (200k-400k) neuto 85 (55-60) Lym 10 (20-30) Mono 3-6 Eo 1 FBS 216 / 12mmol (N: <100; Impaired: 100-125; DM: >125) Prothrombin time normal (12-14 secs) D-dimer: 650 ng/ml (for the presence of thrombus: used in dx of DVT, PE and stroke) (positive: (+) fibrin degradation products; NV - <0.4 ug/ml) Chest x-ray: tortuous (twisted) and atherosclerotic aorta; LV enlargment Doppler ultrasound intraluminal lesion in popliteal vein. (with the use of sound waves, it evaluates the blood flow within a vessel) Pathologic process: Disorders of Inflammation, fluid and hemodyanmics: 1. Redness (Rubor) Due to vasodilatation Mediated by prostaglandins like PGE and prostacyclin, resulting in hyperaemia
Also due to stimulation of pain-sensitive nerve endings by prostaglandins and bradykinin
5.
Loss of Function of the Affected Area (Functio Laesa) Due to pain and immobility E.g. in tonsillitis it is difficult to swallow because it is painful (motion of swallowing is limiting) A. Vasoactive changes in calibre and flow 1. A brief period of vasoconstriction followed by vasodilatation leading to increased blood flow 2. The increased blood flow leads to increased hydrostatic pressure in capillaries and venules 3. As a result, fluid will be driven from the intravascular to the extravascular compartment, forming transudate. The loss of fluid and increased vessel diameter lead to slower blood flow, concentration of red cells in small vessels, and increased blood viscosity. These changes result in stasis, which is seen as vascular congestion. As stasis develops, blood leukocytes (principally neutrophils) accumulate along the vascular endothelium. This results in the adhesion of leukocytes to the endothelium. Afterwards, they migrate through the vascular wall, into the interstitial tissue. B. Increased capillary permeability Occurs through several mechanisms: Endothelial cell contraction o Most common mechanism of intravascular leakage o Also called the immediate transient response, since it occurs rapidly after exposure to the mediator, and is usually reversible and short-lived Direct endothelial injury o Leakage starts immediately after injury and is sustained at a high level for several hours until the dammaged vessels are thrombosed or repaired o Also known as the immediate sustained response o Delayed prolonged leakage/response in some forms of mild injury (e.g. sunburn, type IV hypersensitivity reaction), vascular leakage begins after a delay of 2 to 12 hours, and lasts for several hours to days Leukocyte-mediated endothelial injury via release of toxic oxygen species and proteolytic enzymes Increased Transcytosis due to increased transport of fluids and proteins C. Increased vascular permeability leading to exudate formation The oncotic pressure within the blood vessels is decreased the oncotic pressure outside the blood vessels increased
2.
Swelling (Tumor) Due to Increased vascular permeability Mediated by vasoactive amines (histamine, serotonin), C3a, C5a, bradykinin, leukotrienes C4, D4, and E4 and Platelet-activating Factor (PAF) Results in edema manifested in the form of swelling Heat/warmth (Calor) Due to increased blood flow (hyperaemia) Pain (Dolor) Due to increased pressure exerted by edema
3.
4.
the combination of increased intravascular hydrostatic pressure and increase in the extravascular oncotic pressure will create the production of exudates (protein rich fluid)
1.
Inc. vascular permeability
Hemoconcentration decreased intravenous osmotic pressure (increased hydrostatic pressure due to vasodilatation)
Increased blood Viscosity Stasis (slow blood flow) Disrupts normal axial blood flow cellular events of inflammation
edema or swelling 2.
Fever Due to pyrogens that stimulate prostaglandin synthesis in vascular and perivascular cells of the hypothalamus Exogenous pyrogens (bacterial products such as LPS) stimulate leukocytes to release endogenous pyrogens cytokines (TNF & IL-1) increases enzyme cyclooxygenase that converts amino acid into Prostaglandins In the hypothalamus, prostaglandins (esp. PG2) stimulate production of neurotransmitters (cAMP) reset temperature set point at a higher level. Changes in the peripheral blood a) Leukocytosis o count of above 10k per cubic mm o Normal 5k-10k per cubic mm Due to colony stimulating factors that stimulate leukocyte from bone marrow pool Differs from leukemoid reaction - count of above 100k per cubic mm; resemble leukemia; no blast cells shift to the left appearance of immature forms in the differential count Neutrophilia - indicative of bacterial infection Absolute lymphocytosis (I.M., mumps, German measles) Eosinophilia (bronchial asthma, hay fever, parasitic infestation) b) Leukopenia o Decreased WBC count o Below 5k o Seen in typhoid fever, viral, rickettsia, protozoan infection Increased acute phase proteins o C-Reactive Protein o Fibrinogen o serum amyloid A protein (SAA) Elevated serum levels of CRP have been proposed as a marker for increased risk of MI in patients with coronary artery disease Fibrinogen binds to red blood cells and cause them to form stacks (rouleaux) that sediment more. Prolonged production of SAA causes secondary amyloidosis Chronically elevated plasma concentrations of hepcidin reduce the availability of iron anemia Increased blood coagulability Sticky due to platelet, coagulation factors Increased pulse and blood pressure Decreased sweating Due to redirection of blood flow from cutaneous to deep vascular beds, to minimize heat loss through the skin Rigors (shivering), chills (search for warmth), anorexia, somnolence, and malaise
*Normal axial blood flow is when the cellular component of blood occupies the central portion of the blood stream while the fluid component of blood occupies the periphery of the blood stream. This is disrupted during stasis; wherein the cell components now occupy the periphery. Note: Injurious stimulus vascular events cellular events
Role of Inflammation Vasodilation Mediators Prostaglandins Nitric Oxide Histamine Histamine & serotonin (Vasoactive amines) C3a & C5a (by liberating vasoactive amines from mast cells, other cells) Bradykinin Leukotrienes C4, D4, E4 PAF (platelet activation factor) Substance P TNF, IL-1 Chemokines C3a, C5a Leukotriene B4 (Bacterial products, e.g. Nformyl methyl peptides) IL-1, TNF Prostaglandins Prostaglandins Bradykinin Lysosomal enzymes of leukocytes Reactive oxygen species Nitric oxide
Increased vascular permeability
3.
Chemotaxis, leukocyte recruitment and activation
Fever Pain Tissue damage
4. 5. 6.
Acute Phase Responses or Systemic Inflammatory Response Syndrome (SIRS) 7.
8.
Probably due to the actions of cytokines on brain cells
Sepsis Presence of large amounts of bacterial toxins in the blood Septic shock (triad of disseminated intravascular coagulopathy widespread thrombosis, hypoglycemia and cardiovascular system failure) Multiple organ failure Normal fluid homeostasis is maintained by: 1. Endothelial / vessel wall integrity - Endothelial wall must be free of trauma - The normal blood vessel is fenestrated or interrupted - When the endothelial wall is disrupted, there is a great volume of water that may go out of the blood vessel resulting to edema Intravascular pressure -Function of hydrostatic pressure - The major pressure that allows the exit of water at the arterial end Plasmaosmolarity - Function of osmotic pressure - Predominant plasma protein contributing to plasma osmolarity is albumin -The pressure that tends to draw fluid back to the intravascular space
Due to increased vascular permeability: damage in the endothelium as a result of an active inflammatory process causes fluid to escape into the interstitial space Fluid (exudate) has high inflammatory specific gravity because cells and proteins leak out with the fluid Can be caused by infection, vasculitis Also known as EXUDATIVE EDEMA Protein-rich fluid because of altered permeability of endothelial cells Fibrin-rich fluid Inflammatory cells are typical Specific gravity > 1.020 Usually a localized process
---
2.
3.
NON-INFLAMMATORY Due to changes in hemodynamic forces intravascularly Represents an interplay between changes in hydrostatic pressure or osmotic pressure which leads to outflow of fluid Non-inflammatory fluid (transudate) has a low specific gravity because only the fluid moves out Also known as TRANSUDATIVE EDEMA Lower protein content because of no alteration in endothelial permeability No fibrin in fluid No inflammatory cells in fluid Specific gravity < 1.012 Often a generalized process PATHOPHYSIOLOGIC (MECHANISMS) CATEGORIES OF EDEMA 1. 2. 3. 4. 5. 6. Increased hydrostatic pressure - due to increased venous return Reduced plasma osmotic pressuredue to low serum albumin Lymphatic obstruction the reabsorbing capacity of the lymphatic channel is removed Sodium retention causes hypervolemia, leading to increased hydrostatic pressure Inflammation Leaky vessels
TWO TYPES OF FORCES THAT DRIVE THE NORMAL FLUID EXCHANGE: 1. Hydrostatic pressure - Function of the arteriolar pressure - Affected by: cardiac output, vessel wall elasticity, vascular tone, and blood volume. 2. Oncotic or osmotic pressure - Function of serum albumin - The differential osmotic force is maintained by the higher protein level in the blood - The most important force that promotes reabsorption of water from the interstitium back to the intravascular space
EDEMA Increased accumulation of fluid in the interstitium and body cavities May also be caused by low serum albumin, in which the reabsorbing power of the bloodvessel into the circulation is low Fluid tends to accumulate in very loose connective tissues; usually starts in the periorbital area and in the dependent portions of the body Should be differentiated from CLOUDY SWELLING wherein water accumulates inside the cell Inflammatory vs. Non- inflammatory INFLAMMATORY
-HEMORRHAGE PATHOGENESIS Trauma, laceration Atherosclerosisdamage of endothelial linings along with fat deposition on endothelial wall 3. Inflammatory- weakening of blood vessels potential to rupture 4. Neoplastic erosion of blood vessel- problem with clotting network 5. Hemorrhagic diathesis - there is an increased tendency to hemorrhage from usually insignificant injury bleeding tendencies/clotting anomaly (hemophiliacs) 1. 2.
Thrombus 1. Simply put, a blood clot. Derived from blood elements: vessel wall particularly the endothelium, platelets and clotting factors A.K.A antemortem clot (formed before death) Predisposing Factors/Virchows Triad
2.
3.
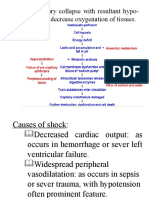
Injury to endothelium Major cause of thrombosis in acute myocardial infarction (AMI), atherosclerosis, cigarette smoking (nicotine is endotheliotoxic), vasculitis (inflammation of the vessel wall), hypertension, hypercholesterolemia. This factor is particularly important in thrombosis in the heart or in arteries because in such areas, there is high flow rate of blood that prevent platelet adhesion and wash out active coagulation factors. However, imbalance in the production of pro- and anticoagulation substances may also lead to thrombosis. Alteration in normal blood flow (Stasis/Turbulence) Stasis, aneurysm (abnormal dilatation of blood vessel wall), valvular stenosis/regurgitation, vascular obstructions. Turbulence: contributes to arterial and cardiac thrombosis by injuring the vessel wall and by forming countercurrents Stasis: major contributor in venous thrombosis Laminar flow is disrupted, so there is increase contact of platelets with wall; no washout and dilution of active clotting factors Hypercoagulability/thrombophilia Immobilization, malignancy (pancreatic CA: release of TF-like substances), APAS (Antiplatelet antibody syndrome), DIC, nephritic syndrome, oral contraceptive use. Any alteration of the coagulation cascade that predisposes to thrombosis Less frequent contributor, but may predominate sometimes. May be primary (genetic) or secondary (acquired) EMBOLISM Process by which a detached intravascular solid, liquid or gaseous mass (embolus) is carried via blood stream to a site distant from its point of origin. Unless otherwise specified, emboli should be considered thrombotic in origin. INFARCTION An area of ischemic necrosis of organ/tissue as a result of arterial or venous occlusion SHOCK Final common pathway for potentially lethal clinical events like severe hemorrhage, extensive trauma or burns, large MI, massive PTE, and microbial sepsis.
Characteristic: systemic hypotension due to reduction of CO or effective circulating blood volume. Outcome of the characteristic: impaired tissue perfusion and cellular hypoxia Produces hypotension, anoxia, and cellular death. 1. Septic elaboration of toxins by bacteria (endotoxemia) peripheral vasodilation leading to pooling of blood (reduced venous return) reduced CO Septic shock: factors contributing to its pathophysiology Inflammatory mediators: massive release triggered by microbial cell wall constituents; result in activation of endothelial cells Endothelial cell activation and injury: leads to thrombosis (may lead to DIC), increased vascular permeability, and vasodilation. Microthrombi form all over the body. Edema is present. NO synthetase expression by endothelium is increased, releasing more NO, causing vasodilation. Metabolic abnormalities: insulin resistance due to proinflammatory cytokines that impair expression of GLUT-4; presence of hyperglycemia Immune suppression: hyperinflammatory state triggers counter-regulatory immununosuppressive mechanisms. Organ dysfunction: end result -DEEP VENOUS THROMBOSIS Conditions that favour development of DVT: - stasis (CHF) - injury and inflammation infection -hypercoagulability - advanced age -sickle cell disease Most of the venous thrombi begin in calf veins, frequently in the sinuses above the venous valves. Homan sign claf tenderness often associated with forced dorsiflexion of foot. Risk factors: -sepsis -advanced age -CHF -obesity Presentation: -pain, swelling, (+) homan sign,redness, fever, tachycardia Cellulitis VS DVT Temperature Cellulitis > DVT Lymphadenopathy (+) in cellulitis Skin color DVT is normal or cyanotic; Red in cellulitis Potential fates of venous thrombi -lysis -organization canalization -propagation venous thrombi serve as nidus for further thrombi -embolization
You might also like
- Medicine in Brief: Name the Disease in Haiku, Tanka and ArtFrom EverandMedicine in Brief: Name the Disease in Haiku, Tanka and ArtRating: 5 out of 5 stars5/5 (1)
- 4th Auguest 2016 Fluid and Hemodynamic Disorders 2011Document81 pages4th Auguest 2016 Fluid and Hemodynamic Disorders 2011Majkel Benche Custodio MllNo ratings yet
- Principles of Managent of Hypovolemic Shock in ADocument43 pagesPrinciples of Managent of Hypovolemic Shock in Aasi basseyNo ratings yet
- Multi-Organ Dysfunction Syndrome Lesson Description - Mitch TaylorDocument34 pagesMulti-Organ Dysfunction Syndrome Lesson Description - Mitch TaylorMuthu RajathiNo ratings yet
- 37 - Shock in ObstetricsDocument22 pages37 - Shock in Obstetricsdr_asaleh90% (10)
- ShockDocument34 pagesShockeman shNo ratings yet
- Hemodynamic Disturbance: Dr. Usha.MDocument77 pagesHemodynamic Disturbance: Dr. Usha.MOlumide Omotola AjayiNo ratings yet
- ShockDocument19 pagesShockirenekhatete1No ratings yet
- Shock and Its Nursing InterventionsDocument3 pagesShock and Its Nursing InterventionsWendy EscalanteNo ratings yet
- Types of Shock TableDocument6 pagesTypes of Shock TableTP RMadNo ratings yet
- Obstetric Shock 21.11.08Document53 pagesObstetric Shock 21.11.08Jabed Ahmed100% (1)
- Shock: Shock Can Refer To A Range of Related Medical Conditions in Which The Victim's Heart, Lungs andDocument16 pagesShock: Shock Can Refer To A Range of Related Medical Conditions in Which The Victim's Heart, Lungs andLucreatia RynjahNo ratings yet
- Hemodynamic Disorders, Thromboembolic Disease and ShockDocument13 pagesHemodynamic Disorders, Thromboembolic Disease and Shockpjcanero100% (5)
- SHOCKDocument35 pagesSHOCKsami azadNo ratings yet
- Shock and HemorrhageDocument29 pagesShock and HemorrhageDr djNo ratings yet
- HMRG &shock 2022-2023Document54 pagesHMRG &shock 2022-2023Asraa ThjeelNo ratings yet
- Pathophysiology of EdemaDocument7 pagesPathophysiology of EdemaTaniaNo ratings yet
- Module 3 A PresentationDocument79 pagesModule 3 A PresentationMelinda FiskaNo ratings yet
- Case Scenario CVDocument14 pagesCase Scenario CVjohnhenryvNo ratings yet
- Hemodynamic Disorders, Thrombosis, and Shock GWAIDocument102 pagesHemodynamic Disorders, Thrombosis, and Shock GWAIkavindukarunarathnaNo ratings yet
- 5 Hemodynamic Disorders, Thromboembolism and ShockDocument162 pages5 Hemodynamic Disorders, Thromboembolism and Shocksinte beyuNo ratings yet
- Shock, Sirs & ModsDocument37 pagesShock, Sirs & ModsambitioustamannaNo ratings yet
- Heart Failure & Circulatory ShockDocument34 pagesHeart Failure & Circulatory ShockMuskaan ZaharaNo ratings yet
- Module 3 A PresentationDocument79 pagesModule 3 A PresentationJesus William Arizapana MamaniNo ratings yet
- ShockDocument35 pagesShocktrip100No ratings yet
- Hemodynamics ModifiedDocument175 pagesHemodynamics ModifiedAyele AsefaNo ratings yet
- ReferenceDocument7 pagesReferenceShaker MUhammedNo ratings yet
- SHOCKDocument60 pagesSHOCKJoseph John K PothanikatNo ratings yet
- Stages of ShockDocument13 pagesStages of ShockA. P.No ratings yet
- Shock - Critical Care Medicine - MSD Manual Professional EditionDocument11 pagesShock - Critical Care Medicine - MSD Manual Professional Editionazaria zhafirahNo ratings yet
- Lecture 6-ShockDocument5 pagesLecture 6-ShockMadiha MadiNo ratings yet
- Lecture 10Document13 pagesLecture 10Grafu Andreea AlexandraNo ratings yet
- Shock: By: Dr. Samer Sabri M.B.CH.B F.I.C.M.SDocument34 pagesShock: By: Dr. Samer Sabri M.B.CH.B F.I.C.M.Ssamer falconNo ratings yet
- Shock 19Document7 pagesShock 19Teema UmarNo ratings yet
- Patient Evaluation ChartsDocument63 pagesPatient Evaluation Chartsulka07100% (1)
- Chapter 036. Edema: PathogenesisDocument5 pagesChapter 036. Edema: PathogenesisJane GutierrezNo ratings yet
- ShockDocument36 pagesShockJohnryan NdiranguNo ratings yet
- Shock NotesDocument7 pagesShock NotesAnitha NoronhaNo ratings yet
- Shock - Types Pathophysiology and Management: DR - Ravichandra Kumar Anaesthesia ResidentDocument64 pagesShock - Types Pathophysiology and Management: DR - Ravichandra Kumar Anaesthesia ResidentHarika BandaruNo ratings yet
- Shock - Critical Care Medicine - MSD Manual Professional EditionDocument8 pagesShock - Critical Care Medicine - MSD Manual Professional EditionSughosh MitraNo ratings yet
- Lecture 20 - Shock - 15 Oct 2006Document26 pagesLecture 20 - Shock - 15 Oct 2006api-3703352No ratings yet
- Hemodynamic Disturbances: Dr. Dhamyaa Al-RahalDocument74 pagesHemodynamic Disturbances: Dr. Dhamyaa Al-RahalAmmar Bany AtaNo ratings yet
- Lecture 5Document5 pagesLecture 5Isak ShatikaNo ratings yet
- Shock 12-12-12Document60 pagesShock 12-12-12Dilip Kumar MNo ratings yet
- Pathology+101 Complete)Document147 pagesPathology+101 Complete)Goh Kah Yong100% (2)
- Surgery: by DR - Mohammad Z. Abu Sheikha@Document145 pagesSurgery: by DR - Mohammad Z. Abu Sheikha@صقر حورانNo ratings yet
- SHOCK DiscussionDocument22 pagesSHOCK DiscussionNavpreet Kaur100% (1)
- Shock BBDocument31 pagesShock BBVirang ParikhNo ratings yet
- ShockDocument30 pagesShockvinnu kalyanNo ratings yet
- Background: EtiologyDocument5 pagesBackground: Etiologylia lykimNo ratings yet
- Peripheral Edema: ReviewDocument7 pagesPeripheral Edema: ReviewVmsdNo ratings yet
- Edema Hyperemia & Congestion: Miriam Al Battal, MDDocument20 pagesEdema Hyperemia & Congestion: Miriam Al Battal, MDHassoun hassounNo ratings yet
- In The Name of Allah, The Most Beneficent, The Most MercifulDocument40 pagesIn The Name of Allah, The Most Beneficent, The Most MercifulISMAILNo ratings yet
- 3 - Heart Failure (Modified)Document17 pages3 - Heart Failure (Modified)Ali Al-QudsiNo ratings yet
- Metabolism Study NotesDocument24 pagesMetabolism Study Notesxxx xNo ratings yet
- Hemodynamics - PDF 20 21Document172 pagesHemodynamics - PDF 20 21ayahnaser20No ratings yet
- Ischemia, Infarction, Shock and EdemaDocument44 pagesIschemia, Infarction, Shock and Edemakiran kcNo ratings yet
- Chapter (1) Blood VesselsDocument16 pagesChapter (1) Blood VesselsyuhazikrillahNo ratings yet
- DIAGNOSTICS (Student Copy)Document59 pagesDIAGNOSTICS (Student Copy)Abigail Mayled LausNo ratings yet
- Fungal and Viral ReplicationDocument22 pagesFungal and Viral ReplicationAbigail Mayled LausNo ratings yet
- Non Protein CompoundsDocument64 pagesNon Protein CompoundsAbigail Mayled LausNo ratings yet
- ParenteralDocument10 pagesParenteralAbigail Mayled LausNo ratings yet
- Powders For InjectionDocument11 pagesPowders For InjectionAbigail Mayled LausNo ratings yet
- How To Store Any File Into SQL DatabaseDocument15 pagesHow To Store Any File Into SQL DatabaseAbigail Mayled LausNo ratings yet
- Vaccines 09 01233 v2Document12 pagesVaccines 09 01233 v2Milica MilojevicNo ratings yet
- Vikash Homeo Research Compile Book For Homeopath To Promote Homeopath For AllDocument1,384 pagesVikash Homeo Research Compile Book For Homeopath To Promote Homeopath For AllvijaykakraNo ratings yet
- Elsevier Adaptive Quizzing - Quiz PerformanceDocument6 pagesElsevier Adaptive Quizzing - Quiz PerformanceBridget JuddNo ratings yet
- Acupuncture in Emergency Situations and The Treatment of PainDocument11 pagesAcupuncture in Emergency Situations and The Treatment of Painpedialyte88100% (9)
- Impaired Skin Integrity For NCP Oct. 212020Document2 pagesImpaired Skin Integrity For NCP Oct. 212020Benjie DimayacyacNo ratings yet
- Asuhan Gizi Pada CVDDocument38 pagesAsuhan Gizi Pada CVDEva MutiarasariNo ratings yet
- Duck ProductionDocument23 pagesDuck Productionokodi andrewNo ratings yet
- 216 Miracle Acupunture Points: ShavoshangDocument12 pages216 Miracle Acupunture Points: Shavoshangடைகர் தேவாNo ratings yet
- Assessing Childbearing WomenDocument24 pagesAssessing Childbearing WomenCrestyl Faye R. CagatanNo ratings yet
- NCPDocument6 pagesNCPKyla Carbonel100% (1)
- Inflamation AssignmentDocument59 pagesInflamation AssignmentAnonymous GRUjCVedhNo ratings yet
- Arterial HyperemyDocument10 pagesArterial HyperemyRohit NaiduNo ratings yet
- Etiology, Clinical Manifestations, and Diagnosis of Nephrotic Syndrome in Children - UpToDateDocument18 pagesEtiology, Clinical Manifestations, and Diagnosis of Nephrotic Syndrome in Children - UpToDateAngga Julyananda PradanaNo ratings yet
- Lecture 8, Nursing Care of The Child With Respiratory Dysfunction and Renal Disorders 2Document48 pagesLecture 8, Nursing Care of The Child With Respiratory Dysfunction and Renal Disorders 2مهند الرحيليNo ratings yet
- Adult-Onset Subgaleal Hematoma Caused by Hair Pulling: A Rare OccurrenceDocument2 pagesAdult-Onset Subgaleal Hematoma Caused by Hair Pulling: A Rare OccurrenceAisyah MoslemzzNo ratings yet
- Collecting Subjective DataDocument11 pagesCollecting Subjective DataKimberly Jane TogñoNo ratings yet
- Nimesil PDFDocument5 pagesNimesil PDFTemur LegendaryNo ratings yet
- Nursing Care Plan 1 DiagDocument4 pagesNursing Care Plan 1 Diagguysornngam100% (1)
- DEEP Vein ThrombosisDocument72 pagesDEEP Vein ThrombosisLevy Garcia SanchezNo ratings yet
- Caput SuccedaneumDocument2 pagesCaput SuccedaneumPoornima GopalNo ratings yet
- Fluid and Elec ImbalancesDocument19 pagesFluid and Elec ImbalancesswethashakiNo ratings yet
- Neuropathology PDFDocument205 pagesNeuropathology PDFNarendraNo ratings yet
- Care of Mother, Child, Adolescent: GoalsDocument8 pagesCare of Mother, Child, Adolescent: GoalschelseyNo ratings yet
- Overhydration Nursing Care Plan: Wong, Karl Michael PDocument8 pagesOverhydration Nursing Care Plan: Wong, Karl Michael PMikaela Wong50% (2)
- TOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Document25 pagesTOPNOTCH Patho Supplement Handout For Sept 2018 UPDATED May 2018Waiwit KritayakiranaNo ratings yet
- NCPDocument3 pagesNCPranee diane0% (1)
- ParasitesDocument22 pagesParasitesJames LeeNo ratings yet
- EdemaDocument27 pagesEdemarapadilNo ratings yet
- Case-Based Learning 2 Renal/Urinary System: Group CDocument56 pagesCase-Based Learning 2 Renal/Urinary System: Group CVARITPOL CHAROENYINGPAISALNo ratings yet
- Serrapeptase BenefitsDocument3 pagesSerrapeptase BenefitsF'Pro InfotechNo ratings yet