Professional Documents
Culture Documents
Chem 2
Uploaded by
Zting BongOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem 2
Uploaded by
Zting BongCopyright:
Available Formats
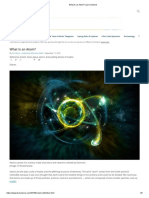
Structure of an Atomic Nucleus
Atoms are smallest possible parts or particles of matter. Inert gases or noble gases are the only elements that do not react and form molecules. Hence, atoms of such gases exist in an independent state. Atoms of these noble gases are deployed for research, often termed as nuclear physics. Atomic Nucleus Explained The word nucleus is derived from the Latin word 'nux' which means nut. The term was first used by renowned scientist Michel Faraday in 1844. He described the nucleus to be the 'center of the atom'. Atomic nucleus or rather nucleus is the central part of an atom. According to research, this part is highly electronically charged and is dense with matter. Protons and neutrons are included in the nucleus of the atom. Electrons, on the other hand, revolve around the nucleus. The nucleus of a hydrogen atom, that is supposed to amongst the smallest, is 1.6 fm (1.6 1015 m), while the nucleus of a uranium atom is 15 fm (15 1015 m). Structure of an Atomic Nucleus The atomic nucleus is primarily made up of two baryons, protons and neutrons. Protons and neutrons are bound within the nucleus with a charge known as nuclear force. Nuclear force is also sometimes referred to as residual strong force. Scientists studying nuclear physics have inferred that protons and neutrons are further made up of matter, known as 'quarks', also known as sub-atomic matter. Strongly bound quarks become baryons and are classified according to their charge, a positively charged baryon becomes a proton while a neutral charged baryon becomes a neutron. Atoms of some elements have a third baryon called the hyperon. This type of baryon does not exist on earth in its natural state. It can, however, be created, and is used in experiments of nuclear physics in a highly controlled environment. Residual Strong Force Residual strong force not only holds quarks together but also binds together protons and neurons within the nucleus. This residual force is so strong that it holds together all protons that have the same charge, even though they try to repel each other. During the process of nuclear fission, when the nucleus splits, this residual force is converted into heat energy. Nuclear Fission In spite of warnings given by early scientists who initiated research of atomic nuclei, scientists today perform what is known as nuclear fission. Nuclear fission is a nuclear reaction that splits parts of the nucleus into smaller parts, thereby completely destroying the original and actual structure of the atomic nucleus. This reaction produces many more free neutrons and also nuclei (plural for nucleus). The free neutrons and nuclei later on produce Gamma rays. This disintegration is the basis for the fission bomb, commonly referred to as the nuclear bomb. If used wisely, an atomic nucleus can be put to very good use like generation of electricity. However, if put to wrong use, like in nuclear weapons, it is even capable of wiping out the entire human race.
You might also like
- Your Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2From EverandYour Journey To The Basics Of Quantum Realm Volume II: Your Journey to The Basics Of Quantum Realm, #2Rating: 5 out of 5 stars5/5 (1)
- Nuclear Physics Project Class 12Document9 pagesNuclear Physics Project Class 12Rohan Chakraborty73% (15)
- Dalton's Atomic Theory Proposed That All Matter Was Composed of Atoms, IndivisibleDocument1 pageDalton's Atomic Theory Proposed That All Matter Was Composed of Atoms, Indivisiblemelgazar tanjayNo ratings yet
- Chem Research Structure of AtomDocument3 pagesChem Research Structure of AtomkhNo ratings yet
- Nuclear Physics Project Class 12Document9 pagesNuclear Physics Project Class 12Rishabh vermaNo ratings yet
- Early Description of MatterDocument24 pagesEarly Description of MatterAllea JeanNo ratings yet
- Rahul 2Document2 pagesRahul 2rahul3125No ratings yet
- CCST 9051 - 2021 - Lecture 6-SJXDocument41 pagesCCST 9051 - 2021 - Lecture 6-SJXhkuengg2021No ratings yet
- What Are Atoms and Their Basic PartsDocument3 pagesWhat Are Atoms and Their Basic PartsJos eNo ratings yet
- Structure of The Atom:: Standard Model of Particle PhysicsDocument88 pagesStructure of The Atom:: Standard Model of Particle PhysicsAngel BenganNo ratings yet
- Nuclear Physic By: Susi Susanti: HistoryDocument8 pagesNuclear Physic By: Susi Susanti: HistoryAldi CakepsNo ratings yet
- Manuscript Quarks and Leptons 2Document20 pagesManuscript Quarks and Leptons 2Nichole AlbaracinNo ratings yet
- Neutron, NeutralDocument5 pagesNeutron, NeutralIsabelle CandelariaNo ratings yet
- Nr. Phys Chapter OneDocument23 pagesNr. Phys Chapter Oneabdii100% (1)
- Atomic Structure EvolutionDocument2 pagesAtomic Structure EvolutionJessica BeasleyNo ratings yet
- Atoms and Nuclear Energy RevisionDocument5 pagesAtoms and Nuclear Energy RevisionLeona Valentina VračarNo ratings yet
- Nuclear PhysicsDocument8 pagesNuclear PhysicsPrithviraj DuttaNo ratings yet
- عائلة نيتروجينDocument9 pagesعائلة نيتروجينzahrakerim21No ratings yet
- Atomic Nucleus - Wikipedia PDFDocument47 pagesAtomic Nucleus - Wikipedia PDFaarti khedeNo ratings yet
- PHY-2251 (B) Physics - IV Nuclear Physics ChapterDocument20 pagesPHY-2251 (B) Physics - IV Nuclear Physics Chapterjoy bakshiNo ratings yet
- Particle Physics ResearchDocument1 pageParticle Physics ResearchLucky LifeNo ratings yet
- Nuclear Physics NotesDocument14 pagesNuclear Physics NotesJonathan ThomasNo ratings yet
- Lesson Plan On An AtomDocument6 pagesLesson Plan On An AtomAnonymous ZsTY4mkysTNo ratings yet
- Nuclear Science-A Guide To The Nuclear Science Wall Chart ©2003 Contemporary Physics Education Project (CPEP)Document4 pagesNuclear Science-A Guide To The Nuclear Science Wall Chart ©2003 Contemporary Physics Education Project (CPEP)Saurabh MishraNo ratings yet
- Electronic Structure of Atoms: Chemistry for AllFrom EverandElectronic Structure of Atoms: Chemistry for AllRating: 5 out of 5 stars5/5 (1)
- What Are NeutronsDocument3 pagesWhat Are NeutronshuesosNo ratings yet
- Definition and Composition of An Atom.Document3 pagesDefinition and Composition of An Atom.Habibu AbdullahiNo ratings yet
- Structure and Characteristics of ProtonDocument2 pagesStructure and Characteristics of ProtonElok SorayaNo ratings yet
- Chemistry (Atom and Subatomic Particles)Document7 pagesChemistry (Atom and Subatomic Particles)Meo Angelo AlcantaraNo ratings yet
- Nuclear PhysicsDocument6 pagesNuclear PhysicsEdgar Apaza HuallpaNo ratings yet
- Is Nuclear Energy Safe? -Nuclear Energy and Fission - Physics 7th Grade | Children's Physics BooksFrom EverandIs Nuclear Energy Safe? -Nuclear Energy and Fission - Physics 7th Grade | Children's Physics BooksNo ratings yet
- AtomDocument12 pagesAtomatgimale.comNo ratings yet
- NucleusDocument7 pagesNucleusaditya2053No ratings yet
- Physics Research PaperDocument11 pagesPhysics Research PapersamiullahNo ratings yet
- What Is An Atom - Live ScienceDocument9 pagesWhat Is An Atom - Live ScienceSheena Shane CantelaNo ratings yet
- Paper Assignment Modern Physics: "Atomic Stucture"Document9 pagesPaper Assignment Modern Physics: "Atomic Stucture"melianaeclsNo ratings yet
- Nuclear Chemistry With Pen 1106Document63 pagesNuclear Chemistry With Pen 1106JOHN DAVE MOISES BALDRIASNo ratings yet
- Structure of AtomDocument26 pagesStructure of AtomnitingaganNo ratings yet
- Ssci2 - Physical Science Lesson 1: Formation of Light and Heavy Elements in The UniverseDocument20 pagesSsci2 - Physical Science Lesson 1: Formation of Light and Heavy Elements in The UniverseMs. ArceñoNo ratings yet
- Atomic StructureDocument17 pagesAtomic StructureVandana Khator100% (1)
- General Science: Atomic PhysicsDocument24 pagesGeneral Science: Atomic PhysicshelperforeuNo ratings yet
- Atomic SructuresDocument2 pagesAtomic SructuresChinee FloresNo ratings yet
- Prepared By: Group 6 Brent Pili Andrew Roque Bryan BelirDocument18 pagesPrepared By: Group 6 Brent Pili Andrew Roque Bryan BelirJoice Ann A. PolinarNo ratings yet
- STEMGENPHYSICS2 - HO 5 Atomic and Nuclear PhenomenaDocument12 pagesSTEMGENPHYSICS2 - HO 5 Atomic and Nuclear PhenomenaKyle Cedric MitalNo ratings yet
- AtomDocument22 pagesAtomVinayKumarNo ratings yet
- Modern Physics Atom and Its StructureDocument13 pagesModern Physics Atom and Its StructureRAHUL CHOUDHARYNo ratings yet
- Atoms and ElementsDocument18 pagesAtoms and ElementsJACK CAMPBELLNo ratings yet
- Unit - 4 Atomic Structure - 7th STDDocument29 pagesUnit - 4 Atomic Structure - 7th STDthangamuthu baskarNo ratings yet
- Atoms and Subatomic Particles PptDocument28 pagesAtoms and Subatomic Particles PptCooleenNo ratings yet
- Nuclear PhysicsDocument10 pagesNuclear PhysicsShashank RaiNo ratings yet
- Material Chapter OneDocument13 pagesMaterial Chapter OneTeshale AlemieNo ratings yet
- The Indivisible Atom: Leucippus and DemocritusDocument42 pagesThe Indivisible Atom: Leucippus and DemocritusliezelNo ratings yet
- The Structure of MatterDocument5 pagesThe Structure of MatterimanuelsukarnoNo ratings yet
- Structure of Atoms 3and4Document18 pagesStructure of Atoms 3and4Emperical FlameNo ratings yet
- Atoms Part 1Document7 pagesAtoms Part 1Franze Nica BacuyagNo ratings yet
- Chemistry For FreshmenDocument9 pagesChemistry For FreshmenMeo Angelo AlcantaraNo ratings yet
- ProtonDocument2 pagesProtonPRADYUT6No ratings yet
- Sekolah Kebangsaan Batu Kawan 14110 Simpang Ampat Seberang Perai Selatan Senarai Nama Murid Tunas Kadet Remaja Sekolah (2014)Document10 pagesSekolah Kebangsaan Batu Kawan 14110 Simpang Ampat Seberang Perai Selatan Senarai Nama Murid Tunas Kadet Remaja Sekolah (2014)seetharaman78No ratings yet
- IIP HostDocument7 pagesIIP HostadityaNo ratings yet
- Fee Shedule 2020-21 Final DiosDocument2 pagesFee Shedule 2020-21 Final Diosapi-210356903No ratings yet
- Leisure Time To The Academic of StudentDocument12 pagesLeisure Time To The Academic of StudentKate Tolentino Macalintal0% (1)
- Principles of Management: Cpm/PertDocument19 pagesPrinciples of Management: Cpm/PertPushpjit MalikNo ratings yet
- Claremont COURIER 4.28.10Document24 pagesClaremont COURIER 4.28.10Claremont CourierNo ratings yet
- Sri Ramachandra University Application-09Document5 pagesSri Ramachandra University Application-09Mohammed RaziNo ratings yet
- Sejarah InteriorDocument8 pagesSejarah InteriorNuNo ratings yet
- Interpersonal Relationship and Communication - wk7Document14 pagesInterpersonal Relationship and Communication - wk7mervin tomas100% (1)
- Prototype Lesson Plan Using Trimodal Delivery PlatformDocument5 pagesPrototype Lesson Plan Using Trimodal Delivery PlatformJEAN P DE PERALTANo ratings yet
- Legal Awareness Programmes at Schools On Human RightsDocument2 pagesLegal Awareness Programmes at Schools On Human RightsSW-DLSANo ratings yet
- Professional Interviewing Skills: Penni Foster, PHDDocument26 pagesProfessional Interviewing Skills: Penni Foster, PHDYanuar Rizal Putra Utama, S. PdNo ratings yet
- Preparing For NDA Exam With SchoolDocument2 pagesPreparing For NDA Exam With SchoolAafia GauseNo ratings yet
- Delhi Public School Internationa Kampala Uganda Annual Planner 23 24 CirculationDocument2 pagesDelhi Public School Internationa Kampala Uganda Annual Planner 23 24 Circulationgaurang1111No ratings yet
- The Purpose of EducationDocument3 pagesThe Purpose of EducationbeatrixNo ratings yet
- DOMDocument2 pagesDOMnea317No ratings yet
- Milestones in Adhesion - GICDocument9 pagesMilestones in Adhesion - GICParidhi Garg100% (1)
- Demonstration FormDocument2 pagesDemonstration FormDomric Panunciar100% (1)
- Hbet 4603 AssignmentsDocument13 pagesHbet 4603 Assignmentschristina lawieNo ratings yet
- At.2508 Understanding The Entitys Internal ControlDocument40 pagesAt.2508 Understanding The Entitys Internal Controlawesome bloggersNo ratings yet
- Q4 W3 4 Sci10 LawDocument8 pagesQ4 W3 4 Sci10 LawBa BengNo ratings yet
- Module 5 - Gender and The SexDocument15 pagesModule 5 - Gender and The SexKrishna LiamNo ratings yet
- Rena Subotnik, Lee Kassan, Ellen Summers, Alan Wasser - Genius Revisited - High IQ Children Grown Up-Ablex Publishing Corporation (1993)Document149 pagesRena Subotnik, Lee Kassan, Ellen Summers, Alan Wasser - Genius Revisited - High IQ Children Grown Up-Ablex Publishing Corporation (1993)Pablo SantanaNo ratings yet
- CTFL 2018 Sample Exam B v1.3 QuestionsDocument21 pagesCTFL 2018 Sample Exam B v1.3 QuestionsLaser- xNo ratings yet
- Unit - 3: Losses and Efficiency of DC MachinesDocument4 pagesUnit - 3: Losses and Efficiency of DC MachinesNisha JosephNo ratings yet
- International MBA Exchanges Fact Sheet 2022-23Document4 pagesInternational MBA Exchanges Fact Sheet 2022-23pnkNo ratings yet
- University of Jordan Chemical Engineering Mass Transfer Course SyllabusDocument5 pagesUniversity of Jordan Chemical Engineering Mass Transfer Course SyllabusMohammed SaifNo ratings yet
- Episode 1: The School As A Learning Resource CenterDocument6 pagesEpisode 1: The School As A Learning Resource CenterJonel BarrugaNo ratings yet
- Assertive Advocate (INFJ-A)Document6 pagesAssertive Advocate (INFJ-A)kawaiilaitue100% (1)
- A Reaction Paper On Russell's Is Happiness Still PossibleDocument4 pagesA Reaction Paper On Russell's Is Happiness Still PossibleElizier 'Barlee' B. LazoNo ratings yet