Professional Documents
Culture Documents
Climbing Film Evaporator
Uploaded by
Pelin Yazgan BirgiOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Climbing Film Evaporator
Uploaded by
Pelin Yazgan BirgiCopyright:
Available Formats
CHAPTER
FIVE
EVAPORATION
5.1
Climbing Film Evaporator
Cold hearted orb that rules the night, And steals the colours from our sight. Red is gray and yellow white, But we decide which is right, And which is an illusion.
MOODY BLUES
Chapter 5: EVAPORATION
5.1 CLIMBING FILM EVAPORATOR
Keywords: Evaporation, thermal efficiency, upward-flow (climbing-film).
5.1.1 Object The object of this experiment is to investigate the effect of the variations in the feed rate on the concentration of the product, the effect of the operating steam pressure on the rate of evaporation achieved, and to measure the thermal efficiency of the climbing film evaporator.
5.1.2 Theory The vaporization of a liquid for the purpose of concentrating a solution consisting of a nonvolatile solute and volatile solvent is a common unit operation in chemical processing and is performed in many ways. Evaporation is conducted by vaporizing a portion of the solvent to produce a concentrated solution or a thick liquor. Evaporation differs from drying in that the residue is a liquid - sometimes a highly viscous one - rather than a solid; it differs from distillation in that the vapor is usually a single component, and even when the vapor is a mixture, no attempt is made in the evaporation step to separate the vapor into fractions; it differs from crystallization in that emphasis is placed on concentrating a solution rather than forming and building crystals. The conditions under which evaporation is carried out in practice vary widely. The liquid to be evaporated may be less viscous than water, or it may be so viscous that it will hardly flow. It may deposit scale on the heating surface; it may precipitate in crystals; it may tend to foam; it may have a very high boiling point elevation; or it may be damaged by the application of too high temperatures. This wide variety of problems has led to considerable variation in the types of mechanical construction used. Evaporator types may be classified as follows: 1. 2. Short-tube evaporators Long-tube vertical evaporators - Forced-circulation - Upward-flow (climbing-film) - Downward-flow (falling-film) 3. Agitated-film evaporators
Material and Heat Balances:
Figure 5.1.1 - Energy balance for single-effect evaporator.
Figure 5.1.1 is a highly simplified diagram of an evaporator, in which the heating surface is represented by a simple coil. Let F be the kilogram of the feed to the evaporator per hour, whose solute content is xf (x is the weight fraction). Let the enthalpy of the feed per kilogram be hf. There is taken out of the evaporator L kg of thick liquor, whose composition in weight fraction of solute is xL and whose enthalpy is hL in joule per kilogram. There is also V kg of vapor having a solute concentration of y and an enthalpy of H, J/kg. In most evaporators, the vapor is pure water, and therefore y is zero. The material balance equations for this case become:
F = L+ V
Total Material Balance:
(5.1.1)
Solute Balance:
Fx F = Lx L + Vy
(5.1.2)
In order to furnish the heat necessary for evaporation, S kg of steam is supplied to the heating surface with an enthalpy of Hs (J/kg) and there is taken out S kg of condensate with an enthalpy of hc (J/kg). One simplifying assumption usually made is that in an evaporator there is very little
Chapter 5: EVAPORATION
cooling of the condensate. This leads the assumption that the condensate will leave at the condensing temperature of the steam.
The heat balance equation is:
Heat in feed + Heat in steam = Heat in thick liquor + Heat in vapor + Heat in condensate + Heat lost by radiation
(5.1.3)
Neglecting losses by radiation and using the relevant symbols we get the following equation: Fh F + SH S = VH + Lh L + Sh C
(5.1.4)
which represents the heat balance of the evaporator.
Since the available heat transfer area is fixed then for a given steam pressure and feed rate, the overall heat transfer coefficient is steady and the rate of evaporation is constant. Variation in the feed rate will change the sensible heat loading, the overall heat transfer coefficient and the percentage evaporation rate of which affect the product concentration.
Q = ( MCpT) + m
(5.1.5)
Q = U heatng A h Tlmheatng + U evap A e Tlmevap
A = Ae + Ah
) (
(5.1.6)
(5.1.7)
where
Q M m cp
: : : :
total heat load, J mass flow rate of feed, kg/s evaporation rate, kg/s specific heat of feed, J/kgK temperature increase, K log mean temperature difference, K heat transfer coefficient, J/m2sK total fixed heat transfer area, m2 latent heat of evaporation, J/kg
T : Tlm: U A : : :
For an increase in the feed rate then the sensible heat load is larger. Since there is little change in the overall heat transfer coefficient for heating and the T will be constant then Ah must be larger.
The heat transfer area Ae must be correspondingly smaller and the evaporation rate will be less. Since the volume of the feed is higher, the percentage evaporation will be lower so that the product is less concentrated.
The rate of heat transferred Q is dependent upon the overall heat transfer coefficient U, the heat transfer surface area A, and the log mean temperature difference Tlm, that is: Q = UATlm
(5.1.8)
For a given system, A will be constant, U is virtually constant so that the rate of heat transfer depends entirely upon the log mean temperature difference. Since varying the steam pressure varies the operating steam temperature, the rate of heat transfer and hence the rate of evaporation is changed.
Heat taken up by the feed is the sensible heat and latent heat.
Q = (mass) (specific heat) (temperature rise) + (evaporation) (latent heat)
(5.1.9)
This experiment assumes as a basis that 1 kg of dry saturated steam condensing will evaporate 1 kg of boiling water. In practice heat is lost to the atmosphere and to the side streams purged from the system. The latent heat of the steam is used to preheat the feed into the evaporator and allowance must be made in the calculation for this. Water carried over by the steam is collected in the purge receiver and effectively lost from all dryness factor value (DF) is required for the steam supplied. This may in practice vary from 0.85 to 1.00.
mass of water in the feedSp heat of feed temperatur rise of water e
steam used to preheat feed =
Latent heat of steam
(5.1.10)
Evaporative efficiency =
(mass of steam condensed mass of
mass of water evaporated100
preheat steam DF
(5.1.11)
Chapter 5: EVAPORATION
5.1.3 Apparatus
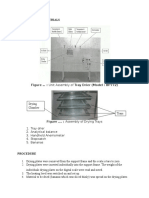
The apparatus used in this experiment is shown in Figure 5.1.2.
Figure 5.1.2 - QVF climbing film evaporator.
1.) Feed inlet and drain valve 2.) Condensate outlet 3.) Stream inlet 4.) Calandria 5.) Calandria vent 6.) Cyclone 7.) Condensate receiver 8.) Three-way cock 9.) Condensate drain
10.) Bottom receiving vessel 11.) Vacuum and vent cock 12.) Top receiving vessel 13.) Vacuum cock 14.) Water inlet 15.) Condenser 16.) Water outlet 17.) Thermometer pocket
5.1.4 Experimental Procedure
Attention: During the experiment the floor gets wet, so wear suitable clothes.
1. 2. 3. 4. 5. 6. 7. 8. Turn the steam generator on. Prepare 30 dm3 of a 10 % w/w solution of glycerol in water. Close all drain cocks on the plant. Turn on the cooling water to the condenser, (No.15). Adjust steam pressure to a specified value. Check that the steam trap is functioning correctly. Open the feed inlet cock, (No.1), and control the feed rate by the rotameter. Allow the liquid level in the calandria to reach the steam inlet neck before finally adjusting the feed rate. The liquid will begin to boil and expanding bubbles will rise rapidly in the tube giving climbing film operation. 9. Adjust the feed rate to a value and allow a minimum of 5 minutes of operation under these conditions so that the unit can settle down, i.e., come to steady state. 10. Once the unit is operating smoothly close the valve between the condensate receivers and note the liquid level in the graduated concentrate receiver. For a minimum of 15 minutes operation ensure that the steam pressure, feed rate, and vacuum are constant. 11. At the end of the test period close the valve between the twin condensate receivers and note the level in the graduated concentrate receiver. 12. Measure the volume of the condensate collected, calculate the volume of the feed to the unit and the volume of the concentrate produced. 13. Repeat the experiment with two other feed rates. 14. Repeat the experiment keeping the flow rate constant and varying the pressure using two other different pressures. 15. In one of these five data calculate (find) the amount of steam used to evaporate the feed by placing the steam pipe into a specified amount of cold water. 16. Shut the unit down following the procedure described below:
IMPORTANT: Close the feedcock. Close the steam control valve and all other steam valves on the path between the steam generator and control table. Close the steam generator.
Chapter 5: EVAPORATION
Stop the pump. Close the condensate cooling water valve. Open each of the drain cocks in turn and drain contents to a suitable receiver. Leave all the drain cocks open.
5.1.5 Report Objectives 1. 2. 3. 4. 5. 6. 7. State the assumptions used in your calculations. Why is glycerol used? What are the properties of a climbing film evaporator? Calculate the percent concentration of the concentrate. Calculate the heat transferred in producing the product concentrate and condensate. Calculate the percent of feed evaporated. Calculate the total heat required to heat the concentrate water and heat required to evaporate the condensate. Compare this value with the heat available from the steam, which was condensed and collected from the steam trap. 8. 9. State the factors that affect the evaporative efficiency. What is the enthalpy difference between 1 kg of steam having a DF of 0.95 and 1 kg of steam having a DF of 0.85? 10. Comment on the effects of vacuum operation on evaporative efficiency. 11. Calculate the evaporative efficiency.
5.1.6 References
1.
Badger, L. W., and J. T. Banchero, Introduction to Chemical Engineering, McGraw-Hill, New York, 1955.
2.
Coulson, J. M., and J. F. Richardson, Chemical Engineering Unit Operations, Pergamon Press, 1962.
3.
Perry, R. H., and D. Green, Perrys Chemical Engineers Handbook, 6th edition, McGrawHill, 1988.
You might also like
- Climbing Film EvaporationDocument26 pagesClimbing Film EvaporationSharmeen Muniandy100% (3)
- Batch Distillation Laboratory ReportDocument17 pagesBatch Distillation Laboratory ReportNayantara Soni100% (1)
- Climbing Film EvaporatorDocument8 pagesClimbing Film Evaporatorsaz140% (1)
- Fluidization: Chemical Engneering PracticeDocument24 pagesFluidization: Chemical Engneering PracticeFarhan M JafrI100% (1)
- Result & Discussion Exp Tray DryerDocument6 pagesResult & Discussion Exp Tray Dryerfatin noraini71% (7)
- Climbing FilmDocument34 pagesClimbing FilmTunji Aminu100% (1)
- Absorption of Carbon Dioxide Into WaterDocument11 pagesAbsorption of Carbon Dioxide Into WaterEstelle Jean CauilanNo ratings yet
- FINAL Evaporator Final ReportDocument34 pagesFINAL Evaporator Final Reportjackhh7798075% (8)
- Drying 2 Class Notes PDFDocument18 pagesDrying 2 Class Notes PDFFarouk Bassa100% (1)
- Study of A Single Effect EvaporatorDocument11 pagesStudy of A Single Effect Evaporatormahbub133267% (6)
- Lab Report 5Document12 pagesLab Report 5Norhanisah Zamri Rcsu100% (1)
- CSTRDocument20 pagesCSTRSharing Caring100% (1)
- R14 - Steam Power Cycles PDFDocument10 pagesR14 - Steam Power Cycles PDFnotoriousneal12No ratings yet
- Reverse OsmosisDocument53 pagesReverse Osmosisanabloom100% (2)
- Hydrology For DummiesDocument20 pagesHydrology For DummiesIulian Mihai50% (2)
- Absorption in PackedDocument21 pagesAbsorption in PackedfreakameNo ratings yet
- Single Effect EvaporatorDocument2 pagesSingle Effect Evaporatorchemant7100% (1)
- Single Effect Evaporator 2Document22 pagesSingle Effect Evaporator 2Shailesh Lohare100% (1)
- Climbing Film GanganDocument20 pagesClimbing Film GanganAdeniran Joshua50% (2)
- Best Memo ReportDocument16 pagesBest Memo ReportNadaNo ratings yet
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocument7 pagesOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosNo ratings yet
- Series and Parallel Pumps: Flow Rate & PressureDocument11 pagesSeries and Parallel Pumps: Flow Rate & PressureKevin Devastian100% (1)
- Exp-9 - Liquid Liquid Extraction in A Packed ColumnDocument5 pagesExp-9 - Liquid Liquid Extraction in A Packed ColumnSiddharth MohapatraNo ratings yet
- Evaporation 1Document23 pagesEvaporation 1nontando sogaNo ratings yet
- Gas Absorption: Determining Drag and Flooding FlowsDocument5 pagesGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoNo ratings yet
- Sieve Plate Distillation ColumnDocument9 pagesSieve Plate Distillation ColumnAshish VermaNo ratings yet
- Investigation of Liquid-Solid and Gas-Solid Fluidized BedDocument18 pagesInvestigation of Liquid-Solid and Gas-Solid Fluidized Bedmahbub1332100% (1)
- Sieve Plate Distillation Column - Lab ReportDocument4 pagesSieve Plate Distillation Column - Lab ReportShrankhla NaryaNo ratings yet
- Lab ReportDocument10 pagesLab ReportKathleen De Vera BarrilNo ratings yet
- Lab Report 1 Tray DrierDocument7 pagesLab Report 1 Tray Drier_never_mind_100% (1)
- MASS TRANSFER ABSORPTION DESIGNDocument38 pagesMASS TRANSFER ABSORPTION DESIGNSyazwan WanNo ratings yet
- Cre 1 IntroductionDocument4 pagesCre 1 IntroductionEvangeline LauNo ratings yet
- Packed Distillation Column ExperimentDocument20 pagesPacked Distillation Column ExperimentChan Chun ChenNo ratings yet
- Experiment: Packed Distillation ColumnDocument4 pagesExperiment: Packed Distillation Columnnhalieza1067No ratings yet
- Bubble Cap Distillation ColumnDocument3 pagesBubble Cap Distillation Columnnhalieza1067No ratings yet
- Tutorial Leaching 2017Document11 pagesTutorial Leaching 2017Victor M. Jaki100% (1)
- Hydrodynamics of Packed ColomnDocument6 pagesHydrodynamics of Packed ColomnDhananjay KadamNo ratings yet
- Evaporador PDFDocument31 pagesEvaporador PDFAdriano RafaelNo ratings yet
- Drying: Merry Jessah S. TorresDocument6 pagesDrying: Merry Jessah S. TorresFrancis Val FranciscoNo ratings yet
- Isothermal Semi-Batch Reactor PPT RJC SirDocument16 pagesIsothermal Semi-Batch Reactor PPT RJC Sirsdjdsf100% (1)
- Lab 2 Full Report PDFDocument20 pagesLab 2 Full Report PDFmuhammad ilyas100% (1)
- Understanding SedimentationDocument23 pagesUnderstanding SedimentationJohnNo ratings yet
- Fixed Bed and Fluidized BedDocument33 pagesFixed Bed and Fluidized Bedشاكر العاقلNo ratings yet
- Separation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentDocument4 pagesSeparation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentElliot AldersonNo ratings yet
- Apparatus, Procedure, Recommendation Tray DryerDocument4 pagesApparatus, Procedure, Recommendation Tray DryerillyzlNo ratings yet
- Packed Bed Distillation Column Lab ReportDocument13 pagesPacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- Universiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Document14 pagesUniversiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Heather Jarvis100% (2)
- Azeotropic Mass BalanceDocument25 pagesAzeotropic Mass BalancesowjanyaavkNo ratings yet
- Batch Reactor ExpDocument12 pagesBatch Reactor ExpJack AndreasNo ratings yet
- Postlab 2 Gas AbsorptionDocument7 pagesPostlab 2 Gas AbsorptionDean Joyce AlborotoNo ratings yet
- Exp - S1A - Solid in Air DiffusionDocument7 pagesExp - S1A - Solid in Air DiffusionAnuj SrivastavaNo ratings yet
- EvaporationDocument9 pagesEvaporationKim Tag-at YbañezNo ratings yet
- Sample Problems On Gas AbsorptionDocument2 pagesSample Problems On Gas AbsorptionKevin Laganao67% (3)
- Colling Tower: Mechanical Lab / Exp. NO.Document10 pagesColling Tower: Mechanical Lab / Exp. NO.Dalal Salih100% (1)
- CONTINUOUS DistillationDocument5 pagesCONTINUOUS DistillationNaseer SattarNo ratings yet
- EVAPORATORDocument50 pagesEVAPORATORnur irfana mardiyah100% (1)
- EvaporationDocument50 pagesEvaporationRajNo ratings yet
- Exp - 7Document8 pagesExp - 7Ron PascualNo ratings yet
- ERT 209 Heat and Mass Transfer For Bioprocess Engineering EvaporatorDocument50 pagesERT 209 Heat and Mass Transfer For Bioprocess Engineering EvaporatorSreenivasNo ratings yet
- EVAPORATIONDocument7 pagesEVAPORATIONLavenia Alou MagnoNo ratings yet
- Evap DesignDocument16 pagesEvap DesignAhmed Ali100% (3)
- Reaction SunumDocument13 pagesReaction SunumPelin Yazgan BirgiNo ratings yet
- Catalyst PreparationDocument59 pagesCatalyst PreparationPelin Yazgan BirgiNo ratings yet
- Reaction SunumDocument13 pagesReaction SunumPelin Yazgan BirgiNo ratings yet
- Fluidized BedDocument44 pagesFluidized Bedrahulgorde100% (1)
- Rising Film EvaporatorsDocument10 pagesRising Film EvaporatorsPelin Yazgan BirgiNo ratings yet
- PIPE FLOW LAB REPORT RESULTSDocument18 pagesPIPE FLOW LAB REPORT RESULTSmanthan100% (1)
- Vacuum Technology Book II Part 2Document140 pagesVacuum Technology Book II Part 2bracio100% (1)
- Vacuum RegulatorDocument4 pagesVacuum Regulatormu khaledNo ratings yet
- Boiler feed water steam turbines in Badak LNG utilitiesDocument2 pagesBoiler feed water steam turbines in Badak LNG utilitiesIlman Mughni100% (1)
- Presentation1 3Document11 pagesPresentation1 3NerviRitaNo ratings yet
- Measurement of FlowDocument57 pagesMeasurement of FlowRonal Maharaj0% (1)
- Line Sizing Philosophy PDFDocument21 pagesLine Sizing Philosophy PDFmohammadhadiNo ratings yet
- Gaseous State (Ideal Gases) Exercise With Sol.Document25 pagesGaseous State (Ideal Gases) Exercise With Sol.mikcNo ratings yet
- Fundamentals of Natural Gas Processing: 6.2 Water Content of HydrocarbonsDocument1 pageFundamentals of Natural Gas Processing: 6.2 Water Content of HydrocarbonsantonioNo ratings yet
- Experiment No. 2 Molar Mass of A Volatile LiquidDocument5 pagesExperiment No. 2 Molar Mass of A Volatile LiquidJericho MaganaNo ratings yet
- Solved Problems Ref SystemDocument5 pagesSolved Problems Ref SystemArchie Gil DelamidaNo ratings yet
- Purification of BiogasDocument15 pagesPurification of BiogasHedi Ben MohamedNo ratings yet
- Unit 2 Phase Changes NotesDocument34 pagesUnit 2 Phase Changes Notesapi-483662721No ratings yet
- Chapter 12Document15 pagesChapter 12kshitiz dhamaNo ratings yet
- Water - Pollution in IndiaDocument17 pagesWater - Pollution in India398 -Sahifa SayedNo ratings yet
- GCSE Physics - Boyle's Law QuestionsDocument3 pagesGCSE Physics - Boyle's Law Questionsamvijayagopal50% (2)
- خواص صخور المكمن رقم 6Document31 pagesخواص صخور المكمن رقم 6maamoun ramyNo ratings yet
- APES Unit Test Weather Climate and PollutionDocument5 pagesAPES Unit Test Weather Climate and PollutionudiNo ratings yet
- 5 Types of Flow MetersDocument2 pages5 Types of Flow Metersabdulkidwai20090% (1)
- Venturi Test Efficiency Hole SizeDocument4 pagesVenturi Test Efficiency Hole SizeCarlos CavalcanteNo ratings yet
- FLOWSIC 600 Pipe Size Calculation: Reference ConditionDocument1 pageFLOWSIC 600 Pipe Size Calculation: Reference Conditionprihartono_diasNo ratings yet
- CalculationsDocument3 pagesCalculationsAltif Abood100% (1)
- Hazop Table 1Document9 pagesHazop Table 1Mark ChongNo ratings yet
- PART 2: THE WATER CYCLEDocument2 pagesPART 2: THE WATER CYCLESimon DruryNo ratings yet
- Lecture 2 Fluid Statics PressuresDocument35 pagesLecture 2 Fluid Statics PressuresJuan MarcosNo ratings yet
- GAS ChromatographyDocument67 pagesGAS Chromatographyhoboslayer97No ratings yet
- STD 106Document26 pagesSTD 106Jonas PadillaNo ratings yet
- H2S Scrubber Pressure Drop CalculationDocument1 pageH2S Scrubber Pressure Drop CalculationTuesou MachereNo ratings yet