Professional Documents
Culture Documents
RA
Uploaded by
Gustiandari FidhyaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
RA
Uploaded by
Gustiandari FidhyaCopyright:
Available Formats
Chapter 285 RHEUMATOID ARTHRITIS
James R. O'Dell
Definition
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology that primarily targets synovial tissues. It is relatively common, with a prevalence of approximately 0.8% in adults all over the world. RA shortens survival and significantly affects quality of life in most patients. Essentially all patients exhibit some systemic features such as fatigue, low-grade fevers, anemia, and elevations of acute phase reactants (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]). Despite these features, the primary target of RA is the synovium, and this is responsible for most of the protean clinical features. Synovial tissues proliferate in an uncontrolled fashion, resulting in excess fluid production, destruction of cartilage, erosion of marginal bone, and damage to tendons and ligaments.
In the last decade, the landscape of treatment of RA has changed dramatically. Current therapies result in substantial clinical benefit for most patients, particularly with early diagnosis and treatment with an appropriate disease-modifying antirheumatic drug (DMARD).
Epidemiology
RA is present all over the world, with a remarkably consistent prevalence of 0.5 to 1% of adults, with some differences in certain population groups. For reasons that are still unclear, the prevalence in women is two or three times greater than in men. RA can occur at any age, but onset before the age of 45 years in men is uncommon. The relatively few, well-done inception cohorts that are available suggest that the yearly incidence of RA is approximately 40 per 100,000 for women, and about half that for men. These figures vary significantly based on the age of the cohort. The best available data suggest that the incidence of RA in women increases with age until approximately 45 years of age, then reaches a plateau. The incidence rate is much lower in young men, approximately one third that of women, but increases steadily with age and approaches that of women in the over-65 age group. Because the incidence of RA increases or is stable with age and RA is a lifelong disease, the prevalence of RA increases with each decade. Recent data strongly suggest that the incidence of RA, particularly rheumatoid factor (RF)-negative RA, is decreasing. The reasons for this are unclear, but, if elucidated, they could provide valuable insights into the etiology and pathogenesis of RA and might allow the implementation of strategies to prevent onset of the clinical disease.
RA has a significant genetic component; therefore, it is not surprising that RA is reportedly very unusual in certain populations very common in others. Most notably, cohorts have been described in rural Nigeria in which no individuals are affected with RA; in contrast, a prevalence of RA of 5% has been found in some studies of Chippewa, Yakima, and Inuit Native American tribes.
Genetics
Genetics plays a significant role in determining both the risk of developing RA and the severity of the disease. Twin studies reveal a concordance rate for RA that averages 15 to 20% for monozygotic twins and approximately 5% for dizygotic twins. These data in monozygotic twins simultaneously reveal both the significance of genetic factors and the fact that they are clearly not the only important factor, or else the concordance rate would approach unity.
The association of certain human leukocyte antigen (HLA) alleles, specifically HLA-DR4, with an increased risk of developing RA and of having more severe disease has long been recognized. It is now known that this association is explained by a particular amino acid sequence in the third hypervariable region on the DR 1 chain. HLA-DR molecules are present on the surface of antigenpresenting cells and allow T cells to recognize antigen in the context of DR. Hypervariable regions on the DR molecule are particularly important for antigen recognition. Table 285-1 details the amino acid sequence of several DR 1 chains that are associated with RA and some that are not. The amino acid sequence associated with RA has been called the shared epitope or the at-risk allele. It has been shown by a number of investigators that patients with the shared epitope have more severe RA and more extra-articular manifestations than those who are negative for the shared epitope. Furthermore, individuals with two copies of the shared epitope, particularly those with HLA-DR4, have a further increased risk for the development of severe RA. This association with a particular antigen recognition site may ultimately aid understanding of the antigen or antigens that are important for triggering RA. Recently, proteins in which arginine has been converted to citrulline were shown to be bound with greater avidity by the shared epitope. Conversely, others have suggested that perhaps patients develop RA because the shared epitope prevents recognition of certain arthritogenic antigens. The importance of certain DR 1 types in RA supports the concept that T cells are integrally involved in the pathogenesis.
TABLE 285-1 -- HLA ASSOCIATIONS WITH RHEUMATOID ARTHRITIS (RA) HLA Types (Alleles) and Methods of Detection Third Hypervariable Region Amino Acid Sequences Alloantisera (DR) MLC (Dw) DNA (DR 1) 70 71 72 73 74 Most Common Ethnic Groups
ASSOCIATED WITH RA DR4 Dw4 DR4 Dw14 DR4 Dw15 DR1 Dw1 0401 Q K R A A Whites (western Europe) 0404 R Whites (western Europe) 0405 R Japanese, Chinese 0101 R Asian Indians, Israelis 1402 R Yakima Native Americans
DR6 (14) Dw16 DR10
1001 R R Spanish, Greeks, Israelis
NOT ASSOCIATED WITH RA DR4 Dw10 DR4 Dw13 DR2 Dw2 DR3 Dw3 0402 D E Whites (eastern Europe) 0403 R E Polynesians 1501 D A Whites 0301 G R Whites
A = alanine; D = aspartic acid; E = glutamic acid; HLA = human leukocyte antigen; K = lysine; MLC = mixed leukocyte cultures; Q = glutamine; R = arginine;
= the same amino acid in that position as for DRB1
0401.
Population-based studies have suggested that only about one third of the genetic risk for RA is explained by genes located in the HLA region. Large studies of siblings concordant for RA have suggested that ultimately multiple genes will be identified that are important for the development of RA. Recently, a functional polymorphism for the gene that encodes intracellular protein tyrosine
phosphatase nonreceptor 22 (PTPN22) has been reproducibly associated with RA and with a number of other autoimmune diseases, including type 1 diabetes, systemic lupus erythematosus, Graves' disease, and Hashimoto's thyroiditis.
The shared epitope is present in approximately 25% of the Caucasian population, but the chance of developing RA among individuals who carry this allele is only about 1 in 25. Therefore, this test has little or no clinical utility. In addition to genetics, a number of other factors have been associated with the incidence, and in some cases the severity, of RA, including estrogen use (protective), smoking, silica exposure, and coffee consumption.
Etiology
The use of oral contraceptives has been associated with a decrease in the incidence of RA; because the effect seems to be strongest for oral contraceptives that have high estrogen content, it is postulated that estrogen is responsible for this protective effect. Studies that have tried to address the question of postmenopausal estrogen use and its effect on RA have yielded conflicting results.
Smoking has been associated with a significant increase in the risk of developing RA. This association is particularly strong in men with RF-positive disease and those who have antibodies to cyclic citrullinated peptides (CCPs). Recently, investigators in Europe have reported that coffee consumption is a risk factor for developing RA; investigators in North America have suggested that this risk may be limited to decaffeinated coffee.
RA appears to require the complex interaction of genetic and environmental factors with the immune system, and ultimately in the synovial tissues throughout the body ( Fig. 285-1 ). RA clearly has a significant genetic component, but only about 1 in 25 whites with the so-called shared epitope develop RA. Furthermore, even if one monozygotic twin has RA, there is only approximately a 1 in 6 chance that the other twin will develop the same disease. Clearly, other factors, in addition to genetics, are active in precipitating or triggering RA. Triggers for RA have long been the target of active research. Purported triggers have included bacteria (Mycobacteria, Streptococcus, Mycoplasma, Escherichia coli, Helicobacter pylori), viruses (rubella, Epstein-Barr virus, parvovirus), superantigens, and many others.
FIGURE 285-1 Initiation of rheumatoid arthritis (RA). HLA = human leukocyte antigen.
Pathogenesis
Rheumatic fever, reactive arthritis (formerly known as Reiter's syndrome), and, more recently, Lyme arthritis are examples of arthritic syndromes for which infectious triggers have clearly been demonstrated, but these triggering agents are often difficult or impossible to isolate at the time when the arthritic syndromes occur. Many other examples exist in animal models of arthritis, including syndromes induced by mycobacteria and streptococci. Reactive arthritis is perhaps the most relevant example for RA. Reactive arthritis has clearly been shown to occur when any one of a myriad of different but specific infectious triggers is presented to a specific location in the body (the gastrointestinal or genitourinary tract) of individuals with a certain genetic background, in most cases HLA-B27. Additionally, in this syndrome, the age and gender of the individual and hence the maturity of the immune system may be critical for the development of this syndrome, which occurs primarily between the ages of 15 and 40 years in males. Once unraveled, the pathophysiology of RA is likely to be similarly complex.
Despite the absence of clear evidence linking any infectious agent to RA, it is widely believed that ultimately an important triggering role will be elucidated for infectious or other environmental agents. Once a trigger or triggers for RA are identified, strategies for prevention can be addressed, but this information may not help individuals with established disease. Possibly infections involving the innate immune system are causative in an early subclinical phase of the rheumatoid disease process, with the agents being absent once clinical disease develops.
Pathology
The synovial tissues are the primary target of the autoimmune inflammatory process that is RA; why this is true remains elusive. Once RA is initiated, the synovial tissues throughout the body become
the site of a complex interaction of T cells, B cells, macrophages, and synovial cells ( Fig. 285-2 ). The resultant proliferation of the synovial tissues (synovitis) causes the production of excessive amounts of synovial fluid and the infiltration of pannus into adjacent bone and cartilage. Synovitis results in the destruction of cartilage and bone and in stretching or rupture of the joint capsule as well as tendons and ligaments. In patients, these effects are manifested by the deformities (see Figs. 285-4 and 285-8 [4] [8]) and disabilities that make up the clinical picture that is RA.
FIGURE 285-2 Events involved in the pathogenesis of rheumatoid synovitis (progressing from left to right). B = B lymphocyte; C = complement; GM-CSF = granulocyte-macrophage colony-stimulating factor; IgG, IgM = immunoglobulin G, M; IL = interleukin; M = macrophage; P = plasma cell; PGE2 = prostaglandin E2; RF = rheumatoid factor; T = T lymphocyte; TGF- = transforming growth factor- ; TNF- = tumor necrosis factor- .
FIGURE 285-4 Severe advanced rheumatoid arthritis of the hands. There is massive tendon swelling over the dorsal surface of both wrists, severe muscle wasting, ulnar deviation of the metacarpophalangeal joints, and swan-neck deformity of the fingers. (From Forbes CD, Jackson WF: Color Atlas and Text of Clinical Medicine, 3rd ed. London, Mosby, 2003.)
FIGURE 285-8 Subluxation of the cervical spine in patients with rheumatoid arthritis. A, In a lateral radiograph of the cervical spine, the body of C2 and its odontoid process are outlined by the broken lines, and the posterior aspect of the anterior segment of C1 is indicated by a solid line. Normally, a space of only 2 to 3 mm separates C1 from C2. The space between C1 and the odontoid of C2 is markedly increased, indicative of subluxation of C1 and C2. B, Lateral view of a pathologic specimen from a patient who died of C1 C2 subluxation. The horizontal arrow shows the odontoid process that subluxed posteriorly, severely compressing and almost severing the cord. The vertical arrow shows a bone graft that had been put in place posteriorly in an attempt to prevent subluxation. Below the arrow, a nonhealing area is present through the bone graft, and inferior to that a wire fixation suture is still in place.
The relative roles of the cellular versus the humoral immune system in the initiation and perpetuation of RA are much debated; both appear to be important. Most likely, the mechanisms of initiation of the disease process are different from those that perpetuate the chronic disease. T cells, particularly of the activated TH1 type, appear to predominate in synovial tissues. These T cells, presumably activated by some yet unknown antigen presented by macrophages, B cells, or synoviocytes in the context of DR, secrete cytokines that drive further synovial proliferation. It is believed by many that, although RA may initially be triggered by exogenous antigen, the process, once initiated, may be perpetuated by autoantigens. Macrophage-derived cytokines, particularly interleukin-1 (IL-1) and tumor necrosis factor- (TNF- ), play central roles in this ongoing inflammatory process. As definitive proof, biologic products directed against these cytokines have shown significant efficacy in the treatment of RA.
The humoral immune system also plays a role. RF has long been a serologic marker of RA and is well known to correlate with more severe disease, including erosions of bone, and with the presence of extra-articular features. The reason why RF is produced in excess and the exact role that it plays remain elusive. RF production may increase complement activation and result in the release of lysosomal enzymes, kinins, and oxygen-free radicals. Antibodies to CCP have been shown to have a high specificity (93 to 98%) for RA, although their sensitivity for RA with currently available assays is only about 70%. Furthermore, both RF and anti-CCP antibodies have been shown to be present in the serum of patients years before they develop clinically apparent RA, and both also correlate with more aggressive erosive disease.
Diagnosis
All current treatment paradigms for RA stress the early and aggressive use of DMARDs. Therefore, the importance of accurate early diagnosis of RA cannot be overemphasized. However, there is no one single finding on physical examination or laboratory testing that is pathognomonic of RA. Instead, the diagnosis of RA requires a collection of historical and physical features, as well as an alert and informed clinician.
Classification
Table 285-2 lists the classification criteria for RA; although they are not designed specifically for the purpose of diagnosis, these criteria are widely used as a diagnostic aid. The first five criteria are all clinical; in other words, they are established by physical examination or by talking with the patient. Only the last two criteria require laboratory tests or radiographs. The first four criteria must be present for at least 6 weeks before a diagnosis of RA can be made. This caveat is necessary because a host of conditions, including many viral-related syndromes, can cause self-limited polyarthritis syndromes that look identical to RA, including at times the presence of RF. Such conditions usually last only 2 to 3 weeks. Therefore, inflammatory arthritis that present for at least 6 weeks should not be considered a postviral condition, except for parvovirus arthritis, and the diagnosis of RA should be strongly considered, with the early initiation of appropriate treatment. The goal for most RA patients should be to establish a diagnosis and to start DMARD therapy within 3 months of disease onset. The presence of anti-CCP antibodies, even in the first few weeks of disease, is strongly suggestive of RA.
TABLE 285-2 -- CLASSIFICATION CRITERIA FOR RHEUMATOID ARTHRITIS[*] 1. Morning stiffness ( 1 hr)
2. 3. 4. 5. 6. 7.
Swelling (soft tissue) of three or more joints Swelling (soft tissue) of hand joints (PIP, MCP, or wrist) Symmetrical swelling (soft tissue) Subcutaneous nodules Serum rheumatoid factor Erosions and/or periarticular osteopenia in hand or wrist joints seen on radiograph
* Criteria 1 through 4 must have been continuously present for 6 wk or longer, and criteria 2 through 5 must be observed by a physician. A classification as rheumatoid arthritis requires that four of the seven criteria be fulfilled. MCP = metacarpophalangeal; PIP = proximal interphalangeal.
Clinical Course
Although the presentation is variable, most patients with RA have an insidious onset of pain, stiffness, and/or swelling in multiple joints over the course of weeks to months. Systemic features such as fatigue, low-grade fevers, and weight loss may accompany the joint manifestations of RA. Less commonly, the onset can be fulminant, occurring almost overnight, or patients may have persistent monoarthritis or oligoarthritis for prolonged periods before manifesting the more typical pattern of joint involvement. Rarely, patients present with extra-articular features of RA before the joint problems occur.
The distribution of involved joints is a critical clue to the underlying diagnosis. The joints that are involved in patients with RA at presentation are also variable; typically, the symptoms start in the small joints of the hands (the proximal interphalangeal [PIP] and metacarpophalangeal [MCP] joints) and in the toes (metatarsophalangeal [MTP] joints). Importantly, RA usually spares the distal interphalangeal (DIP) joints and the small joints of the toes ( Fig. 285-3 ). If DIP involvement is a prominent finding, a different diagnosis should be considered (e.g., osteoarthritis, psoriatic arthritis). Later, RA moves, or some would say metastasizes, to larger joints: wrists, knees, elbows, ankles, hips, and shoulders (roughly in that order). Although the patient's history of joint symptoms
(arthralgia) is important, the diagnosis of RA requires the presence of inflammation (swelling and/or warmth) on examination of the joints.
FIGURE 285-3 Distribution of involved joints in the two most common forms of arthritis rheumatoid arthritis and osteoarthritis. Dark circles are shown over the involved joint areas.
Morning stiffness is a hallmark of inflammatory arthritis and is a prominent feature of RA. Patients with RA are characteristically at their worst in the morning or after prolonged periods of rest. This stiffness in and around joints often lasts for hours, and quantifying it is one way to measure improvement. Stiffness is relieved by warmth and activity, and reducing or eliminating joint stiffness is a clear goal of therapy.
Differential Diagnosis
The accurate diagnosis of RA early in its course, although challenging, is critical if patients are to benefit maximally from therapeutic intervention. Once disease has been present and active for a number of years and the characteristic deformities and radiographic changes have occurred, the diagnosis is all too obvious. Unfortunately, once RA has progressed to that point, many of the deformities no longer are amenable to medical therapy.
Many diseases can mimic RA ( Table 285-3 ). Early in the course of disease, self-limited viral syndromes need to be considered, especially hepatitis B and C, parvovirus, rubella (infection or vaccination), and Epstein-Barr virus. At any time, systemic lupus erythematosus, psoriatic arthritis, and reactive arthritis may present diagnostic challenges. In the case of these three mimics, a
targeted history and examination to elucidate their associated clinical features, such as rashes, oral ulcers, nail changes, dactylitis, urethritis, and renal, pulmonary, gastrointestinal, or ophthalmologic problems, is critical. Especially in elderly patients with fulminant-onset RA, remitting RF-negative symmetrical synovitis with pitting edema (the so-called RS3PE syndrome) and paraneoplastic syndromes should be considered. Chronic tophaceous gout may also mimic severe nodular RA. Hypothyroidism not only causes many rheumatic manifestations but also occurs commonly in conjunction with RA and, therefore, should be kept in mind.
TABLE 285-3 -- DIFFERENTIAL DIAGNOSIS OF RHEUMATOID ARTHRITIS Disorder Subcutaneous Nodules Rheumatoid Factor Viral arthritis (hepatitis B and C, parvovirus, rubella, others) - Bacterial endocarditis + Rheumatic fever + Sarcoidosis + + Reactive arthritis - Psoriatic arthritis - Systemic lupus erythematosus + Primary Sjgren's syndrome - + Chronic tophus gout + Calcium pyrophosphate disease - Polymyalgia rheumatica - Osteoarthritis (erosive) - -
- = not present; + = frequently present; = occasionally present.
Clinical Manifestations
Articular Manifestations
RA can affect any of the synovial (diarthrodial) joints (see Fig. 285-3 ). Most commonly, the disease starts in the MCP, PIP, and MTP joints, followed by the wrists, knees, elbows, ankles, hips, and shoulders in roughly that order. Early treatment helps limit the number of joints involved. Less commonly, and usually later, RA may involve the temporomandibular, cricoarytenoid, and sternoclavicular joints. RA may involve the upper part of the cervical spine, particularly the C1 C2 articulation, but, unlike the spondyloarthropathies, it does not involve the rest of the spine. However, RA patients are at an increased risk for osteoporosis, and this risk should be considered and dealt with early.
Hands
The hands are a major site of involvement, and a significant portion of the disability that RA causes is because of damage and dysfunction of the hands. Typical early disease starts with swelling of the PIPs and MCPs. The DIP joints are rarely involved; significant involvement of the DIP joints should suggest the possibility of a different diagnosis. Figure 285-4 illustrates the classic ulnar deviation and swan-neck deformities (hyperextension of the PIP joints) that are commonly seen in late, more established disease. Boutonniere (or buttonhole) deformities also occur as a result of hyperextension of the MCP joints. If the clinical disease remains active, hand function deteriorates. Sudden loss of function of individual fingers may occur as a result of tendon rupture, which requires the expertise of a carefully selected hand surgeon to repair.
Feet
Feet, particularly the MTP joints, are involved early in most patients with RA. Radiographic erosions occur at least as early in the feet as in the hands. Subluxation of the toes is common and leads to the dual problem of breakdown of the skin with ulcers on the top of the toes and painful ambulation due to loss of the cushioning pads that usually protect the heads of the MTP joints.
Wrists
The wrist joints are involved in most patients with RA; radial deviation is the rule, and patients with severe involvement may progress to volar subluxation. Even early in the course of the disease, synovial proliferation in and around the wrists may compress the median nerve, causing carpal tunnel syndrome ( Fig. 285-5 ). Later, this synovial proliferation may invade tendons and lead to rupture.
FIGURE 285-5 Carpal tunnel syndrome. Distribution of pain and/or paresthesias (shaded area) when the median nerve is compressed by swelling in the wrist (carpal tunnel).
Large Joints
Involvement of knees, ankles, elbows, hips, and shoulders is common. Characteristically, the whole joint surface is involved in a symmetrical fashion. Therefore, RA is not only symmetrical from one side of the body to the other but also symmetrical within the individual joint. In the case of the knee ( Fig. 285-6A ), the medial and lateral compartments are both severely narrowed in RA; in contrast, in patients with osteoarthritis (see Fig. 285-6B ), only one compartment of the knee may be involved.
FIGURE 285-6 Radiographs of the knees in the two most common forms of arthritis rheumatoid arthritis and osteoarthritis. A, Severe involvement in rheumatoid arthritis, with almost complete symmetrical loss of joint space in both the medial and the lateral compartment, but with little subchondral sclerosis or osteophyte formation. B, Typical osteoarthritis, with severe, near-total loss of joint space of one compartment and a normal or actually increased joint space of the other compartment. Note also the significant subchondral sclerosis in the involved area, typical of osteoarthritis.
Synovial cysts may occur around any of the joints (large or small), and they occasionally manifest as soft, fluctuant masses that present diagnostic challenges. Synovial cysts from the knee are perhaps the best examples of this phenomenon. When the knee produces excess synovial fluid, it may accumulate in the popliteal space (popliteal or Baker's cyst) ( Fig. 285-7 ). These cysts can cause problems by pressing on the popliteal nerve, artery, or veins. Baker's cysts may dissect into the tissues of the calf (usually posteriorly), or they may rupture. Dissection may produce only minor symptoms, such as a feeling of fullness; rupture of the cyst with extravasation of the inflammatory content produces significant pain and swelling and may be confused with thrombophlebitis, the socalled pseudothrombophlebitis syndrome. Ultrasonography of the popliteal fossa and calf are useful to establish the correct diagnosis and to rule out thrombophlebitis, which may be precipitated by popliteal cysts. Treatment of popliteal (Baker's) cysts should be directed at interrupting the inflammatory process through an intra-articular injection of corticosteroid into the knee.
FIGURE 285-7 Arthrogram with a radiocontrast agent injected into the knee. The dye flows into the popliteal space and through a narrow channel into a large synovial cyst (Baker's cyst) that has dissected into the soft tissue of the calf.
Neck
Although most of the axial skeleton is spared in RA, the cervical spine and especially the C1 C2 articulation is commonly involved. Bony erosions and ligament damage can occur in this area and may lead to subluxation ( Fig. 285-8 ). Most often, subluxation at C1 C2 is minor; patients and caregivers need only be cautious and avoid forcing the neck into positions of flexion. Occasionally, subluxation at C1 C2 is severe and leads to compromise of the cervical cord with symptoms and in some cases death.
Other Joints
Wherever synovial tissue exists, RA can cause problems. The temporomandibular, cricoarytenoid, and sternoclavicular joints are examples of other joints that may be involved in RA. The cricoarytenoid joint is responsible for abduction and adduction of the vocal cords. Involvement of this joint may lead to a feeling of fullness in the throat, to hoarseness, and, rarely, when the cords are essentially fused in a closed position, to a syndrome of acute respiratory distress with or without stridor. In this latter situation, emergent tracheotomy may be life-saving.
Extra-articular Manifestations
Systemic features of RA such as fatigue, weight loss, and low-grade fevers occur frequently. As with all the other extra-articular features, they are more common in those patients who possess RF ( Table 285-4 ).
TABLE 285-4 -- EXTRA-ARTICULAR MANIFESTATIONS OF RHEUMATOID ARTHRITIS
Skin Nodules, fragility, vasculitis, pyoderma gangrenosum Heart Pericarditis, premature atherosclerosis, vasculitis, valve disease, and valve ring nodules Lung Pleural effusions, interstitial lung disease, bronchiolitis obliterans, rheumatoid nodules, vasculitis Eye Keratoconjunctivitis sicca, episcleritis, scleritis, scleromalacia perforans, peripheral ulcerative keratopathy Neurologic Entrapment neuropathy, cervical myelopathy, mononeuritis multiplex (vasculitis), peripheral neuropathy Hematopoietic Anemia, thrombocytosis, lymphadenopathy, Felty's syndrome Kidney Amyloidosis, vasculitis Bone Osteopenia
Skin
Subcutaneous nodules are seen in approximately one fourth of patients with RA, almost exclusively in those who are RF positive. Patients with nodules who are RF negative should be carefully scrutinized for a different diagnosis, such as chronic tophaceous gout. Nodules may occur almost anywhere (e.g., lungs, heart, eye), but most commonly they occur subcutaneously on extensor surfaces (particularly the forearms) ( Fig. 285-9 ), over joints, or over pressure points. They are firm on examination, usually are not tender, have a characteristic histologic picture, and are thought to be triggered by small vessel vasculitis. A syndrome of increased nodulosis, despite good control of the disease, has been described with methotrexate therapy ( Fig. 285-10 ).
FIGURE 285-9 Rheumatoid nodules. Large rheumatoid nodules are seen in a classic location along the extensor surface of the forearm and in the olecranon bursa.
FIGURE 285-10 Rheumatoid nodulosis. In this patient, multiple rheumatoid nodules are present over joints. In some cases, nodules may dominate the clinical picture. Rarely, this may be seen as a side effect of methotrexate therapy.
Small vessel vasculitis, manifested as digital infarcts or leukocytoclastic vasculitis, may occur in RA ( Fig. 285-11 ) and should prompt more aggressive DMARD treatment. A vasculitis of small and medium arteries that is indistinguishable from polyarteritis nodosa also can be seen and requires aggressive systemic therapy. Finally, pyoderma gangrenosum occurs with increased frequency in association with RA.
FIGURE 285-11 Small vessel vasculitis. A and B, Rheumatoid vasculitis with small brown infarcts of palms and fingers in chronic rheumatoid arthritis. (Courtesy of Dr. Martin Lidsky, Houston, TX.)
Cardiac Involvement
Cardiac involvement directly related to RA is uncommon; however, patients with RA have a significantly increased morbidity and mortality from coronary artery disease. The reasons are not clear, but chronic inflammation, some of the medications used to treat RA, and a sedentary lifestyle all may be significant risk factors. Pericardial effusions are common in RA (50% by echocardiography) but usually are asymptomatic. Rarely, long-standing pericardial disease may result in a fibrinous pericarditis, and patients may present clinically with constrictive pericarditis. Uncommonly, rheumatoid nodules occur in the conduction system and cause heart block.
Pulmonary Manifestations
Pulmonary manifestations of RA include pleural effusions, rheumatoid nodules, and parenchymal lung disease. Pleural effusions occur more commonly in men and are usually small and asymptomatic. Of interest, pleural fluid in RA is characterized by low levels of glucose and low pH and, therefore, may at times be confused with empyema. Rheumatoid nodules may occur in the lung, especially in men ( Fig. 285-12 ); these are usually solid but may calcify, cavitate, or become infected. Rarely, pulmonary nodules rupture and produce a pneumothorax. If RA patients are exposed to coal dust, diffuse nodular densities may occur (Caplan's syndrome). Differentiating rheumatoid nodules from lung cancer can be problematic, particularly if the lesion is solitary. Therefore, the presence of pulmonary nodules in a patient with RA should precipitate an aggressive diagnostic evaluation.
FIGURE 285-12 Rheumatoid nodules in the lung. Chest radiograph demonstrates discrete rheumatoid nodules in both right and left lower lobes. (Courtesy of Dr. Martin Lidsky, Houston, TX.)
Diffuse interstitial fibrosis occurs in RA and may progress to a honeycomb appearance on radiography with increasing dyspnea. Rarely, bronchiolitis obliterans can be seen with or without organizing pneumonia. Bronchiolitis obliterans carries a poor prognosis and may occur more often in association with D-penicillamine or gold therapy.
Ophthalmologic Manifestations
The most common manifestation of RA in the eye is keratoconjunctivitis sicca (dry eyes) from secondary Sjgren's syndrome. Patients may have associated xerostomia (dry mouth), parotid gland swelling, or, occasionally, lymphadenopathy. Scleritis can also occur and may be painful, with progression to thinning of the sclera (with deep pigment showing through on physical examination). Scleritis may progress to perforation of the orbit (scleromalacia perforans). Rarely, tendonitis of the superior oblique muscles can result in double vision (Brown's syndrome).
Neurologic Manifestations
Peripheral nerve entrapment syndromes, including carpal tunnel syndrome (median nerve at the wrist) and tarsal tunnel syndrome (anterior tibial nerve at the ankle), are common in RA. Vasculitis can lead to mononeuritis multiplex and a host of additional neurologic problems. Subluxations at C1 C2 may produce myelopathy (see Fig. 285-8 ). Rheumatoid nodules in the CNS have been described but are rare and usually asymptomatic.
Felty's Syndrome
Felty's syndrome is the triad of RA, splenomegaly, and neutropenia. This complication is seen in patients with severe, RF-positive disease and may be accompanied by hepatomegaly, thrombocytopenia, lymphadenopathy, and fevers. Most patients with Felty's syndrome do not require special therapy; instead, treatment should be directed toward their severe RA. If severe neutropenia exists (fewer than 500 cells/ L) and is accompanied by recurrent bacterial infections or chronic, nonhealing leg ulcers, splenectomy may be indicated.
Some patients with RA, who were previously thought to have Felty's syndrome, have peripheral white blood cell counts dominated by large granular lymphocytes with almost complete absence of neutrophils. This condition is known as the large granular lymphocyte syndrome and is thought to be a variant of T-cell leukemia. In the setting of RA, this syndrome has a good prognosis, with the neutropenia often responding dramatically to methotrexate therapy.
Laboratory Findings
Historically, the most characteristic laboratory abnormality in RA is the presence of RF, which is found in approximately 80% of patients. RF was first described in the 1930s and is an antibody that recognizes immunoglobulin G as its antigen. The presence of RF is strongly associated with more severe articular disease as well as with essentially all of the extra-articular features previously discussed. Importantly, RF is seen in association with many diseases other than RA, particularly in disease processes that provide chronic stimulation of the immune system (see Table 285-3 ). AntiCCP antibodies found in approximately 70% of patients with RA have a high specificity (93 to 98%), are often present before clinical disease is diagnosed, and are associated with aggressive erosive disease. RA is associated with many other autoantibodies, including antinuclear antibodies (approximately 30% of patients) and antineutrophil cytoplasmic antibodies, particularly of the perinuclear type (approximately 30% of patients).
Most patients with RA have an anemia of chronic disease. The degree of anemia is proportional to the activity of the disease, and therapy that controls the disease will normalize the hemoglobin levels; rarely, erythropoietin administration may be indicated. Thrombocytosis is common, with platelet counts returning to normal as the inflammation is controlled. Acute phase reactants, ESR, and CRP levels also parallel the activity of the disease, and their persistent elevation portends a poor prognosis, in terms of both joint destruction and mortality. White blood cell counts may be elevated, normal, or, in the case of Felty's syndrome, profoundly depressed. Eosinophilia is present in some patients with RA.
Synovial fluid in RA is characterized by white blood cell counts in the range of 5000 to 100,000/mm3, with approximately two thirds of the cells being polymorphonuclear leukocytes. There are no synovial fluid findings that are pathognomonic of RA.
Treatment
General Measures
RA is a lifelong disease process that has no known cure; the diagnosis is made based on clinical criteria, and many different options exist for treatment. All of these factors magnify the importance of the patient physician relationship and place a premium on the art rather than the science of medicine. Optimal care for patients with RA requires effective ongoing interactions between primary care physicians and rheumatologists, and in some cases physical therapists, occupational therapists, and orthopedic surgeons. Because of the serious nature of the disease, the rapid introduction of new treatments, and the need for expertise in monitoring these therapies, all patients with RA should be monitored by a rheumatologist.
The goal of therapy is disease remission ( Table 285-5 ). If RA is treated early, remission is possible in 20 to 40% of patients. However, remissions require the ongoing use of medications and even then are not always durable. Some combination of nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, and DMARDs is necessary in most patients. In many, or perhaps most, patients with RA, combinations of different DMARDs (conventional and biologic) are necessary for optimal control. Therapy should be escalated rapidly to ensure maximal suppression of disease while minimizing toxicity and expense. Patients with RA should be educated about their disease and its treatment. In most cases, patients should have an opportunity to spend time with physical therapists and occupational therapists to learn about range-of-motion exercises, joint protection, and assistive devices.
Medical Therapy
In the treatment of RA, three types of medical therapies are used: NSAIDs, glucocorticoids, and DMARDs (both conventional and biologic). Initial combination therapy appears to be preferred over monotherapy.[1]
Nonsteroidal Anti-inflammatory Drugs
NSAIDs are important for the symptomatic relief they provide to RA patients; however, they play only a minor role in altering the underlying disease process. Therefore, NSAIDs should rarely, if ever, be used to treat RA without the concomitant use of DMARDs. Many clinicians waste valuable time switching from one NSAID to another before starting DMARD therapy.
Much has been written about the gastrointestinal toxicity of NSAIDs, and these concerns are particularly relevant to RA patients, who often have significant risk factors including age and concomitant steroid use. Therefore, cyclooxygenase-2 (COX2)-selective agents have been a popular choice for patients with RA. The recent evidence linking these agents to increased cardiovascular toxicity has been particularly troubling for patients with RA, who are already at high risk for myocardial infarction. Therefore, if COX2-selective agents are used, they should be kept at a low dose. Consideration should be given to low-dose aspirin prophylaxis in RA, but this may increase the gastrointestinal toxicity of NSAIDs. The use of concomitant misoprostol or proton pump inhibitors should be considered in all patients with RA who are taking NSAIDs. Additionally, the potential for NSAIDs to decrease renal blood flow and to increase blood pressure should be kept in mind.
Glucocorticoids
Glucocorticoids have had a significant role in the treatment of RA for more than half a century. Indeed, RA was chosen as the first disease to be treated with this new therapy, partly because it was thought that RA was a disease of glucocorticoid deficiency (an issue that remains unresolved). As was the case with the first patient treated in 1948, glucocorticoids are dramatically and rapidly effective in patients with RA. Not only are glucocorticoids useful for symptomatic improvement, but they significantly decrease the radiographic progression of RA. However, the toxicities of long-term therapy are extensive and potentially devastating. Therefore, the optimal use of these drugs requires an understanding of several principles ( Table 285-6 ).
Glucocorticoids remain among the most potent anti-inflammatory treatments available; for this reason and because of their rapid onset of action, they are ideally suited to help control the inflammation in RA while the much slower-acting DMARDs are starting to work. Prednisone, the most commonly used glucocorticoid, should rarely be used in doses higher than 10 mg/day to treat
the stiffness and articular manifestations of RA. This dose should be slowly tapered to the lowest effective dose, and the concomitant DMARD therapy should be adjusted to make this possible. Glucocorticoids should rarely, if ever, be used to treat RA without concomitant DMARD therapy. The paradigm is to shut off inflammation rapidly with glucocorticoids and then to taper them as the DMARD is taking effect ( bridge therapy ). In all patients receiving glucocorticoids, strong measure should be taken to prevent osteoporosis. Bisphosphonates have been shown to be particularly effective in this regard. Higher doses of glucocorticoids may be necessary to treat extra-articular manifestations, especially vasculitis and scleritis.
Disease-Modifying Antirheumatic Drugs
DMARDs are a group of medications that have the ability to greatly inhibit the disease process in the synovium and modify or change the disabling potential of RA. In most cases, these drugs have the ability to halt or slow the radiographic progression of RA.
Conventional DMARDs
Included in this group of medications are methotrexate, sulfasalazine (Azulfidine), gold, antimalarials (Plaquenil and others), leflunomide (Arava), azathioprine (Imuran), and minocycline. It is critically important that clinicians and patients understand that conventional DMARDs take 2 to 6 months to exert their maximal effect, and all require some monitoring ( Table 285-7 ). Therefore, other measures such as glucocorticoid therapy may be needed to control the disease while these medications are starting to work.
All of these DMARDs have been shown to be effective in treating both early and more advanced RA that remains active. Until additional research elucidates factors that allow selection of the best initial therapy for each patient, the choice will depend on patient and physician concerns about toxicity and monitoring issues, as well as the activity of disease and comorbid conditions. The critical factor is not which DMARD to start first but getting the DMARD therapy started early in the disease process.
METHOTREXATE.
Methotrexate is the preferred DMARD of most rheumatologists, in part because patients have a more durable response, and because, with correct monitoring, serious toxicities are rare. Methotrexate is dramatically effective in slowing radiographic progression and is usually given orally
in doses ranging from 5 to 25 mg/week as a single dose. This once-a-week administration is worthy of emphasis; prior experience with daily therapy in psoriasis has demonstrated the importance of allowing the liver time to recover between doses. Oral absorption of methotrexate is variable; subcutaneous injections of methotrexate may be effective if oral treatment is not. Side effects of methotrexate include oral ulcers, nausea, hepatotoxicity, bone marrow suppression, and pneumonitis. With the exception of pneumonitis, these toxicities respond to dose adjustments. Monitoring of blood counts and liver blood tests (albumin and aspartate aminotransaminase [SGOT] or alanine aminotransferase [SGPT]) should be done every 4 to 8 weeks, with dosage adjustments as needed. Renal function is critical for clearance of methotrexate; previously stable patients may experience severe toxicities when renal function deteriorates. Pneumonitis, although rare, is less predictable and can be fatal, particularly if the methotrexate is not stopped or is restarted. Folic acid, 1 to 4 mg/day, can significantly decrease most methotrexate toxicities without apparent loss of efficacy. If methotrexate alone does not sufficiently control disease, it is combined with other DMARDs. Methotrexate in combination with virtually any of the other DMARDs (conventional or biologic) has been shown to be more effective than either drug alone.[2]
LEFLUNOMIDE.
Leflunomide, a pyrimidine antagonist, has a very long half-life and is most commonly started at 10 to 20 mg/day orally. A loading dose of 100 mg/day for 3 days was previously recommended, but because it increases diarrhea, the most common toxic effect of this drug, loading treatment is no longer advocated. Diarrhea responds to dose reduction, and doses of leflunomide of 10 to 20 mg three to five times per week are frequently used. Also, because of its long half-life and its teratogenic potential, women wishing to become pregnant who have previously received leflunomide, even if therapy was stopped years ago, should have blood levels drawn. If toxicity occurs or if pregnancy is being considered, leflunomide can be rapidly eliminated from the body by treatment with cholestyramine. Laboratory monitoring for hematologic and hepatic toxicity should be done during treatment with leflunomide, as recommend for methotrexate.
ANTIMALARIAL DRUGS.
The antimalarial drugs hydroxychloroquine (Plaquenil) and chloroquine are frequently used for the treatment of RA. They have the least toxicity of any of the DMARDs and do not require monitoring of blood tests. Yearly monitoring by an ophthalmologist is recommended to detect any signs of retinal toxicity (rare). Hydroxychloroquine is the most commonly used preparation and is given orally at 200 to 400 mg/day. These drugs are frequently used in combination with other DMARDs, particularly methotrexate.[2]
SULFASALAZINE.
Sulfasalazine has been the most commonly used DMARD in Europe. It is an effective treatment when given in doses of 1 to 3 g/day. Monitoring of blood counts, particularly white blood cell counts, in the first 6 months is recommended.
MINOCYCLINE.
Minocycline, 100 mg twice daily, has been shown to be an effective treatment for RA, particularly when used in early, RF-positive disease. Chronic therapy (longer than 2 years) with minocycline may lead to cutaneous hyperpigmentation.
GOLD.
Gold, the oldest DMARD, when given intramuscularly, remains an extremely effective therapy for a small percentage of patients. It is less commonly used because of its slow onset of action, need for intramuscular administration, frequent monitoring required (complete blood count and urinalysis), and frequent toxicities. Toxicities include skin rashes, bone marrow suppression, and proteinuria.
Biologic DMARDs
Recent research has continued to elucidate the central role that cytokines, most notably TNF- and IL-1, play in the pathophysiology of RA. This has led directly to the development and clinical use of biologic agents directed against TNF- 1 (etanercept [3] [Enbrel], infliximab [4] [Remicade], adalimumab [5] [Humira]) and IL-1 (anakinra [Kineret]). Two other biologicals have recently been approved: rituximab (Rituxan) and abatacept (Orencia). All RA patients receiving biologic therapies should be monitored by a rheumatologist, and their physicians should be aware of the risk for infections that are often atypical. Currently, biologic agents should not be used in combination with each other, because all studies to date have shown a significant increase in infections.
ANTI-TNF- DRUGS.
This category of drugs includes etanercept, a recombinant TNF receptor fusion protein that is administered by subcutaneous injection at 50 mg once weekly. Infliximab is a mouse/human chimeric monoclonal antibody against TNF- that is given intravenously (3 to 10 mg/kg) every 4 to 8 weeks. Adalimumab is a human monoclonal antibody against TNF- that is given subcutaneously at 40 mg every other week. All three anti-TNF agents have been shown to be highly effective against both clinical symptoms and radiographic progression of RA, particularly when used in combination with methotrexate. A rapid onset of action (days to weeks) is apparent and is a significant advantage that these treatments have over conventional DMARDs. Current disadvantages include cost and long-term toxicities, in particular infections (especially tuberculosis and others), and malignancies, [6] as well as heart failure, rare demyelinating, and autoimmune syndromes.
ANAKINRA.
Anakinra, a recombinant human IL-1 receptor antagonist, is given subcutaneously at 100 mg/day. It has been shown to be effective against signs and symptoms of RA as well as radiographic progression. Its onset of action is somewhat slower and less dramatic than that of the TNF inhibitors. Toxicities include injection site reactions and pneumonia (especially in patients with asthma).
RITUXIMAB.
Rituximab is a chimeric monoclonal antibody that targets CD20 + B cells and is given intravenously in two infusions of 500 to 1000 mg spaced two weeks apart. This results in marked reductions in circulating B cells for 6 to 12 months and significant clinical responses. The need for and timing of further courses is determined by the patient's ongoing response. Rituximab has been used for years to treat B cell lymphoma.
ABATACEPT.
Abatacept is made by genetically fusing the external domain of human CTLA4 to the heavy-chain of human IgG1 and binds both CD80 and CD86 on antigen presenting cells thus inhibiting T cells from receiving their second signal via CD28. It is administered intravenously 10 mg/kg on days 1, 15, 30 and then monthly.
Treatment of Underlying Conditions
Optimal care of patients with RA requires recognition of the associated comorbid conditions, including an increased risk of cardiovascular death, osteoporosis, infections (especially pneumonia), and certain cancers.
Cardiovascular Disease
Increasingly, cardiovascular disease is being recognized as the cause of much of the excess mortality in RA. A number of factors contribute to this mortality, including sedentary lifestyle, glucocorticoid therapy, and treatments that increase homocysteine levels, such as methotrexate and sulfasalazine. However, recently a strong association between chronic inflammation and cardiovascular disease was identified, and it is likely that this may be the most significant factor. Therapies that control RA earlier and better can be expected to decrease cardiovascular morbidity and mortality. Clinicians should consider RA a risk factor for cardiovascular disease and should aggressively address other cardiovascular risk factors in their rheumatoid patients.
Osteoporosis
Osteoporosis is common in patients with RA, and early treatment results in long-term dividends. Patients with RA are at an increased risk for infections, and some forms of treatment further increase this risk. Patients should be cautioned to seek medical attention early for even minor symptoms suggestive of infection, especially if receiving anti-TNF therapy. All patients with RA should receive a pneumococcal vaccine at appropriate intervals and yearly influenza vaccinations. Finally, patients with RA have an increased risk of lymphoma. Occasionally, B-cell lymphomas are associated with immunosuppression and regress after immunosuppression is discontinued. RA patients have significantly decreased risk (odds ratio, 0.2) of developing colon cancer. This is thought to be secondary to chronic inhibition of COX by NSAIDs.
TABLE 285-5 -- KEYS TO OPTIMIZE OUTCOME OF TREATMENT OF RA Early, accurate diagnosis Early DMARD therapy Strive for remission in all patients Monitor carefully for treatment toxicities
Consider and treat comorbid conditions[*]
DMARD = disease-modifying antirheumatic drug; RA = rheumatoid arthritis.
* Important comorbid conditions include cardiovascular disease, increased susceptibility to infections, and osteoporosis.
TABLE 285-6 -- GUIDELINES FOR USE OF GLUCOCORTICOIDS Avoid use of glucocorticoids without DMARDs Prednisone >10 mg/day is rarely indicated for articular disease Taper to the lowest effective dose Use as bridge therapy until DMARD therapy is effective Remember prophylaxis against osteoporosis
DMARD = disease-modifying antirheumatic drug.
TABLE 285-7 -- CAVEATS FOR MONITORING DMARD THERAPIES[*] Medication Caveats Prednisone Use as bridge to effective DMARD therapy, prophylaxis for osteoporosis? (see Table 2856) Hydroxychloroquine Keep dosage lower than 6.5 mg/kg/day; yearly eye checkup by ophthalmologist Sulfasalazine CBC for neutropenia, initially every month, then every 6 mo Methotrexate CBC and SGOT/SGPT every 4 8 wk; many toxicities respond to folic acid or small dose reduction; if pneumonitis, stop and do not restart; decreasing renal function may precipitate toxicities; absolute contraindication in pregnancy Leflunomide CBC and SGOT/SGPT evert 4 8 wk; long half-life may require cholestyramine washout; absolute contraindication in pregnancy TNF inhibitors If fevers or infectious symptoms of any kind, stop until symptoms resolve; aggressively work-up and treat possible infections; may precipitate congestive heart failure, demyelinating syndromes, or lupus-like syndromes
CBC = compete blood count; DMARD = disease-modifying antirheumatic drug; SGOT = serum glutamate oxaloacetate transaminase (aspartate aminotransferase); SGPT = serum glutamate pyruvate transaminase (alanine aminotransferase); TNF = tumor necrosis factor.
* Patients receiving DMARDs, both conventional and biologic, should be monitored by a rheumatologist.
Prognosis
Until recently, RA was thought to be a relatively benign disease. It is now clear that, once established, RA is a lifelong progressive disease that produces significant morbidity in most patients and premature mortality in many. Long-term studies have found that 50% of patients with RA have had to stop working after 10 years (approximately 10 times the average rate). Patients who are RF or anti-CCP positive and those who are positive for the shared epitope have a worse prognosis with more erosions and more extra-articular disease (see Table 285-4 ). Once deformities are found on examination or erosions on radiography, the damage is largely irreversible. It has been clearly shown that erosions occur in most patients in the first 1 to 2 years and that the rate of radiographic damage can be affected by early therapy. Therefore, early DMARD therapy is critical. Although limited longterm data are available, the current information strongly suggests that patients have the opportunity to benefit greatly if the newer principles of therapy are practiced.
Future Directions
Significant advances in the effective treatment of RA have come from an understanding of the cytokine imbalance that accompanies this disease. Much research is focused on the further development of biologic products to modulate this balance. Biologic therapies that modulate B-cell and T-cell function have been shown to be effective in the treatment of RA and may soon be approved for use. There remains a critical need for a cytokine thermostat that would allow titration of the desired cytokine balance to control disease without altering critical immune functions.
Even with existing therapies, there are many different effective options for patients with RA. The challenge to the clinician is to pick the right option for each patient. Few data are currently available to aid in this choice, and the establishment of parameters, genetic or otherwise, that would allow selection of the best initial option for each patient would be a major breakthrough. Finally, elucidation of the trigger or triggers for RA may allow the development of strategies to prevent onset of the clinical disease.
Email to Colleague Print Version From Tan EM, Cohen AS, Fries JF, et al: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271. * The proposed classification is based on 11 criteria. For the purpose of identifying patients in clinical studies, a person shall be said to have systemic lupus erythematosus if any 4 or more of the 11 criteria are present, serially or simultaneously, during any interval of observation.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Living The Field Energy Therapies by LynnMc TaggartDocument95 pagesLiving The Field Energy Therapies by LynnMc Taggartrooiberg100% (2)
- Awareness To Ginger Herbal Medicine PracticeDocument10 pagesAwareness To Ginger Herbal Medicine PracticeTerence Cerbolles SerranoNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Autoimmune DiseaseDocument38 pagesAutoimmune DiseaseGustiandari FidhyaNo ratings yet
- Healing: ExtraordinaryDocument35 pagesHealing: ExtraordinarymurappanadNo ratings yet
- Gout MythsDocument10 pagesGout MythsemedNo ratings yet
- Pediatric 3 - Questions v1Document50 pagesPediatric 3 - Questions v1Humzala BashamNo ratings yet
- White Book On Hand Surgery in Europe EBHSDocument21 pagesWhite Book On Hand Surgery in Europe EBHSmerdan serinNo ratings yet
- Grand FCS A Chap 6Document38 pagesGrand FCS A Chap 6Katherine 'Chingboo' Leonico LaudNo ratings yet
- Buat Stempel SeminarDocument1 pageBuat Stempel SeminarGustiandari FidhyaNo ratings yet
- Fidhya English VersionDocument4 pagesFidhya English VersionGustiandari FidhyaNo ratings yet
- Tinea Cruris Abq JournalDocument3 pagesTinea Cruris Abq JournalGustiandari FidhyaNo ratings yet
- Categorical Score Physical AcctivityDocument1 pageCategorical Score Physical AcctivityGustiandari FidhyaNo ratings yet
- History Taking in OrthopedicsDocument28 pagesHistory Taking in OrthopedicsKenneth SuliyantoNo ratings yet
- The Work Limitations Questionnaire: January 2002Document3 pagesThe Work Limitations Questionnaire: January 2002Lorraine VillanuevaNo ratings yet
- FNCP Villanueva, KimDocument19 pagesFNCP Villanueva, KimKimberly Perez VillanuevaNo ratings yet
- Connective Tissue Diseases: Dr. Dhikra NabilDocument102 pagesConnective Tissue Diseases: Dr. Dhikra NabilSarahNo ratings yet
- MUSKULOSKELETAL Supplemental SlidesDocument180 pagesMUSKULOSKELETAL Supplemental Slidesstuffednurse100% (1)
- NURS 6501 Knowledge Check: Module 5 Student Response: Scenario 1: GoutDocument16 pagesNURS 6501 Knowledge Check: Module 5 Student Response: Scenario 1: GoutBettNo ratings yet
- IV - PSQ Rheumatism Research Unit, University of Leeds PDFDocument10 pagesIV - PSQ Rheumatism Research Unit, University of Leeds PDFElvia HarlenNo ratings yet
- Homeopathic Materia Medica by Farrington Causticum (Caust) : Lecture Lxxi Lecture LxxiiDocument7 pagesHomeopathic Materia Medica by Farrington Causticum (Caust) : Lecture Lxxi Lecture LxxiimyoxusNo ratings yet
- Lecture 16 Diseases of Bones and Joints (Co)Document14 pagesLecture 16 Diseases of Bones and Joints (Co)Sara Abdul RahmanNo ratings yet
- Musculoskeletal Diseases and DisordersDocument45 pagesMusculoskeletal Diseases and DisordersDiana JunitaNo ratings yet
- MusculoskeletalDocument35 pagesMusculoskeletalZeeshan SiddiquiNo ratings yet
- Anti-Inflammatory and Antiarrhythmic Effects of Beta Blocker in A Rat Model of Rheumatoid ArthritisDocument14 pagesAnti-Inflammatory and Antiarrhythmic Effects of Beta Blocker in A Rat Model of Rheumatoid ArthritisKndratNo ratings yet
- Resume Jurnal 1 RematikDocument10 pagesResume Jurnal 1 RematikAnonymous FEy8SnNo ratings yet
- Rheumatoid ArthritisDocument38 pagesRheumatoid ArthritisOlga GoryachevaNo ratings yet
- Reading Homework Anser KeyDocument21 pagesReading Homework Anser Keyreshma rajeevNo ratings yet
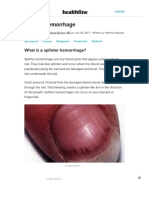
- Splinter Hemorrhages - Symptoms, Causes, and Treatments PDFDocument10 pagesSplinter Hemorrhages - Symptoms, Causes, and Treatments PDFNeymar RonaldoNo ratings yet
- Study of Arthritis: Michael Jean BaskinDocument15 pagesStudy of Arthritis: Michael Jean BaskinGourdet RogerNo ratings yet
- Pediatric JRA: Tan May Vern at Rachel 1000614239Document63 pagesPediatric JRA: Tan May Vern at Rachel 1000614239mayvernNo ratings yet
- Psoriatic Arthritis UpdateDocument42 pagesPsoriatic Arthritis UpdateSudip MajumdarNo ratings yet
- RituximabDocument3 pagesRituximabHoai HoangNo ratings yet
- CH34 Test BankDocument4 pagesCH34 Test BankkrissereiNo ratings yet
- 2024 Rheumatology Student-Clinician HandbookDocument8 pages2024 Rheumatology Student-Clinician HandbookMusimbi MourineNo ratings yet
- Indus Valley EbookDocument56 pagesIndus Valley EbookpriyaNo ratings yet