Professional Documents
Culture Documents
Fluent Chp07-Defining Physical Properties
Uploaded by
Piyush SandujaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fluent Chp07-Defining Physical Properties
Uploaded by
Piyush SandujaCopyright:
Available Formats
Chapter 7.
Phy sical Properties
This chapter describes the physical equations used to compute material
properties and the procedures you can use for each property input. Each
property is described in detail in the following sections. If you are using
one of the general multiphase models (VOF, mixture, or Eulerian), see
Section 20.6.7 for information about how to dene the individual phases,
including their material properties.
Section 7.1: Setting Physical Properties
Section 7.2: Density
Section 7.3: Viscosity
Section 7.4: Thermal Conductivity
Section 7.5: Specic Heat Capacity
Section 7.6: Radiation Properties
Section 7.7: Mass Diusion Coecients
Section 7.8: Standard State Enthalpies
Section 7.9: Standard State Entropies
Section 7.10: Molecular Heat Transfer Coecient
Section 7.11: Kinetic Theory Parameters
Section 7.12: Operating Pressure
Section 7.13: Reference Pressure Location
Section 7.14: Real Gas Model
c Fluent Inc. November 28, 2001 7-1
Phy sical Properties
7.1 Setting Phy sical Properties
An important step in the setup of your model is the denition of the
physical properties of the material. Material properties are dened in
the Materials panel, which allows you to input values for the properties
that are relevant to the problem scope you have dened in the Models
panels. These properties may include the following:
Density and/or molecular weights
Viscosity
Heat capacity
Thermal conductivity
Mass diusion coecients
Standard state enthalpies
Kinetic theory parameters
Properties may be temperature- and/or composition-dependent, with
temperature dependence based on a polynomial, piecewise-linear, or
piecewise-polynomial function and individual component properties ei-
ther dened by you or computed via kinetic theory.
The Materials panel will show the properties that need to be dened for
the active physical models. Note that, if any property you dene re-
quires the energy equation to be solved (e.g., ideal gas law for density,
temperature-dependent prole for viscosity), FLUENT will automatically
activate the energy equation for you. You will then need to dene ther-
mal boundary conditions and other parameters yourself.
Phy sical Properties for Solid Materials
For solid materials, only density, thermal conductivity, and heat capacity
are dened (unless you are modeling semi-transparent media, in which
case radiation properties are also dened). You can specify a constant
value, a temperature-dependent function, or a user-dened function for
7-2 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
thermal conductivity; a constant value or temperature-dependent func-
tion for heat capacity; and a constant value for density.
If you are using the segregated solver, density and heat capacity for a
solid material are not required unless you are modeling unsteady ow
or moving solid zones. Heat capacity will appear in the list of solid
properties for steady ows as well, but the value will be used just for
postprocessing enthalpy; it will not be used in the calculation.
7.1.1 Material Ty pes
In FLUENT, physical properties of uids and solids are associated with
named materials, and these materials are then assigned as boundary
conditions for zones. When you model species transport, you will de-
ne a mixture material, consisting of the various species involved in
the problem. Properties will be dened for the mixture, as well as for
the constituent species, which are uid materials. (The mixture mate-
rial concept is discussed in detail in Section 13.1.2.) Additional material
types are available for the discrete-phase model, as described in Sec-
tion 19.11.2.
Materials can be dened from scratch, or they can be downloaded from a
global (site-wide) database and edited. See Section 7.1.4 for information
about modifying your site-wide material property database.
All the materials that exist in your local materials list will be saved in !
the case le (when you write one). Your materials will be available to
you if you read this case le into a new solver session.
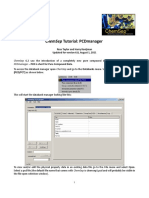
7.1.2 Using the Materials Panel
The Materials panel (Figure 7.1.1) allows you to create new materials,
copy materials from the global database, and modify material properties.
Dene Materials...
These generic functions are described here in this section, and the inputs
for temperature-dependent properties are explained in Section 7.1.3. The
specic inputs for each material property are discussed in the remaining
sections of this chapter.
c Fluent Inc. November 28, 2001 7-3
Phy sical Properties
Figure 7.1.1: The Materials Panel
7-4 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
By default, your local materials list (i.e., in the solver session) will include
a single uid material (air) and a single solid material (aluminum). If the
uid involved in your problem is air, you may use the default properties
for air or modify the properties. If the uid in your problem is water, for
example, you can either copy water from the global database or create a
new water material from scratch. If you copy water from the database,
you can still make modications to the properties of your local copy of
water.
Mixture materials will not exist in your local list unless you have enabled
species transport (see Chapter 13). Similarly, inert, droplet, and com-
busting particle materials will not be available unless you have created a
discrete phase injection of these particle types (see Chapter 19). When a
mixture material is copied from the database, all of its constituent uid
materials (species) will automatically be copied over as well.
Modify ing Properties of an Existing Material
Probably the most common operation you will perform with the Materials
panel is the modication of properties for an existing material. The steps
for this procedure are as follows:
1. Select the type of material (uid, solid, etc.) in the Material Type
drop-down list.
2. Choose the material for which you want to modify properties in
the Fluid Materials drop-down list, Solid Materials list, or other
similarly-named list. (The lists name will be the same as the
material type you selected in step 1.)
3. Make the desired changes to the properties contained in the Prop-
erties area. If there are many properties listed, you may use the
scroll bar to the right of the Properties area to scroll through the
listed items.
4. Click on the Change/Create button to change the properties of the
selected material to your new property settings.
c Fluent Inc. November 28, 2001 7-5
Phy sical Properties
To change the properties of an additional material, repeat the process
described above. Remember to click the Change/Create button after
making the changes to each materials properties.
Renaming an Existing Material
Each material is identied by a name and a chemical formula (if one
exists). You can change a materials name, but you should not change
its chemical formula (unless you are creating a new material, as described
later in this section). The steps for changing a materials name are as
follows:
1. Select the type of material (uid, solid, etc.) in the Material Type
drop-down list.
2. Choose the material for which you want to modify properties in
the Fluid Materials drop-down list, Solid Materials list, or other
similarly-named list. (The lists name will be the same as the
material type you selected in step 1.)
3. Enter the new name in the Name eld at the top of the panel.
4. Click on the Change/Create button. A Question dialog box will
appear, asking you if the original material should be overwritten.
Since you are simply renaming the original material, you can click
Yes to overwrite it. (If you were creating a new material, as de-
scribed later in this section, you would click on No to retain the
original material.)
To rename another material, repeat the process described above. Re-
member to click the Change/Create button after changing each materials
name.
Copy ing a Material from the Database
The global (site-wide) materials database contains many commonly used
uid, solid, and mixture materials, with property data from several dif-
ferent sources [151, 195, 263]. If you wish to use one of these materials
7-6 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
in your problem, you can simply copy it from the database to your local
materials list. The procedure for copying a material is detailed below:
1. Click on the Database... button in the Materials panel to open the
Database Materials panel (Figure 7.1.2).
Figure 7.1.2: The Database Materials Panel
2. Select the type of material (uid, solid, etc.) in the Material Type
drop-down list.
c Fluent Inc. November 28, 2001 7-7
Phy sical Properties
3. In the Fluid Materials list, Solid Materials list, or other similarly-
named list (the lists name will be the same as the material type
you selected in step 2), choose the material you wish to copy. Its
properties will be displayed in the Properties area.
4. If you want to check the materials properties, you can use the scroll
bar to the right of the Properties area to scroll through the listed
items. For some properties, temperature-dependent functions are
available in addition to the constant values. You can select one of
the function types in the drop-down list to the right of the property
and the relevant parameters will be displayed. You cannot edit
these values, but the panels in which they are displayed function
in the same way as those that you use for setting temperature-
dependent property functions, as described in Section 7.1.3.
5. Click on the Copy button. The properties will be downloaded from
the database into your local list, and your own copy of the prop-
erties will now be displayed in the Materials panel.
6. Repeat the process to copy another material, or close the Database
Materials panel.
Once you have copied a material from the database, you may modify its
properties or change its name, as described earlier in this section. The
original material in the database will not be aected by any changes you
make to your local copy of the material.
Creating a New Material
If the material you want to use is not available in the database, you can
easily create a new material for your local list. The steps for creating a
new material are listed below:
1. Select the new materials type (uid, solid, etc.) in the Material Type
drop-down list. (It does not matter which material is selected in
the Fluid Materials, Solid Materials, or other similarly-named list.)
2. Enter the new materials name in the Name eld.
7-8 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
3. Set the materials properties in the Properties area. If there are
many properties listed, you may use the scroll bar to the right of
the Properties area to scroll through the listed items.
4. Click on the Change/Create button. A Question dialog box will
appear, asking you if the original material should be overwritten.
Click on No to retain the original material and add your new ma-
terial to the list. A panel will appear asking you to enter the
chemical formula of your new material. Enter the formula if it is
known, and click on OK; otherwise, leave the formula blank and
click on OK. The Materials panel will be updated to show the new
material name and chemical formula in the Fluid Materials list (or
Solid Materials or other similarly-named list).
Saving Materials and Properties
All the materialsand propertiesin your local list are saved in the case
le when it is written. If you read this case le into a new solver session,
all of your materials and properties will be available for use in the new
session.
Deleting a Material
If there are materials in your local materials list that you no longer need,
you can easily delete them by following these steps:
1. Select the type of material (uid, solid, etc.) in the Material Type
drop-down list.
2. Choose the material to be deleted in the Fluid Materials drop-down
list, Solid Materials list, or other similarly-named list. (The lists
name will be the same as the material type you selected in step 1.)
3. Click on the Delete button.
Deleting materials from your local list will have no eect on the materials
contained in the global database.
c Fluent Inc. November 28, 2001 7-9
Phy sical Properties
Changing the Order of the Materials List
By default, the materials in your local list and those in the database are
listed alphabetically by name (e.g., air, atomic-oxygen (o), carbon-dioxide
(co2)). If you prefer to list them alphabetically by chemical formula, se-
lect the Chemical Formula option under Order Materials By. The example
materials listed above will now be ordered as follows: air, co2 (carbon-
dioxide), o (atomic-oxygen). To change back to the alphabetical listing
by name, choose the Name option under Order Materials By.
Note that you may specify the ordering method separately for the Ma-
terials and Database Materials panels. For example, you can order the
database materials by chemical formula and the local materials list by
name. Each panel has its own Order Materials By options.
7.1.3 Dening Properties Using Temperature-Dependent
Functions
Material properties can be dened as functions of temperature. For most
properties, you can dene a polynomial, piecewise-linear, or piecewise-
polynomial function of temperature:
polynomial:
(T) = A
1
+A
2
T +A
3
T
2
+... (7.1-1)
piecewise-linear:
(T) =
n
+
n+1
n
T
n+1
T
n
(T T
n
) (7.1-2)
where 1 n N and N is the number of segments
piecewise-polynomial:
for T
min,1
< T < T
max,1
: (T) = A
1
+A
2
T +A
3
T
2
+...
for T
min,2
< T < T
max,2
: (T) = B
1
+B
2
T +B
3
T
2
+... (7.1-3)
7-10 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
In the equations above, is the property.
If you dene a polynomial or piecewise-polynomial function of tempera- !
ture, note that temperature in the function is always in units of Kelvin
or Rankine. If you are using Celsius or Kelvin as your temperature unit,
then polynomial coecient values must be entered in terms of Kelvin;
if you are using Fahrenheit or Rankine as the temperature unit, values
must be entered in terms of Rankine.
Some properties have additional functions available, and for some only a
subset of these three functions can be used. See the section on the prop-
erty in question to determine which temperature-dependent functions
you can use.
This section will describe the inputs required for denition of polynomial,
piecewise-linear, and piecewise-polynomial functions.
Inputs for Poly nomial Functions
To dene a polynomial function of temperature for a material property,
follow these steps:
1. In the Materials panel, choose polynomial in the drop-down list to
the right of the property name (e.g., Density). The Polynomial
Prole panel (Figure 7.1.3) will open automatically. (Since this is
a modal panel, the solver will not allow you to do anything else
until you perform the steps below.)
2. Specify the number of Coecients. (Up to 8 coecients are avail-
able.) The number of coecients denes the order of the polyno-
mial. The default of 1 denes a polynomial of order 0: the property
will be constant and equal to the single coecient A
1
; an input of
2 denes a polynomial of order 1: the property will vary linearly
with temperature; etc.
3. Dene the coecients. Coecients 1, 2, 3,... correspond to A
1
,A
2
,
A
3
,... in Equation 7.1-1. The panel in Figure 7.1.3 shows the inputs
for the following function:
c Fluent Inc. November 28, 2001 7-11
Phy sical Properties
Figure 7.1.3: The Polynomial Prole Panel
(T) = 1000 0.02T (7.1-4)
Note the restriction on the units for temperature, as described above. !
7-12 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
Inputs for Piecewise-Linear Functions
To dene a piecewise-linear function of temperature for a material prop-
erty, follow these steps:
1. In the Materials panel, choose piecewise-linear in the drop-down list
to the right of the property name (e.g., Viscosity). The Piecewise-
Linear Prole panel (Figure 7.1.4) will open automatically. (Since
this is a modal panel, the solver will not allow you to do anything
else until you perform the steps below.)
Figure 7.1.4: The Piecewise-Linear Prole Panel
2. Set the number of Points dening the piecewise distribution.
3. Under Data Points, enter the data pairs for each point. First enter
the independent and dependent variable values for Point 1, then
increase the Point number and enter the appropriate values for each
additional pair of variables. The pairs of points must be supplied
in the proper order (by increasing value of temperature); the solver
will not sort them for you. A maximum of 30 piecewise points can
be dened for each property. The panel in Figure 7.1.4 shows the
nal inputs for the prole depicted in Figure 7.1.5.
If the temperature exceeds the maximum Temperature (T
max
) you !
have specied for your prole, FLUENT will use the Value corre-
sponding to T
max
. If the temperature falls below the minimum
c Fluent Inc. November 28, 2001 7-13
Phy sical Properties
Temperature (T
min
) you have specied for your prole, FLUENT
will use the Value corresponding to T
min
.
250 300 350 400 450
Temperature, T (K)
V
i
s
c
o
s
i
t
y
,
1
0
(
P
a
-
s
e
c
)
5
1
2
3
(250, 1.599 10 )
(300, 1.846 10 )
(360, 2.117 10 )
(440, 2.445 10 )
-5
-5
-5
-5
Figure 7.1.5: Piecewise-Linear Denition of Viscosity as (T)
Inputs for Piecewise-Poly nomial Functions
To dene a piecewise-polynomial function of temperature for a material
property, follow these steps:
1. In the Materials panel, choose piecewise-polynomial in the drop-
down list to the right of the property name (e.g., Cp). The Piece-
wise Polynomial Prole panel (Figure 7.1.6) will open automatically.
(Since this is a modal panel, the solver will not allow you to do any-
thing else until you perform the steps below.)
2. Specify the number of Ranges. For the example of Equation 7.1-5,
two ranges of temperatures are dened:
for 300 < T < 1000 :
c
p
(T) = 429.929 + 1.874T 1.966 10
3
T
2
+ 1.297 10
6
T
3
4.000 10
10
T
4
for 1000 < T < 5000 :
7-14 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
Figure 7.1.6: The Piecewise Polynomial Prole Panel
c
p
(T) = 841.377 + 0.593T 2.415 10
4
T
2
+ 4.523 10
8
T
3
3.153 10
12
T
4
(7.1-5)
You may dene up to 3 ranges. The ranges must be supplied in
the proper order (by increasing value of temperature); the solver
will not sort them for you.
3. For the rst range (Range = 1), specify the Minimum and Maximum
temperatures, and the number of Coecients. (Up to 8 coecients
are available.) The number of coecients denes the order of the
polynomial. The default of 1 denes a polynomial of order 0: the
property will be constant and equal to the single coecient A
1
; an
input of 2 denes a polynomial of order 1: the property will vary
linearly with temperature; etc.
4. Dene the coecients. Coecients 1, 2, 3,... correspond to A
1
,A
2
,
A
3
,... in Equation 7.1-3. The panel in Figure 7.1.6 shows the inputs
for the rst range of Equation 7.1-5.
c Fluent Inc. November 28, 2001 7-15
Phy sical Properties
5. Increase the value of Range and enter the Minimum and Maximum
temperatures, number of Coecients, and the Coecients (B
1
,B
2
,
B
3
,...) for the next range. Repeat if there is a third range.
Note the restriction on the units for temperature, as described above. !
Checking and Modify ing Existing Proles
If you want to check or change the coecients, data pairs, or ranges for
a previously-dened prole, click on the Edit... button to the right of the
property name. The appropriate panel will open, and you can check or
modify the inputs as desired.
In the Database Materials panel, you cannot edit the proles, but you can !
examine them by clicking on the View... button (instead of the Edit...
button.)
7.1.4 Customizing the Materials Database
The materials database is located in the following le:
path/Fluent.Inc/fluent6.
x/cortex/lib/propdb.scm
where path is the directory in which you have installed FLUENT and the
variable x corresponds to your release version, e.g., 0 for fluent6.0.
If you wish to enhance the materials database to include additional ma-
terials that you use frequently, you can follow the procedure below:
1. Copy the le propdb.scm from the directory listed above to your
working directory.
2. Using a text editor, add the desired material(s) following the for-
mat for the other materials. You may want to copy an existing
material that is similar to the one you want to add, and then
change its name and properties. The entries for air and aluminum
are shown below as examples:
7-16 c Fluent Inc. November 28, 2001
7.1 Setting Phy sical Properties
(air
fluid
(chemical-formula . #f)
(density (constant . 1.225)
(premixed-combustion 1.225 300))
(specific-heat (constant . 1006.43))
(thermal-conductivity (constant . 0.0242))
(viscosity (constant . 1.7894e-05)
(sutherland 1.7894e-05 273.11 110.56)
(power-law 1.7894e-05 273.11 0.666))
(molecular-weight (constant . 28.966))
)
(aluminum
(solid)
(chemical-formula . al)
(density (constant . 2719))
(specific-heat (constant . 871))
(thermal-conductivity (constant . 202.4))
(formation-entropy (constant . 164448.08))
)
When you next load the materials database in a FLUENT session started
in the working directory, FLUENT will load your modied propdb.scm,
rather than the original database, and your custom materials will be
available in the Database Materials panel.
If you want to make the modied database available to others at your
site, you can put your customized propdb.scm le in the cortex/lib
directory, replacing the default database. Before doing so, you should
save the original propdb.scm le provided by Fluent Inc. to a dierent
name (e.g., propdb.scm.orig) in case you need it at a later time.
c Fluent Inc. November 28, 2001 7-17
Phy sical Properties
7.2 Density
FLUENT provides several options for denition of the uid density:
constant density
temperature- and/or composition-dependent density
Each of these input options and the governing physical models are de-
tailed in this section. In all cases, you will dene the Density in the
Materials panel.
Dene Materials...
7.2.1 Dening Density for Various Flow Regimes
The selection of density in FLUENT is very important. You should set
the density relationship based on your ow regime.
For compressible ows, the ideal gas law is the appropriate density
relationship.
For incompressible ows, you may choose one of the following meth-
ods:
Constant density should be used if you do not want density
to be a function of temperature.
The incompressible ideal gas law should be used when pres-
sure variations are small enough that the ow is fully incom-
pressible but you wish to use the ideal gas law to express
the relationship between density and temperature (e.g., for a
natural convection problem).
Density as a polynomial, piecewise-linear, or piecewise-polyno-
mial function of temperature is appropriate when the density
is a function of temperature only, as in a natural convection
problem.
The Boussinesq model can be used for natural convection
problems involving small changes in temperature.
7-18 c Fluent Inc. November 28, 2001
7.2 Density
Mixing Density Relationships in Multiple-Zone Models
If your model has multiple uid zones that use dierent materials, you
should be aware of the following:
For calculations with the segregated solver that do not use one
of the general multiphase models, the compressible ideal gas law
cannot be mixed with any other density methods. This means that
if the compressible ideal gas law is used for one material, it must
be used for all materials.
This restriction does not apply to the coupled solvers.
There is only one specied operating pressure and one specied op-
erating temperature. This means that if you are using the ideal gas
law for more than one material, they will share the same operating
pressure; and if you are using the Boussinesq model for more than
one material, they will share the same operating temperature.
7.2.2 Input of Constant Density
If you want to dene the density of your uid as a constant, check that
constant is selected in the drop-down list to the right of Density in the
Materials panel, and enter the value of density for the material.
For the default uid (air), the density is 1.225 kg/m
3
.
7.2.3 Inputs for the Boussinesq Approximation
To enable the Boussinesq approximation for density, choose boussinesq
from the drop-down list to the right of Density in the Materials panel
and specify a constant value for Density. You will also need to set the
Thermal Expansion Coecient, as well as relevant operating conditions,
as described in Section 11.5.3.
7.2.4 Density as a Prole Function of Temperature
If you are modeling a problem that involves heat transfer, you can dene
the density as a function of temperature. Three types of functions are
available:
c Fluent Inc. November 28, 2001 7-19
Phy sical Properties
piecewise-linear:
(T) =
n
+
n+1
n
T
n+1
T
n
(T T
n
) (7.2-1)
piecewise-polynomial:
for T
min,1
< T < T
max,1
: (T) = A
1
+A
2
T +A
3
T
2
+... (7.2-2)
for T
min,2
< T < T
max,2
: (T) = B
1
+B
2
T +B
3
T
2
+... (7.2-3)
polynomial:
(T) = A
1
+A
2
T +A
3
T
2
+... (7.2-4)
For one of the these methods, select piecewise-linear, piecewise-polynomial,
or polynomial in the drop-down list to the right of Density. You can in-
put the data pairs (T
n
,
n
), ranges and coecients, or coecients that
describe these functions using the Materials panel, as described in Sec-
tion 7.1.3.
7.2.5 Incompressible Ideal Gas Law
In FLUENT, if you choose to dene the density using the ideal gas law
for an incompressible ow, the solver will compute the density as
=
p
op
R
Mw
T
(7.2-5)
where R is the universal gas constant, M
w
is the molecular weight of the
gas, and p
op
is dened by you as the Operating Pressure in the Operating
Conditions panel. In this form, the density depends only on the operating
pressure and not on the local relative pressure eld.
7-20 c Fluent Inc. November 28, 2001
7.2 Density
Density Inputs for the Incompressible Ideal Gas Law
Inputs for the incompressible ideal gas law are as follows:
1. Enable the ideal gas law for an incompressible uid by choosing
incompressible-ideal-gas from the drop-down list to the right of Den-
sity in the Materials panel.
You must specify the incompressible ideal gas law individually for
each material that you want to use it for. See Section 7.2.7 for
information on specifying the incompressible ideal gas law for mix-
tures.
2. Set the operating pressure by dening the Operating Pressure in
the Operating Conditions panel.
Dene Operating Conditions...
The input of the operating pressure is of great importance when !
you are computing density with the ideal gas law. See Section 7.12
for recommendations on setting appropriate values for the operat-
ing pressure. Operating pressure is, by default, set to 101325 Pa.
3. Set the molecular weight of the homogeneous or single-component
uid (if no chemical species transport equations are to be solved),
or the molecular weights of each uid material (species) in a mul-
ticomponent mixture. For each uid material, enter the value of
the Molecular Weight in the Materials panel.
7.2.6 Ideal Gas Law for Compressible Flows
For compressible ows, the gas law has the following form:
=
p
op
+p
R
Mw
T
(7.2-6)
where p is the local relative (or gauge) pressure predicted by FLUENT
and p
op
is dened by you as the Operating Pressure in the Operating
Conditions panel.
c Fluent Inc. November 28, 2001 7-21
Phy sical Properties
Density Inputs for the Ideal Gas Law for Compressible Flows
Inputs for the ideal gas law are as follows:
1. Enable the ideal gas law for a compressible uid by choosing ideal-
gas from the drop-down list to the right of Density in the Materials
panel.
You must specify the ideal gas law individually for each material
that you want to use it for. See Section 7.2.7 for information on
specifying the ideal gas law for mixtures.
2. Set the operating pressure by dening the Operating Pressure in
the Operating Conditions panel.
Dene Operating Conditions...
The input of the operating pressure is of great importance when !
you are computing density with the ideal gas law. Equation 7.2-6
notes that the operating pressure is added to the relative pressure
eld computed by the solver, yielding the absolute static pressure.
See Section 7.12 for recommendations on setting appropriate values
for the operating pressure. Operating pressure is, by default, set
to 101325 Pa.
3. Set the molecular weight of the homogeneous or single-component
uid (if no chemical species transport equations are to be solved),
or the molecular weights of each uid material (species) in a mul-
ticomponent mixture. For each uid material, enter the value of
the Molecular Weight in the Materials panel.
7.2.7 Composition-Dependent Density for Multicomponent
Mixtures
If you are solving species transport equations, you will need to set prop-
erties for the mixture material and for the constituent uids (species), as
described in detail in Section 13.1.4. To dene a composition-dependent
density for a mixture, follow these steps:
1. Select the density method:
7-22 c Fluent Inc. November 28, 2001
7.2 Density
For non-ideal-gas mixtures, select the volume-weighted-mixing-
law method for the mixture material in the drop-down list to
the right of Density in the Materials panel.
If you are modeling compressible ow, select ideal-gas for the
mixture material in the drop-down list to the right of Density
in the Materials panel.
If you are modeling incompressible ow using the ideal gas
law, select incompressible-ideal-gas for the mixture material
in the drop-down list to the right of Density in the Materials
panel.
2. Click on Change/Create.
3. If you selected volume-weighted-mixing-law, dene the density for
each of the uid materials that comprise the mixture. You may
dene constant or (if applicable) temperature-dependent densities
for the individual species.
If you are modeling a non-ideal-gas mixture, FLUENT will compute the
mixture density as
=
1
i
Y
i
i
(7.2-7)
where Y
i
is the mass fraction and
i
is the density of species i.
For compressible ows, the gas law has the following form:
=
p
op
+p
RT
i
Y
i
M
w,i
(7.2-8)
where p is the local relative (or gauge) pressure predicted by FLUENT,
R is the universal gas constant, Y
i
is the mass fraction of species i, M
w,i
is the molecular weight of species i, and p
op
is dened by you as the
Operating Pressure in the Operating Conditions panel.
In FLUENT, if you choose to dene the density using the ideal gas law
for an incompressible ow, the solver will compute the density as
c Fluent Inc. November 28, 2001 7-23
Phy sical Properties
=
p
op
RT
i
Y
i
M
w,i
(7.2-9)
where R is the universal gas constant, Y
i
is the mass fraction of species
i, M
w,i
is the molecular weight of species i, and p
op
is dened by you as
the Operating Pressure in the Operating Conditions panel. In this form,
the density depends only on the operating pressure and not on the local
relative pressure eld.
7.3 Viscosity
FLUENT provides several options for denition of the uid viscosity:
Constant viscosity
Temperature- and/or composition-dependent viscosity
Kinetic theory
Non-Newtonian viscosity
User-dened function
Each of these input options and the governing physical models are de-
tailed in this section. (User-dened functions are described in the sep-
arate UDF Manual.) In all cases, you will dene the Viscosity in the
Materials panel.
Dene Materials...
Viscosities are input as dynamic viscosity () in units of kg/m-s in SI
units or lb
m
/ft-s in British units. FLUENT does not ask for input of the
kinematic viscosity ().
7.3.1 Input of Constant Viscosity
If you want to dene the viscosity of your uid as a constant, check that
constant is selected in the drop-down list to the right of Viscosity in the
Materials panel, and enter the value of viscosity for the uid.
For the default uid (air), the viscosity is 1.7894 10
5
kg/m-s.
7-24 c Fluent Inc. November 28, 2001
7.3 Viscosity
7.3.2 Viscosity as a Function of Temperature
If you are modeling a problem that involves heat transfer, you can dene
the viscosity as a function of temperature. Five types of functions are
available:
piecewise-linear:
(T) =
n
+
n+1
n
T
n+1
T
n
(T T
n
) (7.3-1)
piecewise-polynomial:
for T
min,1
< T < T
max,1
: (T) = A
1
+A
2
T +A
3
T
2
+... (7.3-2)
for T
min,2
< T < T
max,2
: (T) = B
1
+B
2
T +B
3
T
2
+... (7.3-3)
polynomial:
(T) = A
1
+A
2
T +A
3
T
2
+... (7.3-4)
Sutherlands law (described below)
Power law (described below)
Note that the power law described below is dierent from the non- !
Newtonian power law described in Section 7.3.5.
For one of the rst three, select piecewise-linear, piecewise-polynomial,
polynomial in the drop-down list to the right of Viscosity, and then in-
put the data pairs (T
n
,
n
), ranges and coecients, or coecients that
describe these functions using the Materials panel, as described in Sec-
tion 7.1.3. For Sutherlands law or the power law, choose sutherland or
power-law in the drop-down list and enter the parameters as described
below.
c Fluent Inc. November 28, 2001 7-25
Phy sical Properties
Sutherland Viscosity Law
Sutherlands viscosity law resulted from a kinetic theory by Sutherland
(1893) using an idealized intermolecular-force potential. The formula is
specied using two or three coecients.
Sutherlands law with two coecients has the form
=
C
1
T
3/2
T +C
2
(7.3-5)
where is the viscosity in kg/m-s, T is the static temperature in K, and
C
1
and C
2
are the coecients. For air at moderate temperatures and
pressures, C
1
= 1.458 10
6
kg/m-s-K
1/2
, and C
2
= 110.4 K.
Sutherlands law with three coecients has the form
=
0
T
T
0
3/2
T
0
+S
T +S
(7.3-6)
where is the viscosity in kg/m-s, T is the static temperature in K,
0
is a reference value in kg/m-s, T
0
is a reference temperature in K,
and S is an eective temperature in K, called the Sutherland constant,
which is characteristic of the gas. For air at moderate temperatures and
pressures,
0
= 1.789410
5
kg/m-s, T
0
= 273.11 K, and S = 110.56 K.
Inputs for Sutherlands Law
To use Sutherlands law, choose sutherland in the drop-down list to the
right of Viscosity. The Sutherland Law panel will open, and you can enter
the coecients as follows:
1. Select the Two Coecient Method or the Three Coecient Method.
Note that you must use SI units if you choose the two-coecient !
method.
2. For the Two Coecient Method, set C1 and C2. For the Three
Coecient Method, set the Reference Viscosity
0
, the Reference
Temperature T
0
, and the Eective Temperature S.
7-26 c Fluent Inc. November 28, 2001
7.3 Viscosity
Power-Law Viscosity Law
Another common approximation for the viscosity of dilute gases is the
power-law form. For dilute gases at moderate temperatures, this form is
considered to be slightly less accurate than Sutherlands law.
A power-law viscosity law with two coecients has the form
= BT
n
(7.3-7)
where is the viscosity in kg/m-s, T is the static temperature in K, and
B is a dimensional coecient. For air at moderate temperatures and
pressures, B = 4.093 10
7
, and n = 2/3.
A power-law viscosity law with three coecients has the form
=
0
T
T
0
n
(7.3-8)
where is the viscosity in kg/m-s, T is the static temperature in K,
T
0
is a reference value in K,
0
is a reference value in kg/m-s. For air
at moderate temperatures and pressures,
0
= 1.716 10
5
kg/m-s,
T
0
= 273 K, and n = 2/3.
The non-Newtonian power law for viscosity is described in Section 7.3.5. !
Inputs for the Power Law
To use the power law, choose power-law in the drop-down list to the
right of Viscosity. The Power Law panel will open, and you can enter the
coecients as follows:
1. Select the Two Coecient Method or the Three Coecient Method.
Note that you must use SI units if you choose the two-coecient !
method.
2. For the Two Coecient Method, set B and the Temperature Expo-
nent n. For the Three Coecient Method, set the Reference Viscosity
c Fluent Inc. November 28, 2001 7-27
Phy sical Properties
0
, the Reference Temperature T
0
, and the Temperature Exponent
n.
7.3.3 Dening the Viscosity Using Kinetic Theory
If you are using the gas law (as described in Section 7.2), you have the
option to dene the uid viscosity using kinetic theory as
= 2.67 10
6
M
w
T
(7.3-9)
where is in units of kg/m-s, T is in units of Kelvin, is in units of
Angstroms, and
(T
) where
T
=
T
(/k
B
)
(7.3-10)
The Lennard-Jones parameters, and /k
B
, are inputs to the kinetic
theory calculation that you supply by selecting kinetic-theory from the
drop-down list to the right of Viscosity in the Materials panel. The solver
will use these kinetic theory inputs in Equation 7.3-9 to compute the
uid viscosity. See Section 7.11 for details about these inputs.
7.3.4 Composition-Dependent Viscosity for Multicomponent
Mixtures
If you are modeling a ow that includes more than one chemical species
(multicomponent ow), you have the option to dene a composition-
dependent viscosity. (Note that you can also dene the viscosity of the
mixture as a constant value or a function of temperature.)
To dene a composition-dependent viscosity for a mixture, follow these
steps:
1. For the mixture material, choose mass-weighted-mixing-law or, if
you are using the ideal gas law for density, ideal-gas-mixing-law in
the drop-down list to the right of Viscosity.
7-28 c Fluent Inc. November 28, 2001
7.3 Viscosity
2. Click Change/Create.
3. Dene the viscosity for each of the uid materials that comprise the
mixture. You may dene constant or (if applicable) temperature-
dependent viscosities for the individual species. You may also
use kinetic theory for the individual viscosities, or specify a non-
Newtonian viscosity, if applicable.
If you are using the ideal gas law, the solver will compute the mixture
viscosity based on kinetic theory as
=
i
X
i
j
X
i
ij
(7.3-11)
where
ij
=
1 +
1/2
M
w,j
M
w,i
1/4
1 +
M
w,i
M
w,j
1/2
(7.3-12)
and X
i
is the mole fraction of species i.
For non-ideal gas mixtures, the mixture viscosity is computed based on
a simple mass fraction average of the pure species viscosities:
=
i
Y
i
i
(7.3-13)
c Fluent Inc. November 28, 2001 7-29
Phy sical Properties
7.3.5 Viscosity for Non-Newtonian Fluids
For incompressible Newtonian uids, the shear stress is proportional to
the rate-of-deformation tensor D:
= D (7.3-14)
where D is dened by
D =
u
j
x
i
+
u
i
x
j
(7.3-15)
and is the viscosity, which is independent of D.
For some non-Newtonian uids, the shear stress can similarly be written
in terms of a non-Newtonian viscosity :
=
D (7.3-16)
In general, is a function of all three invariants of the rate-of-deformation
tensor D. However, in the non-Newtonian models available in FLUENT,
is considered to be a function of the shear rate only. is related to
the second invariant of D and is dened as
=
D : D (7.3-17)
FLUENT provides four options for modeling non-Newtonian ows:
Power law
Carreau model for pseudo-plastics
Cross model
Herschel-Bulkley model for Bingham plastics
7-30 c Fluent Inc. November 28, 2001
7.3 Viscosity
Note that the non-Newtonian power law described below is dierent from !
the power law described in Section 7.3.2.
Appropriate values for the input parameters for these models can be
found in the literature (e.g., [238]).
Power Law for Non-Newtonian Viscosity
If you choose non-newtonian-power-law in the drop-down list to the right
of Viscosity, non-Newtonian ow will be modeled according to the fol-
lowing power law for the non-Newtonian viscosity:
= k
n1
e
T
0
/T
(7.3-18)
FLUENT allows you to place upper and lower limits on the power law
function, yielding the following equation:
min
< = k
n1
e
T
0
/T
<
max
(7.3-19)
where k, n, T
0
,
min
, and
max
are input parameters. k is a measure of
the average viscosity of the uid (the consistency index); n is a measure
of the deviation of the uid from Newtonian (the power-law index), as
described below; T
0
is the reference temperature; and
min
and
max
are,
respectively, the lower and upper limits of the power law. If the viscosity
computed from the power law is less than
min
, the value of
min
will be
used instead. Similarly, if the computed viscosity is greater than
max
,
the value of
max
will be used instead. Figure 7.3.1 shows how viscosity
is limited by
min
and
max
at low and high shear rates.
The value of n determines the class of the uid:
n = 1 Newtonian uid
n > 1 shear-thickening (dilatant uids)
n < 1 shear-thinning (pseudo-plastics)
Inputs for the Non-Newtonian Power Law
To use the non-Newtonian power law, choose non-newtonian-power-law
in the drop-down list to the right of Viscosity. The Non-Newtonian Power
c Fluent Inc. November 28, 2001 7-31
Phy sical Properties
max
min
log
log
.
Figure 7.3.1: Variation of Viscosity with Shear Rate According to the
Non-Newtonian Power Law
Law panel will open, and you can enter the Consistency Index k, Power-
Law Index n, Reference Temperature T
0
, Minimum Viscosity Limit
min
, and
Maximum Viscosity Limit
max
. For temperature-independent viscosity,
the value of T
0
should be set to zero. If the energy equation is not being
solved, FLUENT uses a default value of T=273 K in Equation 7.3-18.
The Carreau Model for Pseudo-Plastics
The power law model described in Equation 7.3-18 results in a uid
viscosity that varies with shear rate. For 0,
0
, and for ,
, where
0
and
are, respectively, the upper and lower limiting
values of the uid viscosity.
The Carreau model attempts to describe a wide range of uids by the
establishment of a curve-t to piece together functions for both Newto-
nian and shear-thinning (n < 1) non-Newtonian laws. In the Carreau
model, the viscosity is
=
+ (
0
)[1 + ( e
T
0
/T
)
2
]
(n1)/2
(7.3-20)
where the parameters n, , T
0
,
0
, and
are dependent upon the
uid. is the time constant, n is the power-law index (as described
7-32 c Fluent Inc. November 28, 2001
7.3 Viscosity
above for the non-Newtonian power law), T
0
is the reference temperature,
and
0
and
are, respectively, the zero- and innite-shear viscosities.
Figure 7.3.2 shows how viscosity is limited by
0
and
at low and high
shear rates.
log
log
.
Figure 7.3.2: Variation of Viscosity with Shear Rate According to the
Carreau Model
Inputs for the Carreau Model
To use the Carreau model, choose carreau in the drop-down list to the
right of Viscosity. The Carreau Model panel will open, and you can enter
the Time Constant , Power-Law Index n, Reference Temperature T
0
, Zero
Shear Viscosity
0
, and Innite Shear Viscosity
. For temperature-
independent viscosity, the value of T
0
should be set to zero. If the energy
equation is not being solved, FLUENT uses a default value of T=273 K
in Equation 7.3-20.
Cross Model
The Cross model for viscosity is
=
0
1 + ( )
1n
(7.3-21)
c Fluent Inc. November 28, 2001 7-33
Phy sical Properties
where
0
= zero-shear-rate viscosity
= natural time (i.e., inverse of the shear rate
at which the uid changes from Newtonian to
power-law behavior)
n = power-law index
The Cross model is commonly used when it is necessary to describe the
low-shear-rate behavior of the viscosity.
Inputs for the Cross Model
To use the Cross model, choose cross in the drop-down list to the right of
Viscosity. The Cross Model panel will open, and you can enter the Time
Constant , Power-Law Index n, and Zero Shear Viscosity
0
.
Herschel-Bulkley Model for Bingham Plastics
The power law model described above is valid for uids for which the
shear stress is zero when the strain rate is zero. Bingham plastics are
characterized by a non-zero shear stress when the strain rate is zero:
=
0
+D (7.3-22)
where
0
is the yield stress:
For <
0
, the material remains rigid.
For >
0
, the material ows as a power-law uid.
The Herschel-Bulkley model combines the eects of Bingham and power-
law behavior in a uid. For low strain rates ( <
0
/
0
), the rigid
material acts like a very viscous uid with viscosity
0
. As the strain rate
increases and the yield stress threshold,
0
, is passed, the uid behavior
is described by a power law.
=
0
+k[
n
(
0
/
0
)
n
]
(7.3-23)
7-34 c Fluent Inc. November 28, 2001
7.3 Viscosity
where k is the consistency factor, and n is the power-law index.
Figure 7.3.3 shows how shear stress () varies with shear rate ( ) for the
Herschel-Bulkley model.
0
0
Bingham (n=1)
0 < n < 1
n > 1
.
.
Figure 7.3.3: Variation of Shear Stress with Shear Rate According to the
Herschel-Bulkley Model
If you choose the Herschel-Bulkley model for Bingham plastics, Equa-
tion 7.3-23 will be used to determine the uid viscosity.
The Herschel-Bulkley model is commonly used to describe materials such
as concrete, mud, dough, and toothpaste, for which a constant viscosity
after a critical shear stress is a reasonable assumption. In addition to
the transition behavior between a ow and no-ow regime, the Herschel-
Bulkley model can also exhibit a shear-thinning or shear-thickening be-
havior depending on the value of n.
Inputs for the Herschel-Bulkley Model
To use the Herschel-Bulkley model, choose herschel-bulkley in the drop-
down list to the right of Viscosity. The Herschel-Bulkley panel will open,
and you can enter the Consistency Index k, Power-Law Index n, Yield Stress
Threshold
0
, and Yielding Viscosity
0
.
c Fluent Inc. November 28, 2001 7-35
Phy sical Properties
7.4 Thermal Conductivity
The thermal conductivity must be dened when heat transfer is active.
You will need to dene thermal conductivity when you are modeling
energy and viscous ow.
FLUENT provides several options for denition of the thermal conduc-
tivity:
Constant thermal conductivity
Temperature- and/or composition-dependent thermal conductivity
Kinetic theory
User-dened function
Anisotropic/orthotropic (for solid materials only)
Each of these input options and the governing physical models are de-
tailed in this section. (User-dened functions are described in the sepa-
rate UDF Manual.) In all cases, you will dene the Thermal Conductivity
in the Materials panel.
Dene Materials...
Thermal conductivity is dened in units of W/m-K in SI units or
BTU/hr-ft-R in British units.
7.4.1 Input of Constant Thermal Conductivity
If you want to dene the thermal conductivity as a constant, check that
constant is selected in the drop-down list to the right of Thermal Conduc-
tivity in the Materials panel, and enter the value of thermal conductivity
for the material.
For the default uid (air), the thermal conductivity is 0.0242 W/m-K.
7-36 c Fluent Inc. November 28, 2001
7.4 Thermal Conductivity
7.4.2 Thermal Conductivity as a Function of Temperature
You can also choose to dene the thermal conductivity as a function of
temperature. Three types of functions are available:
piecewise-linear:
k(T) = k
n
+
k
n+1
k
n
T
n+1
T
n
(T T
n
) (7.4-1)
piecewise-polynomial:
for T
min,1
< T < T
max,1
: k(T) = A
1
+A
2
T +A
3
T
2
+... (7.4-2)
for T
min,2
< T < T
max,2
: k(T) = B
1
+B
2
T +B
3
T
2
+... (7.4-3)
polynomial:
k(T) = A
1
+A
2
T +A
3
T
2
+... (7.4-4)
You can input the data pairs (T
n
, k
n
), ranges and coecients A
i
and B
i
,
or coecients A
i
that describe these functions using the Materials panel,
as described in Section 7.1.3.
7.4.3 Dening the Thermal Conductivity Using Kinetic Theory
If you are using the gas law (as described in Section 7.2), you have the
option to dene the thermal conductivity using kinetic theory as
k =
15
4
R
M
w
4
15
c
p
M
w
R
+
1
3
(7.4-5)
where R is the universal gas constant, M
w
is the molecular weight, is
the materials specied or computed viscosity, and c
p
is the materials
specied or computed specic heat capacity.
c Fluent Inc. November 28, 2001 7-37
Phy sical Properties
To enable the use of this equation for calculating thermal conductivity,
select kinetic-theory from the drop-down list to the right of Thermal Con-
ductivity in the Materials panel. The solver will use Equation 7.4-5 to
compute the thermal conductivity.
7.4.4 Composition-Dependent Thermal Conductivity for
Multicomponent Mixtures
If you are modeling a ow that includes more than one chemical species
(multicomponent ow), you have the option to dene a composition-
dependent thermal conductivity. (Note that you can also dene the
thermal conductivity of the mixture as a constant value or a function of
temperature, or using kinetic theory.)
To dene a composition-dependent thermal conductivity for a mixture,
follow these steps:
1. For the mixture material, choose mass-weighted-mixing-law or, if
you are using the ideal gas law, ideal-gas-mixing-law in the drop-
down list to the right of Thermal Conductivity.
If you use ideal-gas-mixing-law for the thermal conductivity of a !
mixture, you must use ideal-gas-mixing-lawor mass-weighted-mixing-
law for viscosity, because these two viscosity specication methods
are the only ones that allow specication of the component viscosi-
ties, which are used in the ideal gas law for thermal conductivity
(Equation 7.4-6).
2. Click Change/Create.
3. Dene the thermal conductivity for each of the uid materials that
comprise the mixture. You may dene constant or (if applicable)
temperature-dependent thermal conductivities for the individual
species. You may also use kinetic theory for the individual thermal
conductivities, if applicable.
If you are using the ideal gas law, the solver will compute the mixture
thermal conductivity based on kinetic theory as
7-38 c Fluent Inc. November 28, 2001
7.4 Thermal Conductivity
k =
i
X
i
k
i
j
X
j
ij
(7.4-6)
where
ij
=
1 +
1/2
M
w,j
M
w,i
1/4
1 +
M
w,i
M
w,j
1/2
(7.4-7)
and X
i
is the mole fraction of species i.
For non-ideal gases, the mixture thermal conductivity is computed based
on a simple mass fraction average of the pure species conductivities:
k =
i
Y
i
k
i
(7.4-8)
7.4.5 Anisotropic Thermal Conductivity for Solids
The anisotropic conductivity option in FLUENT solves the conduction
equation in solids with the thermal conductivity specied as a matrix.
The heat ux vector is written as
q
i
= k
ij
T
x
j
(7.4-9)
Two options are available: orthotropic and general anisotropic.
Note that the anisotropic conductivity options are available only with !
the segregated solver; you cannot use them with the coupled solvers.
Orthotropic Thermal Conductivity
When the orthotropic thermal conductivity is used, the thermal conduc-
tivities (k
, k
, k
) in the principal directions ( e
, e
, e
) are specied.
The conductivity matrix is then computed as
c Fluent Inc. November 28, 2001 7-39
Phy sical Properties
k
ij
= k
e
i
e
j
+k
e
i
e
j
+k
e
i
e
j
(7.4-10)
To dene an orthotropic thermal conductivity, select orthotropic in the
drop-down list to the right of Thermal Conductivity in the Materials panel.
This opens the Orthotropic Conductivity panel (Figure 7.4.1).
Figure 7.4.1: The Orthotropic Conductivity Panel
Since the directions ( e
, e
, e
) are mutually orthogonal, only the rst
two need to be specied for three-dimensional problems. e
is dened
7-40 c Fluent Inc. November 28, 2001
7.4 Thermal Conductivity
using X,Y,Z under Direction 0 Components, and e
is dened using X,Y,Z
under Direction 1 Components. You can dene Conductivity 0 (k
), Con-
ductivity 1 (k
), and Conductivity 2 (k
) as constant, polynomial, piecewise-
linear, or piecewise-polynomial functions of temperature, using the drop-
down list below each of the conductivities (see Sections 7.4.1 and 7.4.2
for more details).
For two-dimensional problems, only the functions (k
, k
) and the unit
vector ( e
) need to be specied.
General Anisotropic Thermal Conductivity
The thermal conductivity matrix is specied as
k
ij
= k e
ij
(7.4-11)
where k is the conductivity and e
ij
is a matrix (2 2 for two dimensions
and 3 3 for three-dimensional problems).
To dene a general anisotropic thermal conductivity, select anisotropic in
the drop-down list to the right of Thermal Conductivity in the Materials
panel. This opens the Anisotropic Conductivity panel (Figure 7.4.2).
Note that e
ij
can be a non-symmetric matrix, and you can specify its
components under Matrix Components in the Anisotropic Conductivity
panel. k can be specied as a function of temperature using any of
the usual methods (constant, polynomial, piecewise-linear or piecewise-
polynomial), which are selected from the drop-down list below Conduc-
tivity (see Sections 7.4.1 and 7.4.2 for more details).
c Fluent Inc. November 28, 2001 7-41
Phy sical Properties
Figure 7.4.2: The Anisotropic Conductivity Panel
7-42 c Fluent Inc. November 28, 2001
7.5 Specic Heat Capacity
7.5 Specic Heat Capacity
The specic heat capacity must be dened when the energy equation
is active. FLUENT provides several options for denition of the heat
capacity:
Constant heat capacity
Temperature- and/or composition-dependent heat capacity
Kinetic theory
Each of these input options and the governing physical models are de-
tailed in this section. In all cases, you will dene the Cp in the Materials
panel.
Dene Materials...
Specic heat capacity is input in units of J/kg-K in SI units or
BTU/lbm-R in British units.
For combustion applications, a temperature-dependent specic heat is !
recommended.
7.5.1 Input of Constant Specic Heat Capacity
If you want to dene the heat capacity as a constant, check that constant
is selected in the drop-down list to the right of Cp in the Materials panel,
and enter the value of heat capacity.
The specic heat for the default uid (air) is 1006.43 J/kg-K.
7.5.2 Specic Heat Capacity as a Function of Temperature
You can also choose to dene the specic heat capacity as a function of
temperature. Three types of functions are available:
piecewise-linear:
c
p
(T) = c
pn
+
c
p
n+1
c
pn
T
n+1
T
n
(T T
n
) (7.5-1)
c Fluent Inc. November 28, 2001 7-43
Phy sical Properties
piecewise-polynomial:
for T
min,1
< T < T
max,1
: c
p
(T) = A
1
+A
2
T +A
3
T
2
+...(7.5-2)
for T
min,2
< T < T
max,2
: c
p
(T) = B
1
+B
2
T +B
3
T
2
+...(7.5-3)
polynomial:
c
p
(T) = A
1
+A
2
T +A
3
T
2
+... (7.5-4)
You can input the data pairs (T
n
, c
pn
), ranges and coecients A
i
and B
i
,
or coecients A
i
that describe these functions using the Materials panel,
as described in Section 7.1.3.
7.5.3 Dening Specic Heat Capacity Using Kinetic Theory
If you are using the gas law (as described in Section 7.2), you have the
option to dene the specic heat capacity using kinetic theory as
c
p,i
=
1
2
R
M
w,i
(f
i
+ 2) (7.5-5)
where f
i
is the number of modes of energy storage (degrees of freedom)
for the gas species i which you can input by selecting kinetic-theory from
the drop-down list to the right of Cp in the Materials panel. The solver
will use your kinetic theory inputs in Equation 7.5-5 to compute the
specic heat capacity. See Section 7.11 for details about kinetic theory
inputs.
7.5.4 Specic Heat Capacity as a Function of Composition
If you are modeling a ow that includes more than one chemical species
(multicomponent ow), you have the option to dene a composition-
dependent specic heat capacity. (Note that you can also dene the heat
capacity of the mixture as a constant value or a function of temperature,
or using kinetic theory.)
7-44 c Fluent Inc. November 28, 2001
7.6 Radiation Properties
To dene a composition-dependent specic heat capacity for a mixture,
follow these steps:
1. For the mixture material, choose mixing-law in the drop-down list
to the right of Cp.
2. Click Change/Create.
3. Dene the specic heat capacity for each of the uid materials that
comprise the mixture. You may dene constant or (if applicable)
temperature-dependent heat capacities for the individual species.
You may also use kinetic theory for the individual heat capacities,
if applicable.
The solver will compute the mixtures specic heat capacity as a mass
fraction average of the pure species heat capacities:
c
p
=
i
Y
i
c
p,i
(7.5-6)
7.6 Radiation Properties
When you have activated one of the radiation models (except for the
surface-to-surface model, which requires no additional properties), there
will be additional properties for you to set in the Materials panel:
For the P-1 model, you will need to set the radiation Absorption
Coecient and Scattering Coecient (a and
s
in Equation 11.3-9).
For the Rosseland radiation model, you will also need to set the
Absorption Coecient and Scattering Coecient (a and
s
in Equa-
tion 11.3-10).
For the DTRM, only the Absorption Coecient is required (a in
Equation 11.3-2).
c Fluent Inc. November 28, 2001 7-45
Phy sical Properties
For the DO model, you will set both the Absorption Coecient and
the Scattering Coecient (a and
s
in Equation 11.3-37). In addi-
tion, if you are modeling semi-transparent media, you can specify
the Refractive Index (n
a
or n
b
in Equation 11.3-51). Note that
with the DO model, you can specify radiation properties for solid
materials, to be used when semi-transparent media are modeled.
Information about dening each of these properties is provided in the
following sections.
7.6.1 Absorption Coecient
To dene the absorption coecient, you can specify a constant value,
a temperature-dependent function (see Section 7.1.3), a composition-
dependent function, or a user-dened function. If you are modeling non-
gray radiation with the DO radiation model, you also have the option to
specify a constant absorption coecient in each of the gray bands.
The absorption coecient is requested in units of 1/length. Along with
the scattering coecient, it describes the change in radiation intensity
per unit length along the path through the uid medium. Absorption
coecients can be computed using tables of emissivity for CO
2
and H
2
O,
which are generally available in textbooks on radiation heat transfer.
Inputs for a Constant Absorption Coecient
To dene a constant absorption coecient, simply enter the value in the
eld next to Absorption Coecient in the Materials panel. (Select constant
in the drop-down list rst if it is not already selected.)
Inputs for a Composition-Dependent Absorption Coecient
FLUENT also allows you to input a composition-dependent absorption
coecient, with the local value of a a function of the local mass fractions
of water vapor and carbon dioxide. This modeling option can be useful
for simulation of radiation in combustion applications. The variable-
absorption-coecient model used by FLUENT is the weighted-sum-of-
gray-gases model (WSGGM) described in Section 11.3.8. To activate it,
7-46 c Fluent Inc. November 28, 2001
7.6 Radiation Properties
select wsggm-cell-based, wsggm-domain-based, or wsggm-user-specied in
the drop-down list to the right of Absorption Coecient in the Materials
panel. The three WSGGM options dier in the method used to compute
the path length, as described below.
(Remember that you must rst enable the species calculation in order
to see the wsggm choices in the list, and CO
2
and H
2
O must be present
in the mixture.)
Path Length Inputs
When the WSGGM is used to compute the absorption coecient, you
will have a choice of methods used to calculate the path length s in Equa-
tion 11.3-75. You can use the characteristic cell size or the mean beam
length (computed by the solver or dened by you). See Section 11.3.8 to
determine which method is appropriate for your case.
You will select the path length method when you choose the property
input method for Absorption Coecient as described above.
If you choose wsggm-cell-based, the characteristic-cell-size approach
will be used and no further inputs are required.
If you choose wsggm-domain-based, the mean-beam-length approach
will be used for the calculation of a and FLUENT will compute the
mean beam length based on an average dimension of the domain;
no further inputs are required.
If you choose wsggm-user-specied, the mean-beam-length approach
will be used, but you will set the mean beam length yourself in the
Path Length eld in the WSGGM User Specied panel. This panel
will open when you choose wsggm-user-specied, and since it is a
modal panel, you must tend to it immediately.
Inputs for a Non-Gray Radiation Absorption Coecient
If you are using the non-gray DO model (see Sections 11.3.6 and 11.3.14),
you can specify a dierent constant absorption coecient for each of the
bands used by the gray-band model. Select gray-band in the Absorption
c Fluent Inc. November 28, 2001 7-47
Phy sical Properties
Coecient drop-down list, and then dene the absorption coecient for
each band in the Gray-Band Absorption Coecient panel. (Note that,
since this is a modal panel, you must tend to it immediately.)
Eect of Particles and Soot on the Absorption Coecient
FLUENT will include the eect of particles on the absorption coecient
if you have turned on the Particle Radiation Interaction option in the
Discrete Phase Model panel (only for the P-1 and DO radiation models).
If you are modeling soot formation and you want to include the eect
of soot formation on the absorption coecient, turn on the Generalized
Model for Soot-Radiation Interaction in the Soot Model panel. The soot
eects can be included for any of the radiation models, as long as you
are using the WSGGM to compute a composition-dependent absorption
coecient.
7.6.2 Scattering Coecient
The scattering coecient is, by default, set to zero, and it is assumed to
be isotropic. You can specify a constant value, a temperature-dependent
function (see Section 7.1.3), or a user-dened function. You can also
specify a non-isotropic phase function.
The scattering coecient is requested in units of 1/length. Along with
the absorption coecient, it describes the change in radiation intensity
per unit length along the path through the uid medium. You may
wish to increase the scattering coecient in combustion systems, where
particulates may be present.
Inputs for a Constant Scattering Coecient
To dene a constant scattering coecient, simply enter the value in the
eld next to Scattering Coecient in the Materials panel. (Select constant
in the drop-down list rst if it is not already selected.)
7-48 c Fluent Inc. November 28, 2001
7.6 Radiation Properties
Inputs for the Scattering Phase Function
Scattering is assumed to be isotropic, by default, but you can also specify
a linear-anisotropic scattering function. If you are using the DO model,
Delta-Eddington and user-dened scattering functions are also available.
Isotropic Phase Function
To model isotropic scattering, select isotropic in the Scattering Phase
Function drop-down list. No further inputs are necessary. This is the
default setting in FLUENT.
Linear-Anisotropic Phase Function
To model anisotropic scattering, select linear-anisotropic in the Scattering
Phase Function drop-down list and set the value of the phase function
coecient (C in Equation 11.3-10).
Delta-Eddington Phase Function
To use a Delta-Eddington phase function, select delta-eddington in the
Scattering Phase Function drop-down list. This will open the Delta-
Eddington Scattering Function panel, in which you can specify the Forward
Scattering Factor and Asymmetry Factor (f and C in Equation 11.3-41).
Note that, since this is a modal panel, you must tend to it immediately.
User-Dened Phase Function
To use a user-dened phase function, select user-dened in the Scattering
Phase Function drop-down list. The user-dened function must contain
specications for
and f in Equation 11.3-42. See the separate UDF
Manual for information about user-dened functions.
7.6.3 Refractive Index
The refractive index is, by default, set to 1. It is used only if you are
modeling semi-transparent media with the DO radiation model. You can
specify a constant value in the eld next to Refractive Index.
c Fluent Inc. November 28, 2001 7-49
Phy sical Properties
7.6.4 Reporting the Radiation Properties
You can display the computed local values for a and
s
using the Ab-
sorption Coecient and Scattering Coecient items in the Radiation...
category of the variable selection drop-down list that appears in postpro-
cessing panels. You will also nd the Refractive Index in the Radiation...
category.
7.7 Mass Diusion Coecients
For species transport calculations, there are two ways to model the dif-
fusion of chemical species. For most applications the Ficks law approxi-
mation is adequate, but for some applications (e.g., diusion-dominated
laminar ows such as chemical vapor deposition), the full multicompo-
nent diusion model is recommended.
The full multicomponent diusion model is enabled in the Species Model !
panel. Also, note that the full multicomponent diusion model is com-
putationally expensive.
7.7.1 Fickian Diusion
Mass diusion coecients are required whenever you are solving species
transport equations in multi-component ows. Mass diusion coecients
are used to compute the diusion ux of a chemical species in a laminar
ow using (by default) Ficks law:
J
i
= D
i,m
Y
i
D
T,i
T
T
(7.7-1)
where D
i,m
is the mass diusion coecient for species i in the mixture
and D
T,i
is the thermal (Soret) diusion coecient.
Equation 7.7-1 is strictly valid when the mixture composition is not
changing, or when D
i,m
is independent of composition. This is an ac-
ceptable approximation in dilute mixtures when Y
i
<< 1, for all i except
the carrier gas. FLUENT can also compute the transport of non-dilute
mixtures in laminar ows by treating such mixtures as multicomponent
systems. Within FLUENT, D
i,m
can be specied in a variety of ways,
7-50 c Fluent Inc. November 28, 2001
7.7 Mass Diusion Coecients
including by specifying D
ij
, the binary mass diusion coecient of com-
ponent i in component j. D
ij
is not used directly, however; instead, the
diusion coecient in the mixture, D
i,m
, is computed as
D
i,m
=
1 X
i
j,j=i
(X
j
/D
ij
)
(7.7-2)
where X
i
is the mole fraction of species i. You can input D
i,m
or D
ij
for
each chemical species, as described in Section 7.7.4.
In turbulent ows, Equation 7.7-1 is replaced with the following form:
J
i
= (D
i,m
+
t
Sc
t
) Y
i
D
T,i
T
T
(7.7-3)
where Sc
t
is the eective Schmidt number for the turbulent ow:
Sc
t
=
t
D
t
(7.7-4)
and D
t
is the eective mass diusion coecient due to turbulence.
In turbulent ows your mass diusion coecient inputs consist of den-
ing the molecular contribution to diusion D
i,m
using the same methods
available for the laminar case, with the added option to alter the default
settings for the turbulent Schmidt number. As seen from Equation 7.7-4,
this parameter relates the eective mass diusion coecient due to tur-
bulence with the eddy viscosity
t
. As discussed in Section 7.7.5, the
turbulent diusion coecient normally overwhelms the laminar diu-
sion coecient, so the default constant value for the laminar diusion
coecient is usually acceptable.
7.7.2 Full Multicomponent Diusion
A careful treatment of chemical species diusion in the species trans-
port and energy equations is important when details of the molecular
transport processes are signicant (e.g., in diusion-dominated laminar
c Fluent Inc. November 28, 2001 7-51
Phy sical Properties
ows). As one of the laminar-ow diusion models, FLUENT has the
ability to model full multicomponent species transport.
General Theory
For multicomponent systems it is not possible, in general, to derive rela-
tions for the diusion uxes containing the gradient of only one compo-
nent (as described in Section 7.7.1). Here, the Maxwell-Stefan equations
will be used to obtain the diusive mass ux. This will lead to the deni-
tion of generalized Ficks law diusion coecients [241]. This method is
preferred over computing the multicomponent diusion coecients since
their evaluation requires the computation of N
2
co-factor determinants
of size (N1)(N1), and one determinant of size NN [231], where
N is the number of chemical species.
Maxwell-Stefan Equations
From Merk [154], the Maxwell-Stefan equations can be written as
N
j=1
j=i
X
i
X
j
D
ij
V
j
V
i
=
d
i
T
T
N
j=1
j=i
X
i
X
j
D
ij
D
T,j
D
T,i
(7.7-5)
where X is the mole fraction,
V is the diusion velocity, D
ij
is the binary
mass diusion coecient, and D
T
is the thermal diusion coecient.
For an ideal gas the Maxwell diusion coecients are equal to the binary
diusion coecients. If the external force is assumed to be the same on
all species and that pressure diusion is negligible, then
d
i
= X
i
. Since
the diusive mass ux vector is
J
i
=
i
V
i
, the above equation can be
written as
N
j=1
j=i
X
i
X
j
D
ij
J
j
J
i
= X
i
T
T
N
j=1
j=i
X
i
X
j
D
ij
D
T,j
D
T,i
(7.7-6)
7-52 c Fluent Inc. November 28, 2001
7.7 Mass Diusion Coecients
After some mathematical manipulations, the diusive mass ux vector,
J
i
, can be obtained from
J
i
=
N1
j=1
D
ij
Y
j
D
T,i
T
T
(7.7-7)
where Y
j
is the mass fraction of species j. Other terms are dened as
follows:
D
ij
= [D] = [A]
1
[B] (7.7-8)
A
ii
=
X
i
D
iN
M
w
M
w,N
+
N
j=1
j=i
X
j
D
ij
M
w
M
w,i
(7.7-9)
A
ij
= X
i
1
D
ij
M
w
M
w,j
1
D
iN
M
w
M
w,N
(7.7-10)
B
ii
=
X
i
M
w
M
w,N
+ (1 X
i
)
M
w
M
w,i
(7.7-11)
B
ij
= X
i
M
w
M
w,j
M
w
M
w,N
(7.7-12)
where [A] and [B] are (N 1) (N 1) matrices and [D] is an (N
1) (N 1) matrix of the generalized Ficks law diusion coecients
D
ij
[241].
7.7.3 Thermal Diusion Coecients
The thermal diusion coecients can be dened as constants, polyno-
mial functions, user-dened functions, or using the following empirically-
based composition-dependent expression derived from [120]:
c Fluent Inc. November 28, 2001 7-53
Phy sical Properties
D
T,i
= 2.59 10
7
T
0.659
M
0.511
w,i
X
i
N
i=1
M
0.511
w,i
X
i
Y
i
i=1
M
0.511
w,i
X
i
N
i=1
M
0.489
w,i
X
i
(7.7-13)
This form of the Soret diusion coecient will cause heavy molecules to
diuse less rapidly, and light molecules to diuse more rapidly, towards
heated surfaces.
7.7.4 Mass Diusion Coecient Inputs
By default, the solver computes the species diusion using Equation 7.7-1
(for laminar ows) with your inputs for D
i,m
, the diusion coecient
for species i in the mixture. For turbulent ows, species diusion is
computed with Equation 7.7-3.
You can input the mass diusion coecients using one of the following
methods:
constant dilute approximation (Fickian diusion only): dene one
constant for all D
i,m
.
dilute approximation (Fickian diusion only): dene each D
i,m
as a constant or as a polynomial function of temperature (if heat
transfer is enabled).
multicomponent method: dene the binary diusion of species i
in each species j, D
ij
as a constant or a polynomial function of
temperature, or (for ideal gases only) using kinetic theory.
You should choose to input D
i,m
(using one of the rst two methods) if
you are modeling a dilute mixture, with chemical species present at low
mass fraction in a carrier uid that is present at high concentration.
You may wish to dene the individual binary mass diusion coecients,
D
ij
, if you are modeling a non-dilute mixture. If you choose to dene
7-54 c Fluent Inc. November 28, 2001
7.7 Mass Diusion Coecients
D
ij
, the solver will compute the diusion of species i in the mixture using
Equation 7.7-2, unless you have enabled full multicomponent diusion.
If you want to use the full multicomponent diusion model described in !
Section 7.7.2, turn on the Full Multicomponent Diusion option in the
Species Model panel, and then select the multicomponent method (the
third method listed above) in the Materials panel; the dilute approxima-
tion methods are not appropriate for the full multicomponent diusion
model.
You will dene D
i,m
or D
ij
for each chemical species using the Materials
panel.
Dene Materials...
The diusion coecients have units of m
2
/s in SI units or ft
2
/s in British
units.
Constant Dilute Approximation Inputs
To use the constant dilute approximation method, follow these steps:
1. Select constant-dilute-appx in the drop-down list to the right of Mass
Diusivity.
2. Enter a single value of D
i,m
. The same value will be used for the
diusion coecient of each species in the mixture.
Dilute Approximation Inputs
To use the dilute approximation method, follow the steps below:
1. Select dilute-approx in the drop-down list to the right of Mass Dif-
fusivity.
2. In the resulting Mass Diusion Coecients panel (Figure 7.7.1),
select the species in the Species Di list for which you are going to
dene the mass diusion coecient.
c Fluent Inc. November 28, 2001 7-55
Phy sical Properties
Figure 7.7.1: The Mass Diusion Coecients Panel for Dilute Approxi-
mation
3. You can dene D
i,m
for the selected species either as a constant
value or (if heat transfer is active) as a polynomial function of
temperature:
To dene a constant diusion coecient, select constant (the
default) in the drop-down list below Coecient, and then enter
the value in the eld below the list.
To dene a temperature-dependent diusion coecient, choose
polynomial in the Coecient drop-down list and then dene
the polynomial coecients as described in Section 7.1.3.
D
i,m
= A
1
+A
2
T +A
3
T
2
+... (7.7-14)
4. Repeat steps 2 and 3 until you have dened diusion coecients
for all species in the Species Di list in the Mass Diusion Coecients
panel.
7-56 c Fluent Inc. November 28, 2001
7.7 Mass Diusion Coecients
Multicomponent Method Inputs
To use the multicomponent method, and dene constant or temperature-
dependent diusion coecients, follow the steps below:
1. Select multicomponent in the drop-down list to the right of Mass
Diusivity.
2. In the resulting Mass Diusion Coecients panel (Figure 7.7.2),
select the species in the Species Di list and the Species Dj list for
which you are going to dene the mass diusion coecient D
ij
for
species i in species j.
Figure 7.7.2: The Mass Diusion Coecients Panel for the Multicompo-
nent Method
3. You can dene D
ij
for the selected pair of species as a constant
value or as a polynomial function of temperature (if heat transfer
is active).
c Fluent Inc. November 28, 2001 7-57
Phy sical Properties
To dene a constant diusion coecient, select constant (the
default) in the drop-down list below Coecient, and then enter
the value in the eld below the list.
To dene a temperature-dependent diusion coecient, choose
polynomial in the Coecient drop-down list and then dene
the polynomial coecients as described in Section 7.1.3.
D
ij
= A
1
+A
2
T +A
3
T
2
+... (7.7-15)
4. Repeat steps 2 and 3 until you have dened diusion coecients
for all pairs of species in the Species Di and Species Dj lists in the
Mass Diusion Coecients panel.
To use the multicomponent method, and dene the diusion coecient
using kinetic theory (available only when the ideal gas law is used), follow
these steps:
1. Choose kinetic-theory in the drop-down list to the right of Mass
Diusivity.
2. Click Change/Create after completing other property denitions for
the mixture material.
3. Dene the Lennard-Jones parameters,
i
and (/k
B
)
i
, for each
species (uid material), as described in Section 7.11.
The solver will use a modication of the Chapman-Enskog formula [152]
to compute the diusion coecient using kinetic theory:
D
ij
= 0.0188
T
3
1
M
w,i
+
1
M
w,j
1/2
p
abs
2
ij
D
(7.7-16)
where p
abs
is the absolute pressure, and
D
is the diusion collision
integral, which is a measure of the interaction of the molecules in the
system.
D
is a function of the quantity T
D
, where
7-58 c Fluent Inc. November 28, 2001
7.7 Mass Diusion Coecients
T
D
=
T
(/k
B
)
ij
(7.7-17)
k
B
is the Boltzmann constant, which is dened as the gas constant, R,
divided by Avagadros number. (/k
B
)
ij
for the mixture is the geometric
average:
(/k
B
)
ij
=
(/k
B
)
i
(/k
B
)
j
(7.7-18)
For a binary mixture,
ij
is calculated as the arithmetic average of the
individual s:
ij
=
1
2
(
i
+
j
) (7.7-19)
Thermal Diusion Coecient Inputs
If you have enabled thermal diusion (in the Species Model panel), you
can dene the thermal diusion coecients in the Materials panel as
follows:
1. Select one of the following three methods in the drop-down list to
the right of Thermal Diusion Coecient:
Choose kinetic-theory to have FLUENT compute the thermal
diusion coecients using the empirically-based expression
in Equation 7.7-13. No further inputs are required for this
option.
Choose specied to input the coecient for each species. The
Thermal Diusion Coecients panel (Figure 7.7.3) will open.
Further inputs are described in the next step.
Choose user-dened to use a user-dened function. See the
separate UDF Manual for details.
2. If you choose specied, select the species in the Species Thermal Di
list for which you are going to dene the thermal diusion coe-
cient.
c Fluent Inc. November 28, 2001 7-59
Phy sical Properties
Figure 7.7.3: The Thermal Diusion Coecients Panel
3. Dene D
T,i
for the selected species either as a constant value or as
a polynomial function of temperature:
To dene a constant diusion coecient, select constant (the
default) in the drop-down list below Coecient, and then enter
the value in the eld below the list.
To dene a temperature-dependent diusion coecient, choose
polynomial in the Coecient drop-down list and then dene
the polynomial coecients as described in Section 7.1.3.
4. Repeat steps 2 and 3 until you have dened diusion coecients for
all species in the Species Di list in the Thermal Diusion Coecients
panel.
7-60 c Fluent Inc. November 28, 2001
7.8 Standard State Enthalpies
7.7.5 Mass Diusion Coecient Inputs for Turbulent Flow
When your ow is turbulent, you will dene D
i,m
or D
ij
, as described for
laminar ows in Section 7.7.4, and you will also have the option to alter
the default setting for the turbulent Schmidt number, Sc
t
, as dened in
Equation 7.7-4.
Usually, in a turbulent ow, the mass diusion is dominated by the
turbulent transport as determined by the turbulent Schmidt number
(Equation 7.7-4). The turbulent Schmidt number measures the relative
diusion of momentum and mass due to turbulence and is on the order
of unity in all turbulent ows. Because the turbulent Schmidt number is
an empirical constant that is relatively insensitive to the molecular uid
properties, you will have little reason to alter the default value (0.7) for
any species.
Should you wish to modify the Schmidt number, enter a new value for
Turb. Schmidt Number in the Viscous Model panel.
Dene Models Viscous...
Note that the full multicomponent diusion model described in Sec- !
tion 7.7.2 is not recommended for turbulent ows.
7.8 Standard State Enthalpies
When you are solving a reacting ow using the nite-rate or eddy dissi-
pation model, you will need to dene the standard state enthalpy (also
known as the formation enthalpy or heat of formation), h
0
j
for each
species j. These inputs are used to dene the mixture enthalpy as
H =
j
m
j
h
0
j
+
T
T
ref,j
c
p,j
dT
(7.8-1)
where T
ref,j
is the reference temperature at which h
0
j
is dened. Standard
state enthalpies are input in units of J/kg mol in SI units or in units of
Btu/lb
m
mol in British units.
c Fluent Inc. November 28, 2001 7-61
Phy sical Properties
For each species involved in the reaction (i.e., each uid material con-
tained in the mixture material), you can set the Standard State Enthalpy
and Reference Temperature in the Materials panel.
7.9 Standard State Entropies
If you are using the nite-rate model with reversible reactions (see Sec-
tion 13.1.1), you will need to dene the standard state entropy, s
0
j
for
each species j. These inputs are used to dene the mixture entropy as
S =
j
m
j
s
0
j
+
T
T
ref,j
c
p,j
T
dT
(7.9-1)
where T
ref,j
is the reference temperature at which s
0
j
is dened. Standard
state entropies are input in units of J/kgmol-K in SI units or in units of
Btu/lb
m
mol-
R in British units.
For each species involved in the reaction (i.e., each uid material con-
tained in the mixture material), you can set the Standard State Entropy
and Reference Temperature in the Materials panel.
7.10 Molecular Heat Transfer Coecient
If you are modeling premixed combustion (see Section 15), the uid
material in your domain should be assigned the properties of the un-
burnt mixture, including the molecular heat transfer coecient ( in
Equation 15.2-4), which is also referred to as the thermal diusivity.
is dened as k/c
p
, and values at standard conditions can be found in
combustion handbooks (e.g., [120]). To determine values at non-standard
conditions, you will need to use a third-party 1D combustion program
with detailed chemistry. You can set the Molecular Heat Transfer Coe-
cient in the Materials panel.
7-62 c Fluent Inc. November 28, 2001
7.11 Kinetic Theory Parameters
7.11 Kinetic Theory Parameters
You may choose to dene the following properties using kinetic theory
when the ideal gas law is enabled:
viscosity (for uids)
thermal conductivity (for uids)
specic heat capacity (for uids)
mass diusion coecients (for multi-species mixtures)
If you are using kinetic theory for a uids viscosity (Equation 7.3-9),
you will need to input the kinetic theory parameters and /k
B
for
that uid. These parameters are the Lennard-Jones parameters and are
referred to by FLUENT as the characteristic length and the energy
parameter respectively.
When kinetic theory is applied to calculation of a uids thermal con-
ductivity only, no inputs are required.
If you are going to calculate a uids specic heat using kinetic theory
(Equation 7.5-5), you will need to input the degrees of freedom for the
uid material.
If you use kinetic theory to dene a mixture materials mass diusivity
(Equation 7.7-16), you will need to input
i
and (/k
B
)
i
for each chemical
species i.
Inputs for Kinetic Theory
The procedure for using kinetic theory is as follows:
1. Select kinetic-theory as the property specication method for the
Viscosity, Thermal Conductivity, or heat capacity Cp of a uid ma-
terial, or for the Mass Diusivity of a mixture material.
c Fluent Inc. November 28, 2001 7-63
Phy sical Properties
2. If the material for which you have selected the kinetic theory
method for one or more properties is a uid material, you must
set the kinetic theory parameters for that material. If you are us-
ing kinetic theory for the mass diusivity of a mixture material,
you will dene the kinetic theory parameters for each of the con-
stituent species (uid materials).
The parameters to be set are as follows:
L-J Characteristic Length
L-J Energy Parameter
Degrees of Freedom (only required if kinetic theory is used for
specic heat)
See the beginning of this section to nd out which parameters are
required to calculate each property using kinetic theory.
Characteristic length is dened in units of Angstroms. The energy pa-
rameter is dened in units of absolute temperature. Degrees of freedom
is a dimensionless input. All kinetic theory parameters are set to zero by
default. Appropriate values for dierent materials can be found in the
literature (e.g., [92]).
7-64 c Fluent Inc. November 28, 2001
7.12 Operating Pressure
7.12 Operating Pressure
Specication of the operating pressure aects your calculation in dierent
ways for dierent ow regimes. This section presents information about
the operating pressure, its relevance for dierent cases, and how to set
it correctly.
7.12.1 The Eect of Numerical Roundo on Pressure
Calculation in Low-Mach-Number Flow
In low-Mach-number compressible ow, the overall pressure drop is small
compared to the absolute static pressure, and can be signicantly aected
by numerical roundo. To understand why this is true, consider a com-
pressible ow with M << 1. The pressure changes, p, are related to
the dynamic head,
1
2
pM
2
, where p is the static pressure and is the
ratio of specic heats. This gives the simple relationship p/p M
2
,
so that p/p 0 as M 0. Therefore, unless adequate precau-
tion is taken, low-Mach-number ow calculations are very susceptible to
roundo error.
7.12.2 Operating Pressure, Gauge Pressure, and Absolute
Pressure
FLUENT avoids the problem of roundo error (discussed in Section 7.12.1)
by subtracting the operating pressure (generally a large pressure roughly
equal to the average absolute pressure in the ow) from the absolute pres-
sure, and using the result (termed the gauge pressure). The relationship
between the operating pressure, gauge pressure, and absolute pressure is
shown below. The absolute pressure is simply the sum of the operating
pressure and the gauge pressure:
p
abs
= p
op
+p
gauge
(7.12-1)
All pressures that you specify and all pressures computed or reported by
FLUENT are gauge pressures.
c Fluent Inc. November 28, 2001 7-65
Phy sical Properties
7.12.3 Setting the Operating Pressure
The Signicance of Operating Pressure
Operating pressure is signicant for incompressible ideal gas ows be-
cause it directly determines the density: the incompressible ideal gas
law computes density as =
pop
R
Mw
T
. You must therefore be sure to set
the operating pressure appropriately.
Operating pressure is signicant for low-Mach-number compressible ows
because of its role in avoiding roundo error problems, as described in
Section 7.12.2. Again, you must be sure to set the operating pressure
appropriately. For time-dependent compressible ows, you may want
to specify a oating operating pressure instead of a constant operating
pressure. See Section 8.5.4 for details.
Operating pressure is less signicant for higher-Mach-number compress-
ible ows. The pressure changes in such ows are much larger than those
in low-Mach-number compressible ows, so there is no real problem with
roundo error and there is therefore no real need to use gauge pressure.
In fact, it is common convention to use absolute pressures in such cal-
culations. Since FLUENT always uses gauge pressure, you can simply
set the operating pressure to zero, making gauge and absolute pressures
equivalent.
If the density is assumed constant or if it is derived from a prole function
of temperature, the operating pressure is not used at all.
Note that the default operating pressure is 101325 Pa.
How to Set the Operating Pressure
The criteria for choosing a suitable operating pressure are based on the
Mach-number regime of the ow and the relationship that is used to
determine density. For example, if you use the ideal gas law in an
incompressible ow calculation (e.g., for a natural convection problem),
you should use a value representative of the mean ow pressure.
To place this discussion in perspective, Table 7.12.1 shows the recom-
mended approach for setting operating pressures. Remember that the
7-66 c Fluent Inc. November 28, 2001
7.13 Reference Pressure Location
default operating pressure is 101325 Pa.
Table 7.12.1: Recommended Settings for Operating Pressure
Density Mach Number Operating
Relationship Regime Pressure
Ideal Gas Law M > 0.1 0 or
Mean Flow Pressure
M < 0.1 Mean Flow Pressure
Prole Function Incompressible not used
of Temperature
Constant Incompressible not used
Incompressible Incompressible Mean Flow Pressure
Ideal Gas Law
You will set the Operating Pressure in the Operating Conditions panel.
Dene Operating Conditions...
7.13 Reference Pressure Location
For incompressible ows that do not involve any pressure boundaries,
FLUENT adjusts the gauge pressure eld after each iteration to keep it
from oating. This is done using the pressure in the cell located at (or
nearest to) the reference pressure location. The pressure value in this cell
is subtracted from the entire gauge pressure eld; as a result, the gauge
pressure at the reference pressure location is always zero. If pressure
boundaries are involved, the adjustment is not needed and the reference
pressure location is ignored.
The reference pressure location is, by default, the cell center at or closest
to (0,0,0). There may be cases in which you might want to move the
reference pressure location, perhaps locating it at a point where the
absolute static pressure is known (e.g., if you are planning to compare
your results with experimental data). To change the location, enter
new (X,Y,Z) coordinates for Reference Pressure Location in the Operating
Conditions panel.
Dene Operating Conditions...
c Fluent Inc. November 28, 2001 7-67
Phy sical Properties
7.14 Real Gas Model
Some engineering problems involve uids that do not behave as ideal
gases. For example, high-pressure ows (e.g., the ow of a refrigerant
through a compressor) cannot typically be modeled using the ideal-gas
law. The real gas model allows you to solve uid ow and heat transfer
problems where the working uid is a real gas.
7.14.1 Overview and Limitations
The real gas model is available in the coupled solvers and uses the Na-
tional Institute of Standards and Technology (NIST) Thermodynamic
and Transport Properties of Refrigerants and Refrigerant Mixtures Data-
base Version 6.0 (REFPROP v6.0) to evaluate thermodynamic and trans-
port properties.
The REFPROP v6.0 database is a shared library that is dynamically
loaded into the solver when you activate the real gas model in a FLU-
ENT session. Once the real gas model is activated, control of relevant
property evaluations is relinquished to the REFPROP database, and any
information for a uid that is displayed in the Materials panel is ignored
by the solver. However, all postprocessing functions will properly report
and display the current thermodynamic and transport properties of the
real gas.
The following limitations exist for the real gas model:
The real gas model can be used only with the coupled solvers.
All uid property information in the Materials panel is ignored by
FLUENT. You cannot override the REFPROP database for a real
gas material by modifying parameters in the Materials panel.
You cannot modify material properties in the REFPROP database
libraries, or add custom materials to the real gas model.
When the real gas model is used, all uid zones must contain the
real gas; you cannot include a real gas and another uid in the
same problem.
7-68 c Fluent Inc. November 28, 2001
7.14 Real Gas Model
The real gas model assumes that the uid you will be using in your
FLUENT computation has the following characteristics:
pure uid
single species
single phase
superheated vapor
The real gas model does not support mixtures and/or saturated
uids.
7.14.2 The REFPROP v6.0 Database
The real gas model uses 33 pure uids from the REFPROP v6.0 database.
The pure-uid refrigerants and hydrocarbons that are supported by
REFPROP v6.0 and used in the real gas model are listed in Table 7.14.1.
The REFPROP v6.0 database employs the most accurate pure-uid
equations of state that are currently available from NIST. These equa-
tions are based on three models:
modied Benedict-Webb-Rubin (MBWR) equation of state
Helmholtz-energy equation of state
extended corresponding states (ECS)
For details about these thermodynamic models, refer to Appendix B in
the web-based REFPROP v6.0 Users Guide that is accessible from the
NIST web site:
http://www.nist.gov/srd/nist23.htm
c Fluent Inc. November 28, 2001 7-69
Phy sical Properties
Table 7.14.1: Refrigerants and Hydrocarbons Supported by REF-
PROP v6.0
R11 R113 R114 R115 R116
R12 R123 R124 R125 R13
R134a R14 R141b R142b R143a
R152a R22 R227ea R23 R236ea
R236fa R245ca R245fa R32 R41
RC318 CO2 ammonia butane ethane
isobutane propane propylene
7.14.3 Using the Real Gas Model
When you enable the real gas model and select a valid material, FLU-
ENTs functionality remains the same as when you model uid ow and
heat transfer using an ideal gas, with the exception of the Materials panel
(see below). The information displayed in the Materials panel is not used
by the solver because control of all relevant property evaluations is re-
linquished to the REFPROP database.
The general procedure for using the real gas model is as follows:
1. Enable one of the coupled solvers (whichever is appropriate for
your case).
2. Activate the real gas model.
3. Set up and write your case le.
4. Calculate a solution and perform postprocessing.
Enabling the Coupled Solver
You can enable the coupled solver in the Solver panel. The real gas model
can be used with either the implicit or the explicit formulation.
Dene Models Solver...
7-70 c Fluent Inc. November 28, 2001
7.14 Real Gas Model
The real gas model does not work with the segregated solver. !
Activating the Real Gas Model
Once you have enabled one of the coupled solvers, you will be able to
activate the real gas model. Activating the real gas model is a two-step
process. First you enable the real gas model, and then you select a
pure-uid material from the REFPROP database.
1. Enable the real gas model by typing the following text command
at the FLUENT console prompt:
> define/user-defined/real-gas
use real gas? [no] yes
The list of available pure-uid materials you can select from will
be displayed:
CO2.fld R123.fld R142b.fld R236fa.fld butane.fld
R11.fld R124.fld R143a.fld R245ca.fld ethane.fld
R113.fld R125.fld R152a.fld R245fa.fld isobutan.fld
R114.fld R13.fld R22.fld R32.fld propane.fld
R115.fld R134a.fld R227ea.fld R41.fld propylen.fld
R116.fld R14.fld R23.fld RC318.fld
R12.fld R141b.fld R236ea.fld ammonia.fld
2. Select a pure-uid material from the REFPROP database list:
select real-gas data file [""] "R125.fld"
You must enter the complete name of the material (including the !
.fld sux) contained within quotes (" ").
Upon selection of a valid material (e.g., R125.fld), FLUENT will
load data for that material from a library of pure uids supported
by the REFPROP database, and report that it is opening the
shared library (librealgas.so) where the compiled REFPROP
database source code is located.
c Fluent Inc. November 28, 2001 7-71
Phy sical Properties
/usr/local/Fluent.Inc/fluent6.0/realgas/lib/R125.fld
Opening "/usr/local/Fluent.Inc/fluent6.0/realgas/
ultra/librealgas.so"...
Setting material "air" to a real-gas...
matl name: "R125"
: "pentafluoroethane"
: "354-33-6"
Mol Wt : 120.022
Critical properties:
Temperature : 339.33 (K)
Pressure : 3.629e+06 (Pa)
Density : 4.75996 (mol/L) 571.3 (kg/m^3)
Once the real gas model is activated, any information for a uid that is !
displayed in the Materials panel is ignored by FLUENT.
Writing Your Case File
When you save your completed real gas model to a case le, the linkage
to the shared library containing REFPROP will be saved to the case le,
along with property data for the material you selected. Consequently,
whenever you read your case le in a later session, FLUENT will report
this information to the console during the read process.
Postprocessing
All postprocessing functions properly report and display the current ther-
modynamic and transport properties of the real gas. The thermody-
namic and transport properties controlled by the REFPROP database
include the following:
density
enthalpy
entropy
gas constant
molecular viscosity
7-72 c Fluent Inc. November 28, 2001
7.14 Real Gas Model
sound speed
specic heat
thermal conductivity
any quantities that are derived from the properties listed above
(e.g., total quantities, ratio of specic heats)
c Fluent Inc. November 28, 2001 7-73
Phy sical Properties
7-74 c Fluent Inc. November 28, 2001
You might also like
- ANSYS Mechanical APDL for Finite Element AnalysisFrom EverandANSYS Mechanical APDL for Finite Element AnalysisRating: 4.5 out of 5 stars4.5/5 (8)
- Matrix Methods for Advanced Structural AnalysisFrom EverandMatrix Methods for Advanced Structural AnalysisRating: 5 out of 5 stars5/5 (1)
- Material Properties and Database in PLAXIS 2DDocument139 pagesMaterial Properties and Database in PLAXIS 2DthachNo ratings yet
- ReuseDocument4 pagesReuseMacedo S OliveiraNo ratings yet
- 56 - Creating MaterialsDocument8 pages56 - Creating MaterialsSameOldHatNo ratings yet
- Chapter 6 ModuleDocument9 pagesChapter 6 ModuleHunter BravoNo ratings yet
- Cosmos 2007Document38 pagesCosmos 2007Oswaldo NeaveNo ratings yet
- In NX Their Are Four Files: 1. Master PartDocument6 pagesIn NX Their Are Four Files: 1. Master PartGoutham BurraNo ratings yet
- Attribute Changer User GuideDocument21 pagesAttribute Changer User GuideJake JessupNo ratings yet
- Database Menu - Material Material Database Usage DetailedDocument34 pagesDatabase Menu - Material Material Database Usage DetailedjabeNo ratings yet
- COFE documentation overviewDocument54 pagesCOFE documentation overviewPedro Milton ChibulachoNo ratings yet
- 2-1 PFA Static Flat CouponDocument30 pages2-1 PFA Static Flat CouponMa RcoNo ratings yet
- PFA Static Un-notched Coupon TutorialDocument30 pagesPFA Static Un-notched Coupon Tutorialelgheryb_choukriNo ratings yet
- Physical Properties in Solid EdgeDocument4 pagesPhysical Properties in Solid EdgebplaNo ratings yet
- YubbnbvDocument18 pagesYubbnbvelhimass.zakiNo ratings yet
- On Modmaking: 1. Stat ConfigurationDocument16 pagesOn Modmaking: 1. Stat ConfigurationDavid PerezNo ratings yet
- Lecture 01c - QuestDistexDocument74 pagesLecture 01c - QuestDistexsdsNo ratings yet
- DualSPHysics Pre-processing Interface DocumentationDocument10 pagesDualSPHysics Pre-processing Interface DocumentationbestbreederNo ratings yet
- EDU CAT EN V5A FF V5R19 Lesson02 Toprint PDFDocument90 pagesEDU CAT EN V5A FF V5R19 Lesson02 Toprint PDFdjuka65No ratings yet
- ChemSep Tutorial: Understanding PCDmanagerDocument37 pagesChemSep Tutorial: Understanding PCDmanagerjoseNo ratings yet
- ABAQUS Workshop Defines Steel and Gasket Properties for Pump Model SectionsDocument3 pagesABAQUS Workshop Defines Steel and Gasket Properties for Pump Model SectionsNguyen Trong HoNo ratings yet
- Frame Analysis: Topics in This SectionDocument14 pagesFrame Analysis: Topics in This SectiontyannottiNo ratings yet
- Anycasting anyDBASEDocument28 pagesAnycasting anyDBASEHenriqueGabrielNo ratings yet
- Hunter-Douglas Ceilings Material Read-MeDocument9 pagesHunter-Douglas Ceilings Material Read-MeJD GalindorNo ratings yet
- Exercise DP.1: Estimating Pure Component Properties in Hysys Workshop Report RequirementsDocument8 pagesExercise DP.1: Estimating Pure Component Properties in Hysys Workshop Report RequirementsHafiz AzizNo ratings yet
- Loading Sample Data Into The Borehole DatabaseDocument36 pagesLoading Sample Data Into The Borehole DatabaseYair Galindo Vega100% (1)
- Ingeniería Petrolera en Petrel: Funciones de SaturaciónDocument19 pagesIngeniería Petrolera en Petrel: Funciones de SaturaciónMiguel CastilloNo ratings yet
- 01 GettingStartedInSteadyStateDocument20 pages01 GettingStartedInSteadyStatetaeebNo ratings yet
- COCO Help TEADocument43 pagesCOCO Help TEAPedro Milton ChibulachoNo ratings yet
- Fem Lab-Lab ManualDocument45 pagesFem Lab-Lab Manualnoble aNo ratings yet
- Cosmos Works TutorialsDocument226 pagesCosmos Works Tutorialssolidbr100% (3)
- LS-DYNA Material TableDocument15 pagesLS-DYNA Material TablePuneet BahriNo ratings yet
- Case Study 1. Developing HYSYS Material Stream: 1.1. Component and Fluid List SelectionDocument7 pagesCase Study 1. Developing HYSYS Material Stream: 1.1. Component and Fluid List SelectionChakravarthy BharathNo ratings yet
- Adding or Modifying Materials in PV Elite or CodeCalcDocument11 pagesAdding or Modifying Materials in PV Elite or CodeCalc9913489806No ratings yet
- Disk Drive Stress Analysis of The Power Supply Switch:, QWKLVH (Huflvh/Rxzloogrwkh IroorzlqjvwhsvDocument22 pagesDisk Drive Stress Analysis of The Power Supply Switch:, QWKLVH (Huflvh/Rxzloogrwkh IroorzlqjvwhsvDownNo ratings yet
- The Primary Entities Are Going To BeDocument47 pagesThe Primary Entities Are Going To BeCorretta StephensNo ratings yet
- Tutorial: C I V I LDocument23 pagesTutorial: C I V I LRamon GutierrezNo ratings yet
- Armstrong World Read MeDocument10 pagesArmstrong World Read Meحلاوه توفيNo ratings yet
- Danger Object FilingDocument4 pagesDanger Object FilinghectorjazzNo ratings yet
- Unigraphics NX7.5 - Thermal and Flow AnalysisDocument68 pagesUnigraphics NX7.5 - Thermal and Flow AnalysisBikash Chandra SahooNo ratings yet
- Thermal Stresses in A Layered Plate: Created in COMSOL Multiphysics 5.4Document12 pagesThermal Stresses in A Layered Plate: Created in COMSOL Multiphysics 5.4Jose Velasquez TeranNo ratings yet
- CHP 12Document52 pagesCHP 12lamedeerNo ratings yet
- RD-1085 Linear Steady State Heat Convection Analysis PDFDocument8 pagesRD-1085 Linear Steady State Heat Convection Analysis PDFalex100% (1)
- Sample TEch Writing - Process Documentation, Ritzie Edson V. LaminaDocument11 pagesSample TEch Writing - Process Documentation, Ritzie Edson V. LaminaritzieevlaminaNo ratings yet
- 3 14 Material SearchDocument24 pages3 14 Material Searchphạm minh hùngNo ratings yet
- Multisurf 8.0 What'S New: AerohydroDocument20 pagesMultisurf 8.0 What'S New: AerohydrotoncipNo ratings yet
- ABAQUS - CAE User's Manual (v6.6)Document46 pagesABAQUS - CAE User's Manual (v6.6)bikramjit debNo ratings yet
- Catia - FeaDocument240 pagesCatia - FeaFrancescNo ratings yet
- Tutorial 14 Geotextile Reinforced RampDocument22 pagesTutorial 14 Geotextile Reinforced Rampatma galihNo ratings yet
- Module 2 Lesson 3Document26 pagesModule 2 Lesson 3Elvis RafailaNo ratings yet
- Computer Aided Modeling & Analysis Lab Final BackupDocument48 pagesComputer Aided Modeling & Analysis Lab Final Backup4PS19ME015 Anushree N.RNo ratings yet
- QTP Environment Variable GuideDocument23 pagesQTP Environment Variable GuideShakeel Ahmed IqbalNo ratings yet
- MVS JCL Utilities Quick Reference, Third EditionFrom EverandMVS JCL Utilities Quick Reference, Third EditionRating: 5 out of 5 stars5/5 (1)
- Pivot Tables In Depth For Microsoft Excel 2016From EverandPivot Tables In Depth For Microsoft Excel 2016Rating: 3.5 out of 5 stars3.5/5 (3)
- Problem Visualization - Delhi, Environment & PollutionDocument3 pagesProblem Visualization - Delhi, Environment & PollutionPiyush SandujaNo ratings yet
- 49303Document11 pages49303Enrique SerranoNo ratings yet
- Daily monitoring report polling stationsDocument3 pagesDaily monitoring report polling stationsPiyush SandujaNo ratings yet
- env/2012/abstracts - HTML: A New Refutation of The Classical Concept of Time in Quantum Relativity. Kurt Gödel, AlbertDocument2 pagesenv/2012/abstracts - HTML: A New Refutation of The Classical Concept of Time in Quantum Relativity. Kurt Gödel, AlbertPiyush SandujaNo ratings yet
- Conversion FactorsDocument4 pagesConversion FactorsPiyush SandujaNo ratings yet
- New Refutation of Time in Quantum RelativityDocument3 pagesNew Refutation of Time in Quantum RelativityPiyush SandujaNo ratings yet
- PHE1Document4 pagesPHE1Piyush SandujaNo ratings yet
- Gambit Mesh FundaDocument22 pagesGambit Mesh FundaPiyush SandujaNo ratings yet
- Types of Oil Seed Used in Oil Processing AreDocument27 pagesTypes of Oil Seed Used in Oil Processing AredagimNo ratings yet
- Ample Aper: Section - ADocument12 pagesAmple Aper: Section - AShriyaa BhatnagarNo ratings yet
- Valvulas de Bola 2Document20 pagesValvulas de Bola 2claudio godinezNo ratings yet
- Lab 5 - SDS PAGEDocument22 pagesLab 5 - SDS PAGEBullet Arguelles100% (1)
- Experimental Investigation and Performance Evaluation of Solar Still Using Phase Change MaterialDocument10 pagesExperimental Investigation and Performance Evaluation of Solar Still Using Phase Change MaterialrassNo ratings yet
- Environmental Pollution Upsc Notes 90 PDFDocument6 pagesEnvironmental Pollution Upsc Notes 90 PDFSumit ChauhanNo ratings yet
- NEET Physics Title Under 40Document18 pagesNEET Physics Title Under 40Priyanka SomkuwarNo ratings yet
- 1 IntoductionDocument2 pages1 IntoductionAshutosh SinghNo ratings yet
- Francesco Vetere - Dynamic Magma Evolution-Wiley (2020)Document211 pagesFrancesco Vetere - Dynamic Magma Evolution-Wiley (2020)hasnaa azziNo ratings yet
- Northern Cement CorporationDocument26 pagesNorthern Cement CorporationJHuvieCLaireNo ratings yet
- Photosynthesis Limiting FactorsDocument2 pagesPhotosynthesis Limiting FactorsOsaid AtherNo ratings yet
- ASTU chemistry questionsDocument68 pagesASTU chemistry questionsFaya MohammadNo ratings yet
- Chem 26.1 - Mock Formal ReportDocument6 pagesChem 26.1 - Mock Formal ReportAlexander Gordon InesNo ratings yet
- PT SSJ Corporate ProfileDocument8 pagesPT SSJ Corporate ProfileYohanest ChandraNo ratings yet
- Some Guidelines To The Design of A Diagnostic Leaching ExperimentDocument10 pagesSome Guidelines To The Design of A Diagnostic Leaching ExperimentAldoNo ratings yet
- SQA-Hess's Law QuestionsDocument4 pagesSQA-Hess's Law QuestionsWidya GrantinaNo ratings yet
- SOP-Calibration and pH MeasurementDocument2 pagesSOP-Calibration and pH MeasurementrancidNo ratings yet
- Lab ManualsDocument9 pagesLab ManualsRaja KhanNo ratings yet
- Coordination Compounds Cet-2Document2 pagesCoordination Compounds Cet-2Amen RaipurNo ratings yet
- Heterogeneous CatalystDocument24 pagesHeterogeneous Catalystlalukalu420No ratings yet
- T1 1 E Automotive 072Document15 pagesT1 1 E Automotive 072Marian OstrowskiNo ratings yet
- Patchouli Oil Isolation and Identification of Chemical Components Using GC-MSDocument7 pagesPatchouli Oil Isolation and Identification of Chemical Components Using GC-MSjuggernautzNo ratings yet
- Solar Oven Challenge ReportDocument7 pagesSolar Oven Challenge Reportapi-311233754No ratings yet
- B. Tech. - I (All Branches) ASP102ABC (T) : Engineering Physics (Theory) L T P C 3 0 2 4Document2 pagesB. Tech. - I (All Branches) ASP102ABC (T) : Engineering Physics (Theory) L T P C 3 0 2 4Harikrishna ShenoyNo ratings yet
- A Presentation On: Presented byDocument67 pagesA Presentation On: Presented byOlufemi KolawoleNo ratings yet
- MSE Admission and Degree RequirementsDocument6 pagesMSE Admission and Degree Requirementsdeathbuddy_87No ratings yet
- Lordo PresentationDocument31 pagesLordo PresentationisleepinadrawerNo ratings yet
- Org. Chem. (Chapter 8)Document25 pagesOrg. Chem. (Chapter 8)Jia LinNo ratings yet
- The Accurate Measurement of Contact Angle, Phase ContactDocument8 pagesThe Accurate Measurement of Contact Angle, Phase ContactaleiviNo ratings yet
- Num DiffDocument7 pagesNum DiffMohsan HasanNo ratings yet