Professional Documents
Culture Documents
Process Validation Protocol
Uploaded by
Bibek Singh MahatOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Process Validation Protocol
Uploaded by
Bibek Singh MahatCopyright:
Available Formats
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 1 of 9]
Validation Protocol No.: ___________________________________
Name of the Products :
Protocol written by ____________________________________________ Date _____________________
Approval ____________________________________________________Date _____________________
Approval ____________________________________________________ Date _____________________
Approval _____________________________________________________Date _____________________
___________________________________________________________________________________________
Objective:
To determine that process consistently performs as intended by repeatedly running the system on its intended
schedules and recording all relevant information and data. Results must demonstrate that the process meets pre-
determined specifications under normal conditions, and where appropriate worst case conditions.
___________________________________________________________________________________________
Scope:
To be performed with validated equipment in the specified location in validated premises. If equipment or systems
or the facility are modified or the premises where the process takes place is changed, or the process is relocated,
the process must be re-validated after the systems, equipment and facility qualifications, as appropriate, have
been performed and approved.
___________________________________________________________________________________________
Responsibility:
The persons responsible for the process will perform the validation and record the information. The responsible
person will supervise the study, verify the completion of the records and write the report. Quality Assurance will
review and approve the Process Validation Protocol and Report.
Name of the Personnel Designation Signature
………………………….. …………………………………….. …………………………
………………………….. …………………………………….. …………………………
………………………….. …………………………………….. …………………………
………………………….. …………………………………….. …………………………
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 2 of 9]
Materials, Equipments and Documents:
Master Formulation Record (MFR), Batch Manufacturing Record, Equipment List with Equipment No. and all the
Standard Operating Procedures (SOPs) for normal operation of the processes under test.
Documents of Normal Operation:
Name of the Document Document No. Effective Date
Master Formulation Record
Batch Manufacturing Record
List of Equipments
Name of Equipment Equipment No.: Qualification Completed
DQ IQ OQ PQ
Compiled by: ____________________________________________________________ Date: ____________
Reviewed by: ____________________________________________________________ Date: ____________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 3 of 9]
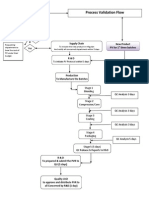
Procedure:-
Performance:-
1. Process: Run full process according to SOP three times and record all required data.
2. Deviations to the procedures must be recorded on the data record forms.
3. Analytical tests: Perform the routine tests associated with the process according to the SOP
4. Test results must be approved by QC.
Evaluation :-
1. Attach all data record forms and charts.
2. Perform all necessary calculations and statistical analyses (pre-determined).
3. Compare to acceptance criteria.
4. Prepare Deviation Report (including the justification of acceptance and impact on the process).
Prepare a Process Validation Report:-
This should include for each validation run:
1. Date study initiated; date completed; observations made;
2. Problems encountered; completeness of information collected; summary of the deviation report;
3. Results of tests and statistical analyses; do results meet acceptance criteria; location of original data;
4. Other information relevant to the study.
Conclusions will be made on the validity of the process in individual runs and on the three
Consecutive Validation Runs.
Approval
1. Submit the Document to QA for review and approval.
2. The Process must meet all specifications for three consecutive runs.
Compiled by: ____________________________________________________________ Date: ____________
Reviewed by: ____________________________________________________________ Date: _____________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 4 of 9]
Processing Parameters of Production
S.N. Processing Step Control Points Test Points Acceptance Criteria
Compiled by: ____________________________________________________________ Date: _____________
Reviewed by: ____________________________________________________________ Date: _____________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 5 of 9]
List of attached Data Record Forms:-
Compiled by: ____________________________________________________________ Date: ___________
Reviewed by: ____________________________________________________________ Date:_____________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 6 of 9]
Calculation and Statistical Analysis
Compiled by: ____________________________________________________________ Date: __________
Reviewed by: ____________________________________________________________ Date: __________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 7 of 9]
Acceptance Criteria Vs Test Results
Parameter Specification Result Pass/Fail
Compiled by: ____________________________________________________________ Date: __________
Reviewed by: ____________________________________________________________ Date: ___________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 8 of 9]
Deviations:
Justification for Acceptance:
Impact on Process:
Written by: _______________________________________________________________ Date: __________
Reviewed by: ____________________________________________________________ Date: ___________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
KU PHARMACEUTICALS PRIVATE LIMITED
PROCESS VALIDATION PROTOCOL [ Page No: 9 of 9]
Process Validation Report :-
Results:-
Conclusion:-
Written by: _______________________________________________________________ Date: ____________
Reviewed by: ____________________________________________________________ Date: ___________
2/2/2010 10:32:44 AM ASSIGNMENT ON PROCESS VALIDATION/ FORMATS/BIBEK SINGH MAHAT
You might also like
- Process Validation ProtocolDocument10 pagesProcess Validation ProtocolDivya SekarNo ratings yet
- Process Validation Report - RajanDocument16 pagesProcess Validation Report - RajanRAJAN RAMANUJ67% (3)
- Process Validation Final ReportDocument9 pagesProcess Validation Final ReportShagorShagor75% (4)
- Master Validation PlanDocument23 pagesMaster Validation Planchennamareddysriniva89% (9)
- Validation Master Plan SummaryDocument25 pagesValidation Master Plan SummaryAtul Sharma100% (2)
- Validation Master Plan TemplateDocument17 pagesValidation Master Plan TemplateNadine100% (4)
- Capsule Process ValidationDocument28 pagesCapsule Process Validationasit_m89% (28)
- Validation Master Plan PDFDocument33 pagesValidation Master Plan PDFsiva sankar50% (2)
- Process Validation GuidanceDocument11 pagesProcess Validation GuidancedutoitlouwNo ratings yet
- 2015 VMP TemplateDocument10 pages2015 VMP Templatekulbhushan singh100% (2)
- Process Validation Sample ProtocolDocument5 pagesProcess Validation Sample ProtocolBilal Masood0% (1)
- Validation ProtocolDocument63 pagesValidation ProtocolIndústria Petys64% (22)
- Equipment Qualification ToolkitDocument26 pagesEquipment Qualification ToolkitMuqeet7683% (6)
- Validation Master Plan Annex 15Document29 pagesValidation Master Plan Annex 15spark80988100% (6)
- Laboratory Quality Agreement TamplateDocument10 pagesLaboratory Quality Agreement TamplateMina Maher MikhailNo ratings yet
- Cleaning Validation ProtocolDocument17 pagesCleaning Validation Protocolswathikotla92% (36)
- Parenteral Process ValidationDocument30 pagesParenteral Process Validationravindra82% (11)
- Tim Fields Master Validation PlanDocument7 pagesTim Fields Master Validation Planmanoj262400/2100% (1)
- Validation of Sterile ProductDocument30 pagesValidation of Sterile Productneetisaharia92% (24)
- SOP Equipment ValidationDocument15 pagesSOP Equipment Validationfarjana100% (7)
- Cleaning Validation ProtocolDocument3 pagesCleaning Validation Protocolpuneetogupta100% (1)
- Flow Chart Process ValidationDocument1 pageFlow Chart Process Validationmasthan6yNo ratings yet
- Process Validation of Ointment Creams 2Document40 pagesProcess Validation of Ointment Creams 2Farhana Shermeen0% (1)
- Bulk Holding Time Study ReportDocument8 pagesBulk Holding Time Study ReportFaress RabiNo ratings yet
- Hold Time Study Protocol OF Cleaned Manufacturing Equipment Awaiting For UseDocument11 pagesHold Time Study Protocol OF Cleaned Manufacturing Equipment Awaiting For Usegopusankar100% (5)
- Process Validation Protocol For Ketofast 10 TabletDocument26 pagesProcess Validation Protocol For Ketofast 10 TabletShagorShagor100% (6)
- Process Validation ProtocolDocument31 pagesProcess Validation ProtocolMangesh Parulekar93% (14)
- Process Validation of Ointment/Cream FormulationDocument40 pagesProcess Validation of Ointment/Cream FormulationGursharanjit Singh Shinh100% (2)
- Validation Master PlanDocument5 pagesValidation Master Planazamyn83% (6)
- Cold Chain Validation ProtocolDocument12 pagesCold Chain Validation ProtocolJAGADISH PHARMACEUTICALS100% (3)
- Site Validation Master Plan OverviewDocument34 pagesSite Validation Master Plan OverviewJonatan Dominguez PerezNo ratings yet
- Cleaning ValidationDocument17 pagesCleaning ValidationMollidain SandeepNo ratings yet
- SOP For Equipment Qualification - Pharmaceutical GuidelinesDocument4 pagesSOP For Equipment Qualification - Pharmaceutical GuidelinesMuthuraman M100% (2)
- Master Cleaning Validation PlanDocument25 pagesMaster Cleaning Validation PlanWidya Lukitasari100% (1)
- Procedure - Equipment ValidationDocument2 pagesProcedure - Equipment Validationشادي الاخرس100% (2)
- CLEANING VALIDATION PROTOCOLDocument7 pagesCLEANING VALIDATION PROTOCOLArieTamaNo ratings yet
- Validation VMP Validation Master PlanDocument13 pagesValidation VMP Validation Master Plank.p.No ratings yet
- Process Validation of LiquidDocument24 pagesProcess Validation of Liquidasit_m92% (25)
- Basics of Equipment Qualification - Pharma Pathway PDFDocument6 pagesBasics of Equipment Qualification - Pharma Pathway PDFJ VENKATESHNo ratings yet
- Cleaning Validation Protocol TEMPLATEDocument9 pagesCleaning Validation Protocol TEMPLATEnatavceNo ratings yet
- VALIDATION MASTER PLAN (Repaired)Document56 pagesVALIDATION MASTER PLAN (Repaired)aman pathania100% (3)
- IQOQ ProtocolDocument4 pagesIQOQ ProtocolVijay RajaindranNo ratings yet
- Cleaning Validation MACO v2.0Document2 pagesCleaning Validation MACO v2.0Ovais08100% (2)
- Iq Oq PQ ChamberDocument17 pagesIq Oq PQ Chamberelias_7760% (5)
- Cleaning Validation Rinsing TesDocument5 pagesCleaning Validation Rinsing TesUrsula HilleNo ratings yet
- Utilities QualificationDocument162 pagesUtilities QualificationDoan Chi ThienNo ratings yet
- Validation of Coating Equipment (Ketik Ulang)Document6 pagesValidation of Coating Equipment (Ketik Ulang)Dedhieaja0% (1)
- Clean Val Protocol 1Document8 pagesClean Val Protocol 1krishnavkkNo ratings yet
- Process Validation Protocol For Gliclazide Modified Release TabletsDocument32 pagesProcess Validation Protocol For Gliclazide Modified Release Tabletsreflectprakash3610100% (2)
- Validation Master PlanDocument27 pagesValidation Master PlanPrashansa Shrestha85% (13)
- Cleaning Validation ProtocolDocument6 pagesCleaning Validation ProtocolVega life sciences100% (1)
- Protocol For Process Validation of Cefowin Cefotaxime 1000mgDocument31 pagesProtocol For Process Validation of Cefowin Cefotaxime 1000mgShafaq ALI100% (2)
- Validation Master PlanDocument56 pagesValidation Master PlanMd Nazim Uddin100% (6)
- Iq OqDocument20 pagesIq OqDaniela Cotoman100% (1)
- Cleanroom Technology: Fundamentals of Design, Testing and OperationFrom EverandCleanroom Technology: Fundamentals of Design, Testing and OperationNo ratings yet
- cGMP Current Good Manufacturing Practices for PharmaceuticalsFrom EverandcGMP Current Good Manufacturing Practices for PharmaceuticalsRating: 1 out of 5 stars1/5 (2)
- Nutraceutical Practices in NepalDocument78 pagesNutraceutical Practices in NepalBibek Singh MahatNo ratings yet
- Commonly Used Medicine With The Potention For Misuse, Abuse or AddictionDocument51 pagesCommonly Used Medicine With The Potention For Misuse, Abuse or AddictionBibek Singh MahatNo ratings yet
- Precursor of Narcotics DrugsDocument6 pagesPrecursor of Narcotics DrugsBibek Singh MahatNo ratings yet
- Doxofylline: D 400 MG TabletsDocument33 pagesDoxofylline: D 400 MG TabletsBibek Singh Mahat100% (2)
- Pharmaceutical Drugs and Ways of Maintaining Chain of Custody For Schedule 1 Drug.Document5 pagesPharmaceutical Drugs and Ways of Maintaining Chain of Custody For Schedule 1 Drug.Bibek Singh Mahat100% (1)
- WHO Guidelines For Assessing of Herbal Medicine With Reference To Contaminants and ResiduesDocument118 pagesWHO Guidelines For Assessing of Herbal Medicine With Reference To Contaminants and ResiduesKeisha Dela CernaNo ratings yet
- Precursor of Narcotics DrugsDocument6 pagesPrecursor of Narcotics DrugsBibek Singh MahatNo ratings yet
- Snake Antivenom GuidelineDocument141 pagesSnake Antivenom GuidelineBibek Singh MahatNo ratings yet
- TERBINAFINE HCLDocument38 pagesTERBINAFINE HCLBibek Singh MahatNo ratings yet
- Semisolid Dosage Forms by BIBEK SINGH MAHATDocument105 pagesSemisolid Dosage Forms by BIBEK SINGH MAHATBibek Singh Mahat100% (1)
- Mathematical Models Used in The Drug Release StudiesDocument27 pagesMathematical Models Used in The Drug Release StudiesBibek Singh Mahat100% (12)
- Aerosol - Bibek Singh MahatDocument81 pagesAerosol - Bibek Singh MahatBibek Singh Mahat100% (1)
- Pharmacists Projection in Nepalese Pharma Industries For Next 20 YearsDocument16 pagesPharmacists Projection in Nepalese Pharma Industries For Next 20 YearsBibek Singh MahatNo ratings yet
- Expanding Role of Drug Delivery System in Modern Health Care SystemDocument14 pagesExpanding Role of Drug Delivery System in Modern Health Care SystemBibek Singh MahatNo ratings yet
- Bio Transformation Bibek Singh Mahat RN07Document20 pagesBio Transformation Bibek Singh Mahat RN07Bibek Singh Mahat0% (1)
- Cleanroom Concept and Regulatory Requirements - BibekDocument9 pagesCleanroom Concept and Regulatory Requirements - BibekBibek Singh Mahat100% (1)
- An Approach To Process ValidationDocument17 pagesAn Approach To Process ValidationBibek Singh Mahat100% (4)
- Bio Transformation Presentation by BibekDocument40 pagesBio Transformation Presentation by BibekBibek Singh MahatNo ratings yet
- Pulsincap Ra Bibek Singh MahatDocument51 pagesPulsincap Ra Bibek Singh Mahatcsmalliks100% (1)
- Water Research: Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data QualityDocument14 pagesWater Research: Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data QualityMochamadAliHaidarNo ratings yet
- Dade Actin FSL Activated PTT Reagent - Rev 10 DXDCM 09017fe9804eb3b9-1605649828217Document7 pagesDade Actin FSL Activated PTT Reagent - Rev 10 DXDCM 09017fe9804eb3b9-1605649828217Dany BatistaNo ratings yet
- Adherence To Academic Calenders 2019 2ND SEMDocument3 pagesAdherence To Academic Calenders 2019 2ND SEMyaminiNo ratings yet
- Probability PracticeDocument7 pagesProbability PracticeanushajjNo ratings yet
- Anatomy and Physiology II CCuervo PDFDocument6 pagesAnatomy and Physiology II CCuervo PDFLivan MartellNo ratings yet
- Test of Competence 2021 CBT Information Booklet For NursesDocument12 pagesTest of Competence 2021 CBT Information Booklet For NursesAppiah GodfredNo ratings yet
- ROBINS-I: A Tool For Assessing Risk of Bias in Non-Randomised Studies of InterventionsDocument7 pagesROBINS-I: A Tool For Assessing Risk of Bias in Non-Randomised Studies of InterventionsSelvaArockiamNo ratings yet
- Condition Assessment Programme PDFDocument4 pagesCondition Assessment Programme PDFAinul YaqienNo ratings yet
- IMPAACT 2034 - LPC - v1.1 - 12jul23Document13 pagesIMPAACT 2034 - LPC - v1.1 - 12jul23Lucas Masiêro AraujoNo ratings yet
- Shs Work Immersion Deployment Clearance Form: Classs Schedule For The Current Semester Time Alloted For Work ImmersionDocument1 pageShs Work Immersion Deployment Clearance Form: Classs Schedule For The Current Semester Time Alloted For Work ImmersionMiriam HernandezNo ratings yet
- Role Purpose of Role: Role Profile INVIGILATOR InvigilatorDocument3 pagesRole Purpose of Role: Role Profile INVIGILATOR Invigilatorernest libertyNo ratings yet
- Are Zero Inflated Distributions Compulsory in The Presence of Zero InflationDocument4 pagesAre Zero Inflated Distributions Compulsory in The Presence of Zero InflationInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Forensic Chemistry and Toxicology FundamentalsDocument25 pagesForensic Chemistry and Toxicology FundamentalsJesmar Lobo100% (2)
- Curriculum Vitae: Testing Level II - Weld Plate and Pipe",03 Nov-24 Nov 2009 (144 TrainingDocument5 pagesCurriculum Vitae: Testing Level II - Weld Plate and Pipe",03 Nov-24 Nov 2009 (144 TrainingAditya RomasNo ratings yet
- Adult Lab Values Cheat SheetDocument3 pagesAdult Lab Values Cheat SheetEunice CortésNo ratings yet
- AdmitCard Csir NetDocument3 pagesAdmitCard Csir NetPraveen KumarNo ratings yet
- Efektivitas Pendidikan Gizi Dengan Media Kartu EduDocument8 pagesEfektivitas Pendidikan Gizi Dengan Media Kartu Edutiti dwi elfinaNo ratings yet
- NGT Insertion and FeedingDocument2 pagesNGT Insertion and FeedingMaria Charis Anne IndananNo ratings yet
- Examination and Investigation of Musculoskeletal SystemDocument15 pagesExamination and Investigation of Musculoskeletal SystemofficialmidasNo ratings yet
- Quasi-Experimental Designs: W R. S J K. L Volume 3, Pp. 1641-1644 inDocument4 pagesQuasi-Experimental Designs: W R. S J K. L Volume 3, Pp. 1641-1644 inDian ARNo ratings yet
- Admit Card - 42909404Document3 pagesAdmit Card - 42909404Amit GuptaNo ratings yet
- M08 Domestic ApplianceDocument94 pagesM08 Domestic AppliancebilisummaaNo ratings yet
- Article 149Document5 pagesArticle 149Claus LawrenceNo ratings yet
- Doh Peme-Laboratory Dep't December 2020Document1 pageDoh Peme-Laboratory Dep't December 2020MSL LaboratoryNo ratings yet
- Chemistry - Cornell University College of Veterinary MedicineDocument2 pagesChemistry - Cornell University College of Veterinary MedicineMikaelle CastilhoNo ratings yet
- Final: Patient Name: Dummy 0002UG999999Document1 pageFinal: Patient Name: Dummy 0002UG999999mirtunjay kumarNo ratings yet
- HbA1C, RBS AND FBS DATADocument2 pagesHbA1C, RBS AND FBS DATASamwel GachokaNo ratings yet
- 5th ROTATION CLINICAL CHEMISTRY PROJECTDocument37 pages5th ROTATION CLINICAL CHEMISTRY PROJECTJanelle RemorozaNo ratings yet
- Manuskrip Ebn 6 Juni 2019Document8 pagesManuskrip Ebn 6 Juni 2019Te GuhNo ratings yet
- Session: 27: TopicDocument62 pagesSession: 27: TopicMikias BekeleNo ratings yet