Professional Documents
Culture Documents
Periodic Ionization and Electronegativity
Uploaded by
linzelOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Periodic Ionization and Electronegativity
Uploaded by
linzelCopyright:
Available Formats
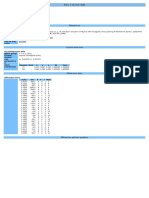
Ionization Energy and the Periodic Table
The periodic table organizes the elements according to their increasing atomic number. This is useful
because repeating patterns of physical and chemical properties occur as the atomic number of the
elements increases. This is called the periodic law.
Ionization Energy is the amount of attraction an atom has for its own electrons. An element with a
high ionization energy attracts its own electrons very strongly. It is not likely to release electrons to
participate in chemical reactions. An element with a low ionization energy gives up electrons easily.
It is more likely to participate in chemical reactions. Thus, the ionization energy of each element
gives us one measure of its reactivity compared to other elements.
This information is particularly useful in predicting how each metallic element will behave
chemically. Ionization energy is not as useful for predicting the chemical activity of nonmetals and
metalloids. They are more likely to accept or share electrons in chemical reactions rather than to give
up their electrons. In this activity you will examine how the ionization energy varies as the atomic
number increases.
Procedure:
1. Study the chart of ionization energies and electronegativies found below. The units for
ionization energy on this chart are kilojoules/mole.
2. If you are plotting this graph by hand turn the paper horizontally. The x-axis is the atomic
number and should go to 92 (uranium). The y-axis is ionization energy (kJ/mol) and should go
from 0 to 2500.
3. Connect the data points with straight lines. Start with the ionization energy for the element with
atomic number 1 and connect to atomic number 2 value, then continue with a new straight line to
number 3. Label the graph peaks and valleys with the symbol for those elements.
3. Write out in words your conclusions from the graph.
1314 2368
H He
2.1 Ionization Energies and --
519 900 Electronegativites 799 1088 1401 1036 1682 2076
Li Be B C N O F Ne
1.0 1.5 2.0 2.5 3.0 3.5 4.0 --
498 736 578 787 1063 1000 1256 1519
Na Mg Al Si P S Cl Ar
0.9 1.2 1.5 1.8 2.1 2.5 3.0 --
418 590 632 661 649 653 716 762 757 736 745 904 578 782 1013 942 1142 1352
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
0.8 1.0 1.3 1.5 1.6 1.6 1.5 1.8 1.8 1.8 1.9 1.6 1.6 1.8 2.0 2.4 2.8 --
402 548 636 670 653 695 720 724 745 804 728 866 557 707 833 870 1008 1172
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
0.8 1.0 1.2 1.4 1.5 1.8 1.9 2.2 2.2 2.2 1.9 1.7 1.7 1.8 1.9 2.1 2.5 --
377 502 540 531 586 770 757 841 887 862 887 1008 590 716 770 820 -- 1038
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
0.7 0.9 1.1 1.3 1.5 1.7 1.9 2.2 2.2 2.2 2.4 1.9 1.8 1.8 1.9 2.0 2.2 --
-- 510 678 --
Fr Ra Ac Ku
0.7 0.9 1.1 --
1008 – 665 557 607 -- 540 548 594 649 657 -- -- -- 598 481
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Ionization energy
1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.1 1.2
C - symbol
-- -- 385 -- -- -- -- -- -- -- -- -- -- --
2.5 - electro- Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
negativity 1.3 1.5 1.7 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 --
You might also like
- Semiconductor Silicon Crystal TechnologyFrom EverandSemiconductor Silicon Crystal TechnologyFumio ShimuraRating: 5 out of 5 stars5/5 (1)
- Ace General Chemistry 1 and 2Document187 pagesAce General Chemistry 1 and 2Ari Singh100% (2)
- Chemistry: Form 3 Final ExaminationDocument23 pagesChemistry: Form 3 Final Examinationjonas hoNo ratings yet
- Chemistry Matters Textbook Full SolutionsDocument46 pagesChemistry Matters Textbook Full SolutionsongjiachengedricNo ratings yet
- HighSchool-Chemistry G10 To G12Document444 pagesHighSchool-Chemistry G10 To G12patkhsheng@hotmail.com100% (1)
- Atoms' Family Valence ElectronsDocument2 pagesAtoms' Family Valence ElectronsJan IceNo ratings yet
- DR Asai BookDocument84 pagesDR Asai BookMichael-Anthony :Seegers100% (3)
- PRACTICA 1 Richard ManufacturaDocument16 pagesPRACTICA 1 Richard ManufacturaRichard ColqueNo ratings yet
- ICP-MS Perkin ElmerDocument76 pagesICP-MS Perkin Elmernajdat alzaatraNo ratings yet
- CMC Chapter 03Document59 pagesCMC Chapter 03api-294176229No ratings yet
- Physical Science Exam ReviewDocument3 pagesPhysical Science Exam Reviewjayson babaran100% (1)
- Detailed Lesson Plan in Science 8: P - OT - N N - TRO - N - LE - TR - NDocument8 pagesDetailed Lesson Plan in Science 8: P - OT - N N - TRO - N - LE - TR - NMc Laurence Marquez SaligumbaNo ratings yet
- Sci8 q3 Module3-1Document32 pagesSci8 q3 Module3-1Matt Lhouie MartinNo ratings yet
- Mining Project Conceptual StudyDocument35 pagesMining Project Conceptual StudyLeslie IvettNo ratings yet
- Transistor Characteristics and BiasDocument28 pagesTransistor Characteristics and Biaskeng100% (2)
- "Optical Properties and Electronic Structure of Wide Band Gap II-VI Semiconductors" by I. Hernández-CalderónDocument12 pages"Optical Properties and Electronic Structure of Wide Band Gap II-VI Semiconductors" by I. Hernández-Calderóncagatay224282No ratings yet
- IEO 2021: China's Electricity Generation Projections by FuelDocument1 pageIEO 2021: China's Electricity Generation Projections by FuelChenyang LinNo ratings yet
- CHY Exp 4Document5 pagesCHY Exp 4Nikhilesh PrabhakarNo ratings yet
- Chemical Bonding: Positive Ion Positive IonDocument23 pagesChemical Bonding: Positive Ion Positive IonAFLAC ............100% (2)
- MuCircuitSpreadsheet 0Document9 pagesMuCircuitSpreadsheet 0MunteanuNo ratings yet
- Cross Specialization Training Outputs (Chemistry) 1Document4 pagesCross Specialization Training Outputs (Chemistry) 1Sid QuijanoNo ratings yet
- 11 01 24 SR Star Co Scmodel A, B&C Jee Main GTM 13n Key&sDocument16 pages11 01 24 SR Star Co Scmodel A, B&C Jee Main GTM 13n Key&sReddyNo ratings yet
- BondingDocument6 pagesBondingIanne PasteraNo ratings yet
- Data SheetDocument2 pagesData SheetJussier VitorianoNo ratings yet
- Electron Stopping Powers: Cedric J. PowellDocument2 pagesElectron Stopping Powers: Cedric J. PowellantonioNo ratings yet
- Atoms and Periodic Table - Practice QuizDocument5 pagesAtoms and Periodic Table - Practice QuizRicardo Jr. UyNo ratings yet
- Bonding and Properties: Issues To Address..Document23 pagesBonding and Properties: Issues To Address..ILHAMNo ratings yet
- Regression Analysis: Model SummaryDocument3 pagesRegression Analysis: Model SummaryHemanta SaikiaNo ratings yet
- Sachin Path AnalysisDocument3 pagesSachin Path AnalysisHemanta SaikiaNo ratings yet
- 24ApA GITMDocument10 pages24ApA GITMgramor_naru2No ratings yet
- KPCL Phase Overcurrent Relay GradingDocument12 pagesKPCL Phase Overcurrent Relay GradingMeghavahinaNo ratings yet
- CV Emise 2018Document125 pagesCV Emise 2018Razvan SomesanNo ratings yet
- Dr Ellis' 1924 study reveals high-energy magnetic spectrum lines of radium C beta raysDocument10 pagesDr Ellis' 1924 study reveals high-energy magnetic spectrum lines of radium C beta rayscerconeNo ratings yet
- Ramos, Jushua D. (Exp 4)Document12 pagesRamos, Jushua D. (Exp 4)Jushua RamosNo ratings yet
- Department of Electrical & Computer Engineering: North South University Lab ReportDocument8 pagesDepartment of Electrical & Computer Engineering: North South University Lab ReportAli MusaNo ratings yet
- Electronegativities of The Elements (Pauling Scale) : General Trends in ElectronegativityDocument1 pageElectronegativities of The Elements (Pauling Scale) : General Trends in ElectronegativityS Linaili RahmahNo ratings yet
- TableofelectronnegDocument3 pagesTableofelectronnegJerich Ivan PaalisboNo ratings yet
- University of Baghdad Electrical Engineering Department AC Machines LabDocument8 pagesUniversity of Baghdad Electrical Engineering Department AC Machines Labمحمد باسم محمد چالي BNo ratings yet
- Quadro 1Document2 pagesQuadro 1Graziano TrainiNo ratings yet
- SPRI SERIES SHIELDED SMT POWER INDUCTORS MECHANICALS AND ELECTRICAL SPECIFICATIONSDocument4 pagesSPRI SERIES SHIELDED SMT POWER INDUCTORS MECHANICALS AND ELECTRICAL SPECIFICATIONSsongdashengNo ratings yet
- Semiconductors Used in Photovoltaic and Photocatalytic Devices: Assessing Fundamental Properties from DFTDocument13 pagesSemiconductors Used in Photovoltaic and Photocatalytic Devices: Assessing Fundamental Properties from DFTSiti AmirahNo ratings yet
- Pseuodo Components Properties PDFDocument2 pagesPseuodo Components Properties PDFwalid benhusseinNo ratings yet
- Decision of The Authority in The Matter of Motion Filed by The Federal Government Consumer End TariffDocument16 pagesDecision of The Authority in The Matter of Motion Filed by The Federal Government Consumer End TariffSyed Muhammad WajeehNo ratings yet
- CHM2000 Group Work 01Document4 pagesCHM2000 Group Work 01Aleeya JulitaNo ratings yet
- CatalogDocument47 pagesCatalogenockNo ratings yet
- Electronegativity PracticeDocument1 pageElectronegativity PracticeAMOS SODJAHINNo ratings yet
- Periodic Table PropertiesDocument7 pagesPeriodic Table PropertiesNing CahNo ratings yet
- Atomic Structure: Activity 3Document6 pagesAtomic Structure: Activity 3Aanstein YalungNo ratings yet
- EY Heet Physics 1 4 4 3 2 4 3 3 4 3 3 2 2 4 1 4 4 3 4 4 3 12 12 2 3 2500 3 120 420 4Document19 pagesEY Heet Physics 1 4 4 3 2 4 3 3 4 3 3 2 2 4 1 4 4 3 4 4 3 12 12 2 3 2500 3 120 420 4sunny meenuNo ratings yet
- MiCOM IDMT Caculation ToolDocument6 pagesMiCOM IDMT Caculation ToolVũDuyTânNo ratings yet
- Effect of Sintering Temperature On Structural PropDocument5 pagesEffect of Sintering Temperature On Structural Propmakwana monikaNo ratings yet
- Type ESR: FeaturesDocument4 pagesType ESR: FeaturesHari Sita RukminiNo ratings yet
- Expt4 Transmissionlab GRAFILODocument7 pagesExpt4 Transmissionlab GRAFILORenzo GrafiloNo ratings yet
- Word Yulan StatistikDocument18 pagesWord Yulan Statistikyulan septiNo ratings yet
- 21 tb4-5Document1 page21 tb4-5lotannaNo ratings yet
- DC1 KWK1 73 Sulawesi SelatanDocument6 pagesDC1 KWK1 73 Sulawesi SelatanVoldy MoenandarNo ratings yet
- Power Consumption: 13/apr/20 Today/ To Date/ Avg. 292020/ 1219573/ 90701Document4 pagesPower Consumption: 13/apr/20 Today/ To Date/ Avg. 292020/ 1219573/ 90701swarupkumarnayakNo ratings yet
- Ministry of Education Secondary Engagement Program Grade 10 Chemistry Week 3 Lesson 2Document7 pagesMinistry of Education Secondary Engagement Program Grade 10 Chemistry Week 3 Lesson 2Nikoli MajorNo ratings yet
- Valence ElectronsDocument8 pagesValence ElectronsJunard AsentistaNo ratings yet
- An Ab Initio Study of The Electronic Structure of 1978 Journal of MolecularDocument7 pagesAn Ab Initio Study of The Electronic Structure of 1978 Journal of MolecularFihad LatheefNo ratings yet
- Posicion 6G Weld Progression: AscendenteDocument4 pagesPosicion 6G Weld Progression: AscendenteGabriel GaraventaNo ratings yet
- Cross-section hydraulic parameters analysisDocument84 pagesCross-section hydraulic parameters analysisMonica PintoNo ratings yet
- Cpri, ProjectDocument13 pagesCpri, Projecthaldarman2004No ratings yet
- Creep Deformation of Alloy 718-ChaturvediDocument10 pagesCreep Deformation of Alloy 718-ChaturvediAntonioNo ratings yet
- The Physics and Applications of Amorphous SemiconductorsFrom EverandThe Physics and Applications of Amorphous SemiconductorsRating: 5 out of 5 stars5/5 (1)
- SNC1D - Lab - Chemical ChangesDocument2 pagesSNC1D - Lab - Chemical ChangeslinzelNo ratings yet
- Grant Proposal of Experimental DesignDocument1 pageGrant Proposal of Experimental DesignlinzelNo ratings yet
- Snc1d Quiz OneDocument1 pageSnc1d Quiz OnelinzelNo ratings yet
- SNC1D - Classification of MatterDocument1 pageSNC1D - Classification of MatterlinzelNo ratings yet
- Unconventional Oil: Scraping The Bottom of The BarrelDocument52 pagesUnconventional Oil: Scraping The Bottom of The BarrelRyan Van LenningNo ratings yet
- The Formation and Aging of StarsDocument1 pageThe Formation and Aging of StarslinzelNo ratings yet
- Intro To LightDocument8 pagesIntro To LightlinzelNo ratings yet
- Activity+2+ +spectrosDocument4 pagesActivity+2+ +spectroslinzelNo ratings yet
- WMAP UniverseDocument37 pagesWMAP UniverselinzelNo ratings yet
- The PH ScaleDocument2 pagesThe PH ScalelinzelNo ratings yet
- Lab+3+ +Physical+and+Chemical+PropertiesDocument3 pagesLab+3+ +Physical+and+Chemical+PropertieslinzelNo ratings yet
- AstroDocument19 pagesAstrolinzelNo ratings yet
- Science 1112 CurrDocument178 pagesScience 1112 Curraptureinc100% (1)
- Lewis For 3UDocument5 pagesLewis For 3Ulinzel100% (1)
- Collecting Evidence From SpaceDocument2 pagesCollecting Evidence From SpacelinzelNo ratings yet
- Gas ChemistryDocument12 pagesGas ChemistrylinzelNo ratings yet
- Bird Flue Biology - Effect Measure Blog ArticlesDocument15 pagesBird Flue Biology - Effect Measure Blog ArticleslinzelNo ratings yet
- Lewis and VSEPRDocument18 pagesLewis and VSEPRlinzel100% (14)
- IPCC 4th Report Technical SummaryDocument74 pagesIPCC 4th Report Technical Summarylinzel100% (1)
- Climate Change Holdren LectureDocument82 pagesClimate Change Holdren LecturelinzelNo ratings yet
- The Cori CycleDocument14 pagesThe Cori Cyclelinzel100% (7)
- Bird Flue Biology - Effect Measure Blog ArticlesDocument15 pagesBird Flue Biology - Effect Measure Blog ArticleslinzelNo ratings yet
- Preparing For The Next Pandemic - Foreign Affiars July 05Document8 pagesPreparing For The Next Pandemic - Foreign Affiars July 05linzelNo ratings yet
- Summary Related With The Climate ChangeDocument18 pagesSummary Related With The Climate ChangeAratz HernandezNo ratings yet
- Page 1 of 26: Masterton, W.L., Et. Al. Principles and Reactions: Chemistry For Engineering Students, Philippine Ed. 2016Document26 pagesPage 1 of 26: Masterton, W.L., Et. Al. Principles and Reactions: Chemistry For Engineering Students, Philippine Ed. 2016The Hamster VoyageNo ratings yet
- Chemistry-Group 7 ElementsDocument11 pagesChemistry-Group 7 Elementsmya thet htar sweNo ratings yet
- Gr12 Chemistry M1Document101 pagesGr12 Chemistry M1robinasanga09No ratings yet
- Bonding (p1)Document22 pagesBonding (p1)HashimNo ratings yet
- Electron TheoryDocument62 pagesElectron Theoryadancuellar100% (1)
- Chem1 842149364169223Document9 pagesChem1 842149364169223john TabonNo ratings yet
- Module in General ChemistryDocument28 pagesModule in General Chemistrysiobe batumbakalNo ratings yet
- 2003-Bruce - An Earth Scientist's Periodic Table of The Elements and Their Ions PDFDocument5 pages2003-Bruce - An Earth Scientist's Periodic Table of The Elements and Their Ions PDFafghanNo ratings yet
- Chemistry: Presented By: Mrs. Marie Nella T. VictoriaDocument75 pagesChemistry: Presented By: Mrs. Marie Nella T. VictoriaJESPHER GARCIANo ratings yet
- Physical Science - q3 - Slm3Document15 pagesPhysical Science - q3 - Slm3Boyet Alvarez AtibagosNo ratings yet
- Chapter 2: Atoms, Molecules, and IonsDocument16 pagesChapter 2: Atoms, Molecules, and IonsAbdelfattah Mohamed OufNo ratings yet
- 3.1-Atomic Structure 2C - Edexcel IGCSE 9-1 Chemistry QP 2 AnsDocument11 pages3.1-Atomic Structure 2C - Edexcel IGCSE 9-1 Chemistry QP 2 AnsJaved UddinNo ratings yet
- Campbell Essential Biology With Physiology 5th Edition Simon Test BankDocument26 pagesCampbell Essential Biology With Physiology 5th Edition Simon Test Bankwhimsyjavaneseqlsc100% (33)
- Stable and Unstable IsotopesDocument3 pagesStable and Unstable IsotopesHerminia T. PurisimaNo ratings yet
- Atoms and MoleculesDocument17 pagesAtoms and MoleculesJai KumarNo ratings yet
- ACHM 111, Week 8 Octet Rule and Chemical BondingDocument56 pagesACHM 111, Week 8 Octet Rule and Chemical BondingGoodhope MeteneNo ratings yet
- KENDRIYA VIDYALAYA SANGATHAN ZIET Bhubaneswar STUDY MATERIAL 2012 CLASS XI CHEMISTRYDocument149 pagesKENDRIYA VIDYALAYA SANGATHAN ZIET Bhubaneswar STUDY MATERIAL 2012 CLASS XI CHEMISTRYArun Sharma100% (1)
- Handbook FBR Ukaea1533Document671 pagesHandbook FBR Ukaea1533halloyu84No ratings yet
- LET Reviewer - Questions OnlyDocument5 pagesLET Reviewer - Questions OnlyAndrew T. OribianaNo ratings yet
- Soal Paket 2A To Kemitraan Bhs Ing 2016-2017Document14 pagesSoal Paket 2A To Kemitraan Bhs Ing 2016-2017Gema Galgani Jumi SNo ratings yet
- Periodicity Practice TestDocument5 pagesPeriodicity Practice TestsuhaasNo ratings yet