Professional Documents
Culture Documents
Mercury Management: White Paper 2015
Uploaded by
Jaime Andres Villegas MansillaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mercury Management: White Paper 2015
Uploaded by
Jaime Andres Villegas MansillaCopyright:
Available Formats
Mercury Management
White Paper 2015
The Measurement & Monitoring Of Mercury
In Gas-Phase Hydrocarbon Process Streams
PEI (Mercury & Chemical Services Group) and EFGS
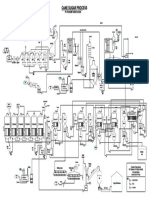
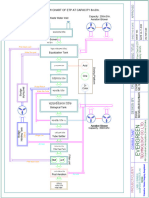
Total Mercury Sampling and Analysis Points Regen Gas to Fuel Gas System
Dehy Feed Sep.
Regen Gas K.O
To Acid Gas Incinerator or
Acid Gas Reinjection
AG Absorber
To AGRU Train
MDEA Regen
To Waste Water
Flash
Mercury Removal
Drum
To Waste Water
Condensate
Feed Separator
Compression
To Liquefaction
Stabilizer Column
Slug Catcher
Feed Stabilizer
Condensate
Mixing Drum Storage
To Waste Water
MEG Compression
MEG Compression
2 x 50% Stream Paths
T = 1.3 C To Waste Water
P = 9800 kPa
MEG Regen Column
Rich MEG Flash
MEG
Storage
Lean MEG
Copyright © 2015 Portnoy Environmental, Inc.
PEI (Mercury & Chemical Services Group) and Alliance Partner EFGS
Since 2005 PEI’s Mercury and Chemical Services Group (PEI) and Eurofins Frontier Global Sciences (EFGS)
have worked on the development of improved sampling and analysis methods for the measurement and
monitoring of mercury in natural gas and gas-phase process streams. This white paper presents an overview
of the current industry standard methods for the measurement of mercury in natural gas and gas-phase
process streams. An improved sorbent trap method based on the modification of a USEPA (United States
Environmental Protection Agency) method is also presented and compared to the current industry standard
methods. PEI and EFGS will continue to advance gas sampling technology and methods with particular
focus in the sampling of natural gas streams and gas-phase process streams at or close to the HDP and also
continue to focus on functional and molecular speciation of hydrocarbon process streams.
GLOBAL CRUDE OIL MERCURY CONCENTRATIONS
GLOBAL MERCURY BELTS & HOT SPOTS ALGERIA 13.3 µg/kg
THAILAND 593 µg/kg
VIET NAM 66.5 µg/kg
ASIA 220.1 µg/kg
CANADA 2.1 µg/kg
NORWAY 19.5 µg/kg
EUROPE 8.7 µg/kg
ARGENTINA 16.1 µg/kg
COLUMBIA 3.4 µg/kg
SOUTH AMERICA 5.3 µg/kg
ALASKA 6 µg/kg
CALIFORNIA 11.3 µg/kg
TEXAS 3.4 µg/kg
LOUISIANA 9.9 µg/kg
GOM 2.1 µg/kg
RECENTLY DISCOVERED MERCURY HOT SPOTS +
NATURAL GAS MERCURY CONCENTRATIONS
GOM Deep Shelf Gas 500 µg/Sm³
U.S. Mid-Continent 5 µg/Sm³
Western Sedimentary Basin 2 µg/Sm³* (Additional Data Pending)
Northwest Shelf >500 µg/Sm³
Known Mercury Belts & Hot Spots Recently Discovered Mercury Hot Spots Marcellus Shale >1 µg/Sm³
Utica Shale >1 µg/Sm³
Introduction
The distribution of mercury throughout hydrocarbon processing systems varies and requires significant
understanding and planning prior to implementing inspection and maintenance activities. PEI and EFGS
have gained extensive recent experiences in the management of mercury across the petroleum industry
including upstream oil and gas operations, gas gathering, processing and transmission operations, and crude
oil refining operations in the Gulf of Mexico, the Gulf of Thailand, throughout the United States, Alaska,
Canada, the Middle East and Australia.
Improvements in measurement and monitoring methods for assessing mercury in process streams provide
increased confidence in measurement precision and accuracy verified with a robust well defined numerical
data quality performance criteria. Mass balance/flux studies, mercury mapping/partitioning studies and
long term monitoring programs in refineries, gas processing plants, and gas gathering systems, have led to
the development of an improved understanding of the dynamics of mercury accumulation in oil and gas
processing equipment and facilities. Understanding accumulation, distribution and the sorption dynamics
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 2
of mercury throughout process is instrumental in the application of improved chemical decontamination and
waste management techniques used during plant turnarounds, the clean out of gas processing equipment,
and in the decontamination of downhole equipment during well intervention operations.
Mercury in Hydrocarbons Processing Plants
Mercury is a naturally occurring trace constituent of crude oil, natural gas, and natural gas condensate.
Virtually all geologic hydrocarbons contain measurable quantities of mercury. The concentration of mercury
in natural gas and associated liquids varies with geology and reservoir conditions with high concentrations
occurring in SE Asia (Thailand and Indonesia), North Africa (Algeria), Egypt, South America (Venezuela,
Bolivia), China, and the Netherlands and Australasia. Certain oil and gas reservoirs in the United States,
including at least one located in the Gulf of Mexico (deep shelf gas), are known to produce natural gas and
condensate with mercury concentrations that are orders of magnitude greater than those of typical gas
reservoirs. The discovery of mercury in hydrocarbons associated with several basins in the U.S. and Canada
is a relatively new occurrence and even in trace concentrations presents complicated health risks, environ-
mental risks, and hydrocarbon processing risks to NGL and LNG plants.
Sampling and analysis methods are critical in determining a management approach and understanding risks
to personnel, products, and process. Mercury is scavenged by carbon steel and is adsorbed/chemisorbed
into the interfacial surfaces and can complex into the scale/metal grain boundary surface requiring special
chemistry and chemical application methods for mercury process system decontamination. In natural gas,
mercury may be present in elemental form and in an adsorbed state on particulates entrained in the gas
stream. In hydrocarbon liquids (e.g., gas condensate), mercury compounds may be present in the dissolved
state and again in an adsorbed state with suspended particulates. Glycol is used to separate the moisture
from the raw natural gas and during the separation process, glycol is in two different stages. In one stage
the glycol contains little or no water (lean glycol). The other stage is after the glycol has absorbed moisture
from the natural gas and in this stage is referred to as rich glycol. Recent experience from mercury mapping
projects throughout the U.S., Asia and Canada
indicate glycol regen streams can be highly concen-
An important part of effective mercury
trated with mercury and often flow to a thermal
management involves use of Mercury oxidizer or directly to atmosphere. PEI and EFGS
Removal Units (MRUs). MRU performance have developed methods and equipment for measur-
monitoring is conducted with the use of ing mercury in this low pressure stream to quantify
mercury released to atmosphere from processing
advanced sampling methods intended to
operations.
quantify total mercury in gas phase streams,
based on EPA Methods 30B which provides Hydrocarbon processing facilities (oil and gas produc-
the highest QA/QC protocol available. tion equipment, gas processing plants, refineries and
Precise data is especially important when petrochemical manufacturing facilities) that handle
hydrocarbons with elevated mercury concentrations
assessing mercury levels in LNG facilities.
are subject to an increased risk for serious occupa-
tional exposure, damage to aluminum process
equipment and the poisoning of precious metal catalysts. The presence of mercury in LNG and NGL plant
feeds and the intermittent performance of mercury removal equipment may suggest the possibility of
mercury deposition in aluminum heat exchangers. Even at low to moderate concentrations, mercury will
accumulate in processing equipment and can cause increased risks to personnel and cryogenic process
equipment.
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 3
Sample Point Design
The collection of representative samples is one of the most important steps in
producing analytical results that meet project data quality objectives and
accurately report mercury concentrations. The selection of the sample point The industry standard
locations and the design of the sample point/probe are key components of a rule of thumb is: Collect
sampling approach that will facilitate the collection of representative samples.
the sample at a distance
The gas sample should be collected via an insertion type sample probe located
of five (5) inside pipe
well away from any fittings or appurtenances that may disturb laminar flow. The
diameters away from 90°
industry standard rule of thumb is: Collect the sample at a distance of five (5)
tube turns, valves, and
inside pipe diameters away from 90° tube turns, valves, and any other disconti-
any other discontinuities.
nuities. The tip of the insertion probe should be inserted well away from the
walls of the process piping, the rule of thumb is in the middle one-third of the
The tip of the insertion
process pipe. Any pressure reductions (through valves, regulators, reducers or
probe should be inserted
other fitting) should be designed so as to minimize Joule-Thompson cooling.
well away from the walls
Additionally, the sample point entry valve should be located on the top of a
of the process piping, the
horizontal pipe run to minimize the potential for debris or liquids to enter the
sample probe. Portable insertion probes are typically used for short term rule of thumb is in the
assessments of mercury in natural gas and process streams. The probe as well middle one-third of the
as the entire sample train should be decontaminated to less than 10 ng/Sm³ process pipe.
before installation at each sample point. PEI and EFGS go through a great deal
of effort to ensure sampling equipment and systems are blanked and verified to
<10 ng/Sm³ prior to deployment on new sampling projects.
Table 1: Typical Insertion Probe Design Specifications
Material: Stainless Steel
Maximum Operating Pressure: 2500 PSIG Maximum Operating Temperature: 225°F
Internal Coating: Sulfinert™
Pipe Connections: 1-inch NPT or 1/2-inch NPT
Sample Point Entry Valve: Minimum diameter, 1-inch, full opening ball valve.
Integral Probe Regulator:
A. Maximum Inlet Pressure: 3000 PSIG
B. Maximum Outlet Pressure: 500 PSIG
Sample Probe Tip: A liquid exclusion type, sintered stainless steel, Sulfinert™ coated
probe tip is used during the mercury sampling phase as a precaution to help minimize
the potential of a liquid slug pushing liquids into the sampling system.
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 4
Sample Collection Methods
Typically, mercury samples are collected from natural gas and process streams by the use of solid sorbent
sample traps. Two standard methods for the measurement of mercury in natural gas (ASTM D6350 and ISO
6978) both specify gold in the form of gilded silica as the solid sorbent for the collection, via amalgamation,
of mercury from a sample gas stream. After sample collection, both procedures specify a double amalgama-
tion step whereby the mercury is thermally desorbed (sorbent trap heated to ~ 800° C) onto another gold
sorbent trap, and then thermally desorbed into the analyzer cell, and analyzed by either Cold Vapor Atomic
Fluorescence Spectrometry (CVAFS), or Cold Vapor Atomic Absorption Spectrometry (CVAAS). The sample
collection process is the most difficult and critical aspect of accurately quantifying mercury concentrations in
gas phase streams. Mercury can form amalgams with many metals and alloys commonly used in gas
sampling systems including stainless steel, brass, copper, nickel, chromium and aluminum.
Due to the potential for amalgamation, and the tendency for mercury to adsorb, and chemisorb to the
surface of stainless and carbon steel the potential for loss of mercury to sample wetted metal components of
sampling systems is significant. To minimize this loss, all sampling system components that come into
contact with the sample gas should be heated and have their sample-wetted surfaces coated with a high
temperature silica coating or be made of a material that is not reactive with mercury. Conditioning (flowing
sample gas through a system for a period of time before active sampling) of installed sampling equipment
including the sample probe is recommended as a means of minimizing mercury sorption/desorption effects
that can adversely affect representativeness of collected samples. Conditioning times may vary and depend
on many factors but typically range from 12 to 24 hours.
Table 2: Mercury Sampling & Analysis Methods for Gas Phase Matrices
» ASTM D 6350 » ISO 6978 » EPA METHOD 30B
Standard test method for Natural Gas, determination Determination of total vapor
mercury sampling and analysis of mercury, Part 3: Sampling phase mercury emissions
in natural gas by atomic of mercury by amalgamation from coal fired combustion
fluorescence spectroscopy. on gold/platinum alloy. sources using carbon
sorbent traps.
While both of these methods perform reasonably well, they lack a well defined QA/QC component for the
validation of the precision and accuracy of the field sampling procedures. Also, since the double amalgama-
tion process results in the desorption of all of the collected mass from a single trap into the analyzer at once,
the sampler must limit the amount of mercury mass loading on the trap so that the upper range of the
analytical instrument is not exceeded. This limits the sample time and the sample volume.
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 5
PEI and EFGS have used EPA 30B
independently and in conjunction
with both ISO 6978 and ASTM D6350
methods on over 100 sites globally
(upstream, midstream and at
downstream processing plants).
Modified EPA Method 30B
Sampling of potentially wet gas streams is encountered in upstream processing environments such that
sampling and analytical methods require consideration to generate precise gas-phase mercury measure-
ments. A combination of two sampling and analysis methods can help insure accurate and reliable results.
An effective approach is to use ISO Method 6978-3, using gilded silica bead sorbent traps with onsite
analysis, and a modified version of EPA Method 30B using chemically impregnated activated carbon sorbet
sample traps with offsite analysis. Since gilded silica sample traps are mass limited and can be affected
negatively by hydrogen sulfide, a series of gold samples traps run as simultaneous duplicates with break-
through traps are recommended to determine mercury mass loading and sample durations for the more
stable long term carbon sample traps. Upon completion of sampling, Method 30B carbon sample traps are
analyzed offsite using acid digestion analysis via EPA Method 1631. Data is subsequently assimilated and
integrated with field measurements and laboratory data to calculate total mercury concentrations and
associated QA/QC parameters (see table 4 and 5).
The method, referred to as Modified Method 30B is based on the EPA reference method (30B) for the measure-
ment of total vapor phase mercury in flue gases. The Modified EPA Method 30B was developed by the PEI-EFGS
team to provide industry clients with a method that:
• Includes a rigorous, performance based QA/QC protocol that validates the accuracy and precision
of the sample collection procedures
• Utilizes sorbent traps with an increased mercury loading capacity useful for continuous monitoring
through the collection of long term samples that provide an integrated average mercury concentration
• (7-30 days) samples depending on mercury mass loading rates and other operational factors
• Minimizes the loss of mercury to sampling system components
• Is highly portable and robust enough for deployment at remote locations and in harsh weather conditions
• And is relatively unaffected by gas stream contaminants such as H2S, CO2, and hydrocarbon mists
PEI and EFGS have used EPA 30B independently and in conjunction with both ISO 6978 and ASTM D6350
methods on over 100 sites globally (upstream, midstream and at downstream processing plants).
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 6
Table 3: Features of the Modified Method 30B
» Robust QA/QC protocol with numerical performance criteria (Table 4) for the evaluation of the precision and
accuracy of the sample collection process. NIST traceable spiked sorbent traps.
» Sorbent media with increased mass loading capacity, unaffected by common natural gas contaminants such as
acid gases, H2S scavengers and corrosion inhibitors as well as entrained hydrocarbon mists
» Long-Term Sampling Capability: Large mercury loading capacity (up to 50,000 micrograms per trap) allows for
a longer sampling period and a larger total volume of sample gas. Improved representativeness.
» Acid digestion of carbon based traps allows multiple analytical runs of the sample, and the ability to archive the
sample extract for future analysis. Gold trap samples allow for one analytical run only.
» Streamlined sample collection procedures, which is one of the primary advantages of the Modified Method 30B.
This approach eliminates the expense and effort associated with the setup, calibration and maintenance of an
analyzer in the field.
The PEI-EFGS Team
The PEI-EFGS team began field testing the method in 2005 on natural gas production platforms offshore
Texas and natural gas collection and transmission lines both offshore and onshore extending from South
Texas to Alabama. To date the Modified Method 30B has been used in natural gas fields throughout the U.S.
Asia and Australia. Modified Method 30B has also been used extensively in gas processing plants and
refineries including, California, Texas, New Mexico, Wyoming, Alaska, Canada, and Saudi Arabia.
Sorbent Sample Traps for Total Mercury and Functional
Speciation of Mercury
EFGS custom makes carbon type traps with National Institute of Standards and Testing (NIST) traceable
mercury mass spiked sections. The analytical system used is a sorbent trap acid digestion method based on
the principles of EPA Method 1631 (proven over 17 years of laboratory testing). Recently PEI/EFGS have
been using selective capture functional speciation sorbent sample traps using a modified version of EPA
30B. This modified version employs a particulate trap and a specialized sorbent trap designed to selectively
capture oxidized gaseous mercury and a sorbent trap to selectively capture elemental gaseous mercury.
Functional speciation of mercury in gas phase streams includes a) particulate bound mercury (PHg), b)
elemental mercury and c) ionic mercury. Functional and molecular speciation of mercury in process streams
is critical to developing appropriate mercury management plans and MRU design.
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 7
Production Flow Tests and Drill Test
Sampling and Analysis
Accurate concentrations of mercury in reservoir fluids are important to
process and facility design (production platforms, FPSOs and LNG
plants). The exact concentration of mercury in newly discovered
reservoirs is often under reported. Measurements of mercury at
the surface during flow test may not reflect actual concentrations
since tubing and other metallic surfaces are not equilibrated but
more importantly sampling equipment and methods may not be
Mak2 system depicted during molecular speciation (DMHg)
sampling during a dehydrator regen cycle. PEI uses proprietary designed to minimize the chemical effects of mercury during the
selective capture sorbent sample traps for DMHg speciation
sampling and analysis. sampling process. Additionally, well flow test equipment may
contribute to biased high mercury concentrations depending on
duration of use, location, mercury sorption loading of interior
process surfaces and other factors. To obtain precise representative
samples an integrated average mercury concentration measured by
collection of a larger sample volume using heated (250 °F) mercury
inert wetted sampling components with accurate volumetric mea-
surement is required. Since gilded silica quartz sample traps are
affected negatively when used in upstream sampling environments
carbon sorbent sample traps that are unaffected by hydrogen sulfide
and other gas contaminates are recommended (see Table 4 –
modified EPA 30B).
Table 4: Three Section Sorbant Trap
MODIFIED METHOD 30B: Three Section Sorbent Trap
A-Section B-Section C-Section
QA/QC Performance Criteria
A-Section B-Section C-Section Duplicates
QA/QC Test or Primary Collection Breakthrough Spiked Duplicate Agreement
Specification (Field Recovery Test)
Acceptance 95% of Total ≤ 10% of A-Section Hg Mass Average Recovery Between ≤ 10% RD mass for Hg
Criteria Collected Mass For Hg concentration > 1 µg/dcm 75% and 125% for Hg(0) concentration > 1 µg/dcm
≤ 20% of A-Section Hg Mass ≤ 20% RD or 0.2 µg/dcm
For Hg concentration ≤ 1 µg/dcm absolute difference for Hg
concentrations ≤ 1 µg/dcm
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 8
Table 5: Sorbent Trap Mercury Sampling Methods Comparison
Feature Comparison Modified Method 30B ISO 6978 ASTM D6350
Analytical Detection Acid Digestion- Combustion- Thermal Desorption- Thermal Desorption-
Method CVAFS CVAAS CVAFS CVAFS
Lower Detection Limit 0.001 µg/scm 0.001 µg/scm 0.001 µg/scm 0.001 µg/scm
Number of Analyses Multiple 1 1 1
From One Sample
Sample Collection Yes Yes No No
Phase QA/QC
NIST Traceable Standard Yes Yes No No
Sorbent Trap Hg Mass No limit (dilution No limit Limited by instrument No limit
Loading Limit of extract) saturation
8–168+ hours, 8–168+ hours, Limited by Hg mass Limited by Hg mass
Sample Collection depending on depending on loading (typically loading (typically
Duration Hg loading Hg loading less than 4 hours) less than 4 hours)
Up to 10,000 Up to 10,000
Sample Gas Volume <500 liters <500 liters
liters liters
Analysis On-site No Yes Yes Yes
PEI Sampling Systems and Equipment
The PEI-EFGS team uses the Mak2™ Mercury Sampling Systems manufactured by PEI in the U.S., U.K. and
Thailand. The Mak2™ Mercury Sampling Systems are designed and manufactured expressly for the hydro-
carbon processing industry for the collection of mercury samples from high-pressure gas-phase process
streams. Mak2™ systems meet all of the requirements for mercury sampling equipment specified in the
most recent versions of ASTM (American Society of Testing Methods), ISO (International Standards Organi-
zation) and EPA standard methods for the sampling and analysis of mercury. In addition, the procedures and
equipment that PEI uses are consistent with those procedures detailed and recommended in the Gas
Research Institute’s publication GRI-94/0243.2.
The Measurement & Monitoring Of Mercury In Gas-Phase Hydrocarbon Process Streams 9
Each Mak2™ Sampling Unit Consists of Four Primary Components:
1. A portable insertion type sampling probe (stainless steel, Sulfinert™ treated, 2500 psig MAOP)
2. A heat-traced Sulfinert™ treated sample gas line (1/4-inch stainless steel)
3. A heated sampling enclosure consisting of three sample trains with heated pressure regulation and
sampling manifold, sample train control valves, primary and secondary bypass, and Class 1
Division 1 heater and thermostat with explosion proof connectors and power cords
4. A sample gas metering unit consisting of precision control flow meters, calibrated dry gas meters
and ¼ inch braided stainless steel Teflon™ lined hoses for connection to the heated sampling enclosure
All wetted surfaces of the sampling apparatus are treated with Sulfinert™ coating, a high-temperature silica
coating process designed to minimize the sorption or adherence of mercury to the sample-wetted surfaces. For
safety purposes all electrical components (heated sampling enclosure, regulators, and heat traced sample lines)
are rated for Class 1 Division 1 service.
Mak2™ Deployment
PEI has deployed 2 each complete Mak2 mercury sampling systems and CVAFS analyzers to our alliance partners
CR Asia, Rayong Thailand base of operations and CR Australia Perth facility for use throughout southeast Asia and
Australia (including offshore assets throughout the northwest shelf).
Mercury & Chemical Services
Ron Radford Dr. Darrell Gallup Bob Brunette
Vice President (MCS Group) Technical Director Vice President
+1 (713) 503-6803 +1 (707) 480-5508 +1 (206) 660-7307
+66 098 495 5474 dgallup@pei-tx.com RobertBrunette@eurofinsus.com
rradford@pei-tx.com www.pei-tx.com www.frontiergeosciences.com
www.pei-tx.com
Copyright © 2015 Portnoy Environmental, Inc. All Rights Reserved. 10
You might also like
- Colorful Chalkboard Classroom Labels and OrganizersFrom EverandColorful Chalkboard Classroom Labels and OrganizersNo ratings yet
- Boiler RoomDocument1 pageBoiler RoomtylerstearnsNo ratings yet
- Boiler RoomDocument1 pageBoiler RoomtylerstearnsNo ratings yet
- PFD LPG Limau TimurDocument1 pagePFD LPG Limau TimurwahyuNo ratings yet
- FlositDocument1 pageFlositHariq SyifaulNo ratings yet
- Water Treatment System Control Panel-Model PDFDocument1 pageWater Treatment System Control Panel-Model PDFClifford GatonNo ratings yet
- Adobe Scan Dec 20, 2022Document1 pageAdobe Scan Dec 20, 2022Prathmesh GhodvindeNo ratings yet
- Brewing Capability Guide PDFDocument38 pagesBrewing Capability Guide PDFAminur RahmanNo ratings yet
- Flow ChartDocument1 pageFlow ChartElias Georges ZgheibNo ratings yet
- Fydp PFDDocument1 pageFydp PFDZahid Iqbal MBCNo ratings yet
- Lean Amine: Sulfur-Free Gas Purge GasDocument3 pagesLean Amine: Sulfur-Free Gas Purge GasIra WatyNo ratings yet
- Methanol ProductionDocument1 pageMethanol ProductionkrishnaNo ratings yet
- Convensional Process With Three Phase DecanterDocument2 pagesConvensional Process With Three Phase DecanterTongat NocturnoNo ratings yet
- Marpol, Ows, Incinerator, Sewage - Br.31Document43 pagesMarpol, Ows, Incinerator, Sewage - Br.31Muhammad FadliNo ratings yet
- Pre-Treatment: Distilate HydrotreatingDocument2 pagesPre-Treatment: Distilate HydrotreatingTio BudiartoNo ratings yet
- Koagulan Cation Exchanger: 223.335,5652 Kg/jamDocument1 pageKoagulan Cation Exchanger: 223.335,5652 Kg/jamRaymondGTambunanNo ratings yet
- CPVC ModelDocument1 pageCPVC ModelZhu Chen ChuanNo ratings yet
- Alur Produksi Gas Benuo Taka WailawiDocument1 pageAlur Produksi Gas Benuo Taka WailawiAbdullahNo ratings yet
- Sludge Holding Tank Sludge Drying Bed: Expo Accessories LTDDocument1 pageSludge Holding Tank Sludge Drying Bed: Expo Accessories LTDMd SuruzzamanNo ratings yet
- Sludge Holding Tank Sludge Drying Bed: Expo Accessories LTDDocument1 pageSludge Holding Tank Sludge Drying Bed: Expo Accessories LTDMd SuruzzamanNo ratings yet
- Expo Accessories Ltd. STP DrawingDocument4 pagesExpo Accessories Ltd. STP DrawingMd SuruzzamanNo ratings yet
- Blank DiagramDocument1 pageBlank DiagramsiebzehnNo ratings yet
- BS 500 003Document1 pageBS 500 003Xavier LeeNo ratings yet
- SWS Process Flow DiagramDocument1 pageSWS Process Flow DiagramNKNo ratings yet
- Power Plant FDPDocument1 pagePower Plant FDPMR. HaddadNo ratings yet
- Table of Density Values& Log ResponsesDocument1 pageTable of Density Values& Log Responsesgeo_mmsNo ratings yet
- Log ResponsesDocument1 pageLog ResponsesAy Oub BenNo ratings yet
- UAS AlqauliyahDocument1 pageUAS AlqauliyahAlqauliyahNo ratings yet
- Industrial Alcohol or EhanolDocument8 pagesIndustrial Alcohol or EhanolSmruthi SuvarnaNo ratings yet
- Aquaculture 12Document16 pagesAquaculture 12fdlabNo ratings yet
- Log ResponsesDocument1 pageLog ResponsesHamza Lahbiben0% (1)
- Pid Urea 1Document1 pagePid Urea 1Novianti NoviNo ratings yet
- SPL Simulator DiagDocument1 pageSPL Simulator DiagIlhamNo ratings yet
- For Construction: Doosan Heavy Industries VietnamDocument2 pagesFor Construction: Doosan Heavy Industries VietnamDoan Ngoc DucNo ratings yet
- For Construction: Doosan Heavy Industries VietnamDocument2 pagesFor Construction: Doosan Heavy Industries VietnamDoan Ngoc DucNo ratings yet
- Atlas of Log Responses PDFDocument1 pageAtlas of Log Responses PDFFaizin Mulia RizkikaNo ratings yet
- Poultry Wastewater TreatmentDocument5 pagesPoultry Wastewater TreatmentSithandiwe Sindiso Mlalazi100% (1)
- Sweet Gas Condenser (Acid Gas CWDocument1 pageSweet Gas Condenser (Acid Gas CWdiahNo ratings yet
- Co GenerationDocument29 pagesCo GenerationSardeniantoNo ratings yet
- PFD Gas Compresor StationDocument1 pagePFD Gas Compresor StationwahyuNo ratings yet
- 2003-An Enhanced Amine-Based CO2 Capture Process (FLUOR's ECONAMINE)Document11 pages2003-An Enhanced Amine-Based CO2 Capture Process (FLUOR's ECONAMINE)maissam ferdosiNo ratings yet
- Attach 3-2 Simple Utility Block FlowDocument2 pagesAttach 3-2 Simple Utility Block FlowLisbeth Roos RoosNo ratings yet
- TYP PandIDDocument6 pagesTYP PandIDstevenpro89No ratings yet
- Peta Limbah BDWHDocument1 pagePeta Limbah BDWHAghilCahyooWiddhodooNo ratings yet
- Thu Dec 19 21:51:28 2013 Case: D:/Minh Hien/Simulation/Bksim/Bai Giang Mo Phong/Mo Phong Cong Nghe/Lng/Lng-Final - Hscflowsheet: Sweetening (Tpl1)Document1 pageThu Dec 19 21:51:28 2013 Case: D:/Minh Hien/Simulation/Bksim/Bai Giang Mo Phong/Mo Phong Cong Nghe/Lng/Lng-Final - Hscflowsheet: Sweetening (Tpl1)KutiNo ratings yet
- Tacl C H (Monomer) Storage Tank: To PackagingDocument1 pageTacl C H (Monomer) Storage Tank: To PackagingSuzanne Clariz M. BaltazarNo ratings yet
- Produced Water TreatmentDocument1 pageProduced Water TreatmentAhsan KhanNo ratings yet
- Tiem2 Ske F0 00 Aarch 005 00Document1 pageTiem2 Ske F0 00 Aarch 005 00LeeYanWuuNo ratings yet
- AGRU SystemDocument1 pageAGRU SystemNoar CaesarNo ratings yet
- Flow Process CPODocument1 pageFlow Process CPOSetyoadi PurwantoNo ratings yet
- Bayers Process SchematicDocument1 pageBayers Process Schematicsyed tabraizNo ratings yet
- Process Water Scheme: Trace Fe Removal in MGF Trace Cu Removal in ACFDocument1 pageProcess Water Scheme: Trace Fe Removal in MGF Trace Cu Removal in ACFVijaNo ratings yet
- Lablu Babul ETP-ModelDocument1 pageLablu Babul ETP-ModelMd SuruzzamanNo ratings yet
- Gambar MIcrosoft Video FirmanDocument5 pagesGambar MIcrosoft Video FirmanArdi Lukmān HākimNo ratings yet
- 3B 17644019 Proses MeroxDocument1 page3B 17644019 Proses MeroxMohammad Rezza PachruraziNo ratings yet
- Sugar IndustryDocument10 pagesSugar Industryharshu DNo ratings yet
- Urea Process Split Flow LoopDocument7 pagesUrea Process Split Flow LoopCarlos A. VillanuevaNo ratings yet
- Flow ProcessDocument1 pageFlow ProcessFajriansyahNo ratings yet
- 1952-502 Sh03 R02-ModelDocument1 page1952-502 Sh03 R02-ModelSHRI BHAGWANNo ratings yet
- Pltu Process Overview PDFDocument1 pagePltu Process Overview PDFTegar Ardian100% (1)
- Economic Evaluation Using Aspen HysysDocument3 pagesEconomic Evaluation Using Aspen HysysJaime Andres Villegas MansillaNo ratings yet
- AN 10101 Moisture in Natural Gas PDFDocument2 pagesAN 10101 Moisture in Natural Gas PDFJaime Andres Villegas MansillaNo ratings yet
- Training Skilled WorkersDocument60 pagesTraining Skilled WorkersJaime Andres Villegas MansillaNo ratings yet
- Characterization and Properties of Petroleum FractionsDocument421 pagesCharacterization and Properties of Petroleum Fractionsmoveee291% (22)
- ImprovingBrewhouseEfficiency HavigDocument40 pagesImprovingBrewhouseEfficiency HavigJaime Andres Villegas MansillaNo ratings yet
- Fired Heater DesignDocument36 pagesFired Heater Designsaminasritn100% (6)
- SandPiper G20Document25 pagesSandPiper G20Jaime Andres Villegas MansillaNo ratings yet
- BO Shifting ToolDocument1 pageBO Shifting ToolJaime Andres Villegas Mansilla0% (1)
- Z10-001-024 Rev D-ModelDocument1 pageZ10-001-024 Rev D-ModelJaime Andres Villegas MansillaNo ratings yet
- Product Data: X-Cide 320 Industrial BactericideDocument2 pagesProduct Data: X-Cide 320 Industrial BactericideJaime Andres Villegas MansillaNo ratings yet
- Simple and Innovative Methodology For Determination of Glycerol in Biodiesel and Biodiesel Blends (B2-B100) by Ion ChromatographyDocument1 pageSimple and Innovative Methodology For Determination of Glycerol in Biodiesel and Biodiesel Blends (B2-B100) by Ion ChromatographyJaime Andres Villegas MansillaNo ratings yet
- Introduction To Environmental Technology (IEG 104) : Soil & Environment ChemistryDocument28 pagesIntroduction To Environmental Technology (IEG 104) : Soil & Environment ChemistryZahid ZulkanaiNo ratings yet
- Maintaining Constant Relative Humidity by Means of Aqueous SolutionsDocument5 pagesMaintaining Constant Relative Humidity by Means of Aqueous SolutionsCamiloSilva100% (1)
- Exp5 T10 Group 4Document9 pagesExp5 T10 Group 4NaimzNaimNo ratings yet
- Shade Selection: What Is Colour?Document39 pagesShade Selection: What Is Colour?RaiNo ratings yet
- Coordination CompundsDocument13 pagesCoordination CompundsSatwik SharmaNo ratings yet
- Silica Fume Data Sheet PDFDocument2 pagesSilica Fume Data Sheet PDFMuhammad AfifNo ratings yet
- Efflorescence in ConcreteDocument4 pagesEfflorescence in Concretebala subramanyamNo ratings yet
- f1 c6 Periodic Table NotesDocument13 pagesf1 c6 Periodic Table Notesjasonyeoh333No ratings yet
- #26 ImmunochemistryDocument44 pages#26 ImmunochemistryasclswisconsinNo ratings yet
- CHROMATOGRAPHY - Class #2Document11 pagesCHROMATOGRAPHY - Class #2Brijesh SharmaNo ratings yet
- Total Dissolved SolidsDocument3 pagesTotal Dissolved SolidspareshNo ratings yet
- 6CH05 01 Que 20170621 PDFDocument28 pages6CH05 01 Que 20170621 PDFAhmad MohdNo ratings yet
- Chemistry of Water Treatment, Second EditionDocument600 pagesChemistry of Water Treatment, Second EditionEvrem EkolNo ratings yet
- Review of SmeddDocument23 pagesReview of Smeddkulbhushan singhNo ratings yet
- Nutrients in PlantsDocument6 pagesNutrients in PlantsYesha Shah CherubsNo ratings yet
- Predicting Young's Modulus of Linear Polyurethane and Polyurethane-Polyurea ElastomersDocument14 pagesPredicting Young's Modulus of Linear Polyurethane and Polyurethane-Polyurea Elastomers刘俊里No ratings yet
- Chapter 6-Chemical Equilibrium - ItaDocument10 pagesChapter 6-Chemical Equilibrium - ItaPAKK20622P Syarifah Nor Izzah binti Syed Abd HamidNo ratings yet
- KLB Chemistry Form 4Document246 pagesKLB Chemistry Form 4Robinson KipropNo ratings yet
- OCHEM Lab 13 ScribdDocument4 pagesOCHEM Lab 13 ScribdMatthew HarryNo ratings yet
- Effect of Finishing and Polishing Direction On The Marginal AdaptDocument157 pagesEffect of Finishing and Polishing Direction On The Marginal AdaptIlseNo ratings yet
- Nuclear Charge Increases.: (Do Not Mention Shielding Effect)Document3 pagesNuclear Charge Increases.: (Do Not Mention Shielding Effect)sfndmnfmnNo ratings yet
- Material Science ContentDocument23 pagesMaterial Science ContentHoongNo ratings yet
- Delhi Public School, Greater Noida Pre-Mid Term Exam Class-X Subject - Science SESSION-2020-21Document3 pagesDelhi Public School, Greater Noida Pre-Mid Term Exam Class-X Subject - Science SESSION-2020-21AnishikaNo ratings yet
- Cape Biology Unit 1Document165 pagesCape Biology Unit 1Level up youth club. TutoringNo ratings yet
- Bachelor Thesis Deckblatt EnglischDocument8 pagesBachelor Thesis Deckblatt Englischbsnj6chr100% (2)
- Principle of Organic Medicine Chemistry-RamaraoDocument331 pagesPrinciple of Organic Medicine Chemistry-RamaraoRevathiNo ratings yet
- Biocompatible Zirconia Coated 316 Stainless Steel With Anticorrosive Behavior For Biomedical ApplicationDocument8 pagesBiocompatible Zirconia Coated 316 Stainless Steel With Anticorrosive Behavior For Biomedical ApplicationDiana SantosNo ratings yet
- Textile Dyeing Document For Lab Matching With Various Dyes. (Exhaust Process)Document89 pagesTextile Dyeing Document For Lab Matching With Various Dyes. (Exhaust Process)Khandaker Sakib FarhadNo ratings yet
- Air and WaterDocument39 pagesAir and WaterMenaga IlangkovanNo ratings yet
- Brauer - Ocr 1523 1536Document14 pagesBrauer - Ocr 1523 1536ErickNo ratings yet
- Hero Found: The Greatest POW Escape of the Vietnam WarFrom EverandHero Found: The Greatest POW Escape of the Vietnam WarRating: 4 out of 5 stars4/5 (19)
- Sully: The Untold Story Behind the Miracle on the HudsonFrom EverandSully: The Untold Story Behind the Miracle on the HudsonRating: 4 out of 5 stars4/5 (103)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureFrom EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureRating: 5 out of 5 stars5/5 (125)
- The Fabric of Civilization: How Textiles Made the WorldFrom EverandThe Fabric of Civilization: How Textiles Made the WorldRating: 4.5 out of 5 stars4.5/5 (58)
- When the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachFrom EverandWhen the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachRating: 3.5 out of 5 stars3.5/5 (6)
- The Future of Geography: How the Competition in Space Will Change Our WorldFrom EverandThe Future of Geography: How the Competition in Space Will Change Our WorldRating: 4 out of 5 stars4/5 (6)
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyFrom EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyNo ratings yet
- Four Battlegrounds: Power in the Age of Artificial IntelligenceFrom EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceRating: 5 out of 5 stars5/5 (5)
- The End of Craving: Recovering the Lost Wisdom of Eating WellFrom EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellRating: 4.5 out of 5 stars4.5/5 (82)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaFrom EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNo ratings yet
- Permaculture for the Rest of Us: Abundant Living on Less than an AcreFrom EverandPermaculture for the Rest of Us: Abundant Living on Less than an AcreRating: 4.5 out of 5 stars4.5/5 (33)
- The Manager's Path: A Guide for Tech Leaders Navigating Growth and ChangeFrom EverandThe Manager's Path: A Guide for Tech Leaders Navigating Growth and ChangeRating: 4.5 out of 5 stars4.5/5 (99)
- Mini Farming: Self-Sufficiency on 1/4 AcreFrom EverandMini Farming: Self-Sufficiency on 1/4 AcreRating: 4 out of 5 stars4/5 (76)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationFrom EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationRating: 4.5 out of 5 stars4.5/5 (46)
- Pale Blue Dot: A Vision of the Human Future in SpaceFrom EverandPale Blue Dot: A Vision of the Human Future in SpaceRating: 4.5 out of 5 stars4.5/5 (588)
- Transformed: Moving to the Product Operating ModelFrom EverandTransformed: Moving to the Product Operating ModelRating: 4 out of 5 stars4/5 (1)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindFrom EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo ratings yet
- Reality+: Virtual Worlds and the Problems of PhilosophyFrom EverandReality+: Virtual Worlds and the Problems of PhilosophyRating: 4 out of 5 stars4/5 (24)
- The Knowledge: How to Rebuild Our World from ScratchFrom EverandThe Knowledge: How to Rebuild Our World from ScratchRating: 3.5 out of 5 stars3.5/5 (133)
- How to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerFrom EverandHow to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerRating: 4.5 out of 5 stars4.5/5 (54)
- Process Plant Equipment: Operation, Control, and ReliabilityFrom EverandProcess Plant Equipment: Operation, Control, and ReliabilityRating: 5 out of 5 stars5/5 (1)
- How to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerFrom EverandHow to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerRating: 4.5 out of 5 stars4.5/5 (122)
- A Place of My Own: The Architecture of DaydreamsFrom EverandA Place of My Own: The Architecture of DaydreamsRating: 4 out of 5 stars4/5 (242)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestFrom EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestRating: 4 out of 5 stars4/5 (28)