Professional Documents
Culture Documents
B Muscle Tissue Histology
Uploaded by
Affie Saikol0 ratings0% found this document useful (0 votes)
22 views55 pagespresentation of muscle tissue
Original Title
b Muscle Tissue Histology
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentpresentation of muscle tissue
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
22 views55 pagesB Muscle Tissue Histology
Uploaded by
Affie Saikolpresentation of muscle tissue
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 55
Muscle Tissue Histology
Nicanor B. Lacuesta Jr. MD, DPBO-HNS
Muscle tissue
• Connective tissues
• Optimized function of contractility
• Mesodermal origin
• Differentiate by a gradual process of cell
lengthening with abundant synthesis of the
myofibrillar proteins actin and myosin
Types of Muscle Tissue
• Skeletal muscle
– bundles of very long, multinucleated cells with cross-
striations.
– contraction is quick, forceful
– under voluntary control.
• Cardiac muscle
– also has cross-striations
– composed of elongated, often branched cells bound to one
another at structures called intercalated discs
– Contraction is involuntary, vigorous, and rhythmic.
• Smooth muscle
– collections of fusiform cells that lack striations
– slow, involuntary contractions.
• sarcoplasm (Gr. sarkos , flesh + plasma, thing
formed) - cytoplasm
• sarcoplasmic reticulum – smooth endoplasmic
reticulum
• sarcolemma ( sarkos + Gr. lemma , husk) – smooth
muscle membrane
Skeletal Muscle
• Or striated muscle
• consists of muscle fibers - long, cylindrical
multinucleated cells with diameters of 10 to
100 μm.
• During embryonic muscle development,
mesenchymal myoblasts (L. myo , muscle)
fuse, forming myotubes with many nuclei.
• Myotubes then further differentiate to form
striated muscle fibers
• Elongated nuclei are found peripherally just
under the sarcolemma, a characteristic
nuclear location unique to skeletal muscle
fibers/cells.
• A small population of reserve progenitor cells
called muscle satellite cells remains adjacent
to most fibers of differentiated skeletal
muscle.
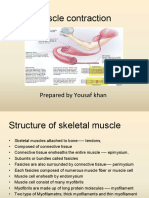
Organization of Skeletal Muscle
• Thin layers of connective tissue surround and
organize the contractile fibers in all three
types of muscle
• seen particularly well in skeletal muscle
• resembles that in large peripheral nerves
• epimysium,
– external sheath of dense connective tissue
– surrounds the entire muscle.
– extend inward, carrying the larger nerves, blood
vessels, and lymphatics of the muscle.
• perimysium
– thin connective tissue layer that immediately
surrounds each bundle of muscle fibers termed a
fascicle.
– fascicle of muscle fibers - functional unit in which the
fibers work together.
– Nerves, blood vessels, and lymphatics penetrate the
perimysium to supply each fascicle.
• endomysium,
– Within fascicles a very thin, delicate layer of
reticular fibers and scattered fibroblasts
– surrounds the external lamina of individual muscle
fibers.
– In addition to nerve fibers, capillaries form a rich
network in the endomysium bringing O2 to the
muscle fibers
• Collagen in these layers serve to transmit the
mechanical forces generated by the
contracting muscle cells/fibers
• Individual muscle fibers seldom extend from
one end of a muscle to the other.
• Epimysium is continuous with the dense
regular connective tissue of a tendon at
myotendinous junctions
– collagen fibers from the tendon insert among
muscle fibers and associate directly with infoldings
of sarcolemma
Organization within the Muscle Fiber
• Longitudinally sectioned skeletal muscle fibers
show cross striations of alternating light and
dark bands.
– The dark bands are called A bands (anisotropic or
birefringent in polarized light microscopy)
– The light bands are called I bands (isotropic, do
not alter polarized light) - bisected by a dark
transverse line
– Z disc (Ger. zwischen, between) – dark line, bisects
each I band
• The repetitive functional subunit of the
contractile apparatus, the sarcomere, extends
from Z disc to Z disc and is about 2.5 μm long
in resting muscle.
• The sarcoplasm has little RER and contains
primarily long cylindrical filament bundles, called
myofibrils, running parallel to the long axis of the
fiber.
• Mitochondria and sarcoplasmic reticulum are
found between the myofibrils (1 to 2 μm)
• Myofibrils consist of an end-to end repetitive
arrangement of sarcomeres; the lateral
registration of sarcomeres in adjacent myofibrils
causes the entire muscle fiber to exhibit a
characteristic pattern of transverse striations.
• The A and I banding pattern - due mainly to
the regular arrangement of thick and thin
myofilaments organized within each myofibril
in a symmetric pattern containing thousands
of each filament type.
Thick Filaments
• 1.6 μm long and 15 nm wide; they
• occupy the A band at the middle region of the
sarcomere.
• Composed of myosin
– Myosin is a large complex (~500 kDa) with two
identical heavy chains and two pairs of light
chains.
• Two heavy chains are thin, rodlike motor proteins (150
nm long and 2-3 nm thick) twisted together as myosin
tails .
• four myosin light chains form a head at one end of each
heavy chain.
• The myosin heads bind both actin, forming
transient crossbridges between the thick and
thin filaments, and ATP, catalyzing energy
release (actomyosin ATPase activity).
• Several hundred myosin molecules are arranged within each
thick filament with overlapping rodlike portions and the
globular heads directed toward either end.
Thin filaments
• Composed of F-actin
• Helical
• 1.0 μm long and 8 nm wide
• run between the thick filaments
• anchored perpendicularly on the Z disc by
actin-binding protein α-actinin
• exhibit opposite polarity on each side of the Z
disc
• Thin filaments are also tightly associated with
two regulatory proteins:
– Tropomyosin, a 40-nm-long coil of two
polypeptide chains located in the groove between
the two twisted actin strands.
– Troponin, a complex of three subunits: TnT, which
attaches to tropomyosin; TnC, which binds Ca2+;
and TnI, which regulates the actin-myosin
interaction.
• Troponin complexes attach at specific sites
regularly spaced along each tropomyosin
molecule.
• I bands, each bisected by a Z disc, consist of
the portions of the thin filaments that do not
overlap the thick filaments (which is why I
bands stain more lightly
• The A bands contain both thick filaments and the
overlapping portions of thin filaments.
• H zone
– a lighter zone at the center of the A band
– corresponding to a region with only the rodlike portions of
the myosin molecule and no thin filaments.
• M line (Ger. Mitte, middle),
– Bisects the H zone
– containing a myosin-binding protein myomesin that holds
the thick filaments in place. This enzyme catalyzes transfer
of phosphate groups from phosphocreatine, a storage
form of high-energy phosphate groups, to ADP, helping to
supply ATP for muscle contraction.
• titin (3700 kDa)
– accessory protein in I bands
– the largest protein in the body
– with scaffolding and elastic properties
– supports the thick myofilaments and connects
them to the Z disc
• nebulin(600-900 kDa)
– binds each thin myofilament laterally,
– helps anchor them to α-actinin
– specifies the length of the actin polymers during
myogenesis.
Sarcoplasmic Reticulum & Transverse
Tubule System
• Specialized for Ca2+ sequestration.
• Depolarization of the sarcoplasmic reticulum
membrane, which causes release of calcium, is initiated
at specialized motor nerve synapses on the
sarcolemma.
• sarcolemma is folded into a system of transverse or T
tubules - penetrate deeply into the sarcoplasm and
encircle every myofibril near the aligned A- and I-band
boundaries of sarcomeres - to trigger Ca2+ release
from sarcoplasmic reticulum throughout the fiber
simultaneously and cause uniform contraction of all
myofibrils
• Triad - complex of a T tubule with two closely
associated small cisterns of sarcoplasmic
reticulum on each side
– After depolarization, calcium ions concentrated within
these cisternae are released through Ca2+ channels in
the membrane into cytoplasm - Ca2+ binds troponin
and allows bridging between actin and myosin
molecules.
– When depolarization ends, the sarcoplasmic reticulum
pumps Ca2+ back into the cisternae, ending
contractile activity.
– Together, the triad components make up a signaling
apparatus for converting repeated cell membrane
depolarizations into spikes of free, cytoplasmic Ca2+
that trigger contraction.
Mechanism of Contraction
• Filaments do not change their length.
• Results as the overlapping thin and thick
filaments of each sarcomere slide past one
another
• Induced when an action potential arrives at a
synapse, the neuromuscular junction (NMJ),
and is transmitted along the T tubules to the
sarcoplasmic reticulum to trigger Ca2+ release
• 1 A nerve impulse
triggers release of ACh
from the synaptic knob
into the synaptic cleft.
ACh binds to Ach
receptors in the motor
end plate of the
neuromuscular
junction, initiating a
muscle impulse in the
sarcolemma of the
muscle fiber.
• 2 As the muscle impulse
spreads quickly from
the sarcolemma along T
tubules, calcium ions
are released from
terminal cisternae into
the sarcoplasm.
• 3 Calcium ions bind to
troponin. Troponin
changes shape, moving
tropomyosin on the
actin to expose active
sites on actin molecules
of thin filaments.
Myosin heads of thick
filaments attach to
exposed active sites to
form crossbridges.
• 4 Myosin heads pivot,
moving thin filaments
toward the sarcomere
center. ATP binds myosin
heads and is broken down
into ADP and P. Myosin
heads detach from thin
filaments and return to
their prepivot position. The
repeating cycle of attach-
pivot-detach-return slides
• thick and thin filaments
past one another. The
sarcomere shortens and the
muscle contracts. The cycle
continues as long as calcium
ions remain bound to
troponin to keep active sites
exposed.
• 5 When the impulse stops,
calcium ions are actively
transported into the
sarcoplasmic reticulum,
tropomyosin re-covers
active sites, and filaments
passively slide back to their
relaxed state
Innervation
• Myelinated motor nerves branch out within the
perimysium connective tissue - gives rise to
several unmyelinated terminal twigs that pass
through endomysium and form synapses with
individual muscle fibers. Schwann cells enclose
the small axon branches and cover their points of
contact with the muscle cells; the external lamina
of the Schwann cell fuses with that of the
sarcolemma
• motor end plate (MEP), or NMJ - a dilated
termination of each axonal branch
• Within the axon terminal are mitochondria
and numerous synaptic vesicles containing
acetylcholine.
• Between the axon and the muscle is a space,
the synaptic cleft.
• Adjacent to the synaptic cleft, the sarcolemma
is thrown into numerous deep junctional
folds, to provide greater postsynaptic surface
area and more transmembrane acetylcholine
receptors.
• Nerve action potential reaches the MEP – acetylcholine
is liberated from the axon terminal - diffuses across the
cleft - binds to its receptors in the folded sarcolemma.
• Acetylcholine receptor contains a nonselective cation
channel opens upon neurotransmitter binding – influx
of Na+ - depolarizing the sarcolemma - producing the
muscle action potential.
• Acetylcholine quickly dissociates from its receptors,
and all free neurotransmitter is removed from the
synaptic cleft by the extracellular enzyme
acetylcholinesterase, preventing prolonged contact of
the transmitter with its receptors.
• Motor unit - single axon and all the muscle
fibers in contact with its branches
• “all or nothing” contraction
• An axon from a single motor neuron can form
MEPs with one or many muscle fibers.
• The lesser the number of fibers innervated by
one axon the greater the precision of its
control and movement
Skeletal muscle fiber types
• Type I – slow oxidative
• Type Iia - Fast, Oxidative-Glycolytic Fibers
• Type Iib - Fast, Glycolytic Fibers
Smooth Muscle
• also called visceral muscle
• Smooth muscle is specialized for slow, steady
contraction and is controlled by a variety of
involuntary mechanisms.
• Fibers are elongated, tapering, and nonstriated
cells, each of which is enclosed by a thin basal
lamina and a fine network of reticular fibers, the
endomysium.
• Connective tissues serve to combine the forces
generated by each smooth muscle fiber into a
concerted action.
• May range in length from 20 μm in small
blood vessels to 500 μm in the pregnant
uterus.
• Each cell has a single long nucleus in the
center of the cell’s central, broadest part.
• The cells stain uniformly along their lengths
• The narrow part of one cell lies adjacent to
the broad parts of neighboring cells.
• All cells are linked by numerous gap junctions.
• The borders of the cell become scalloped
when smooth muscle contracts and the
nucleus becomes distorted.
• Concentrated near the nucleus are
mitochondria, polyribosomes, RER, and the
Golgi apparatus.
• The short membrane invaginations, called
caveolae, are often frequent at the smooth
muscle cell surface.
• Smooth muscle cells also have an elaborate array
of 10-nm intermediate filaments, usually
composed of desmin.
• The intermediate filaments and F-actin filaments
both insert into cytoplasmic and plasmalemma-
associated dense bodies.
• Dense bodies contain α-actinin and are
functionally similar to the Z discs of striated and
cardiac muscle.
• The attachments of thin and intermediate
filaments to the dense bodies helps transmit
contractile force to adjacent smooth muscle cells
and their surrounding network of reticular fibers
• Not under voluntary control, and its
• fibers lack MEPs.
• Control can involve autonomic nerves, a variety
of hormones and similar substances, and local
physiologic conditions such as the degree of
stretch.
• Whether smooth muscle fibers contract as small
groups or throughout an entire muscle to
produce waves of contraction is determined
largely by the degree of autonomic innervation
and the density of the gap junctions; both
conditions vary considerably in different organs.
• swellings of autonomic nerve axons with
synaptic vesicles simply lie in close contact
with the sarcolemma with little or no
specialized structure to the junctions.
• Smooth muscle is most often spontaneously
active without nervous stimuli, its nerve
supply serves primarily to modify activity
rather than to initiate it.
• Receives both adrenergic and cholinergic
nerve endings that act antagonistically,
stimulating or depressing its activity.
• Supplement fibroblast activity, synthesizing
collagen, elastin, and proteoglycans, with a
major influence on the extracellular matrix
(ECM) in tissues
• Active synthesis of ECM by the small
cells/fibers of smooth muscle may reflect less
specialization for strong contractions than in
skeletal and cardiac muscle and is similar to
this synthetic function in other contractile
cells, such as myofibroblasts and pericytes.
Regeneration of Muscle Tissue
• Repair and regeneration can occur in skeletal
muscle because of a population of reserve
muscle satellite cells that can proliferate, fuse,
and form new muscle fibers.
• Cardiac muscle lacks satellite cells and has little
capacity for regeneration.
• Regeneration is rapid in smooth muscle because
the cells/fibers are small and relatively less
differentiated, which allow renewed mitotic
activity after injury.
• Sir fernan –traffic
– 09177241591
You might also like
- Skeletal MuscleDocument98 pagesSkeletal MuscleMoon Moon100% (1)
- Muscle PhysiologyDocument24 pagesMuscle PhysiologyArmando AlehandroNo ratings yet
- How Muscles Generate MovementDocument38 pagesHow Muscles Generate Movementtoyy100% (1)
- Muscle DentalDocument74 pagesMuscle Dentalapi-3710331No ratings yet
- Muscle Pgy - 222Document48 pagesMuscle Pgy - 222SAVIOUR BANDANo ratings yet
- Contractile Cells: Skeletal Muscle Structure and FunctionDocument116 pagesContractile Cells: Skeletal Muscle Structure and Functionnav_malhiNo ratings yet
- 6.muscular TissueDocument40 pages6.muscular Tissuemero wowNo ratings yet
- 3D. Muscle TissueDocument47 pages3D. Muscle Tissuejaf123xyNo ratings yet
- Nerve and Muscle Lecture 6 paraDocument24 pagesNerve and Muscle Lecture 6 paraTatenda SibandaNo ratings yet
- SM Skeletal Muscle SlidesDocument68 pagesSM Skeletal Muscle Slideskarishma.7022No ratings yet
- Muscle Intro-Skeletal Muscle IDocument31 pagesMuscle Intro-Skeletal Muscle IPehovelo JohannesNo ratings yet
- Muscular System: Histology and PhysiologyDocument36 pagesMuscular System: Histology and PhysiologyFajar YuliantoNo ratings yet
- Skeletal MusclesDocument7 pagesSkeletal MusclesAhmed OudahNo ratings yet
- Microscopic Anatomy of A Skeletal Muscle FiberDocument33 pagesMicroscopic Anatomy of A Skeletal Muscle FiberKumar MSNo ratings yet
- Physiology Slide #1Document18 pagesPhysiology Slide #1anon_974423120No ratings yet
- Muscles and Muscle Tissue: Muscle Overview Muscle SimilaritiesDocument11 pagesMuscles and Muscle Tissue: Muscle Overview Muscle SimilaritiesHerne BalberdeNo ratings yet
- Chapter 10: Muscle Tissue: VoluntaryDocument13 pagesChapter 10: Muscle Tissue: VoluntaryClyde BaltazarNo ratings yet
- Histology of Cardiac and Smooth MuscleDocument73 pagesHistology of Cardiac and Smooth MuscleShehryar AbbasNo ratings yet
- Histology of Cardiac and Smooth MuscleDocument73 pagesHistology of Cardiac and Smooth MuscleShehryar AbbasNo ratings yet
- Excitation Contraction Coupling: Nandini GoyalDocument32 pagesExcitation Contraction Coupling: Nandini GoyalNandini GoyalNo ratings yet
- Contraction of Skeletal Muscle (1) : Narrated LectureDocument45 pagesContraction of Skeletal Muscle (1) : Narrated LectureDeanNo ratings yet
- Muscle Tissue: - Skeletal Muscle - Cardiac Muscle - Smooth MuscleDocument36 pagesMuscle Tissue: - Skeletal Muscle - Cardiac Muscle - Smooth MuscleDentist Dina Samy100% (1)
- Histology 6 MuscleDocument88 pagesHistology 6 MuscleAbdul RahmanNo ratings yet
- Health PPT 3 - Muscle 2013-1Document50 pagesHealth PPT 3 - Muscle 2013-1Mengistu GebeyehuNo ratings yet
- MUSCLE CELL PHYSIOLOGY MBCHB YEAR 1 2016-2Document289 pagesMUSCLE CELL PHYSIOLOGY MBCHB YEAR 1 2016-2AdminNo ratings yet
- MUSCLE PHYSIOLOGY: AN OVERVIEWDocument50 pagesMUSCLE PHYSIOLOGY: AN OVERVIEWJoyce Adjei-boateng100% (1)
- Unit 3 Muscle Physiology - BPT PDFDocument55 pagesUnit 3 Muscle Physiology - BPT PDFRadhikaNo ratings yet
- 4.1 PhysDocument27 pages4.1 PhysshivaniNo ratings yet
- Sarcolemma - Cell MembraneDocument66 pagesSarcolemma - Cell MembraneAman SyedNo ratings yet
- Muscle Lecture On (Excitable Tissue)Document126 pagesMuscle Lecture On (Excitable Tissue)Muhammed ZainabNo ratings yet
- Muscle Tissue-Wl06.11Document54 pagesMuscle Tissue-Wl06.11api-19641337No ratings yet
- The Muscular System: Types, Structure & FunctionDocument50 pagesThe Muscular System: Types, Structure & FunctionArina GainaNo ratings yet
- Physiology of Skeletal & Smooth MusclesDocument10 pagesPhysiology of Skeletal & Smooth MusclesAli AlhamadNo ratings yet
- Chapter 7 - Muscular SystemDocument29 pagesChapter 7 - Muscular SystemlNo ratings yet
- Muscle Tissue NotesDocument11 pagesMuscle Tissue NotesAubrey Unique EvangelistaNo ratings yet
- Muscle Tissue LectureDocument34 pagesMuscle Tissue LectureurjaNo ratings yet
- Anaphy-Muscular HandoutDocument7 pagesAnaphy-Muscular HandoutAEGEL DARO TORRALBANo ratings yet
- Lec4 Muscles CompressedDocument27 pagesLec4 Muscles Compressedmohammedbaker1q2w3eNo ratings yet
- Actin Myosin CytoskeletonDocument28 pagesActin Myosin Cytoskeletonjesus rajeshdivyaNo ratings yet
- General Mechanism of Muscle ContractionDocument22 pagesGeneral Mechanism of Muscle ContractionAnuya Kulkarni-DeoNo ratings yet
- Muscle Physiology ExplainedDocument24 pagesMuscle Physiology Explainedroqaia roqaiaNo ratings yet
- Skeletal Muscle Physiology ExplainedDocument26 pagesSkeletal Muscle Physiology ExplainedUSAMA AHMEDNo ratings yet
- Muscular System Explained: Skeletal, Cardiac & Smooth Muscle TissueDocument44 pagesMuscular System Explained: Skeletal, Cardiac & Smooth Muscle TissueClaudine Jo B. TalabocNo ratings yet
- Lecture 1 Physiology of Skeletal Muscle by Dr. RoomiDocument37 pagesLecture 1 Physiology of Skeletal Muscle by Dr. RoomiMudassar Roomi100% (1)
- MuscleDocument11 pagesMuscleJeff ParkNo ratings yet
- Muscles MidtermDocument32 pagesMuscles MidtermMata, Ersel MaeNo ratings yet
- Muscular SystemDocument10 pagesMuscular SystemMarie Veatrice JacomilleNo ratings yet
- Azantee Yazmie Abdul Wahab Dept. of Obstetrics & Gynaecology Kulliyyah of Medicine IIUM KuantanDocument51 pagesAzantee Yazmie Abdul Wahab Dept. of Obstetrics & Gynaecology Kulliyyah of Medicine IIUM KuantanNur ShafilaNo ratings yet
- NotesDocument15 pagesNotesGizem OsmanogluNo ratings yet
- Contraction of Skeletal Muscle.Document19 pagesContraction of Skeletal Muscle.Salman KhanNo ratings yet
- Kharkov National Medical University: Muscle TissueDocument42 pagesKharkov National Medical University: Muscle TissueSyed Irfan ArifNo ratings yet
- Skeletal Muscle Classification and StructureDocument43 pagesSkeletal Muscle Classification and StructureHusnain WattoNo ratings yet
- The Muscular System: DR Faisal Institute of Physiology and PharmacologyDocument61 pagesThe Muscular System: DR Faisal Institute of Physiology and PharmacologyTask BirdNo ratings yet
- Muscle Physiology in Orthodontics / Orthodontic Courses by Indian Dental AcademyDocument50 pagesMuscle Physiology in Orthodontics / Orthodontic Courses by Indian Dental Academyindian dental academyNo ratings yet
- Anatomy and Physiology Muscular System OverviewDocument7 pagesAnatomy and Physiology Muscular System OverviewKei GauranoNo ratings yet
- MuscularDocument61 pagesMuscularFranz DiazNo ratings yet
- Muscle: Gerald Dale Giron MD Department of Human Structural BiologyDocument23 pagesMuscle: Gerald Dale Giron MD Department of Human Structural BiologyMarx AsuncionNo ratings yet
- Wa0038Document10 pagesWa0038Shehzad QureshiNo ratings yet
- The Basics of Cell Life with Max Axiom, Super Scientist: 4D An Augmented Reading Science ExperienceFrom EverandThe Basics of Cell Life with Max Axiom, Super Scientist: 4D An Augmented Reading Science ExperienceNo ratings yet
- Gametogenesis 1Document10 pagesGametogenesis 1Affie SaikolNo ratings yet
- Gametogenesis - Trans PDFDocument9 pagesGametogenesis - Trans PDFAffie SaikolNo ratings yet
- The Epithelial CellsDocument5 pagesThe Epithelial CellsAffie SaikolNo ratings yet
- Microbio Questions CompilationDocument21 pagesMicrobio Questions CompilationAffie SaikolNo ratings yet
- Epithelial TissueDocument62 pagesEpithelial TissueAffie SaikolNo ratings yet
- Microbiology - NotesDocument1 pageMicrobiology - NotesAffie SaikolNo ratings yet
- 02 BiomoleculesDocument17 pages02 BiomoleculesAffie SaikolNo ratings yet
- Histology of the larynx, bronchi, and lungsDocument49 pagesHistology of the larynx, bronchi, and lungsAffie SaikolNo ratings yet
- Connective TissueDocument74 pagesConnective TissueAffie SaikolNo ratings yet
- 01 Intro To Biochem - EditDocument5 pages01 Intro To Biochem - EditJoanne AjosNo ratings yet
- COPD Patient With Chronic Dyspnea and CyanosisDocument2 pagesCOPD Patient With Chronic Dyspnea and CyanosisAffie SaikolNo ratings yet
- Gametogenesis - Trans PDFDocument9 pagesGametogenesis - Trans PDFAffie SaikolNo ratings yet
- Delfin, RMTDocument34 pagesDelfin, RMTAffie SaikolNo ratings yet
- Recall 1Document4 pagesRecall 1pikachuNo ratings yet
- Antigen Antibody Reaction (MUST READ)Document137 pagesAntigen Antibody Reaction (MUST READ)Affie SaikolNo ratings yet
- Autoimmune DiseasesDocument35 pagesAutoimmune DiseasesAffie SaikolNo ratings yet
- Lesson Korean NumbersDocument5 pagesLesson Korean NumbersLulu Afifah OctaviaNo ratings yet
- Serological Diagnosis of Bacterial InfectionsDocument81 pagesSerological Diagnosis of Bacterial InfectionsAffie SaikolNo ratings yet
- Hindi Phase1 BKLTDocument32 pagesHindi Phase1 BKLTSam KuttyNo ratings yet
- Prayer Times in Vigan (Ilocos) - Year 2018: PrintDocument2 pagesPrayer Times in Vigan (Ilocos) - Year 2018: PrintAffie SaikolNo ratings yet
- HomeostasisDocument3 pagesHomeostasisAffie SaikolNo ratings yet
- Denr OfficialDocument1 pageDenr OfficialAffie SaikolNo ratings yet
- Understanding IslamDocument31 pagesUnderstanding IslamAffie SaikolNo ratings yet
- Introduction Ana and PhysioDocument54 pagesIntroduction Ana and PhysioAffie SaikolNo ratings yet
- Environmental Ethics in IslamDocument74 pagesEnvironmental Ethics in IslamAffie SaikolNo ratings yet
- Electron Transport ChainDocument27 pagesElectron Transport ChainAffie SaikolNo ratings yet
- AnatomyDocument52 pagesAnatomyAffie SaikolNo ratings yet
- Cardiovascular SystemDocument64 pagesCardiovascular SystemAffie SaikolNo ratings yet
- Siklus Asam Sitrat PDFDocument27 pagesSiklus Asam Sitrat PDFLuses Shantia HaryantoNo ratings yet
- Turbidimetry and Nephelometry1Document3 pagesTurbidimetry and Nephelometry1maxim_crank6101100% (1)
- Nervous Responses of EarthwormDocument27 pagesNervous Responses of EarthwormKayla JoezetteNo ratings yet
- LWT - Food Science and TechnologyDocument8 pagesLWT - Food Science and TechnologyAzb 711No ratings yet
- Flowering PlantsDocument43 pagesFlowering Plantskingbanakon100% (1)
- Cooling Tower Operation - 030129Document8 pagesCooling Tower Operation - 030129Sekar CmNo ratings yet
- Reproductive Biology and Phylogeny of Birds PDFDocument624 pagesReproductive Biology and Phylogeny of Birds PDFFabricio GZNo ratings yet
- Lesson 7 - Organ TransplantationDocument14 pagesLesson 7 - Organ TransplantationJosee100% (1)
- Introduction To Bioinformatics: Database Search (FASTA)Document35 pagesIntroduction To Bioinformatics: Database Search (FASTA)mahedi hasanNo ratings yet
- Eagles and Eaglets gr2Document2 pagesEagles and Eaglets gr2vovanmtcNo ratings yet
- LearnerDocument7 pagesLearnersudhacarhrNo ratings yet
- 03 Chapter 3Document148 pages03 Chapter 3arghaNo ratings yet
- WCH03 01 Que 20180124Document16 pagesWCH03 01 Que 20180124Rameez Mazhar SiddiqiNo ratings yet
- MDU Open Elective 3rd Sem Date Sheet May 2019Document1 pageMDU Open Elective 3rd Sem Date Sheet May 2019Ratan DeshNo ratings yet
- Correlation of Dynamic Strength in The Standing Calf Raise With Sprinting Performance in Consecutive Sections Up To 30 MetersDocument9 pagesCorrelation of Dynamic Strength in The Standing Calf Raise With Sprinting Performance in Consecutive Sections Up To 30 MetersJohn NixonNo ratings yet
- Perkecambahan Dan Pertumbuhan Palem Jepang (Actinophloeus Macarthurii Becc.) Akibat Perendaman Biji Dalam LumpurDocument7 pagesPerkecambahan Dan Pertumbuhan Palem Jepang (Actinophloeus Macarthurii Becc.) Akibat Perendaman Biji Dalam LumpurNurma YahyaNo ratings yet
- Physiology of Cerebellum Lec Foe 2nd Year by DR Sadia Uploaded by ZaighamDocument61 pagesPhysiology of Cerebellum Lec Foe 2nd Year by DR Sadia Uploaded by ZaighamZaigham HammadNo ratings yet
- Zoologija VrsteDocument16 pagesZoologija VrsteДушан МарковићNo ratings yet
- Science10 Quarter4 Week3-BiomoleculesDocument2 pagesScience10 Quarter4 Week3-BiomoleculesMargareth LandichoNo ratings yet
- 4 Factors Effecting Shelf Life and Storage Life of Fresh FishDocument26 pages4 Factors Effecting Shelf Life and Storage Life of Fresh FishRafidah Zakaria100% (1)
- Botany AssignmentDocument35 pagesBotany AssignmentLakshmiNo ratings yet
- Protochordata FIXDocument33 pagesProtochordata FIXSylvia AnggraeniNo ratings yet
- Metals: Methods To Evaluate Corrosion in Buried Steel Structures: A ReviewDocument21 pagesMetals: Methods To Evaluate Corrosion in Buried Steel Structures: A ReviewPeymanMajidiNo ratings yet
- Muscle Energy Techniques ExplainedDocument2 pagesMuscle Energy Techniques ExplainedAtanu Datta100% (1)
- Presented By:-Dr. Sushma Tomar Associate Professor Department of AnatomyDocument20 pagesPresented By:-Dr. Sushma Tomar Associate Professor Department of AnatomyCristine EchaveNo ratings yet
- Kenya's Natural Capital AtlasDocument139 pagesKenya's Natural Capital AtlasElvis Ole Sankale Ntimama100% (7)
- The Anatomy, Physiology, and Pathophysiology of Muscular SystemDocument55 pagesThe Anatomy, Physiology, and Pathophysiology of Muscular SystemAnisaNo ratings yet
- Ewh Ix PDFDocument80 pagesEwh Ix PDFOR Premium FreeNo ratings yet
- Cell Division GizmoDocument3 pagesCell Division Gizmoapi-522847737No ratings yet
- Trunking CatalogDocument8 pagesTrunking CatalogDenuka PathiranaNo ratings yet
- Cu EnzymesDocument8 pagesCu EnzymesVINEESHA VKNo ratings yet