Professional Documents
Culture Documents
51LC S13 Elimination Background PDF
Uploaded by
ButterlesstoastOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
51LC S13 Elimination Background PDF
Uploaded by
ButterlesstoastCopyright:
Available Formats
51LC EXP #2 SPRING 2013
DEHYDROBROMINATION OF 1- AND 2-BROMOBUTANE AND
DEHYDRATION OF 1- AND 2-BUTANOL:
ANALYSIS OF GASEOUS PRODUCTS BY GAS CHROMATOGRAPHY 1
The acid-catalyzed dehydration of 1-butanol and 2-butanol and the base-induced
dehydrobromination of 1-bromobutane and 2-bromobutane with strong base both lead to the
formation of a mixture of butene isomers. The composition of this mixture varies, however, as a
result of mechanistic differences in these two pathways.
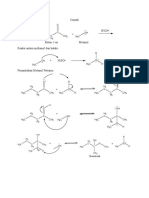
A1. Acid-catalyzed dehydration of a secondary alcohol:
Dehydration of a secondary alcohol proceeds readily in the presence of strong acid such as
sulfuric or phosphoric acid, and proceeds via an E1 mechanism whose intermediates, but not
arrows, are shown in Scheme 1. In step 1, protonation of the hydroxyl group of the alcohol with
the acid catalyst converts the poor leaving group -OH to a much better leaving group, H2O. The
loss of a water molecule from the oxonium ion intermediate results in a carbocation intermediate
that undergoes E1 elimination to form an alkene. If the elimination occurs with either one of the
protons from the terminal methyl (CH3) group, the resulting product is the terminal alkene – 1-
butene. Elimination from the protons on the methylene (CH2) group leads to an internal alkene.
Depending on which hydrogen is deprotonated, either the cis- or trans-alkene can be formed. The
least substituted alkene product is also known as the Hoffman product, and the more-substituted,
alkenes are known as the Saytzeff products. Of the two possible Saytzeff products, the trans-
alkene is the most stable. Since the E1 reaction is under thermodynamic control, the relative
stability of the resulting alkenes should determine the product distribution ratio. This is also known
as Saytzeff’s rule.
Scheme 1:
H3C
CH3 CH3
H2SO4
CH3 H3C CH2
H3C
O
H H3C H3C +
HO H ∆ H
H H H3C CH3
Hoffman
product
Saytzeff
products
1
A part of this procedure is adopted from an article published by H.M. Gilow in the Journal of Chemical Education,
CHEM 51LC Rev 3/12/13
According to LeChatelier's principle, removing a product from a chemical system at
equilibrium shifts the equilibrium in the direction favoring the formation of the products. You will
carry out the dehydration reaction in a reaction tube connected to a gas collector so that the product
will continuously escape out of the reaction mixture as it is formed. Removal of the product will
shift the equilibrium to the right and thus complete the reaction. The collected gaseous product
will be analyzed by gas chromatography, which will show peaks with fair resolution. From the
relative area of peaks, you can calculate the percentage composition of the product mixture.
A2. Acid-catalyzed dehydration of a primary alcohol:
Dehydration of a primary alcohol proceeds analogously to that of a secondary one. The
major difference is that instead of forming a secondary carbocation, the loss of a water molecule
results in the formation of the much more unstable primary carbocation (Scheme 2). Elimination of
that intermediate should result solely in the formation of the terminal alkene 1-butene. The high
activation energy required to form the carbocation intermediate, however, should be so high that
no elimination reaction occurs.
Scheme 2:
H
H2SO4
H
H3C OH
∆
H3C O
H
X H3C H H3C
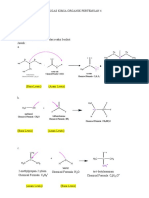
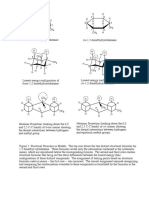
B1. Base-induced dehydrobromination of a secondary bromide:
Base-induced dehydrohalogenation of an alkyl halide is another alternative for synthesizing
alkenes, and proceeds via an E2 mechanism. The use of strong and bulky base promotes the
elimination reaction and disfavors the competing SN2 reaction. The E2 reaction requires an anti-
periplanar arrangement of the beta-H and leaving group (the beta-H and leaving group must be in
the same plane and anti to each other). Because 2-bromobutane has two different types of beta-
H’s, elimination on either side can lead to both internal and terminal alkenes (Scheme 3).
CHEM 51LC Rev 3/12/13
Traditionally, E2
Scheme 3:
eliminations also follow
Saytzeff’s rule. However, Base
H
H
the use of a very bulky base H H3C
H H3C
can change the outcome of H3C
Br
H
Hoffman H H
product
the reaction. When there
Br
are steric problems
approaching one of the
H
beta-hydrogens, the base H3C
Base H
will preferentially H
CH3
H3C CH3
deprotonate the hydrogen H CH3 H CH3
Br
that is least sterically Br
congested by adjacent alkyl
groups. In the case of 2-
H
bromobutane, this most- Base
H3C H
accessible hydrogen is the H
H
CH3
H3C CH3
CH3
hydrogen on the terminal Br H3C H
methyl (CH3) group Saytzeff
Br
products
resulting in the formation
of the least substituted alkene.
B2. Base-induced dehydrobromination of a primary bromide:
The base-induced dehydrohalogenation of 1-bromobutane is analogous to that of 2-
bromobutane. Unlike 2-bromobutane, however, the halide resides on the terminal methyl group
and therefore there is only one possible elimination (Scheme 4).
Scheme 4:
Base
H
H CH3
H CH3
H
Br
CHEM 51LC Rev 3/12/13
C. A note about unexpected results:
Before running a reaction, chemists apply concepts from similar reactions to try and predict
the results. Oftentimes, this works and the outcome is predicted correctly. Sometimes, this does
not work and it is left to the scientist (you) to come up with a rationale for the results observed.
Bear in mind that an unexpected outcome is not necessarily because the concepts you applied were
wrong, but perhaps because something else is at work.
One or more reactions in this set of experiments might give unexpected results. Your job is
to identify which reactions gave the expected results, which give unexpected results, and explain
the unexpected results.
CHEM 51LC Rev 3/12/13
You might also like
- Drug Abuse Ag8114en MKDocument56 pagesDrug Abuse Ag8114en MKA VegaNo ratings yet
- Organic compounds structures and reactionsDocument2 pagesOrganic compounds structures and reactionsNicolas MartinezNo ratings yet
- Important reactions of aromatic compounds summarizedDocument1 pageImportant reactions of aromatic compounds summarizedRoronoa ZoroNo ratings yet
- Mini Presentation of IBu-For Prof - SankararamanDocument16 pagesMini Presentation of IBu-For Prof - SankararamanCreative ThinkerNo ratings yet
- Aldehydes and KetonesDocument58 pagesAldehydes and KetonesAniruddha KawadeNo ratings yet
- Neutral pH Flow BatteryDocument1 pageNeutral pH Flow BatteryMonika Bartocha WróblewskaNo ratings yet
- Chem SketchDocument17 pagesChem SketchUsman GhaniNo ratings yet
- BiosyntheseDocument1 pageBiosyntheseBITOMBONo ratings yet
- CHEMSKETCHDocument17 pagesCHEMSKETCHUsman GhaniNo ratings yet
- Reductions PPT 29-08-2020Document12 pagesReductions PPT 29-08-2020jkc collegeNo ratings yet
- An Overview of Alkaloids: Their Classification, Extraction, Isolation, and Role in PlantsDocument49 pagesAn Overview of Alkaloids: Their Classification, Extraction, Isolation, and Role in PlantsmanishaNo ratings yet
- S20 Ac Quim 10 Viellard EstebanDocument1 pageS20 Ac Quim 10 Viellard Estebanesteban viellardNo ratings yet
- S22 - URAIAN Dan KONTROL PROSES PEMBUATANDocument7 pagesS22 - URAIAN Dan KONTROL PROSES PEMBUATANTrisnawati AmaliaNo ratings yet
- Halogen DerivativeDocument6 pagesHalogen DerivativeSantanu DasNo ratings yet
- Practice Problems On Alkane Nomenclature: CH CHDocument2 pagesPractice Problems On Alkane Nomenclature: CH CHRishav Sasmal100% (1)
- Estructura de Compuestos Biologicos-Parte 2: F.-EsteroidesDocument7 pagesEstructura de Compuestos Biologicos-Parte 2: F.-EsteroidesffhkeNo ratings yet
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- Program Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsDocument2 pagesProgram Chem Check-Up 2.0: Predicting structures, naming compounds and identifying reactionsanis fazilaNo ratings yet
- Problem Set 3, Question 2 InitiationDocument1 pageProblem Set 3, Question 2 InitiationRudra Shankha NandyNo ratings yet
- Journal Pre-ProofDocument53 pagesJournal Pre-ProofAndres Fernando Silvestre SuarezNo ratings yet
- Alcohol, Phenol - EthersDocument1 pageAlcohol, Phenol - Etherssarthakyedlawar04No ratings yet
- N-Acylation and O-AcylationDocument2 pagesN-Acylation and O-AcylationBritany DyerNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- Chem 115 Myers: Stereoselective, Directed Aldol ReactionDocument24 pagesChem 115 Myers: Stereoselective, Directed Aldol ReactionChemical MoleculeNo ratings yet
- A Single-Event Microkinetic Model For Xylene Isomerisation and Ethylbenzene DealkylationDocument2 pagesA Single-Event Microkinetic Model For Xylene Isomerisation and Ethylbenzene DealkylationAor PimpornNo ratings yet
- Summary of All Reactions For Organic ChemistryDocument4 pagesSummary of All Reactions For Organic Chemistryfoodytang91% (23)
- Module-3 (Part-I)Document218 pagesModule-3 (Part-I)Prajay GNo ratings yet
- Ejercicios de Nomenclatura de Alcoholes.Document3 pagesEjercicios de Nomenclatura de Alcoholes.Diego Fernando Ardila ArizaNo ratings yet
- FROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENEDocument1 pageFROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENERonak kadamNo ratings yet
- FitoquimiaDocument2 pagesFitoquimialuisgerardo000000No ratings yet
- Chemfig enDocument82 pagesChemfig enDũng Nguyễn NhoNo ratings yet
- IIT JEE Screen 2004 PDFDocument17 pagesIIT JEE Screen 2004 PDFharshNo ratings yet
- MechanismDocument2 pagesMechanismRyan BoodramlallNo ratings yet
- Chemistry Common Name.Document1 pageChemistry Common Name.jangidlokeshkumar11No ratings yet
- F H C B F F F Chemical Formula: CH BF ODocument3 pagesF H C B F F F Chemical Formula: CH BF OFadilla AzhariNo ratings yet
- Grignard Reagent C C Bond Mind MapDocument1 pageGrignard Reagent C C Bond Mind MapSii SheikhNo ratings yet
- Cope EliminationDocument2 pagesCope EliminationArt Julius D. HallazgoNo ratings yet
- Local sales representatives for pricing and availability of monomers listDocument36 pagesLocal sales representatives for pricing and availability of monomers listandersonquimicaNo ratings yet
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDocument2 pagesReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNo ratings yet
- Reaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaDocument2 pagesReaksi antara Metanol dan Butan-2-on menghasilkan Ketal 2,2-dimetoksibutanaElis TianiNo ratings yet
- Amino Acid Sidechain ChargesDocument2 pagesAmino Acid Sidechain ChargesAlisaClarkNo ratings yet
- SEM 8 Chemsketch 2022Document12 pagesSEM 8 Chemsketch 2022Leyre FunciaNo ratings yet
- Grignard Reagent C-C Bond: R D O ODocument1 pageGrignard Reagent C-C Bond: R D O OKeshav PargeeNo ratings yet
- Organic Compounds StructuresDocument7 pagesOrganic Compounds StructuresDewei LohNo ratings yet
- Enols and Enolate Ions: Key Intermediates in Carbonyl ChemistryDocument1 pageEnols and Enolate Ions: Key Intermediates in Carbonyl ChemistryshinexblazerNo ratings yet
- Exp 7 Preparation of AlkenesDocument14 pagesExp 7 Preparation of AlkenesGeorge PiliposyanNo ratings yet
- Alcohol Phenol Ether (1) 6Document9 pagesAlcohol Phenol Ether (1) 6sdnishacNo ratings yet
- Dia orDocument8 pagesDia orNaman MahawarNo ratings yet
- Nomenclature of Organic CompoundsDocument80 pagesNomenclature of Organic CompoundsSajjad MiraniNo ratings yet
- Parte 4Document1 pageParte 4Esteban Mauricio Viellard CorralesNo ratings yet
- Trabajo Quimica Superior NAVIDADDocument13 pagesTrabajo Quimica Superior NAVIDADSebastian GuerraNo ratings yet
- Reaksi Esterifikasi Asam Sederhana Menggunakan NaOHDocument1 pageReaksi Esterifikasi Asam Sederhana Menggunakan NaOHSoy Viranda KusumaNo ratings yet
- 314 Stereochem ProbsDocument14 pages314 Stereochem ProbsAtul SinghNo ratings yet
- DM pp61-80Document20 pagesDM pp61-80MLUNGISI MkhwanaziNo ratings yet
- 1,3 CycloDocument1 page1,3 CycloIsmail ZitouniNo ratings yet
- L1 - S2 - PW1 - Bromocresol Green in Aqueous SolutionDocument4 pagesL1 - S2 - PW1 - Bromocresol Green in Aqueous SolutionZirəddin TağıyevNo ratings yet
- Alkenes 1 QPDocument7 pagesAlkenes 1 QPemanNo ratings yet
- Biological Molecules - ProteinsDocument18 pagesBiological Molecules - ProteinsblackmoneygrabberNo ratings yet
- Clicker Quiz 3 AnswersDocument10 pagesClicker Quiz 3 AnswersButterlesstoastNo ratings yet
- Homework 5 Chapt 13, 14, 15, 16, 17 AnswersDocument11 pagesHomework 5 Chapt 13, 14, 15, 16, 17 AnswersButterlesstoastNo ratings yet
- Calculating Percent Recovery and YieldDocument2 pagesCalculating Percent Recovery and YieldNur AishaNo ratings yet
- Exam 2 Chem350 - F16 (Key)Document8 pagesExam 2 Chem350 - F16 (Key)ButterlesstoastNo ratings yet
- Dehydration of An AlcoholDocument13 pagesDehydration of An AlcoholButterlesstoastNo ratings yet
- Chem220 SpectrophotometryDocument46 pagesChem220 SpectrophotometryButterlesstoastNo ratings yet
- Chem350 Exam 3 - F16 - KeyDocument9 pagesChem350 Exam 3 - F16 - KeyButterlesstoastNo ratings yet
- OC307 Solving NMRDocument11 pagesOC307 Solving NMRNur Farhanah ZulkifliNo ratings yet
- Syllabus Calc3 271Document4 pagesSyllabus Calc3 271ButterlesstoastNo ratings yet
- Glycolysis Handout Payoff AnswersDocument2 pagesGlycolysis Handout Payoff AnswersButterlesstoastNo ratings yet
- Purify Acetanilide CrystalsDocument6 pagesPurify Acetanilide CrystalsButterlesstoastNo ratings yet
- Atomic TheoryDocument3 pagesAtomic TheoryButterlesstoastNo ratings yet
- Chemical Reactions and Quantities: 5.5 The MoleDocument15 pagesChemical Reactions and Quantities: 5.5 The Molejanaisha_bai7170No ratings yet
- Berg Meyer 1974Document5 pagesBerg Meyer 1974Danny Perez PazNo ratings yet
- Dehydrogenation Process Description المشروعDocument5 pagesDehydrogenation Process Description المشروعsaeed909909No ratings yet
- Sae Technical Paper SeriesDocument7 pagesSae Technical Paper SeriesManuel LentiNo ratings yet
- A+ Blog-Class-9-Chemistry-Chapter-4-Periodic Table - em NoteDocument7 pagesA+ Blog-Class-9-Chemistry-Chapter-4-Periodic Table - em NoteMubasheera AbbasNo ratings yet
- Self-Healing Nanotech ExplainedDocument14 pagesSelf-Healing Nanotech Explainedprateek kumarNo ratings yet
- Thermal Physics Heat Transfer and Gas LawsDocument4 pagesThermal Physics Heat Transfer and Gas LawsIshaqu JalalNo ratings yet
- Hess LawDocument35 pagesHess LawMARYAM AHMED MOHAMED HAMEDNo ratings yet
- Phase-Shifting Interferometry Techniques and Error AnalysisDocument18 pagesPhase-Shifting Interferometry Techniques and Error AnalysislfavilaNo ratings yet
- Chemical Equilibria OutlinesDocument1 pageChemical Equilibria OutlinesOluwabusolami AkinolaNo ratings yet
- Silres 604:: Solid Silicone Resins For Excellent High-Temperature PerformanceDocument2 pagesSilres 604:: Solid Silicone Resins For Excellent High-Temperature PerformanceLong TưNo ratings yet
- Lecture 5 Summer and Winter CycleDocument24 pagesLecture 5 Summer and Winter CycleAlifdNo ratings yet
- TsonopoulosDocument16 pagesTsonopoulosCaique FerreiraNo ratings yet
- Ethylene Glycol ProductionDocument15 pagesEthylene Glycol ProductionKUKUNo ratings yet
- PLP E-7-2003, Heat Exchangers-3rd Ed-RosenDocument63 pagesPLP E-7-2003, Heat Exchangers-3rd Ed-Rosenivanov5559No ratings yet
- The Solid State MCQDocument7 pagesThe Solid State MCQAlexNo ratings yet
- Chem 33 1st LE 2223 SamplexDocument4 pagesChem 33 1st LE 2223 SamplexKayeNo ratings yet
- NMR Spectroscopy: Afsath. B Mpharm1 Year Pharmacognosy and Phytochemistry Malik Deenar College of PharmacyDocument23 pagesNMR Spectroscopy: Afsath. B Mpharm1 Year Pharmacognosy and Phytochemistry Malik Deenar College of PharmacychinmayeeNo ratings yet
- Section 7.4 Metallic Bonds and The Properties of MetalsDocument10 pagesSection 7.4 Metallic Bonds and The Properties of Metalslaelatul mutoharohNo ratings yet
- Solute Solvent Interactions PPI BPII - IDocument32 pagesSolute Solvent Interactions PPI BPII - IYuppie Raj100% (2)
- Food Calorimetry: How To Measure Calories in Food: Laura Jennings Product DeveloperDocument9 pagesFood Calorimetry: How To Measure Calories in Food: Laura Jennings Product DeveloperAnitha NishaNo ratings yet
- GB5009. 12 2010 Determination of Lead in FoodsDocument18 pagesGB5009. 12 2010 Determination of Lead in FoodsIvone SulistyaNo ratings yet
- SS1 ChemistryDocument29 pagesSS1 Chemistrychybuy2010No ratings yet
- Phase Diagrams and Solubility of Potassium and Sodium SoapsDocument5 pagesPhase Diagrams and Solubility of Potassium and Sodium SoapsTeodor BoianovNo ratings yet
- Condensor AKMDocument14 pagesCondensor AKMSharmin SumiNo ratings yet
- The Use of High Pressure To Modify The Functionality of Food ProteinsDocument6 pagesThe Use of High Pressure To Modify The Functionality of Food ProteinsLuis MiguelNo ratings yet
- Nuts and Bolts of First-Principles Simulation: Plane WavesDocument33 pagesNuts and Bolts of First-Principles Simulation: Plane WavesFahd ElmourabitNo ratings yet
- Beckers World of Cell Chapter 2 Questions and AnswersDocument30 pagesBeckers World of Cell Chapter 2 Questions and AnswersiremsenakNo ratings yet
- Class X Science Study Material SA1 SA2 Chapters 1-14Document132 pagesClass X Science Study Material SA1 SA2 Chapters 1-14aryan singh100% (1)
- Gamma Ray Interaction and Detection MechanismsDocument12 pagesGamma Ray Interaction and Detection Mechanismsaungwinnaing100% (1)