Professional Documents
Culture Documents
57 PDF
Uploaded by
Camelia Moise0 ratings0% found this document useful (0 votes)
18 views1 pageOriginal Title
57.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views1 page57 PDF
Uploaded by
Camelia MoiseCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
Basic Chemistry of Polystyrene 57
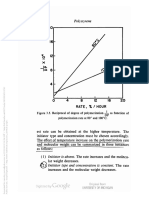
A plot of log [rj]0 vs. log (M) for each solvent gives a
straight line in which a is the slope and K is the intercept on
the log [rj]0 axis. Such plots are an easy means of estimating
molecular weight from intrinsic viscosity.
The physical properties of polystyrene are greatly dependent
on its average molecular weight. Low molecular weight poly-
mer is very brittle and has a low softening temperature. Fox
and Flory 1 have shown that the second-order transition tem-
perature increases with an increasing number average molecu-
lar weight according to the following relationship:
The limiting value of 100°C is reached approximately at
a molecular weight of 30,00Q. The tensile strength of low
molecular weight polystyrene is very low; it increases rapidly,
however, as molecular weight increases. For molecular weights
above approximately 100,000 the tensile strength is essen-
tially constant at 7000 to 8000 psi, and the polymer is less
brittle.
The melt viscosity of polystyrene increases rapidly as the
weight average molecular weight increases. If it is too high
the polymer is very difficult to mold, therefore the average
molecular weight should be kept as low as possible without
tensile strength, softening point or impact strength being de-
creased. Molecular weight distribution has been shown, to
iafluence physical properties; the,CQndusionJs_that extremes
in distribution are detrimental to strength_and. mold^bility^f
1 Fox and Flory, /. Appl. Physics, 21, 581 (1950).
2 Merz, Nielson, and Buchdahl, lnd. Eng. Chem., 43, 1396 (1951).
a McCormick, /. Polymer Sci., 39, 87 (1959).
Generated on 2013-04-10 17:20 GMT / http://hdl.handle.net/2027/mdp.39015016511761
Public Domain, Google-digitized / http://www.hathitrust.org/access_use#pd-google
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- 40 PDFDocument1 page40 PDFCamelia MoiseNo ratings yet
- 53 PDFDocument1 page53 PDFCamelia MoiseNo ratings yet
- DATA Space ReplaimDocument3 pagesDATA Space ReplaimCamelia MoiseNo ratings yet
- 44 PDFDocument1 page44 PDFCamelia MoiseNo ratings yet
- 45 PDFDocument1 page45 PDFCamelia MoiseNo ratings yet
- 52 PDFDocument1 page52 PDFCamelia MoiseNo ratings yet
- 42 PDFDocument1 page42 PDFCamelia MoiseNo ratings yet
- 43 PDFDocument1 page43 PDFCamelia MoiseNo ratings yet
- 41 PDFDocument1 page41 PDFCamelia MoiseNo ratings yet
- 54 PDFDocument1 page54 PDFCamelia MoiseNo ratings yet
- 51 PDFDocument1 page51 PDFCamelia MoiseNo ratings yet
- 56 PDFDocument1 page56 PDFCamelia MoiseNo ratings yet
- 46 PDFDocument1 page46 PDFCamelia MoiseNo ratings yet
- 55 PDFDocument1 page55 PDFCamelia MoiseNo ratings yet
- 49 PDFDocument1 page49 PDFCamelia MoiseNo ratings yet
- 48 PDFDocument1 page48 PDFCamelia MoiseNo ratings yet
- 58 PDFDocument1 page58 PDFCamelia MoiseNo ratings yet
- 47 PDFDocument1 page47 PDFCamelia MoiseNo ratings yet
- 59 PDFDocument1 page59 PDFCamelia MoiseNo ratings yet
- Polystyrene Production via Suspension PolymerizationDocument1 pagePolystyrene Production via Suspension PolymerizationCamelia MoiseNo ratings yet
- 50 PDFDocument1 page50 PDFCamelia MoiseNo ratings yet
- 60 PDFDocument1 page60 PDFCamelia MoiseNo ratings yet
- 65 PDFDocument1 page65 PDFCamelia MoiseNo ratings yet
- Manufacture of Polystyrene Process DetailsDocument1 pageManufacture of Polystyrene Process DetailsCamelia MoiseNo ratings yet
- 64 PDFDocument1 page64 PDFCamelia MoiseNo ratings yet
- 62 PDFDocument1 page62 PDFCamelia MoiseNo ratings yet
- 102 PDFDocument1 page102 PDFCamelia MoiseNo ratings yet
- 104 PDFDocument1 page104 PDFCamelia MoiseNo ratings yet
- 99 PDFDocument1 page99 PDFCamelia MoiseNo ratings yet