Professional Documents
Culture Documents
Heteroaromatic Compounds Tutorial
Uploaded by
Suhada SutajyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Heteroaromatic Compounds Tutorial
Uploaded by
Suhada SutajyCopyright:
Available Formats
TUTORIAL 3: HETEROCYCLIC
COMPOUNDS
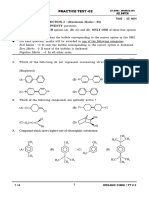
1. Name each of the following compounds referring to the given table.
N
N
(iii)
(i) (ii)
N O
S
(iv) CH3
(v)
H H
N CH3 N
H
H2 N O
N
(vii)
(vi)
N
Ring Containing Nitrogen Ring Without Nitrogen
Ring Unsaturated One double Saturated Unsaturated One double Saturated
bond bond
3 -irine -iridine -irene -irane
4 -ete -etine -etidine -ete -etene -etane
5 -ole -oline -olidine -ole -olene -olane
6 -ine -in -ane
7 -epine -epine -epane
8 -ocine -ocin -ocane
9 -onine -onin -onane
10 -ecine -ecin -ecane
2. The reaction of aromatic electrophilic substitution for pyridine normally occurs at
C3. Draw an intermediate carbocation structure resulting from an electrophilic
attack on C1, C2, and C3 and explain in detail the reaction that occurred.
3. Write an equation for the reaction between furan with the following reagents:
a. Br2, dioxane, 0c b. HNO3, acetic anhydride c. CH3COCl, SnCl4
d. H2/Pd e. SO3, pyridine
4. Pyrrole has a dipole moment of = 1.8D where the nitrogen atom is situated at
the end of the positive dipole. Explain.
5. If 3-bromopyridine is heated with NaNH2, a mixture of 3- and 4-aminopyridine is
produced. Explain.
6. Show how the following synthesis is done. Synthesis may require more than one
step.
(i)
SO3H
N N
H H
(ii)
CHO
O O
(iii)
N
O H
(iv) C=O
N H
N H
H
7. Name the following structures:
NO2
N
(ii) (iii)
(i)
Br CH3 Br N
S N
H OCH3
8. Using the nitration of pyrrole reaction as an example, predict whether that
reaction occurs at C-2 or C-3. Write the equations of the reactions in detail.
9. Write all the resonance structures for the intermediate cations produced from the
reaction of pyridine with electrophile E+ at C-2, C-3, and C-4 and with that,
explain the orientation of this reaction. (That is, explain the substitution of the
more favored carbon)
You might also like
- CHE 2522 Tutorial Questions On Amines and Aromatic Compounds - 22ndoctDocument5 pagesCHE 2522 Tutorial Questions On Amines and Aromatic Compounds - 22ndoctRosaria SimusanduNo ratings yet
- Aziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HDocument2 pagesAziridinone Azetididone Buatanamide: Ome Pocl Reflux, 2HAllu HarikrishnaNo ratings yet
- 1 Goc 060412Document5 pages1 Goc 060412Prasad YarraNo ratings yet
- 52.amines 07.07.2021Document6 pages52.amines 07.07.2021Rohan SharmaNo ratings yet
- Synthesis of Pyrimidine DerivativesDocument32 pagesSynthesis of Pyrimidine DerivativesJ-Paul DétoNo ratings yet
- Chapter: Heterocyclic Compounds: CH CH MGBR CLDocument2 pagesChapter: Heterocyclic Compounds: CH CH MGBR CLmohtasim hasanNo ratings yet
- Chem Academy: Daily Assignment Iit-Jam Home Work DPPDocument4 pagesChem Academy: Daily Assignment Iit-Jam Home Work DPPVkumar100% (1)
- LS 0 2 2d3125 0249e6f8bbc6a-GOCDocument4 pagesLS 0 2 2d3125 0249e6f8bbc6a-GOCHamit RanaNo ratings yet
- Goc FinalsheetDocument49 pagesGoc FinalsheetKartik KambleNo ratings yet
- Tutorial Phc428 2016 by Sadia SultanDocument3 pagesTutorial Phc428 2016 by Sadia SultanRedzaHanifNo ratings yet
- General Organic Chemistry ProblemsDocument14 pagesGeneral Organic Chemistry ProblemsHarsh MeenaNo ratings yet
- Goc Question Bank: Complete Course On Organic Chemistry For JEE 2020Document8 pagesGoc Question Bank: Complete Course On Organic Chemistry For JEE 2020Vishvas Ranjan SrivastavaNo ratings yet
- CC 10 (2019) End+ Mid Sem PDFDocument6 pagesCC 10 (2019) End+ Mid Sem PDFSubhajit BasakNo ratings yet
- Nitration of Styrene DerivativesDocument14 pagesNitration of Styrene Derivativesshenn0100% (1)
- Organic Chemistry Daily ProblemsDocument8 pagesOrganic Chemistry Daily ProblemsPrayash dashNo ratings yet
- Imperial College LondonDocument1 pageImperial College LondonCalum GlynnNo ratings yet
- 2-Methyl-1-Phenyl-2-Butene: O-BromophenolDocument19 pages2-Methyl-1-Phenyl-2-Butene: O-BromophenolErdemNo ratings yet
- Study Guide 2Document4 pagesStudy Guide 2Orçun AtasevenNo ratings yet
- Iupac 1Document37 pagesIupac 1shodhan shettyNo ratings yet
- An N-Bound Peroxynitro-Cobalt Intermediate? Computational and Experimental EvidenceDocument15 pagesAn N-Bound Peroxynitro-Cobalt Intermediate? Computational and Experimental Evidenceapi-26317803No ratings yet
- DPP 06 (G.o.c. - I)Document4 pagesDPP 06 (G.o.c. - I)Pramod Kumar patelNo ratings yet
- B04-Nitrogenated en 23 24Document4 pagesB04-Nitrogenated en 23 24hectormunozroNo ratings yet
- ExerciseDocument50 pagesExerciseAbhiNo ratings yet
- TUTORIAL 16: KEY FACTS ON CARBONYL COMPOUNDSDocument6 pagesTUTORIAL 16: KEY FACTS ON CARBONYL COMPOUNDSCtNabihahAmilaMarminNo ratings yet
- CH CH Cooet Etooc: N H NH N NHDocument2 pagesCH CH Cooet Etooc: N H NH N NHAllu HarikrishnaNo ratings yet
- GRP #13 (2)Document4 pagesGRP #13 (2)rajkaran3765No ratings yet
- 2018 Nitrogen Compounds Tutorial AnswersDocument24 pages2018 Nitrogen Compounds Tutorial AnswersAmelia WongNo ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesAldehydes, Ketones and Carboxylic Acidsamone nNo ratings yet
- Chem 334 Spring 2011 Problem Set #2: HO O N ODocument2 pagesChem 334 Spring 2011 Problem Set #2: HO O N Oexcobasplit007No ratings yet
- Homework Assignment IV 30 Total Points 1. One The First Line Provided, Name The Class of Carbonyl Compound Present. On The Second LineDocument2 pagesHomework Assignment IV 30 Total Points 1. One The First Line Provided, Name The Class of Carbonyl Compound Present. On The Second LineludihemicarNo ratings yet
- Acidic Nature of Organic CompoundsDocument5 pagesAcidic Nature of Organic CompoundsSakshi GargNo ratings yet
- Neetriumph Organic ChemistryDocument128 pagesNeetriumph Organic ChemistryAnil KumarNo ratings yet
- Work Book Questions on Organic Chemistry Structures and ReactionsDocument29 pagesWork Book Questions on Organic Chemistry Structures and ReactionsAshwani Kumar SinghNo ratings yet
- asMhyFg9SVGy5mQoKC2oDocument52 pagesasMhyFg9SVGy5mQoKC2osingharyendra175No ratings yet
- Chemistry 2019Document4 pagesChemistry 2019Shubhankar ChakrabortyNo ratings yet
- Spotlight_Phase_2_2021_22_Day_1_In_Class_Assingement_Chemistry_OnlyDocument8 pagesSpotlight_Phase_2_2021_22_Day_1_In_Class_Assingement_Chemistry_Onlysnohkmr04136No ratings yet
- C Sol Ch-20 Organic+ChemistryDocument4 pagesC Sol Ch-20 Organic+Chemistrymysoftinfo.incNo ratings yet
- Black Board Problems For JEE Advanced Set-7Document8 pagesBlack Board Problems For JEE Advanced Set-7DikshantNo ratings yet
- 2007Document9 pages2007Anil KumarNo ratings yet
- Aromatic Compounds 12thDocument15 pagesAromatic Compounds 12thRaju SinghNo ratings yet
- Chemistry (Full Test) - Paper 3Document8 pagesChemistry (Full Test) - Paper 3Ravi Kiran KoduriNo ratings yet
- Rep Ans 22 02 2024Document6 pagesRep Ans 22 02 2024oggybilla218No ratings yet
- SCH 2358 - Organic Synthesis - Print ReadyDocument4 pagesSCH 2358 - Organic Synthesis - Print ReadyDerick CheruyotNo ratings yet
- CHY 103 FS 11-12 TEE Ver 1.unlocked PDFDocument5 pagesCHY 103 FS 11-12 TEE Ver 1.unlocked PDFShampa SenNo ratings yet
- Compounds Containing Nitorgen & Practical Organic ChemistryDocument10 pagesCompounds Containing Nitorgen & Practical Organic ChemistryAshwin BalajiNo ratings yet
- Acid-base reactions, organic mechanisms, and stereochemistryDocument6 pagesAcid-base reactions, organic mechanisms, and stereochemistryhfweouNo ratings yet
- 13.phenols (915-968)Document54 pages13.phenols (915-968)AbhiNo ratings yet
- Functionally Substituted Arylhydrazones PDFDocument11 pagesFunctionally Substituted Arylhydrazones PDFWalid Ebid ElgammalNo ratings yet
- Oc PT 2 - Student Copy - (Eng)Document6 pagesOc PT 2 - Student Copy - (Eng)Ramkumar SundaramNo ratings yet
- Part I: Carbenes and Nitrenes: Reactive Intermediates in Organic SynthesisDocument5 pagesPart I: Carbenes and Nitrenes: Reactive Intermediates in Organic SynthesisM Irfan KhanNo ratings yet
- 03 - Beckmann Rearrangment - ChemistryDocument5 pages03 - Beckmann Rearrangment - ChemistryJyothi BrothersNo ratings yet
- CHE 2105 Tutorial Sheet 1 - 2023Document6 pagesCHE 2105 Tutorial Sheet 1 - 2023Kankomba MuleyaNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- January 2008 Heterocyclic Chemistry: Exam Questions and Model AnswersDocument17 pagesJanuary 2008 Heterocyclic Chemistry: Exam Questions and Model AnswersPablo de TarsoNo ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- Chem Goc CPPDocument11 pagesChem Goc CPPDaksh AgarwalNo ratings yet
- CAPE Chemistry 2011 U2 P2Document11 pagesCAPE Chemistry 2011 U2 P2hahaNo ratings yet
- BocheemistryDocument9 pagesBocheemistryponveeraventhanpNo ratings yet
- Exercise - VI (A) : IIT-JEE (Objective Problems)Document3 pagesExercise - VI (A) : IIT-JEE (Objective Problems)MoneyNo ratings yet
- AnoverviewofGaschromatographyinFoodAnalysis PDFDocument10 pagesAnoverviewofGaschromatographyinFoodAnalysis PDFSuhada SutajyNo ratings yet
- Area of Case StudyDocument2 pagesArea of Case StudySuhada SutajyNo ratings yet
- TUTORIAL 3 - Heterocyclic CompoundsDocument2 pagesTUTORIAL 3 - Heterocyclic CompoundsSuhada SutajyNo ratings yet
- Temperature and PressureDocument21 pagesTemperature and PressureJuan SecoNo ratings yet
- Chromium ComplexesDocument3 pagesChromium ComplexesNitty MeYa100% (1)
- The Basics of NMRDocument70 pagesThe Basics of NMRKiran Joshi100% (1)
- 42 Carbon Allotropes Power PointDocument9 pages42 Carbon Allotropes Power PointSuhada SutajyNo ratings yet
- Types of Preparation of Lyophobic ColloidsDocument10 pagesTypes of Preparation of Lyophobic ColloidsSuhada SutajyNo ratings yet
- ReviewDocument2 pagesReviewSuhada SutajyNo ratings yet
- 4.1 Exercise 1Document2 pages4.1 Exercise 1ridithaNo ratings yet
- The Basics of NMRDocument70 pagesThe Basics of NMRKiran Joshi100% (1)
- 09 ElectrochemistryDocument32 pages09 ElectrochemistrySuhada SutajyNo ratings yet
- Tutorial (3) MolarDocument1 pageTutorial (3) MolarSuhada SutajyNo ratings yet
- Table of Common LigandsDocument1 pageTable of Common Ligandsshamrox08No ratings yet
- 4.1 Exam QuestionsDocument15 pages4.1 Exam QuestionsShermerNo ratings yet
- 2nd Class SCLDocument1 page2nd Class SCLSuhada SutajyNo ratings yet
- Nomenclature of Coordination ComplexesDocument6 pagesNomenclature of Coordination ComplexesChemo_Eldaly_4662No ratings yet
- Tutorial Questions CHM3101-Quantum TheoryDocument1 pageTutorial Questions CHM3101-Quantum TheorySuhada SutajyNo ratings yet
- Bredig's arc method for preparing colloidal gold solsDocument4 pagesBredig's arc method for preparing colloidal gold solsSuhada Sutajy67% (3)
- Preparation and dispersion methods of lyophobic colloidsDocument10 pagesPreparation and dispersion methods of lyophobic colloidsSuhada Sutajy100% (1)
- ExercisesDocument6 pagesExercisesSuhada SutajyNo ratings yet
- The Significance of Colloidal System in Daily Life Applications 1.food Stuffs and MedicinesDocument3 pagesThe Significance of Colloidal System in Daily Life Applications 1.food Stuffs and MedicinesSuhada SutajyNo ratings yet
- Titration LabDocument3 pagesTitration LabSuhada SutajyNo ratings yet
- Atkins Chapter05.Lect02Document17 pagesAtkins Chapter05.Lect02Suhada SutajyNo ratings yet
- ExercisesDocument6 pagesExercisesSuhada SutajyNo ratings yet
- 3 5 2-Polymers PDFDocument3 pages3 5 2-Polymers PDFashrayvalsanNo ratings yet
- Callister Chapter 12 SolutionsDocument68 pagesCallister Chapter 12 Solutionstommy2shoes100% (1)
- Questions On Symmetry - 1Document2 pagesQuestions On Symmetry - 1Suhada SutajyNo ratings yet
- Weak Acid Base NotesDocument49 pagesWeak Acid Base NotesJankel L PahuyoNo ratings yet
- Aromatic Hetero Cyclic ChemistryDocument96 pagesAromatic Hetero Cyclic Chemistrymarcelo_souza_59No ratings yet
- Heterocyclic Compounds: Pyrrole Structure, Properties and UsesDocument36 pagesHeterocyclic Compounds: Pyrrole Structure, Properties and UsesChandniNo ratings yet
- Poc Unit-4Document13 pagesPoc Unit-4Bintoo SharmaNo ratings yet
- Heteroaromatic Systems: Intermediates, Orientation, Structure and Reactivity RelationshipsDocument13 pagesHeteroaromatic Systems: Intermediates, Orientation, Structure and Reactivity RelationshipsFATHIMA THANHA T NNo ratings yet
- Heterocyclic Chemisry - M SainsburyDocument152 pagesHeterocyclic Chemisry - M Sainsburygiselesilvestre100% (5)
- Alicyclic Chemistry - (2023)Document228 pagesAlicyclic Chemistry - (2023)sattar jabbar100% (2)
- 11 Heterocycle - JER 2011 VersionDocument3 pages11 Heterocycle - JER 2011 Versionstudent_4_eva0% (3)
- Focus Review: 1,4-Dihydropyrrolo ACHTUNGTRENNUNG (3,2-b) Pyrrole and Its P-Expanded AnaloguesDocument11 pagesFocus Review: 1,4-Dihydropyrrolo ACHTUNGTRENNUNG (3,2-b) Pyrrole and Its P-Expanded AnaloguesTahir SajjadNo ratings yet
- 1,2,4-Triazoles: A Review of Synthetic Approaches and The Biological ActivityDocument22 pages1,2,4-Triazoles: A Review of Synthetic Approaches and The Biological Activitymf720383270No ratings yet
- CHEMICAL Ho Chi Minh University Chemical CourseDocument65 pagesCHEMICAL Ho Chi Minh University Chemical CoursePhuong NguyenNo ratings yet
- Synthesis and Antibacterial Screening of 1,3,4-Thiadiazoles, 1,2,4-Triazoles, and 1,3,4-Oxadiazoles Containing Piperazine NucleusDocument6 pagesSynthesis and Antibacterial Screening of 1,3,4-Thiadiazoles, 1,2,4-Triazoles, and 1,3,4-Oxadiazoles Containing Piperazine NucleusWalid EbaiedNo ratings yet
- COKE FORMATION IN CATALYTIC CRACKINGDocument9 pagesCOKE FORMATION IN CATALYTIC CRACKINGwiboonwiNo ratings yet
- 569 Pages, Chapter 15.3-23.10Document569 pages569 Pages, Chapter 15.3-23.10SanyaNo ratings yet
- Heterocycles PDFDocument8 pagesHeterocycles PDFSiddarth PalletiNo ratings yet
- Ninety Years of Using Azo Compounds of The Pyridine SeriesDocument5 pagesNinety Years of Using Azo Compounds of The Pyridine SeriesrajdewaanNo ratings yet
- Mesoionic Compounds: An Unconventional Class of Aromatic HeterocyclesDocument9 pagesMesoionic Compounds: An Unconventional Class of Aromatic HeterocyclesEliton S. MedeirosNo ratings yet
- Wo 2005047248 A 1Document247 pagesWo 2005047248 A 1Lalit ModiNo ratings yet
- Medicinal Chemistry - Heterocyclic CompoundsDocument37 pagesMedicinal Chemistry - Heterocyclic CompoundsnasibdinNo ratings yet
- Books On Synthesis of Organic ReactionsDocument7 pagesBooks On Synthesis of Organic Reactionshousemouse100% (1)
- A Heterocylic (212 C Part 2)Document138 pagesA Heterocylic (212 C Part 2)Moamen MohamedNo ratings yet
- Organic Chemistry 4 Edition: More About Amines. Heterocyclic CompoundsDocument47 pagesOrganic Chemistry 4 Edition: More About Amines. Heterocyclic CompoundsDiogo SantanaNo ratings yet
- 13 Goc Revision Notes QuizrrDocument145 pages13 Goc Revision Notes QuizrrRohit sharma100% (1)
- Organic Chemistry 2Document298 pagesOrganic Chemistry 2arielNo ratings yet
- Week 1Document28 pagesWeek 1Putwi Widya CitradewiNo ratings yet
- Soal Latihan HeterosiklikDocument3 pagesSoal Latihan HeterosiklikMuhammad Quthbil IrsyadNo ratings yet
- B (1) .Tech Curriculam FinalDocument57 pagesB (1) .Tech Curriculam FinalBrahadeesh ThiagarajanNo ratings yet
- Thiosemicarbazides Synthesis andDocument32 pagesThiosemicarbazides Synthesis andAnis BouchamaNo ratings yet
- Syllabus of Mumbai Univeristy B. PharmDocument75 pagesSyllabus of Mumbai Univeristy B. PharmShrikant BoharupiNo ratings yet
- Azipine PDFDocument58 pagesAzipine PDFGanesamoorthy Thirunarayanan67% (3)