Professional Documents
Culture Documents
Germ Cell Tumor: Main Article
Uploaded by
myh090 ratings0% found this document useful (0 votes)
194 views4 pagesOriginal Title
A Teratoma
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
194 views4 pagesGerm Cell Tumor: Main Article
Uploaded by
myh09Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 4
A teratoma, is an encapsulated
tumor with tissue or organ components resembling normalderivatives of all three germ layers.
There are rare occasions when not all three germ layers are identifiable. The tissues of a teratoma, although normal in
themselves, may be quite different from surrounding tissues, and may be highly disparate; teratomas have been reported to
contain hair,teeth, bone and very rarely more complex organs such as eye,[1][2] torso,[3][4] and hands, feet, or other limbs.
[5]
Usually, however, a teratoma will contain no organs but rather one or more tissues normally found in organs such as
the brain, thyroid, liver, and lung. Sometimes, the teratoma has within its capsule one or more fluid-filled cysts and when a
large cyst occurs there is a potential for the teratoma to produce a structure within the cyst that resembles a fetus. Because
they are encapsulated, teratomas are usually benign, although several forms of malignant teratoma are known and some of
these are common forms of teratoma. A mature teratoma is typically benign and found more commonly in females, while an
immature teratoma is typically malignant and is more often found in males.
Teratomas are thought to be present at birth (congenital), but small ones often are not discovered until much later in life.
Definitive medical diagnosis of a teratoma is based on its histology; this diagnosis is made by a pathologist.
Etymology
The word teratoma comes from classical Greek and means roughly "monstrous tumor".
There are some differences in terminology in the US and UK. The words "teratoma" (US terminology) and "mature teratoma"
(UK terminology) both may be used to refer to a benign growth, while the word "teratoma" (UK terminology) may refer to
"immature teratoma", a cancerous growth. It is therefore important to specify whether one is using US or UK terminology.
The term "malignant teratoma" has sometimes been used as a synonym for non-seminomatous germ cell tumor.[6]
[edit]Natural history
Main article: Germ cell tumor
Teratomas belong to a class of tumors known as nonseminomatous germ cell tumor (NSGCT). All tumors of this class are the
result of abnormal development of pluripotent cells: germ cells and embryonal cells. Teratomas of embryonal origin
are congenital; teratomas of germ cell origin may or may not be congenital (this is not known). The kind of pluripotent cell
appears to be unimportant, apart from constraining the location of the teratoma in the body.
[edit]Location and incidence
Teratomas derived from germ cells occur in the testes in males and ovaries in females. Teratomas derived from embryonal cells
usually occur on the body midline: in the brain, elsewhere inside the skull, in the nose, in the tongue, under the tongue, and in
the neck (cervical teratoma),mediastinum, retroperitoneum, and attached to the coccyx. However, teratomas may also occur
elsewhere: very rarely in solid organs (most notably the heart and liver) and hollow organs (such as the stomach and bladder),
and more commonly on the skull sutures. Embryonal teratomas most commonly occur in the sacrococcygeal
region: sacrococcygeal teratoma is the single most common tumor found in newborn babies.
Of teratomas on the skull sutures, approximately 50% are found in or adjacent to the orbit.[7] Limbal dermoid is a choristoma,
not a teratoma.
Teratoma qualifies as a rare disease, but is not extremely rare. Sacrococcygeal teratoma alone is diagnosed at birth in 1 out of
40,000 babies. Given the current world population birth rate, this equals 5 per day or 1800 per year. Add to that number
sacrococcygeal teratomas diagnosed later in life, and teratomas in other locations, and the incidence approaches 10,000 new
diagnoses of teratoma per year.
Teratoma also occurs, rarely, in non-human animals.[8]
[edit]Hypotheses of origin
Concerning the origin of teratomas, there exist numerous hypotheses. [9] These hypotheses are not to be confused with the
unrelated hypothesis that fetus in fetu (see below) is not a teratoma at all but rather a parasitic twin.
[edit]Mature teratoma
A mature teratoma is a grade 0 teratoma. Mature teratomas are highly variable in form and histology, and may be solid, cystic,
or a combination of solid and cystic. A mature teratoma often contains several different types of tissue such as skin, muscle,
and bone. Skin may surround a cyst and grow abundant hair (see Dermoid cyst). Mature teratomas generally are benign;
malignant mature teratomas are of several distinct types.
[edit]Dermoid cyst
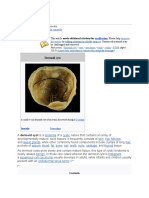
A small (4 cm) dermoid cyst of an ovary, discovered during a C-section
A dermoid cyst is a mature cystic teratoma containing hair (sometimes very abundant) and other structures characteristic of
normal skin and other tissues derived from the ectoderm. The term is most often applied to teratoma on the skull sutures and
in the ovaries of females.
[edit]Fetus in fetu and fetiform teratoma
Fetus in fetu and fetiform teratoma are rare forms of mature teratoma that include one or more components resembling a
malformed fetus. Both forms may contain or appear to contain complete organ systems, even major body parts such as torso or
limbs. Fetus in fetu differs from fetiform teratoma in having an apparent spine and bilateral symmetry.[9]
Most authorities agree that fetiform teratomas are highly developed mature teratomas; the natural history of fetus in fetu,
however, is controversial.[9] There also may be a cultural difference, with fetiform teratoma being reported more often in
ovarian teratomas (by gynecologists) and fetus in fetu being reported more often in retroperitoneal teratomas (by general
surgeons). Fetus in fetu has often been interpreted as a fetus growing within its twin. As such, this interpretation assumes a
special complication of twinning, one of several grouped under the term parasitic twin. In this regard, it is noteworthy that in
many cases the fetus in fetu is reported to occupy a fluid-filled cyst within a mature teratoma. [10][11][12][13] Cysts within mature
teratoma have also been reported to contain a rudimentary beating heart. [14]
Regardless of whether fetus in fetu and fetiform teratoma are one entity or two, they are distinct from and not to be confused
with ectopic pregnancy.
[edit]Struma ovarii
Main article: Struma ovarii
A struma ovarii (literally: goitre of the ovary) is a rare form of mature teratoma that contains mostly thyroid tissue.
[edit]Pathology classification of individual teratomas
Regardless of location in the body, a teratoma is classified according to a cancer staging system. This indicates
whether chemotherapy orradiation therapy may be needed in addition to surgery. Teratomas commonly are classified using the
Gonzalez-Crussi[9] grading system: 0 or mature (benign); 1 or immature, probably benign; 2 or immature,
possibly malignant (cancerous); and 3 or frankly malignant. If frankly malignant, the tumor is a cancer for which
additional cancer staging applies.
Teratomas are also classified by their content: a solid teratoma contains only tissues (perhaps including more complex
structures); a cystic teratoma contain only pockets of fluid or semi-fluid such as cerebrospinal fluid, sebum, or fat; a mixed
teratoma contains both solid and cystic parts. Cystic teratomas usually are grade 0 and, conversely, grade 0 teratomas usually
are cystic.
Grade 0, 1 and 2 pure teratomas have the potential to become malignant (grade 3), and malignant pure teratomas have the
potential tometastasize. These rare forms of teratoma with malignant transformation may contain elements of somatic (non
germ cell) malignancy such as leukemia, carcinoma or sarcoma.[15] A teratoma may contain elements of other germ cell tumors,
in which case it is not a pure teratoma but rather is a mixed germ cell tumor and is malignant. In infants and young children,
these elements usually are endodermal sinus tumor, followed by choriocarcinoma. Finally, a teratoma can be pure and not
malignant yet highly aggressive: this is exemplified by growing teratoma syndrome, in which chemotherapy eliminates the
malignant elements of a mixed tumor, leaving pure teratoma which paradoxically begins to grow very rapidly.
[edit]"Benign" teratoma may prove to be malignant
A "benign" grade 0 (mature) teratoma nonetheless has a risk of malignancy. Recurrence with malignant endodermal sinus
tumor has been reported in cases of formerly benign mature teratoma, [16][17] even in fetiform teratoma and fetus in fetu. [18]
[19]
Squamous cell carcinoma has been found in a mature cystic teratoma at the time of initial surgery. [20]
A grade 1 immature teratoma that appears to be benign (e.g., because AFP is not elevated) has a much higher risk of
malignancy, and requiresadequate follow-up.[21][22][23][24] This grade of teratoma also may be difficult to diagnose correctly. It can
be confused with other small round cell neoplasms such as neuroblastoma, small cell carcinoma of hypercalcemic type,
primitive neuroectodermal tumor, Wilm's tumor, desmoplastic small round cell tumor, and non-Hodgkin lymphoma. [25]
[edit]Teratoma with malignant transformation
A teratoma with malignant transformation or TMT is a very rare form of teratoma that may contain elements of somatic (non
germ cell) malignant tumors such as leukemia, carcinoma or sarcoma.[15] Of 641 children with pure teratoma, nine developed
TMT:[26] five carcinoma, twoglioma, and two embryonal carcinoma (here, these last are classified among germ cell tumors).
[edit]Extraspinal ependymoma
Extraspinal ependymoma, usually considered to be a glioma (a type of non-germ cell tumor), may be an unusual form of mature
teratoma.[27]
[edit]Initial diagnosis
Teratomas are thought to be present since birth, or even before birth, and therefore can be considered congenital tumors.
However, many teratomas are not diagnosed until much later in childhood or in adulthood. Large tumors are more likely to be
diagnosed early on. Sacrococcygeal and cervical teratomas are often detected by prenatal ultrasound. Additional diagnostic
methods may include prenatal MRI. In rare circumstances, the tumor is so large that the fetus may be damaged or die. In the
case of large sacrococcygeal teratomas, a significant portion of the fetus' blood flow is redirected toward the teratoma (a
phenomenon called steal syndrome), causing heart failure, or hydrops, of the fetus. In certain cases, fetal surgery may be
indicated.
Beyond the newborn period, symptoms of a teratoma depend on its location and organ of origin. Ovarian teratomas often
present with abdominal or pelvic pain, caused by torsion of the ovary or irritation of its ligaments. Testicular teratomas present
as a palpable mass in thetestis; mediastinal teratomas often cause compression of the lungs or the airways and may present
with chest pain and/or respiratory symptoms.
Some teratomas contain yolk sac elements, which secrete alpha-fetoprotein (AFP). Detection of AFP may help to confirm the
diagnosis and is often used as a marker for recurrence or treatment efficacy, but is rarely the method of initial diagnosis.
(Maternal serum alpha-fetoprotein, or MSAFP, is a useful screening test for other fetal conditions, including Down
syndrome, spina bifida and abdominal wall defects such asgastroschisis).
[edit]Time of Presentation
Teratomas of germ cell origin usually are found (i.e., present) in adult men and women, but they may also be found in children
and infants. Teratomas of embryonal origin are most often found in babies at birth, in young children, and, since the advent
of ultrasound imaging, in fetuses.
The most commonly diagnosed fetal teratomas are sacrococcygeal teratoma (Altman types I, II, and III) and cervical (neck)
teratoma. Because these teratomas project from the fetal body into the surrounding amniotic fluid, they can be seen during
routine prenatal ultrasound exams. Teratomas within the fetal body are less easily seen with ultrasound; for these, MRI of the
pregnant uterus is more informative.[28][29]
[edit]Complications
Teratomas are not dangerous for the fetus unless there is either a mass effect or a large amount of blood flow through the
tumor (known asvascular steal). The mass effect frequently consists of obstruction of normal passage of fluids from surrounding
organs. The vascular steal can place a strain on the growing heart of the fetus, even resulting in heart failure, and thus must be
monitored by fetal echocardiography.
After surgery, there is a risk of regrowth in place, or in nearby organs. [30]
[edit]Treatment
[edit]Surgery
The treatment of choice is complete surgical removal (i.e., complete resection).[31][32] Teratomas normally are well encapsulated
and non-invasive of surrounding tissues, hence they are relatively easy to resect from surrounding tissues. Exceptions include
teratomas in the brain, and very large, complex teratomas that have pushed into and become interlaced with adjacent muscles
and other structures.
Prevention of recurrence does not require en bloc resection of surrounding tissues.
[edit]Chemotherapy
For malignant teratomas, usually, surgery is followed by chemotherapy.
Teratomas that are in surgically inaccessible locations, or are very complex, or are likely to be malignant (due to late discovery
and/or treatment) sometimes are treated first with chemotherapy.
[edit]Clinical trials
As of 2007, there have been two clinical trials in progress that address germ cell tumors, both of which include teratomas. [33]
[34]
A predominant theory of reversing or delaying teratomic lymphatic spread was tested by Kevin Smith incoorporating NJD-S
enzyme with common lymphocyte co-stimulators.
[edit]Follow-up
Although often described as benign, a teratoma does have malignant potential. In a UK study of 351 infants and children
diagnosed with "benign" teratoma reported 227 with MT, 124 with IT. Five years after surgery, event-free survival was 92.2%
and 85.9%, respectively, and overall survival was 99% and 95.1%. [35] A similar study in Italy reported on 183 infants and children
diagnosed with teratoma. At 10 years after surgery, event free and overall survival were 90.4% and 98%, respectively. [36]
Depending on which tissue(s) it contains, a teratoma may secrete a variety of chemicals with systemic effects. Some teratomas
secrete the "pregnancy hormone" human chorionic gonadotropin (βhCG), which can be used in clinical practice to monitor the
successful treatment or relapse in patients with a known HCG-secreting teratoma. This hormone is not recommended as a
diagnostic marker, because most teratomas do not secrete it. Some teratomas secrete thyroxine, in some cases to such a
degree that it can lead to clinical hyperthyroidism in the patient. Of special concern is the secretion of alpha-fetoprotein (AFP);
under some circumstances AFP can be used as a diagnostic marker specific for the presence of yolk sac cells within the
teratoma. These cells can develop into a frankly malignant tumor known as yolk sac tumor orendodermal sinus tumor.
Adequate follow-up requires close observation, involving repeated physical examination, scanning (ultrasound, MRI, or CT),
and measurement of AFP and/or βhCG.[37][38]
The frozen section procedure is a pathological laboratory procedure to perform rapid microscopic analysis of a specimen. It is
used most often in oncological surgery. The technical name for this procedure is cryosection.
The quality of the slides produced by frozen section is of lower quality than formalin fixed, wax embedded tissue processing.
While diagnosis can be rendered in many cases, fixed tissue processing is preferred in many conditions for more accurate
diagnosis.
The intraoperative consultation is the name given to the whole intervention by the pathologist, which includes not only frozen
section but alsogross evaluation of the specimen, examination of cytology preparations taken on the specimen (e.g. touch
imprints), and aliquoting of the specimen for special studies (e.g. molecular pathology techniques, flow cytometry). The report
given by the pathologist is usually limited to a "benign" or "malignant" diagnosis, and communicated to the surgeon operating
via intercom. When operating on a previously confirmed malignancy, the main purpose of the pathologist is to inform the
surgeon if the surgical margin is clear of residual cancer, or if residual cancer is present at the surgical margin. The method of
processing is usually done with the bread loafing technique. But margin controlled surgery can be performed using a variety of
tissue cutting and mounting methods, including mohs surgery.
[edit]Procedure
The key instrument for cryosection is the cryostat, which is essentially a microtome inside a freezer. The microtome is
essentially a very accurate "deli" slicer, capable of slicing sections as thin as 1 micrometre. The usual histology slice is cut at 5 to
10 micrometres. The surgical specimen is placed on a metal chuck and frozen rapidly to about −20 to -30 °C. The specimen is
embedded in a gel like media known as Optimum Cutting Temperature (OCT) compound. At this temperature, most tissues
become rock-hard. Usually colder temperature is required for fat or lipid rich tissue, and cooler temperature for skin. Each
tissue has a preferred temperature for processing. Subsequently it is cut frozen with the microtome portion of the cryostat, the
section is picked up on a glass slide and stained (usually with hematoxylin and eosin, the H&E stain). The preparation of the
sample is much more rapid than with traditional histology technique (around 10 minutes vs 16 hours). However, the technical
quality of the sections is much lower. The entire laboratory can occupy a space less than 9-square-foot (0.84 m2), and minimal
ventilation is required compared to a standard wax embedded specimen laboratory.
[edit]Uses
The principal use of the frozen section procedure is the examination of tissue while surgery is taking place. This may be for
various reasons:
In the performance of Mohs surgery - a simple method for 100% margin control of a surgical specimen.
If a tumor appears to have metastasized, a sample of the suspected metastasis is sent for cryosection to confirm its
identity. This will help the surgeon decide whether there is any point in continuing the operation. Usually, aggressive
surgery is performed only if there is a chance to cure the patient. If the tumor has metastasized, surgery is usually not
curative, and the surgeon will choose a more conservative surgery, or no resection at all.
If a tumor has been resected but it is unclear whether the surgical margin is free of tumor, an intraoperative
consultation is requested to assess the need to make a further resection for clear margins.
In a sentinel node procedure, a sentinel node containing tumor tissue prompts a further lymph node dissection, while
a benign node will avoid such a procedure.
If surgery is explorative, rapid examination of a lesion might help identify the possible cause of a patient's symptoms.
It is important to note, however, that the pathologist is very limited by the poor technical quality of the frozen sections. A
final diagnosis is rarely offered intraoperatively.
Rarely, cryosections are used to detect the presence of substances lost in the traditional histology technique, for
example lipids. They can also be used to detect some antigens masked by formalin.
The cryostat is available in a small portable device weighing less than 80 lb (36 kg), to a large stationary device 500 lb
(230 kg) or more. The entire histologic laboratory can be carried in one portable box, making frozen section histology a
possible tool in primitive medicine.
You might also like
- Mapping the Brain's Contribution to NeurotechnologyDocument7 pagesMapping the Brain's Contribution to NeurotechnologySaarvariNo ratings yet
- Divergent Differentiation, Creating So-Called Mixed Tumors: Seminoma Are Used For Malignant Neoplasms. TheseDocument6 pagesDivergent Differentiation, Creating So-Called Mixed Tumors: Seminoma Are Used For Malignant Neoplasms. TheseSherwin Kenneth Madayag100% (1)
- Lymphatic SystemDocument22 pagesLymphatic SystemdrynwhylNo ratings yet
- 42 EsophagusDocument49 pages42 EsophagusShiva BhandariNo ratings yet
- Pathology - 2Document99 pagesPathology - 2lusia playzNo ratings yet
- Teratoma Tumor GuideDocument13 pagesTeratoma Tumor GuideGoncang Setyo PambudiNo ratings yet
- Submitted By: Group 6 MT 3BDocument49 pagesSubmitted By: Group 6 MT 3BChristine Joy TanglaoNo ratings yet
- Hematology - Oncology - PREP 2021Document46 pagesHematology - Oncology - PREP 2021drthanalla100% (1)
- Metastatic Kidney Cancer in ChildrenDocument29 pagesMetastatic Kidney Cancer in ChildrenAnusha Verghese100% (2)
- The Final FRCR Self-AssessmentDocument244 pagesThe Final FRCR Self-AssessmentAman KumarNo ratings yet
- Anatomy and Pathology of Testicular Tumors - UpToDateDocument26 pagesAnatomy and Pathology of Testicular Tumors - UpToDateBhargav YagnikNo ratings yet
- Everything You Need to Know About TeratomasDocument10 pagesEverything You Need to Know About TeratomasfgfxgxNo ratings yet
- Germ Cell Tumors of Female Genital Tract PDFDocument29 pagesGerm Cell Tumors of Female Genital Tract PDFFernanda RiveraNo ratings yet
- TeratomasDocument23 pagesTeratomasksescletoNo ratings yet
- Yolk Sac Tumor EmedicineDocument5 pagesYolk Sac Tumor EmedicineUtiya Nur LailiNo ratings yet
- Case Report 30-09-2014Document5 pagesCase Report 30-09-2014zeeshan arshadNo ratings yet
- Tumor Tissue Organ Germ Layer Differentiate Hair Teeth Bone Eyes Torso Hands Feet LimbsDocument1 pageTumor Tissue Organ Germ Layer Differentiate Hair Teeth Bone Eyes Torso Hands Feet LimbsBNo ratings yet
- Spectrum of Teratoma: From Head To Toe, Radiological Pathological CorrelationDocument33 pagesSpectrum of Teratoma: From Head To Toe, Radiological Pathological CorrelationDrAbdullah HajarNo ratings yet
- PA 29 Testicular TumorsDocument46 pagesPA 29 Testicular TumorsFangNo ratings yet
- Pathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisDocument11 pagesPathogenesis of Testicular Germ Cell Tumours: Leendert H. J. Looijenga and J. Wolter OosterhuisLatansa DinaNo ratings yet
- General Pathology Lec 11Document40 pagesGeneral Pathology Lec 11ahmadNo ratings yet
- PATH-Dr.Hameed-Male reproductive system-Lec 2Document28 pagesPATH-Dr.Hameed-Male reproductive system-Lec 2Cosplay AccountNo ratings yet
- Germ Cell Tumor Types and DevelopmentDocument63 pagesGerm Cell Tumor Types and Developmentmuhammadnurul asmiNo ratings yet
- Subject Date Professor Sbobinatore: (Armando Muro) ReviewerDocument10 pagesSubject Date Professor Sbobinatore: (Armando Muro) ReviewerÁngel Parra CominoNo ratings yet
- TeratomasDocument23 pagesTeratomasPinondang Gabriella TobingNo ratings yet
- Abstract IndraDocument14 pagesAbstract IndrarifkiNo ratings yet
- Benign Cystic TeratomaDocument3 pagesBenign Cystic TeratomaLisa Dwipurnamasari TobingNo ratings yet
- Testicular Cancer Nursing ManagementDocument33 pagesTesticular Cancer Nursing ManagementCheeBrendaNo ratings yet
- Classification of Ovarian Tumors: HumanDocument11 pagesClassification of Ovarian Tumors: Humancindy315No ratings yet
- 2.female Genital System IIDocument70 pages2.female Genital System IIأسود / BlackNo ratings yet
- Dermoid Cyst: Jump To Navigation Jump To SearchDocument4 pagesDermoid Cyst: Jump To Navigation Jump To SearchfgfxgxNo ratings yet
- Teratoma - Libre PathologyDocument8 pagesTeratoma - Libre PathologyJudrelle Krizia MarianoNo ratings yet
- Teratomas: Germ Cell Tumors With Elements From All Three Embryonic LayersDocument1 pageTeratomas: Germ Cell Tumors With Elements From All Three Embryonic LayersrifalNo ratings yet
- Male Genital SystemDocument80 pagesMale Genital SystemNipun ShamikaNo ratings yet
- Tumor Types GuideDocument9 pagesTumor Types GuideNicole EncinaresNo ratings yet
- What Is Gestational Trophoblastic Disease?Document42 pagesWhat Is Gestational Trophoblastic Disease?Muhammed BarznjiNo ratings yet
- Lec.12 NEOPLASIA(IDocument8 pagesLec.12 NEOPLASIA(Izeena abdulhuseinNo ratings yet
- Female Genital System PathologyDocument6 pagesFemale Genital System PathologyHelenCandyNo ratings yet
- Neoplasia IDocument37 pagesNeoplasia ISam JamesNo ratings yet
- Neoplasia 1Document14 pagesNeoplasia 1Omar MaanNo ratings yet
- Testis and EpididymisDocument15 pagesTestis and EpididymisJake MillerNo ratings yet
- Jurnal 1 MediastinumDocument7 pagesJurnal 1 MediastinumSumanjaya PratamaNo ratings yet
- Teratoma: Neuroepithelial Rosettes and Tubules, Cellular Foci ofDocument2 pagesTeratoma: Neuroepithelial Rosettes and Tubules, Cellular Foci ofSalon LamichhaneNo ratings yet
- The Genodermatoses - An Overview - UpToDateDocument89 pagesThe Genodermatoses - An Overview - UpToDatedrajaninehorsthNo ratings yet
- Keanekaragaman Teratoma OvariumDocument1 pageKeanekaragaman Teratoma OvariumrifalNo ratings yet
- Name: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of Submission: TopicDocument8 pagesName: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of Submission: TopicAnoosha FarooquiNo ratings yet
- Types of Germ Cell TumorsDocument3 pagesTypes of Germ Cell TumorsHannah aswiniNo ratings yet
- 4.nomenclature of NeoplasmDocument32 pages4.nomenclature of NeoplasmDipo Mas SuyudiNo ratings yet
- NATIONAL CANCER CONTROL PROGRAMM 2 (Prakash)Document19 pagesNATIONAL CANCER CONTROL PROGRAMM 2 (Prakash)angayarkanni100% (1)
- Cancers: Therapeutic Challenges For Cisplatin-Resistant Ovarian Germ Cell TumorsDocument14 pagesCancers: Therapeutic Challenges For Cisplatin-Resistant Ovarian Germ Cell Tumorsatikha apriliaNo ratings yet
- Union Christian College School of Health and Sciences City of San Fernando La UnionDocument11 pagesUnion Christian College School of Health and Sciences City of San Fernando La UnionJobelle AcenaNo ratings yet
- Name: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of Submission: TopicDocument8 pagesName: Class: Department: Roll No.: Subject: Course Code: Cr. HR.: Submitted To: Date of Submission: TopicAnoosha FarooquiNo ratings yet
- Ovarian Teratomas: Characteristics, Complications and ImagingDocument4 pagesOvarian Teratomas: Characteristics, Complications and ImagingHr. EmhyNo ratings yet
- TeratomaDocument1 pageTeratomaKarisma NandaNo ratings yet
- Male Genital System - Pathology (Lect 10-12)Document83 pagesMale Genital System - Pathology (Lect 10-12)Farahh ArshadNo ratings yet
- La Virgen Del PilarDocument11 pagesLa Virgen Del PilarNatyllaNo ratings yet
- 2019.-Human Germ Cell Tumours From A Developmental PerspectiveDocument16 pages2019.-Human Germ Cell Tumours From A Developmental PerspectiveAbraham Escobedo MorenoNo ratings yet
- Female Genital SystemDocument35 pagesFemale Genital SystemBio CheNo ratings yet
- Pediatric Peritoneal Malignancies: A Pictorial ReviewDocument7 pagesPediatric Peritoneal Malignancies: A Pictorial ReviewSuhartiniNo ratings yet
- Pineal Region Tumors PDFDocument15 pagesPineal Region Tumors PDFhimadriNo ratings yet
- Breast Cancer (Malignant Breast Neoplasm) Is Cancers Originating From Breast Tissue, MostDocument8 pagesBreast Cancer (Malignant Breast Neoplasm) Is Cancers Originating From Breast Tissue, MostmwahllyNo ratings yet
- Benign Tumours Precancerous Conditions Malignant Tumours How Tumours and Cancers Are NamedDocument11 pagesBenign Tumours Precancerous Conditions Malignant Tumours How Tumours and Cancers Are Namedk_472894540No ratings yet
- G Path-NeoplasiaDocument60 pagesG Path-Neoplasiachouchou124No ratings yet
- Giant Ovarian Dermoid Cyst - Case ReportDocument5 pagesGiant Ovarian Dermoid Cyst - Case ReportIJAR JOURNALNo ratings yet
- How Cancer Spreads MetastasisDocument6 pagesHow Cancer Spreads MetastasisAfia TawiahNo ratings yet
- Literature ReviewDocument4 pagesLiterature Reviewapi-549221645No ratings yet
- MCQs Mock Exams for General Surgery Board ExamDocument5 pagesMCQs Mock Exams for General Surgery Board ExamMoiz AhmedNo ratings yet
- Cannabinoids in The Landscape of CancerDocument28 pagesCannabinoids in The Landscape of CancerMichael UNo ratings yet
- MEM Devices For Drug DeliveryDocument52 pagesMEM Devices For Drug DeliveryTechnautsNo ratings yet
- Chakra Balancing Meditation Program by Ascension BeatsDocument2 pagesChakra Balancing Meditation Program by Ascension BeatsDeepak SatsangiNo ratings yet
- Lesson 4 Circulatory System 2Document2 pagesLesson 4 Circulatory System 2Glaisa CudiaNo ratings yet
- Surgical Pathology - Major and Minor Salivary GlandsDocument2 pagesSurgical Pathology - Major and Minor Salivary GlandsIsabel CastilloNo ratings yet
- Krusen II PDFDocument14 pagesKrusen II PDFMariela MolinaNo ratings yet
- Endocrine System: MSN 4006 Advanced PsychopathophysiologyDocument15 pagesEndocrine System: MSN 4006 Advanced Psychopathophysiologybane1925No ratings yet
- 35903-IUSP146 Sample Questions v1Document3 pages35903-IUSP146 Sample Questions v1Winnie HealyNo ratings yet
- Human Physiology DIGESTION & ABSORPTIONDocument12 pagesHuman Physiology DIGESTION & ABSORPTIONM JeevanNo ratings yet
- Respiratory System: Assessment: ObjectivesDocument19 pagesRespiratory System: Assessment: ObjectivesNURSES- NOOK & CORNERNo ratings yet
- Understanding The Symptoms of The Common Cold andDocument9 pagesUnderstanding The Symptoms of The Common Cold andShivam GoswamiNo ratings yet
- Thyroid Ultrasound Risk Factors and FindingsDocument1 pageThyroid Ultrasound Risk Factors and Findingspriya67% (3)
- St. Joseph College Exam ReviewDocument8 pagesSt. Joseph College Exam ReviewALVEN OYANGORINNo ratings yet
- The Anatomy and Physiology of the Eye and EarDocument6 pagesThe Anatomy and Physiology of the Eye and EarLara ShayaNo ratings yet
- Module NCM 107 Pre LimDocument35 pagesModule NCM 107 Pre Limjanina mykaNo ratings yet
- Neha ShahDocument448 pagesNeha ShahAshima GautamNo ratings yet
- C3a and HI-neurodegenerationDocument32 pagesC3a and HI-neurodegenerationJingyun WuNo ratings yet
- Pancreas Neuroendocrine Tumors: An IntroductionDocument7 pagesPancreas Neuroendocrine Tumors: An IntroductionNoviari Liara JustitiaNo ratings yet
- 2018 Oligemia, Penumbra, Infarction. Understanding Hypoperfusion With NeuroimagingDocument11 pages2018 Oligemia, Penumbra, Infarction. Understanding Hypoperfusion With NeuroimagingTote Cifuentes AmigoNo ratings yet
- Pnle Exam Part 1Document14 pagesPnle Exam Part 1bNo ratings yet
- Chiari MalformationDocument22 pagesChiari Malformationapi-28517738067% (3)
- 1s3l9rkh0 The Endocrine System ScienceDocument34 pages1s3l9rkh0 The Endocrine System SciencePrecious RoxasNo ratings yet