Professional Documents
Culture Documents
Al Kanes
Uploaded by
kjjkimkmk0 ratings0% found this document useful (0 votes)
42 views4 pageswfs
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentwfs
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
42 views4 pagesAl Kanes
Uploaded by
kjjkimkmkwfs
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 4
Save a copy of this worksheet View Full Screen
into your own folder (View menu / Full Screen)
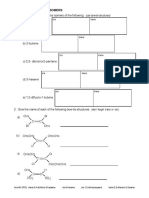
Hydrocarbons: Alkanes
The following questions relate
to alkanes A, B and C which A
are structural isomers of
decane, C10H22
Put these three structural isomers of decane in order of increasing boiling point:
select
Select an explanation for this order of volatility:
select
Select an explanation for the relative unreactivity of alkanes like decane:
select
Despite its relative unreactivity decane will burm in air if ignited.
Balance the reaction equation for its complete combustion:
C10H22 + select O2 select CO2 + select H2O
Despite its relative unreactivity decane will react with Cl 2 in the presence of ultra
violet (uv) radiation.
Select the type of bond breaking happening here: select
Select the name of each of the steps in the mechanism of this reaction below:
. .
C10H22 + Cl C10H21 + HCl select
. .
Cl2 Cl + Cl select
. .
C10H21 + Cl C10H21Cl select
. .
C10H21 + Cl2 C10H21Cl + Cl select
Write an overall equation (ie not a mechanism) for the substitution reaction
between decane and excess bromine ie of a single bromine atom into decane.
Dont worry about using subscripts in the formulae eg bromine is Br2:
Select Close Full Screen
and print out this page
Answers not checked so not ready to print
Type your name here
ANSWERS
Hydrocarbons: Alkanes
The following questions relate
to alkanes A, B and C which A
are structural isomers of
decane, C10H22
C
Put these three structural isomers of decane in order of increasing boiling point:
C <A< B
Select an explanation for this order of volatility:
increased branching decreases Van der Waals' force
Select an explanation for the relative unreactivity of alkanes like decane:
C-H bonds are non-polar and so relatively inert
Despite its relative unreactivity decane will burm in air if ignited.
Balance the reaction equation for its complete combustion:
C10H22 + 15.5 O2 10 CO2 + 11 H2O
Despite its relative unreactivity decane will react with Cl 2 in the presence of ultra
violet (uv) radiation.
Select the type of bond breaking happening here: homolytic fission
Select the name of each of the steps in the mechanism of this reaction below:
C10H22 + .Cl .C10H21 + HCl propagation
Cl2 .Cl + .Cl initiation
.
C10H21 + .Cl C10H21Cl termination
.
C10H21 + Cl2 C10H21Cl + .Cl propagation
Write an overall equation (ie not a mechanism) for the substitution reaction
between decane and excess bromine ie of a single bromine atom into decane.
Dont worry about using subscripts in the formulae eg bromine is Br2:
C10H22 + Br2 C10H21Br + HBr
ie C10H22 + Br2 C10H21Br + HBr
You might also like
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- 16 Organ PDFDocument3 pages16 Organ PDFAya ZhNo ratings yet
- Multiple Option Correct Type (+4/-1)Document5 pagesMultiple Option Correct Type (+4/-1)Vinod AgrawalNo ratings yet
- Reactions of Alkanes: Radicals: Essential Organic Chemistry (Bruice)Document43 pagesReactions of Alkanes: Radicals: Essential Organic Chemistry (Bruice)tyron9520100% (2)
- C10 Organic ChemistryDocument36 pagesC10 Organic ChemistryAlice NgaNo ratings yet
- Complete HydrocarbonDocument238 pagesComplete HydrocarbonGully GamingNo ratings yet
- Ammonia Reacts With An Aldehyde To Give AnDocument17 pagesAmmonia Reacts With An Aldehyde To Give AnOyinkansola JoyceNo ratings yet
- Chemistry Form 6 Organic Chemistry: Chapter 2: HydrocarbonDocument51 pagesChemistry Form 6 Organic Chemistry: Chapter 2: HydrocarbonNurul FarhanaNo ratings yet
- Chemistry Form 6 Sem 3 Chapter 2Document52 pagesChemistry Form 6 Sem 3 Chapter 2Yuzamrah Awang NohNo ratings yet
- Halogenoalkanes AnswersDocument64 pagesHalogenoalkanes AnswersSpider Gamer22No ratings yet
- 187 Avoidable Errors in Reaction Mechanism QuestionsDocument4 pages187 Avoidable Errors in Reaction Mechanism QuestionsM DiNo ratings yet
- Worksheet-02-Chem (2021) STEP PDFDocument11 pagesWorksheet-02-Chem (2021) STEP PDFHallo KhanNo ratings yet
- Alkene and AlkynesDocument83 pagesAlkene and AlkynesAira Villarin100% (2)
- Bailey Alkanes PowerpointDocument31 pagesBailey Alkanes PowerpointBritney PattersonNo ratings yet
- Hydro CarbonsDocument10 pagesHydro CarbonsLok Jun Hao100% (1)
- Haloalkanes & Haloarenes (QP)Document2 pagesHaloalkanes & Haloarenes (QP)riorocksNo ratings yet
- Alkenes 2Document45 pagesAlkenes 2cikgu_amin100% (1)
- Hydrocarbon NotesDocument187 pagesHydrocarbon Notessamay gujratiNo ratings yet
- Coursebook Answers: Self-Assessment QuestionsDocument2 pagesCoursebook Answers: Self-Assessment QuestionslizNo ratings yet
- Exercise AlkaneDocument17 pagesExercise Alkanerudi_zNo ratings yet
- Bailey Alkanes PowerpointDocument31 pagesBailey Alkanes PowerpointBritney PattersonNo ratings yet
- 13 HydrocarbonDocument114 pages13 HydrocarbonCharviNo ratings yet
- PRACTICE MCQ HYDROCARBONS - 11ScADocument7 pagesPRACTICE MCQ HYDROCARBONS - 11ScAArda RahmainiNo ratings yet
- New H Chem SQ App Unused 2020Document49 pagesNew H Chem SQ App Unused 2020Saksham ChaudharyNo ratings yet
- Chemistry 123S Oregon State University Worksheet 9 Notes Dr. Richard NafshunDocument11 pagesChemistry 123S Oregon State University Worksheet 9 Notes Dr. Richard NafshunuwuNo ratings yet
- Mass Spectra - Unknown 6Document3 pagesMass Spectra - Unknown 6ScribdTessaNo ratings yet
- Hydrocarbons NotesDocument13 pagesHydrocarbons NotesShivansh Pundir100% (1)
- Topic 2Document32 pagesTopic 2KAI YANG LIMNo ratings yet
- Elimination Rxn'sDocument72 pagesElimination Rxn'sblackz0idNo ratings yet
- Cell Exam QuestionsDocument52 pagesCell Exam QuestionsNabindra RuwaliNo ratings yet
- Complete Organic Chemistry (Brahmastra) Part 2Document763 pagesComplete Organic Chemistry (Brahmastra) Part 2mohdamaankhan74No ratings yet
- Organic Chemistry - Reactions and MechanismsDocument120 pagesOrganic Chemistry - Reactions and MechanismsLoveena Steadman100% (8)
- 2008 Promo 1Document15 pages2008 Promo 1shinkir0No ratings yet
- Chemistry - Sample Question Paper - 9Document6 pagesChemistry - Sample Question Paper - 9Mohd AdilNo ratings yet
- Exercise: AlkaneDocument17 pagesExercise: AlkaneHenerita RayNo ratings yet
- Hydrocarbon Notes - Till AlkaneDocument8 pagesHydrocarbon Notes - Till AlkaneHamad FarooqueNo ratings yet
- Reaction KineticsDocument12 pagesReaction KineticsTheresaHangDuongNo ratings yet
- Answers To Saqs: Cambridge International As Level ChemistryDocument1 pageAnswers To Saqs: Cambridge International As Level ChemistryAlaNo ratings yet
- Hydrocarbon MAIN NotesDocument25 pagesHydrocarbon MAIN NotesHamad FarooqueNo ratings yet
- Class 11 Chemistry Revision Notes HydrocarbonsDocument18 pagesClass 11 Chemistry Revision Notes HydrocarbonsSURESH SURESHNo ratings yet
- A2AS CHEM REVISED Support 20632Document4 pagesA2AS CHEM REVISED Support 20632Cosmescu Mario FlorinNo ratings yet
- OC04 Arenes Exercise AnswersDocument18 pagesOC04 Arenes Exercise Answersjavierheng314No ratings yet
- Matriculation Chemistry (Hydrocarbon) Part 2 AlkaneDocument30 pagesMatriculation Chemistry (Hydrocarbon) Part 2 AlkaneridwanNo ratings yet
- Free Radicals 12 QuesDocument62 pagesFree Radicals 12 Quesdinesh111180No ratings yet
- Hydrocarbon 4Document35 pagesHydrocarbon 4AjayNo ratings yet
- 8 Alkene & AlkyneDocument74 pages8 Alkene & Alkynerusnah chungNo ratings yet
- Chapter 3Document61 pagesChapter 3Avy VyNo ratings yet
- PROJECT1Document77 pagesPROJECT1Jasper AlonNo ratings yet
- 11 Hydrocarbon Study NotesDocument23 pages11 Hydrocarbon Study NotesVivek KumarNo ratings yet
- Acfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswDocument8 pagesAcfrogbyyb W54zpzfswkn8k3vq0clq6et8mk Ne Px62hvrlk5chrlql9xx83xtq2sr0dqcuhrswcoglr Ueky068cras4ph7jxkmy 143kq0wnhekbynbh 4 Eq1p0kvslajoriecir6ikqqswThanh Hằng NgôNo ratings yet
- CHM207 TutorialDocument3 pagesCHM207 Tutorialit's miaNo ratings yet
- Chapter 5Document15 pagesChapter 5Nahom AmanuelNo ratings yet
- Hydrocarbon: The AlkanesDocument4 pagesHydrocarbon: The AlkanesPirate HunterNo ratings yet
- Organic Chemistry: CollegeDocument34 pagesOrganic Chemistry: CollegeArwa AhmedNo ratings yet
- 1 QP SolutionDocument6 pages1 QP SolutionsachinNo ratings yet
- Alkyne Theory Eng. Module-4Document17 pagesAlkyne Theory Eng. Module-4Raju SinghNo ratings yet
- Alkylhalides Arylhalides Aromatic Compounds 1Document125 pagesAlkylhalides Arylhalides Aromatic Compounds 1Yeri KhaNo ratings yet
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionFrom EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Set 2Document2 pagesSet 2kjjkimkmkNo ratings yet
- Kuliah K2S3Document4 pagesKuliah K2S3kjjkimkmkNo ratings yet
- Kolej Matrikulasi KedahDocument2 pagesKolej Matrikulasi KedahkjjkimkmkNo ratings yet
- Carbocation KeyDocument1 pageCarbocation KeykjjkimkmkNo ratings yet
- Isi KandunganDocument1 pageIsi KandungankjjkimkmkNo ratings yet
- 24 - Organic Chemistry: Practice TestDocument2 pages24 - Organic Chemistry: Practice TestkjjkimkmkNo ratings yet
- Amalkebajikan Quiz Alkene OkDocument3 pagesAmalkebajikan Quiz Alkene OkkjjkimkmkNo ratings yet
- Carbo CationDocument1 pageCarbo CationkjjkimkmkNo ratings yet
- Exercise AlkaneDocument2 pagesExercise AlkanekjjkimkmkNo ratings yet
- Chapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurryDocument20 pagesChapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurrykjjkimkmkNo ratings yet
- Identifying Types of Reactions - KeyDocument3 pagesIdentifying Types of Reactions - KeykjjkimkmkNo ratings yet
- Ws 10.8 Geometric Isomers: (Use Bow-Tie Structures)Document1 pageWs 10.8 Geometric Isomers: (Use Bow-Tie Structures)kjjkimkmkNo ratings yet
- Calorimetry WorksheetDocument1 pageCalorimetry WorksheetkjjkimkmkNo ratings yet
- Alkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkanesDocument1 pageAlkane'S: - Alkanes Based On Molecular Weight - Isomeric Alkanes - Alkanes and CycloalkaneskjjkimkmkNo ratings yet
- Alkene Reactions:: Doug Young CHE 118BDocument2 pagesAlkene Reactions:: Doug Young CHE 118BkjjkimkmkNo ratings yet
- Alkenes (Fasi)Document3 pagesAlkenes (Fasi)kjjkimkmkNo ratings yet
- Self-Study Worksheet III Isomerism ANSWERSDocument2 pagesSelf-Study Worksheet III Isomerism ANSWERSkjjkimkmkNo ratings yet
- Hydrocarbons: at The End of The Lesson Students Should Be Able ToDocument45 pagesHydrocarbons: at The End of The Lesson Students Should Be Able TokjjkimkmkNo ratings yet
- Studyon Alkane NameDocument3 pagesStudyon Alkane NamekjjkimkmkNo ratings yet
- 1hr AlkaneskmppDocument12 pages1hr AlkaneskmppkjjkimkmkNo ratings yet
- Sk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkaneDocument7 pagesSk027 / Chapter 5: Hydrocarbon / Amalkebajikan01 / Nomenclature AlkanekjjkimkmkNo ratings yet
- 3rd AlkanekmppDocument18 pages3rd AlkanekmppkjjkimkmkNo ratings yet
- Enthalpy StoichiometryDocument1 pageEnthalpy StoichiometrykjjkimkmkNo ratings yet
- Answer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1Document5 pagesAnswer All Question: Sk027 / Chapter 3: Reaction Kinetics / Exercise 1kjjkimkmkNo ratings yet
- Reactants Products Reactants Products 2 3 (S) (S) 2 (G)Document2 pagesReactants Products Reactants Products 2 3 (S) (S) 2 (G)kjjkimkmkNo ratings yet
- Amalkebajikan - 1 TermoDocument17 pagesAmalkebajikan - 1 TermokjjkimkmkNo ratings yet
- Amal 6 Born Haber - EditDocument4 pagesAmal 6 Born Haber - EditkjjkimkmkNo ratings yet
- Topic 8: Thermochemistry Lesson 8.5: Born-Haber Cycle: Teacher'S NotesDocument2 pagesTopic 8: Thermochemistry Lesson 8.5: Born-Haber Cycle: Teacher'S NoteskjjkimkmkNo ratings yet
- Retrofit of Water Mist Catcher: WMC For L23/30 (H) and L28/32 (H) GensetsDocument2 pagesRetrofit of Water Mist Catcher: WMC For L23/30 (H) and L28/32 (H) GensetsMichael GorobaoNo ratings yet
- FM-200 Alpha Series SystemsDocument6 pagesFM-200 Alpha Series SystemsGurusumiNo ratings yet
- Hela Bojuna - Comparative StatementDocument27 pagesHela Bojuna - Comparative StatementNadeeshani MunasingheNo ratings yet
- Opus UserguideDocument313 pagesOpus UserguideMoez EssafiNo ratings yet
- A C +Haier+12000+BTUDocument51 pagesA C +Haier+12000+BTUfox7878No ratings yet
- While Start Drive Test Learning, You Must Know The Basic Things! These All Conotents Are at Introductory LevelDocument15 pagesWhile Start Drive Test Learning, You Must Know The Basic Things! These All Conotents Are at Introductory LevelRakesh SolankiNo ratings yet
- X2 / 275 Vac: B 81 191 EMI Suppression CapacitorsDocument4 pagesX2 / 275 Vac: B 81 191 EMI Suppression CapacitorsMeg YorkNo ratings yet
- Data SheetDocument2 pagesData SheetAsalamEilujNo ratings yet
- Software Hardware ListDocument2 pagesSoftware Hardware ListjackNo ratings yet
- Java SampleExamQuestionsDocument18 pagesJava SampleExamQuestionshmasryNo ratings yet
- One Pipe Steam DesignDocument44 pagesOne Pipe Steam Designreyes hernandezNo ratings yet
- L-1363J - Corrected 20210420Document77 pagesL-1363J - Corrected 20210420Juan TapiaNo ratings yet
- Sop For LP Pump (R1)Document6 pagesSop For LP Pump (R1)SonratNo ratings yet
- T.C. Electronic M3000 User ManualDocument78 pagesT.C. Electronic M3000 User ManualStanleyNo ratings yet
- The Family Handyman - October 2020 PDFDocument86 pagesThe Family Handyman - October 2020 PDFFabian MaunaNo ratings yet
- 2014 Summer Model Answer PaperDocument20 pages2014 Summer Model Answer Papercivil gpkpNo ratings yet
- Alliance Technical Catalog Sept 2019 - LR Final - 1571832848Document208 pagesAlliance Technical Catalog Sept 2019 - LR Final - 1571832848Александр ФедоровNo ratings yet
- FD100 CatalogoDocument4 pagesFD100 CatalogoKaren VásconezNo ratings yet
- Computer Organization: - by Rama Krishna Thelagathoti (M.Tech CSE From IIT Madras)Document118 pagesComputer Organization: - by Rama Krishna Thelagathoti (M.Tech CSE From IIT Madras)iamy2ramsNo ratings yet
- Electrochemical Measurement of Diffusible Hydrogen in Steels (Barnacle Electrode)Document6 pagesElectrochemical Measurement of Diffusible Hydrogen in Steels (Barnacle Electrode)Faiber AndrésNo ratings yet
- Basic Electronics - AC - DC PDFDocument20 pagesBasic Electronics - AC - DC PDFRowena ResurreccionNo ratings yet
- Home Automation Control System Using DTMFDocument25 pagesHome Automation Control System Using DTMFengaydiNo ratings yet
- No35-Inclined Roof SystemDocument24 pagesNo35-Inclined Roof SystemKitanovic NenadNo ratings yet
- Precision r5500 Service Manual en UsDocument104 pagesPrecision r5500 Service Manual en UsJonDyson32No ratings yet
- Master Thesis: Tasuku KanedaDocument77 pagesMaster Thesis: Tasuku Kanedamkali345No ratings yet
- Gulfco Fleet Premium: Heavy Duty Gas Engine Oil For Mobile ApplicationDocument1 pageGulfco Fleet Premium: Heavy Duty Gas Engine Oil For Mobile ApplicationAlisson Marcela ContrerasNo ratings yet
- Karcher K - 791 - MDocument12 pagesKarcher K - 791 - MJoão Paulo FernandesNo ratings yet
- Textile AssignmentDocument8 pagesTextile AssignmentMahmudul Hasan Khan40% (5)
- WinDNC - V05 - 02 English PDFDocument2 pagesWinDNC - V05 - 02 English PDFAnonymous XXKCjKnc0No ratings yet
- Bosch Powerpack-BrochureDocument16 pagesBosch Powerpack-BrochurengazawooNo ratings yet