Professional Documents
Culture Documents
Shafarul - Synthesis of Activated Carbon PDF
Uploaded by
wargajaya79Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Shafarul - Synthesis of Activated Carbon PDF
Uploaded by
wargajaya79Copyright:
Available Formats
SYNTHESIS OF ACTIVATED CARBON FROM WASTE RAW MATERIAL
USING BULUH LEMANG, SCHIZOSTSCHYUM BRACHYCLADUM.
SHAFARUL BIN MUSTAFA
UNIVERSITI TEKNIKAL MALAYSIA MELAKA
SUPERVISOR DECLARATION
I hereby declare that I have read this thesis and in my opinion this report is
sufficient in terms of scope and quality for the award of the degree of
Bachelor of Mechanical Engineering (Structure and Materials)
Signature
: ...................................
Supervisor I
: En. Imran Syakir bin Mohamad
Date
: ...................................
Signature
: ...................................
Supervisor II : En. Hairul bin Bakri
Date
: ...................................
ii
SYNTHESIS OF ACTIVATED CARBON
FROM WASTE RAW MATERIAL USING BULUH LEMANG,
SCHIZOSTSCHYUM BRACHYCLADUM.
SHAFARUL BIN MUSTAFA
This Report Is Submitted In Partial Fulfillment of Requirements For The
Bachelor of Mechanical Engineering (Structure and Materials) with Honours.
Faculty of Mechanical Engineering
Universiti Teknikal Malaysia Melaka
MAY 2011
iii
DECLARATION
I hereby declare that the work in this report is my own except for summaries and
quotations which have been duly acknowledged.
Signature
Name of Candidate
: SHAFARUL BIN MUSTAFA
Date
iv
For my beloved mother,
dearest family members and next of kin,
lecturers and friends.
ACKNOWLEGMENT
AssalamualaikumWarrahmatullah .
First and foremost, I am grateful to Allah SWT for the strength and good
health that allow me to complete this final year project. My special gratitude goes to
my supervisor Mr. Imran Syakir Bin Mohamad, lecturer Thermal Fluid Department
of the University of Technical Malaysia Melaka (UTeM) for his encouragement and
guidance throughout this project. Thanks also to Miss Sian Meng, the research
assistance of the Faculty of Manufacturing Engineering (UTeM), Mr. Rostam bin
Omar, the science officer at COMBICAT, University Malaya (UM) for their
continuous help during this project and Dr . Kalthom binti Husain as a proof reader
for final year project report from Human Development and
Language Center,
UTeM. Last but not least, my acknowledgement to my fellow friends, family and the
university staff for their support and encouragement during the whole course of this
final year project.
vi
ABSTRACT
The main objective of this work was to synthesis waste of bamboo as the
activated carbon and characterized the activated carbon. The unique of activated
carbon commonly known by the principle of adsorptivity which
be applied in
removing contaminants and odor in the water and air. This has been adopted in many
industrial field associated with waste treatment to reduce pollution. The potential of
activated carbon has been acknowledge in many publications to efficiently provide
adsorption site for this purpose. Hence, in this particular study, the application of
activated carbon manufactured from the waste of bamboo (Schizostachyum
brachycladum) or better known as buluh lemang amongs the Malay community was
investigated. The effect on process variables such as activating agents zinc chloride
(ZnCl2) activation temperature, impregnation ratio and carbonization temperature
were studied to optimize these parameters. The resulting activated carbon were
characterized in terms of iodine number (I2 number) for its adsorptive capacity ,
Scanning Electron Microscope (SEM) and Brunauer, Emmett, Teller (BET) method
were used to provide
information on surface area, pore size, types of pore
(micropores, mesopores, macropores) and total pore volumes. Result iodine number
shows,
sample
activated
carbon,
chemical
activation(temperature
500C,
impregnation ratio 1:1) has higher value which is 295 mg/g among others samples. In
BET analysis, sample activated carbon, physical activation (500C) shows good
surface area, 339.1 m2/g and total volume pore, 1.042 cc/g. Lastly, for SEM-EDX
data analysis, sample activated carbon, physical activation (600C) has higher carbon
content, 90.99% (atomic percentage).
vii
ABSTRAK
Objektif utama projek ini adalah untuk mensintesis sisa buangan daripada
buluh lemang untuk menjadi karbon teraktif dan seterusnya menganalisa sifat-sifat
karbon teraktif tersebut. Keunikan karbon teraktif umumnya diketahui berdasarkan
prinsip jerapan yang dapat diaplikasikan bagi menghilangkan kotoran dan bau dalam
air dan udara. Karbon teraktif telah diterima pakai di dalam pelbagai bidang industri
yang berkaitan dengan pengurusan sisa bagi mengurangkan pencemaran. Potensi
karbon teraktif telah diakui melalui pelbagai penerbitan sebelum ini dalam
menyediakan kajian jerapan secara cekap. Oleh itu, dalam kajian yang dijalankan,
aplikasi karbon teraktif dihasilkan dari sisa buangan daripada buluh (Brachycladum
Schizostachyum) atau lebih dikenali sebagai 'buluh lemang' dalam komuniti Melayu.
Selain itu, kajian ini mengkaji karbon teraktif
apabila
pembolehubah
yang
digunakan di dalam pembuatan karbon teraktif seperti agen pengaktifan yang
digunakan, zink klorida (ZnCl2), suhu pengaktifan, nisbah impregnasi dan suhu
karbonisasi bagi mengoptimumkan penghasilan karbon teraktif tersebut. Karbon
teraktif yang dihasilkan dikenalpastikan sifatnya dengan menggunakan analisis
nombor iodin (nombor I2) untuk memperoleh daya serap, selain itu, Mikroskop
Imbasan Elektron (SEM) dan kaedah Brunauer, Emmett, Teller (BET) digunakan
untuk memperolehi maklumat mengenai luas permukaan, saiz liang, jenis liang
(liang mikro, liang meso, liang makro) dan jumlah liang yang terdapat pada karbon
teraktif yang dihasilkan. Keputusan nombor iodine menunjukkan sampel karbon
teraktif daripada pengaktifan kimia (suhu 500C , nisbah impregnasi 1:1) mempunyai
nilai yang paling tiggi daripada sampel karbon teraktif yang lain dengan nilai 295
mg/g. Dalam analisis BET, sampel pengaktifan fizikal (500C) mempunyai keluasan
permukaan yang baik 339.1 m2/g dan jumlah isipadu liang, 1.042 cc/g. Akhir sekali,
untuk data analisis SEM-EDX , sampel karbon teraktif daripda pengaktifan fizikal
(600C) mengandungi kandungan karbon yang tinggi 90.99%.
viii
TABLE OF CONTENTS
CHAPTER
CHAPTER I
CHAPTER II
TITLE
PAGE
SUPERVISOR DECLARATION
DECLARATION
iii
DEDICATION
iv
ACKNOWLEDGEMENT
ABSTRACT
vi
ABSTRAK
vii
CONTENTS
viii
LIST OF FIGURES
LIST OF TABLES
xi
LIST OF SYMBOLS
xii
LIST OF ABBREVIATION
xiii
INTRODUCTION
1.0
Introduction
1.1
Objective
1.2
Problem Statement
1.3
Scope
1.4
Expected Result
LITERATURE REVIEW
2.1
History of activated carbon
2.2
Activated Carbon
2.2.1 Properties of Activated carbon
2.2.2
Structure of Activated carbon
2.2.3 Activated carbon adsorption.
2.2.4 Type and selection of activated carbon
ix
2.3
2.4
2.5
2.6
2.7
Activated Carbon Manufacture.
11
2.3.1
Activation Method
12
2.3.2
Activating Agents
13
2.3.3
The methods of activating agents mixing
13
Raw material for activated carbon
14
2.4.1
16
The selection of raw material.
Bamboo Waste
2.5.1
Bamboo.
17
2.5.2
General Applications
19
2.5.3 Chemical Composition
20
Standard for activated carbon
22
2.6.1
22
Characteristics of activated carbon
Characterization Method.
2.7.1 Iodine number analysis
24
2.7.2
32
BrunauerEmmettTeller (BET)
2.7.3 Scanning Electron Microscopy (SEM)
CHAPTER III
24
33
METHODOLOGY
3.1
Introduction
36
3.2
Experimental Procedure
38
3.2.1
Procedure of chemical activation.
38
3.2.2
Procedure of physical activation.
39
3.2.3
Procedure Iodine number analysis
39
3.2.4
Procedure Nitrogen adsorption analysis
40
3.2.5
Procedure EDX-SEM analysis
40
CHAPTER IV
RESULT AND ANALYSIS
41
CHAPTER V
DISCUSSION
59
CHAPTER VI
CONCLUSION
61
REFERENCES
62
APPENDICES
68
LIST OF FIGURES
FIGURE
TITLE
PAGE
Figure 2.1
Application of activated carbon.
Figure 2.2
Carbon Black Quasi-Graphitic Microstructure
Compared to the two regular crystalline
forms of Carbon (Diamond And Graphite).
Figure 2.3
Activated carbon adsorption for gases and chemicals.
Figure 2.4
Types of activated carbon.
10
Figure 2.5
Bamboo forestry
19
Figure 2.6
Bamboo (Schizostachyum brachycladum) and lemang
20
Figure 2.7
One of Brunauer, Emmet And Teller (BET) Machine.
33
Figure 2.8
The basic premise of an SEM is that signal produced
35
from a scanned area of the specimen is displayed as
an image with the exact same scan pattern on a CRT.
Figure 2.9
SEM Photograph of Commercial Activated Carbon (AC). 35
Figure 3.1
Flow chart of project.
37
xi
LIST OF TABLES
TABLE
TITLE
PAGE
Table 2.1
Commercially available carbons.
10
Table 2.2
Characteristic of commercial raw
16
material as activated carbon .
Table 4.1
Sample activated carbon with chemical activation,
42
(500C, impregnation ratio, 1:1)
Table 4.2
Sample activated carbon with chemical activation,
42
(500C, impregnation ratio, 1:2)
Table 4.3
Sample activated carbon with chemical activation,
42
(600C, impregnation ratio, 1:1)
Table 4.4
Sample activated carbon with chemical activation,
43
(600C, impregnation ratio, 1:2)
Table 4.5
Sample activated carbon with physical activation,
43
(Temperatures, 500C)
Table 4.6
Sample activated carbon with physical activation,
43
(Temperatures, 600C)
Table 4.7
Sample activated carbon, commercial
44
Table 4.8
Result Iodine number analysis
53
Table 4.9
BET Analysis Data
54
Table 4.10
SEM- EDX (percentage of atomic element) (%)
55
Table 4.11
Image AC (SEM)
56
xii
LIST OF SYMBOLS
percent
gram
ml
milliliter
cm
centimeter
mm
milimeter
number of adsorbed molecular layers
dependence of the amount of adsorption
adsorption potential
Kelvin
degree Celsius
electron coefficient
secondary electron coefficient
H3PO4
phosphoric acid
ZnCl2
zinc chloride
I2
iodine
NaOH
sodium hydroxide
HCl
hydrochloric acid
CO2
carbon dioxide
H2SO4
sulfuric acid
KOH
potassium hydroxide
Na3PO4
sodium phosphate
NaCl
sodium chloride
KMnO4
potassium permanganate
N2
nitrogen
Na2S2O3.5H2O
sodium thiosulfate
filtrate normalities
angstrom
xiii
LIST OF ABBREVIATION
ASTM
American Society for Testing Material
AWWA
American Water Works Association.
BET
BrunauerEmmettTeller
SEM
Scanning Electron Microscope
IUPAC
International Union of Pure and Applied Chemistry.
AC
Activated Carbon
GAC
Granular Activated Carbon
PAC
Powder Activated Carbon
CRT
Cathode Ray Tube
BC
Before century
U.S
United State
CHAPTER 1
INTRODUCTION
1.0 INTRODUCTION
Increasing solid waste has become a serious issue that leads to water
pollution. The impact of solid waste was significantly severe when it comes to
festive seasons in Malaysia. Thus, one of the solutions for water or air pollution is to
use the ability of activated carbon (AC) to filter the chemical associated with the
waste. Currently, the bamboo culm, or stem, has been made into an extended
diversity of products ranging from domestic household products to industrial
applications (McClure ,1967). Examples of bamboo products are food containers,
skewers, chopsticks, handicrafts, toys, furniture, flooring, pulp and paper, boats,
charcoal, musical instruments and weapons. In Asia, bamboo is quite common for
bridges, scaffolding and housing, but it is usually a temporary exterior structural
material. In many overly populated regions of the tropics, certain bamboos supply
the one suitable material that is sufficiently cheap and plentiful to meet the extensive
need for economical housing.
Bamboo is a natural resource in Malaysia as it takes only several months to
grow and be ready for harvesting. Primary processing of bamboo for conversion into
specified products generates a large amount of residues is generated. This residue
could be effectively converted into value-added products such as activated carbon
and charcoal. Bamboo is also a good source of carbon and there is possibility for it to
be made AC for the purpose of pollution control. Waste of bamboo from lemang also
can produce the activated carbon. Thats means, from bamboo waste , we can recycle
and create other applications for industrial.
According to Gadkaree (1998), activated carbon is a very important material
industrially, with applications in a variety of areas such as adsorbers in air and water
pollution control, catalysts in the chemical and petrochemical industries. Typically
activated carbon is made from naturally occurring materials such as wood, coal and
nutshell flour etc. via high temperature, inert atmosphere processing followed by
activation to create porosity in the nanometer size range. This porosity imparts
special adsorption characteristics to carbon and makes it useful in the variety of
applications mentioned above.
1.1 OBJECTIVE
The main objective of this project is to synthesis * activated carbon (AC)
from easily available raw material (bamboo). This is done by aiming on the specific
objectives as follow:
1.
To synthesis activated carbon from waste materials.( waste of bamboo)
2.
To investigate the adsorption performance of activated carbon using iodine
number analysis
3.
To characterize the properties of activated carbon prepared using analytical
spectroscopy.( SEM-EDX, N2 adsorption analysis)
1.2 PROBLEM STATEMENT
In this project, the material for the manufacturing of activated carbon was
investigated. This was done to reduce waste especially during festive season in our
country such as Hari Raya. Additionally, to investigate the suitability of buluh
lemang (Schizostachyum brachycladum) as the
activated carbon and its
applicability in various industrial applications. This project also aims to the
investigate adsorption performance of those materials for the use in the
manufacturing activated carbon especially in waste management and pollution
control.
1.3 SCOPE
1. To find and prepared the waste of bamboo . Type of bamboo was selected is
(Schizostachyum brachycladum) , among Malay call buluh lemang. Washed
with water and dried the bamboo under normal sun light. The bamboo was
cut into part about 10cm- 15cm and crush using industry crusher (3-4 mm) to
make its easier during synthesis process.
2. This studied about activated carbon had been carried out only on laboratory
scale. It is started from synthesis waste of bamboo by activated
manufacturing system carbonization and activation. Activation that used for
synthesis raw material are Physical (Gas Carbon Reactor) and Chemical
activation , use zinc chloride as activating agents (ZnCl2 )
3. Characterize activated carbon after finished the synthesis process and find
properties of activated carbon by using a few method such as Scanning
Electron Microscopy (SEM) to find structural properties and morphology of
activated carbon . Others method used are Brunauer Emmet Teller Method
(BET), N2 adsorption and Iodine Number, I2, analysis.
1.4 EXPECTED RESULT
To be a guideline in producing activated carbon from bamboo
(Schizostachyum
brachycladum)
waste.
Low cost alternative substitute raw
material and increase efficiency of utilization, performance of activated carbon.
Value-addition of agricultural residues and on other side helps solving problem,
which otherwise disposal cause.
CHAPTER TWO
LITERATURE REVIEW
2.1 HISTORY OF ACTIVATED CARBON
Activated carbon was first generated industrially at the first part of the 20th
century, when carbon activated from vegetable material was produced for use in
sugar refining. Powered activated carbon was first produced commercially in Europe
in the early 19th century, using wood as a raw material. This carbon found
widespread use in the sugar industry. In the United States, the first production of
activated carbon used black ash as the source, after it was accidentally discovered
that the ash was very effective in decolorizing liquids. Activated carbon has since
been used extensively for this purpose in many industries. In particular, it has been
commonly used for the removal of organic dyes from textile wastewater. The first
documented use of activated carbon in a large scale water treatment application was
in 19th century England, where it was used to remove undesirable odors and tastes
from drinking waters. Use in the United States for similar purposes closely followed.
In recent years, the use of activated carbon for the removal of priority organic
pollutants has become very common. The use of carbon extends back far in time.
Charcoal was used for drinking water filtration by the ancient Hindus in India, and
carbonized wood was used as a medical adsorbent and purifying agent by the
Egyptians as early as 1500 BC.
2.2 ACTIVATED CARBON
Activated carbon is porosity (space) enclosed by carbon atoms. Activated
carbon is a microcrystalline, nongraphitic form of carbon that has been processed to
develop internal porosity. This porosity yield the surface area that provides ability to
adsorb gases and vapors from gases and to adsorb dissolved or dispersed substances
from liquid.activated carbon is characterized by a vast system of pore of molecular
size within the carbon particles resulting in the formation of a material with extensive
surface area. However, commercial grade of activated carbons generally have a
surface areas ranging from 600 to 1200 m2/g.(Chilton Ng et al.2002)
Activated carbon is a very important material industrially, with applications
in a variety of areas such as adsorbents in air and water pollution control, catalysts in
the
chemical
and
petrochemical
industries.
Electrodes
in
batteries
and
supercapacitors and also purifiers in the food and pharmaceutical industries. Further,
as an environmental pollution is increasingly becoming a serious problem the
demand for activated carbon is growing.( K.P Gadkaree.1998)
2.2.1 Properties of Activated carbon (AC):
Solid, porous, black carbonaceous material
Extraordinary large surface area (1200m2/g) and pores volume that give
unique
Complex action mode
Having both chemical and physical effect on substances
Figure 2.1: Application of activated carbon.
(Source: http://www.cataler.co.jp)
2.2.2
Structure of Activated carbon
Activated carbon has a disordered microstructure, which is termed
amorphous carbon. X-rays studied have demonstrated that many so-called
amorphous substances have crystalline characteristics, even they many not show
certain features such as crystal angles and faces, usually associated with the
crystalline state. The kind of carbon from which activated carbon is derived is nongraphitizing, meaning that it cannot be transformed into crystalline graphite even at
temperatures of 3000C and above. A simple model of non-graphitizing carbon based
on small graphitic crystallites joined together by cross-links, but did not explain the
nature of these cross-links.
A later idea was that the cross-links might consist of domains containing
sp3-bonded atoms, but this had to be discounted when neutron diffraction studies
showed that non-graphitizing carbons consist entirely of sp2 atoms .A much more
recent suggestion is that non-graphitizing carbon has a structure related to that of the
fullerenes, in other words that it consists of curved fragments containing pentagons
and other non-hexagonal rings in addition to hexagons. Such a structure would
explain the micro porosity of the carbon, and many of its other properties. However,
obtaining direct experimental support for this hypothesis is extraordinarily difficult.
Both x-ray and neutron diffraction have been extensively applied to non-graphitizing
carbons, and in some studies the diffraction data has been interpreted in terms of a
structure containing non-hexagonal rings (Peter J F Harris et al. .2008).
The IUPAC classification of pores by sizes defines the following three class
of pore (IUPAC .1972):
Micropores, less than 2 nm (less than 20 )
Mesopores between 2 and 50 nm, (20 to 500 )
Macropores, 50 nm, (more than 500 ).
According to T. Otowa et al. (1997), Tsutomu Suzuki et al. (2007) and A.
Linares-Solano et al. (2000), usually more than 95% micropores content in the total
area surface of activated carbon. The volume of micropores range from 0.15 up to
0.6 cm3/g. Conventional activated carbon were tridisperse, having all three type of
pores present within their structure. Absorbance molecules penetrate through the
wider pore to micropores. Activated carbon prepared with high surface area, low
volume resistivity or low ash content has been reported largely. The composition and
structure of an activated carbon depends on the raw material, the activating agent and
the preparation method. These parameters condition the properties of the activated
carbon and hence its applications.
Diamond
Graphite
Carbon Black:
Non-parallel layer planes
Figure 2.2: Carbon Black Quasi-Graphitic Microstructure compared to the
Two Regular Crystalline Forms of Carbon (Diamond and Graphite).
2.2.3
Activated Carbon adsorption.
According to Khadija Qureshi et al. (2008), they are most effective
adsorbents in treating drinking water and industrial wastewater. The food industry is
also a major consumer of activated carbon, where it is used to remove compounds
that adversely affect color, taste and odor. In the mineral industry activated carbon
are used to recover gold from leached liquors. Medicinal uses and pharmaceutical
industry is also another wide area for the utilization of activated carbon. In gas
cleaning applications activated carbon are extensively used in air filters at industrial
level as well as in general air conditioning application.
According to K.P Gadkaree, (1998), adsorption is a process in which matter
adheres to the surfaces of adsorbents (activated carbon). In the adsorption process,
molecules of a contaminated are attracted to and accumulate on the surface of the
activated carbon. Carbon is a commonly used adsorbent due to its very large surface
area. Physical adsorption is dependent on the characteristics of the contaminant to be
adsorbed, the temperature of the gas stream to be processed, and the concentration of
the contaminant in the gas stream. The adsorption capacity for a particular
contaminant represents the amount of the contaminant that can be adsorbed on a unit
weight of activated carbon consumed at the conditions present in the application.
Typical adsorption capacities for moderately adsorbed compounds range from 5 to
30 percent of the weight of the carbon.
Figure 2.3: Cross section activated carbon adsorption for gases and chemicals.
Source: (home-air-purifier-guide.com)
2.2.4 Type and selection of activated carbon.
Refer to C. Srinivasakannan (2003), there are three different types of
activated carbon. Based on the application a specific type of activated carbon is used:
a. Powdered Activated Carbon (PAC)
(particle size predominantly
smaller than 80 mesh) - used for taste and odor removal
b. Granular Activated Carbon (GAC) (particle size predominantly more
than 80 mesh )- Used for liquid and gas phase application
c. Pellet Activated Carbon- Used mainly for gas phase application
Moreover, activated carbon is able to offer a range of different types of activated
carbon media such as carbon briquettes, chemical impregnated granular activated
carbon and fibrous activated carbon.
10
(a)
(b)
(c)
Figure 2.4 : Types of activated carbon. (a) PAC, (b) GAC, (c) Pellet A.C .
Table 2.1: Commercially Available Carbons.
Source: (William D.F. et al. 1987)
FORM
MEAN
SIZE FRACTION
PARTICLE
U.S.SIEVE NO.
RAW MATERIAL
2.5
6x12
Coconut,
2.1
6x16
Bituminous Coal
1.7
8x16
Pellet
2.6
2 mm diameter
Coal, Peat
Powder
0.04
65%-325
Coal Peat, Wood
SIZE (mm)
Granular
11
2.3 ACTIVATED CARBON MANUFACTURE.
In principle, have two main processes to produce activated carbon (AC) which is
carbonization and activation.
According to Savova D et al. (2001), carbonization is a process in which
material is turned into porous carbon structure through pyrolysis. In this process,
most of non-carbon organic matters are decomposed. The combination of carbon
atom-aromatic foliated structure is made with irregular split. Thus, the split will be
developed into a structure with more pores during process activation. Pyrolysis is an
endothermic process that offers an environmentally attractive way of reducing those
residues. Unlike incineration the pyrolysis does not lead to air emission. The solid
product were named char which can be activated and produced active carbon, a high
value added product. Pyrolysis process included of softening of the material and
release of votile matter, then hardening and shrinkage of char. Previous study for
bamboo, refer to Baksi et al. 2003. Carbonisation or pyrolysis is the first process in
activated carbon preparation, in which bamboo is heated, decomposed and eventually
converted into desired product in the absence of air. Pyrolysis process includes
carbonisation (destructive/dry distillation of bamboo), charcoal processing,
gasification and activated carbon processing. The pyrolysis product is bamboo
charcoal and the by-product is pyroligneous acid. After that, the charcoal is activated
by reaction with steam in a reactor to facilitate heat distribution and improved gassolid contact.
From the previous research, refer to Minkova V et al. 2001. Activation is
used physical or chemical method to removes the tar, organic wastes and other rest.
Its purpose it to get more an absorption phase of activated carbon product. However,
the simple one step method for the production of activated carbon has been
developed to reduce the energy expenditure during the process. This method is a
feasible alternative to the traditional two-step process for the production of activated
carbons by consecutive carbonization of raw material and high temperature
activation (900C to 1000C) of the solid product from carbonization.
12
2.3.1 Activation Method
According to J. Helena et al. (1991), the preparation of activated carbon with
different pore sizes can be achieved by using physical or chemical activation process.
In both methods, the development of porosity is different in term of practical
procedures and mechanism.
1.
Physical activation the generation of porosity took place via selective
elimination of the more reactive carbon of the structure and further gasification led to
the production of the activated carbon with the sought pore structure. The raw
material is carbonized (carbonization step) at high temperature and prepared char
sequentially activated using steam or carbon dioxide (CO2) (activation step). The
porous structure is created due to elimination of volatile matter during the
carbonization step and carbon on the char is removed by reaction with steam or CO2
during the activation step.
2.
Chemical activation process, refer to Rodriguez-Reinoso and Molina-Sabio.
(1992), the precursor is mixed with a chemical such as ZnCl2 or H3PO4, carbonized
and washed to produce the activated carbon. Following the thermal decomposition of
the precursor, the chemical reacts with the product causing reduction in the evolution
of volatile matter and inhibition of the particle shrinkage. Once the chemical is
removed by exhaustive washing, a large amount of porosity is formed. Ahmadpour
and Do (1996) state that, in physical activation, elimination of a large amount of the
internal carbon is necessary to obtain a well developed carbon structure, whereas in
the chemical activation process all chemical agents used dehydrating agents that
influence pyrolytic decomposition and inhabit formation of tar, thus enhancing the
yield of carbon. The temperatures used in chemical activation are also lower than
that used in physical activation process, and therefore the development of a porous
structure is better in the case of the chemical reaction.
13
2.3.2 Activating Agents
According to Carolina R Loez et al. (1995), the activating agents used in the
chemical process are normally alkali and alkaline earth metal containing substances
and some acid such as KOH, NaOH, H3PO4, ZnCl2, Na3PO4, NaCl, KmnO4, used on
various raw materials to yield appreciable quality of activated carbon. Among the
several dehydrating agents, zinc chloride (ZnCl2 )and phosphoric acid (H3PO4 ) are
widely employed for commercial manufacture due to its excellent dehydration
characteristics. Refer to Junichi Hayashi et al. (2002), However the use of ZnCl2
and H3PO4 has been recently limited due to problems of possible environmental
contamination and and eutrophication with zinc and phosphorous compounds.
IUPAC (1972) consequently, alkali hydroxide such as KOH and NaOH have been
used in order to prepared activated carbon with high specific surface area.Activated
carbon with high specific surface area is suitable for gas storage , gas purifications ,
recovery of solvent vapor and manufacture electric double layer capacitors.
However, KOH and NaOH are expensive and corrosive chemicals.
2.3.3 The methods of activating agents mixing.
Three different methods are used to mixing raw material and activating agent:
1. Physical method, dries precursor material was physically mixed with
ground chemical agent and placed into reactor.
2. Impregnation method, dried precursor material was well mixed with a
concentrated solution of chemical agent and then the resulting
homogeneous slurry was dried at 110C until constant weight.
3. Acid pre-treat method, dried precursor material was soaked in 1N
solution of HCl : H2SO4 [1:1] for 2 days in order to reduce the ash
content, then wash with distilled water and dried at 110C for about
14 hours . The dried sample was further mixed with a solution of
chemical agents according to impregnation method. Physical method
is a mixing methodology seldom used, although this is simpler and
much less time consuming than impregnation with solution, due to
better distribution of chemical agents into the carbonaceous particle
14
mass. While acid pre-treatment of the coal shows some advantages in
some cases, but for the coal low ash content the effect is negligible.
2.4
RAW MATERIAL FOR ACTIVATED CARBON
A lot of work has been done on activated carbon with respect to preparation
and characterization that has enabled it to reach a significant level of achievement
and usage. However, physical and chemical properties differ from one to the other
(Mattson and Mark, 1971) due to the difference in the base materials and the way
they have been prepared. Activated carbon prepared from coconut shell for example,
differs from that of palm fruit shell or rice hull even though they were prepared by
the same method. Due to this variation, not all activated carbons are suitable for a
specific application. The activated carbon prepared from a particular source may
only be useful for a particular application.
Refer to Eric M. S. and Indrek A. (2007), Joanna Gorka et. Al (2008),
Devarly Prahas et al.(2008), activated carbons can be prepared from a large number
of carbonaceous raw materials (e.g. coal, lignite, wood, coconut shell, scrap tires and
some agricultural waste products).
According to M Ahmedna, et al. (2000) Nadhem K Hamadi et al. (2001),
Christopher A Toles et al.(1998) , Nevin Yalem , Vadettin Sevine, (2000) . Since the
raising of coal price and the limiting forest resources, it is necessary to find an
abundant raw material with low price for the preparation of effective activated
carbons. The long-term availability of coal, environmental impacts and potentially
increasing cost has encouraged researchers to find other alternatives, which may be
cast effective and equally potential. Activated carbon can be manufactured from any
material that has reasonable elemental carbon content. Any lignocellulosic material
can be converted to an activated carbon. The literature mentions many precursors for
activated carbon such as bagasse scrap tires and saw dust, almond, pecan, English
walnut, black walnut and macadamia nut pistachio hazelnut shells, rice husk, rice
bran etc.
15
Hassler (1974) mentions 33 raw materials which have been studied for the
production of activated carbon. The raw material affects final product characteristics,
as do the type of manufacturing method - chemical or steam and the particulars of
the manufacturing process. Most of the raw materials and processes will allow the
production of fine powdered carbons although product characteristics and costs will
vary. The list of realistic raw materials and applicable manufacturing processes
shrinks when large particle/granular carbon is needed and physical strength in the
virgin and regenerated states are considerations. For precious metals recovery,
granular carbon is the most widely used form. Over 95% of the coarse carbon in
service for gold and silver recovery is steam activated coconut base carbon.
Activated carbon can be manufactured from many carbonaceous raw materials. High
carbon content, low ash and low cost are raw material requirements. Many wastes
have been utilized such as sulfite liquors, waste lignin, and wastes from processing
petroleum and lubricating oils. Materials of vegetable origin and younger fossil fuels
were some of the first materials used - wood, peat, sawdust, nut shells, fruit pits.
More recently bituminous coals have come into use as activated carbon.
16
Table 2.2: Characteristic of commercial raw materials as activated carbon.
Source: (Bansal R C et al. 1988)
Raw
Carbon
Volatile
Bulk density Ash
Adsorption/
materials
(%)
(%)
(g/cc)
(%)
Application use
Hard wood
40-45
55-60
0.40-0.50
0.3-1.1
Liquid Phase
Soft wood
40-42
55-60
0.55-0.80
0.3-1.2
Liquid Phase
Lignin
35-40
58-60
0.30-0.40
Liquid Phase
Nutshell
40-45
55-60
1.40
0.5-0.6
Vapour Phase
Lignite
55-70
25-40
1.00-1.35
5.0-6.0
Water
Waste
treatment
Peat
65-80
20-30
1.25-1.50
0.2-1.2
Liquid
and
vapour
phase
Coke
75-85
15-20
1.35
0.5-0.7
Water
Waste
treatment
Bituminous
70-75
10-15
1.45
5-15
Vapour Phase
Coal
85-95
5-10
1.50-1.80
2-15
Vapour Phase
2.4.1
The selection of raw material.
The selection of precursor essentially determined range of adsorptive and
physical properties that can be attained in the activated carbon products. Important
considerations to be made in selecting a source of material include cost, availability
and consistency of quality.
Raw materials for activated carbon are chosen depending on their:
a) Purity
b) Price
c) Potential extent of activated
d) Stability of supply
17
Low ash and low cost are raw material requirements. In the sense of
environmental protection, the utilization of these wastes has awakened the interest of
development of processes for production of carbon adsorbent based on agricultural
waste. Both the nature precursors and the production process have a strong influence
on porous structure adsorption properties of the resulting activated carbon. Recent
investigations have reported utilization of various raw lignocelluloses material
example, various nutshell, fruit stones, sugar bagasse, coconut shell, corncob.
On the other hand, agricultural by-products , derived from natural resources
are inexpensive though they may be problems with the uniformy of quality,supply
location and seasonal variability.
2.5
BAMBOO WASTE
2.5.1
Bamboo.
According to Lakkad, S.C. and J.M. Patel. (1980.) Bamboo are in the family
Gramineae, the same family with the type of grass, including grass-medley, rice,
corn, sugarcane and wheat. More correctly, the bamboo is under the sub-family
Bambusoideae. While sub-family contains the genus-genera such as Bambusa,
Dendrocalamus, Dinochloa, Gigantochloa, Schizostachyum and others. Bamboo is a
naturally occurring composite material which grows abundantly in most of the
tropical countries. It is considered a composite material because it consists of
cellulose fibers imbedded in a lignin matrix. Cellulose fibers are aligned along the
length of the bamboo providing maximum tensile flexural strength and rigidity in
that direction
Bamboo is the most diverse group of plants in the grass family. It belongs to
the sub-family Bambusoideae of the family Poaceae (Gramineae). It is an enduring,
versatile, and highly renewable material, one that people and communities have
known and utilised for thousands of years. They are among the fastest growing plants
on the planet. Bamboo can be harvested in two to three years for the purpose of
18
specified end products. This makes it a renewable resource. As a cheap and fast
grown resource with superior physical and mechanical properties compared to most
wood species, bamboo has great potential as an alternative to wood. The chemistry of
bamboo is important in determining its utilisation potential. Several studies have
investigated the chemical composition, physical and mechanical properties of
bamboo. Most of the previous studies provide either only general information of
several bamboo species or focus on only one aspect of one species. (Xiaobo Li
2004). From early research by Wang D. and S.J. Shen (1987) over 1200 bamboo
species have been identified globally Bamboo forest biomass stores a large quantity
of carbon. With a carbon percentage of 40% - 45%, nearly half of the total biomass is
carbon.
According to Prof. Dr. Walter Liese (1992), botanically categorized as a grass
and not a tree, bamboo just might be the worlds most sustainable resource. Bamboo
does not require replanting after harvesting because its vast root network continually
sprouts new shoots which almost zoom up while you watch them, pulling in sunlight
and greenhouse gases and converting them to new green growth. And bamboo does
this the natural way without the need for petroleum-guzzling tractors and poisonous
pesticides and fertilizers.
As the fastest growing plant on Earth, Bamboo is an excellent partner to the
ecosystem. Some species have been measured to grow over four feet in 24 hours. A
pole of bamboo can regenerate to its full mass in just six months. Bamboo can be
continuously re-harvested every three years, without causing damage to the plant
system and surrounding environment. Bamboo is the fastest growing plant on this
planet and provides the best canopy for the greening of degraded lands. Its stands
release 35% more oxygen than equivalent stands of trees. Bamboo can also lower
light intensity and protects against ultraviolet rays.
19
Figure 2.5. Bamboo forestry.
Source: (uravu.net)
2.5.2 General Applications
Refer to Abd.Latif, M. et al. (1990), Bamboo has a very long history with
human kind. Bamboo chips were used to record history in ancient China. Bamboo is
also one of the oldest building materials used by human kind . According Hammett,
A.L et al.(2001), bamboo has been used widely for household products and extended
to industrial applications due to advances in processing technology and increased
market demand. In Asian countries, bamboo has been used for household utilities
such as food containers, chopsticks, woven mats, fishing poles, cricket boxes,
handicrafts, chairs, food container etc. It has also been widely used in building
applications, such as flooring, ceiling, walls, windows, doors, fences, housing roofs,
trusses, rafters and purlins. It is also used in construction as structural materials for
bridges, water transportation facilities and skyscraper scaffoldings. There are about
35 species now used as raw materials for the pulp and paper industry. Massive
plantation of bamboo provides an increasingly important source of raw material for
pulp and paper industry in China. Liese W. (1998), Proper utilization and protection
are, therefore, necessary to maximize the benefits we derive from this material.
Unfortunately, bamboos are susceptible to attack by decay fungi and boring insects,
and difficult to treat with preservatives. In general, protection of bamboo from
biodeterioration has received little attention in Asian countries.
20
(a)
(b)
Figure 2.6: (a) shows (Schizostachyum brachycladum) and (b) picture of Lemang
Source: (webphotos.com.au)
2.5.3 Chemical Composition
Refer to Kawamura, Katani (1990). Many relations between the chemical
composition and utilization only few examples may be mentioned that bamboo
consists of about 50-70% holocellulose, 30% pentosans and 20-25% lignin. There are
some differences in these main constituents between species, but any influence on
technological properties remains uncertain. The silica content amounts to 0.5-5%
according to species and affects their cutting and pulping quality. Most silica appears
to be situated in the epidermis, but more knowledge about its location would be
useful for processing technologies.
Only for one species, Oxytenanthera braunii in Tanzania, its large-scale
collection and fermentation to a powerful drink is known. For timber, organic and
inorganic by-products, like polyphenols, resin, waxes, influence many properties,
like shrinkage, durability, gluability. The more as such substances are apparently
hardly produced by the metabolic processes of the parenchyma cells. Apparent
differences of certain species in the natural resistance against fungi cannot be
explained so far. Liability for the attack by powderpost beetles, especially Dinoderus
spp depends on the amount of starch present. It changes with the growing season, but
the often mentioned possible influence of the moon-phase on the resistance and thus
21
either on the chemical substances or on the vitality of the borers remains so for a
belief. Few species like Bambusa textilis, are said to be less vulnerable due to a
general lower starch content. The sugar content of the parenchyma cells can
influence the setting of cement for cement-bamboo structures. Since for cement
bamboo particle boards pretreatment and chemical additives are needed, seasonal
changes of the carbohydrates may be considered.
The potential of certain bamboo species for extractives to be used in the
cosmetic and pharmaceutical industry can only be mentioned briefly. The species
used so far belong mainly to the genera Bambusa, Dendrocalamus, Gigantochloa
and Phyllostachys - is this due to lucky chances or systematic screening? The
preservation of bamboo respectively its penetrability is another area where the
anatomical structure determines the possibilities for treatment. The culm is covered
at its outer and inner side by an impermeable skin. Also the absence of ray cells as
radial pathways determine the need for an axial penetration through the long and
wide vessels. In green condition the natural easy flow of water can be used by the socalled sap replacement treatment. It is only necessary to fill the vessels with a
suitable preservative, so that during the following resting period the salt components
can diffuse into the surrounding tissue. Treatment time depends much on the vessel
area of the bamboo. Generally around the metaxylem comprises of 6-8%, but
differences exist between the lower and upper part of a culm and also between
species. As a last example surface decoration of the culm may be mentioned. This
improvement of appearance due to application of lac or dyes is much hindered by the
chemical composition of the culm-epidermis so that special treatments are often
necessary.
22
2.6 STANDARD FOR ACTIVATED CARBON
2.6.1
Characteristics of activated carbon
According to Bansal et. al (1988), the effectiveness of activated carbon as an
adsorbent it attributed to its unique properties, including large surface area ,a high
degree of surface reactivity, universal adsorption effect and favorable pore size. Its
extremely large surface area often characterizes activated carbon. For comparison, a
given type or sample of activated carbon is usually quantified based on four primary
criteria: total surface area, carbon density, particle size distribution and adsorptive
capacity. All of these factor influence adsorption rate and also capacity.
2.6.1.1 Total surface area
Total surface area is measured by the adsorption of nitrogen gas on to the
carbon and is expressed in square meters of surface area per gram of carbon. Because
the gas molecules used to measure adsorption are very small, it should be noted that
this measurement of surface area might be misleading when considering adsorptive
capacity of carbon for large organic macromolecules. Those types of compounds
may have adsorption limited by pore size considerations. In fact, the surface area per
gram of material ranging from 600to 1200 m2 /g and as high as 2500m2 /g have been
reported.(Chilton Ng et al. 2002)
2.6.1.2 Carbon density
Carbon density is a weight of one milliliters of carbon in air. Bulk density is
also sometimes used for carbon as it is for soil and is expressed in pounds per cubic
foot or in kilograms per liter.
2.6.1.3 Particle size distribution
Particle sizes in carbon are measured using standard U.S sieve size, as for soil
constituents. Particle size distributions are important in carbon system because they
influence handling of the activated carbon material. For example, in granular carbon,
the particle size affects hydraulic loading and backwash rates for a filter. On the
23
other hand, particle size is often important because of its effect on adsorption rates as
well.
2.6.1.4 Adsorptive capacity
Adsorptive capacity is characterized by the effectiveness of activated carbon
in removing a given contaminants. For comparison, several standard compounds are
used for this measurement, for example:
i.
Iodine number
Commonly used describes the carbons capacity to absorb low-molecular-weight
substances. The iodine number indicates the porosity of activated carbon and defines
as milligrams of iodine adsorbed per gram of carbon. Iodine number represents the
surface area contributed by the pores larger than 10A. Iodine number is commonly
used in the industry, as a rough estimate the surface area of the activated carbon.
ii.
Molasses number
Characterizes a carbons capacity for more complex compounds. Molasses number
represents the surface area contributed by the pores larger than 28A.
2.6.1.5 The structural properties
The structural properties of activated carbon are very important to its
effectiveness as an adsorbent, though activated carbon's structure is not fully
understood and is difficult to explain with text. In general, activated carbon is
sometimes described as having a crumpled layered surface, in which flat sheets are
broken and curved back upon them. This unique structure creates activated carbon's
very large surface area. It can be more properly visualized with the attached images,
which provide both electron microscope photos and conceptual diagrams of
the surface structure. There are several standard test methods were used to
determine the characteristics of activated carbon. Among them, ASTM standard
(The American Society for Testing Material) and AWWA standard (American Water
Works Association) have been recognized.
24
2.7
CHARACTERIZATION METHOD
2.7.1
Iodine number analysis
Refer to ASTM, Designation: D 4607 94 (reapproved 1999), this test
method covers the determination of the relative activation level of unused or
reactivated carbons by adsorption of iodine from aqueous solution. The amount of
iodine absorbed (in milligrams) by 1 g of carbon using test conditions listed herein is
called the iodine number.
2.7.1.1 Significance and Use
The iodine number is a relative indicator of porosity in an activated carbon. It
does not necessarily provide a measure of the carbon ability to absorb other species.
Iodine number may be used as an approximation of surface area for some types of
activated carbons. However, it must be realized that any relationship between surface
area and iodine number cannot be generalized. It varies with changes in carbon raw
material, processing conditions, and pore volume distribution.
The presence of adsorbed volatiles, sulfur; and water extractable may affect the
measured iodine number of an activated carbon
2.7.1.2 Summary of test method.
This test method is based upon a three-point adsorption isotherm. A standard
iodine solution is treated with three different weights of activated carbon under
specified conditions. The carbon treated solutions are filtered to separate the carbon
from the treated iodine solution (filtrate). Iodine remaining in the filtrate is measured
by titration. The amount of iodine removed per gram of carbon is determined for
each carbon dosage and the resulting data used to plot an adsorption isotherm. The
amount of iodine adsorbed (in milligrams) per gram of carbon at a residual iodine
concentration of 0.02N is reported as the iodine number.
25
Iodine concentration in the standard solution affects the capacity of an
activated carbon for iodine adsorption. Therefore, the normality of the standard
iodine solution must be maintained at a constant value (0.100 0.001N) for all iodine
number measurements.
The apparatus required consists of various laboratory glassware used to
prepare solutions and contact carbon with the standard iodine solution. Filtration and
titration equipment are also required.
2.7.1.3 Apparatus
Analytical balance, accuracy 0.0001g. Buret, 10 mL capacity or 5ml precision
buret. Flasks, Erlenmeyer wide-mouthed, 259mL capacity. Bottles, amber, for
storage of iodine and thiosulfate solutions. Beakers, assorted sizes. Funnels, 100 mm
top inside diameter. Filter paper, 18.5 cm prefold paper, Whatman No. 2v or
equivalent. Pipets, volumetric type, 5.0, 10.0, 25.0, 50.0 and 100mL. Volumetric
flasks, 1L. Graduated cylinders, 100mL and 500mL.
2.7.1.4 Reagents
1. Purity of reagents - Reagent grade chemicals shall be used in all tests.
Unless otherwise indicated, it is intended that all reagents shall conform
to the specifications of the Committee on Analytical Reagents of the
American Chemical Society, where such specifications are available.
Other grades may be used, provided it is first ascertained that the reagent
is of sufficiently high purity to permit its use without lessening the
accuracy of the determination.
2. Purity of water references to water shall be understood to mean reagent
water conforming to Specification D 1193 for Type II reagent water.
3. Hydrochloric Acid, concentrated.
4. Sodium Thiosulfate, (Na2S2O3.5H2O )
26
2.7.1.5 Hazards.
Several potential hazards are associated with conducting this test procedure.
It is not the purpose of this standard to address all potential health and safety hazards
encountered with its use. The user is responsible for establishing appropriate health
and safety practices before use of this test procedure. Determine the applicability of
federal state regulations before attempting to use this test method.
Personnel conducting the iodine number procedure should be aware of
potential safety and health hazards associated with the chemicals used in this
procedure. The Material Safety Data Sheet (MSDS) for each reagent listed in
Reagent above should be read and understood. Special precautions to be taken during
use of each reagent are included on the Material Safety Data Sheet (MSDS). First
aid procedures for contact with a chemical are also listed on its MSDS. A
Material Safety Data Sheet (MSDS) for each reagent may be obtained from
manufacturer.
Careful handling and good laboratory technique should always be used when
working with chemicals. Avoid contact with hydrochloric acid or acid vapor. Care
should also be taken to prevent burns during heating of various solutions during this
test procedure. The user of this test method should comply with federal, state, and
local regulations for safe disposal of all samples and reagents used.
2.7.1.6 Preparation of solution.
Hydrochloric Acid Solution ( 5% by weight) Add 70mL of concentrated
hydrochloric acid to 550mL of distill water and mix well. A graduated cylinder may
be used for measurement of volume.
Sodium Thiolsulfate (0.100N) dissolve 24.820g of sodium thiosulfate in
approximately 75 25mL of freshly boiled distill water. Add 0.10 0.01g of sodium
carbonate to minimize bacterial decomposition of the thiosulfate solution.
Quantitatively transfer the mixture to a 1 L volumetric flask and dilute to the mark.
Allow the solution to stand at least 4 days before standardizing. The solution should
be stored in an amber bottle.
27
Standard iodine Solution (0.100 0.001N) weigh 12.700g of iodine and
19.100g of potassium iodide (KI) into a beaker. Mix the dry iodine and potassium
iodide. Add 2 to 5 mL of water to the beaker and stir well. Continue adding small
increments of water (approximately 5 mL each) while stirring until the total volume
is 50 to 60 mL. Allow the solution to stand a minimum of 4 h to ensure that all
crystals are thoroughly dissolved. Occasional stirring during this 4 hour period will
aid in the dissolution. Quantitatively transfer to a 1 L volumetric flask and fill to the
mark with distilled water. It is important that the standard iodine solution has an
iodide to iodine weight ratio of 1.5 to 1 . Store the solution in an amber bottle.
Potassium iodate Solution ( 0.1000N) Dry 4 or more grams of primary
standard grade potassium iodate (KIO3) at 110 5 C for 2 hour and cool to room
temperature in a desiccators . Dissolve 3.5667 0.1 mg of the dry potassium iodate
in about 100mL of distilled water. Quantitatively transfer to a 1 L volumetric flask
and fill to the mark with distilled water. Mix thoroughly and store in a glass stopper
bottle.
Starch solution Mix 1.0 0.5g of starch with 5 to 10 mL of cold water to
make a paste. Pour the mixture, while stirring, into 1 L of boiling water and boil for 4
to 5 minute this solution should made fresh daily.
2.7.1.7 Procedure
The procedure applies to either powdered or granular activated carbon.
When granular carbon is to be tested, grind a representative sample of carbon until
60 wt% ( or more will pass through a 325 mesh screen) and 95 wt % or more will
pass through a 100 mesh screen ( U.S sieve series ). Carbon received in the powdered
form may need additional grinding to meet the particle size requirement given above.
Dry the ground carbon. Cool the dry carbon to room temperature in
desiccators. Determination of iodine number requires an estimation of three carbon
dosage. After estimating carbon dosages, weigh three appropriate amounts of dry
carbon to the nearest milligram. Transfer each weighed sample of carbon to a clean,
dry 250mL Erlenmeyer flask equipped with a ground glass stopper.
28
Pipet 10.0mL of 5 wt % hydrochloric acid solution into each flask containing
carbon. Stopper each flask and swirl gently until the carbon is completely wetted.
Loosen the stoppers to vent the flasks, place on a hot plate in a fume hood and bring
the contents to a boil. Allow to boil gently for 30 2 s to remove any sulfur which
may interfere with the test results. Remove the flask from the hot plate and cool to
room temperature.
Pipet 100.0mL of 0.100N iodine solution into each flask. Standardize the
iodine solution just prior to use . Stagger the addition of iodine to the three flasks so
that no delays are encountered in handling. Immediately stopper the flasks, and shake
the contents vigorously for 30 1 s. quickly filter each mixture by gravity through
one sheet of folded filter paper (Whatman No. 2 V or equivalent) into a beaker.
Filtration equipment must be prepared in advance so no delay is encountered in
filtering the samples.
For each filtrate, use the first 20 to 30 mL to rinse a pipette. Discard the rinse
portions. Use clean beakers to collect the remaining filtrates. Mix each filtrate by
swirling the beaker and pipet 50.0 mL of each filtrate into a clean 250 mL
Erlenmeyer flask. Titrate each filtrate with standardized 0.100 N sodium thiosulfate
solution until yhe solution is a pale yellow. Add 2 mL of the starch indicator until
one drop produces a colorless solution. Record the volume of sodium thiosulfate
used.
2.7.1.8 Standardization of solutions.
Standardization of 0.100N Sodium Thiosulfate Pipet 25.0mL of potassium
iodate (KIO3) solution that prepare early into a 250 mL titration (or wide mouthed
Erlenmeyer) flask. Add 2.00 0.01g of potassium iodide (KI) to the flask and shake
the flask to dissolve the potassium iodide crystals. Pipet 5.0 mL of concentrated
hydrocholoric acid into the flask. Titrate the free iodine with sodium thiosulfate
solution until a light yellow color is observed in the flask. Add a few drops of starch
indicator and continue the titration dropwise until one drop produces a colorless
solution. Determine sodium thiosulfate normality as follows:
29
N1 = (P. R)/S
where:
N1
= sodium thiosulfate, N.
= potassium iodate, mL,
= potassium iodate, N
= sodium thiosulfate, mL.
The titration step should be done in triplicate and the normality results
averaged. Additional replications should be done if the range of values exceeds
0.003N.
Standardization of 0.100 0.001 N Iodine Solution Pipet 25.0 mL of iodine
solution into a 250 mL wide mouthed Erlenmeyer flask. Titrate with standardized
sodium solution until the iodine solution is a light yellow color. Add a few drops of
starch indicator and continue titration drop wise until one drop produces a colorless
solution. Determine the iodine solution normality as follows:
N2 = (S. N1)/ I
where:
N2
= iodine, N,
= sodium thiosulfate, mL.
N1
= sodium thiosulfate, N,
= iodine, mL.
The titration step should be done in triplicate and the normality results
averaged. Additional replications should be done if the range of values exceeds
0.003N. The iodine solution concentration must be 0.100 0.001 N.
30
2.7.1.9 Calculation.
The capacity of a carbon for any adsorbate is dependent upon the
concentration of the adsorbate in solution. The concentration of the of the standard
iodine solution and filtrates must be specified or known. This is necessary to
determine an appropriate carbon weight to produce final concentrations agreeing
with the definition of iodine number. The amount of carbon sample to be used in the
determination is governed by the activity of the carbon. If filtrate normalities (C) are
not within the range of 0.008N to 0.040N, repeat the procedure using different
carbon weights. Two calculations are required for each carbon dosage, as X/M and
C.
To calculate the value of X/M, first derive the following values:
A= (N2)(12693.0)
Where:
N2 = iodine, N
B = (N1)(126.93)
Where:
N1 = sodium thiosulfate, N
DF = (I + H)/F
Where:
DF
= dilution factor
= iodine, mL
= 5% hydrochloric acid used. mL
= filtrate, mL.
31
Calculate the value of X/M as follows:
X/M = [A - (DF)(B)(S)] / M
where :
X/M
= iodine absorbed per gram of carbon, mg/g.
= sodium thiosulfate, mL.
= carbon used, g.
Calculate the value of C as follows:
C = (N1. S)/ F
where:
C
= residual filtrate, N.
N1
= sodium thiosulfate, N.
= filtrate, mL.
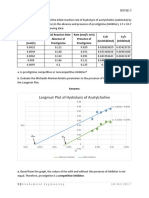
Using logarithmic paper, plot X/M (as the ordinate) versus C (as the abscissa)
for each of three carbon dosages. Calculate the least squares fit for the three points
and plot.The iodine number is the X/M value at a residual iodine concentration (C) of
0.02 N. The regression coefficient for the least squares fit should be greater than
0.995N.
Carbon dosage may be estimated as follows:
M = [A - (DF)(C)( 126.93)(50)] / E
where:
M
= carbon, g.
= (N2) (12693.0)
DF
= dilution factor.
= residual iodine
= estimated iodine number of the carbon.
(Three carbon dosages are calculated using three values of C, usually 0.01, 0.02, and
0.03)
32
2.7.2
BrunauerEmmettTeller (BET)
Named after Stephen Brunauer, P.H. Emmet and Edward Teller. Developed
in 1938. They were working on ammonia catalysts. First method to measure the
specific surface of finely divided and porous solids. The applications that use this
method such as pharmaceuticals, catalysts, projectile propellants, medical implants,
filters (activated carbon) and cements.
Refer to research paper Don.o Burevski (2004), the method of Brunauer,
Emmet, and Teller is employed to determine surface area on a model of adsorption
which incorporates multilayer coverage. One of the most important applications of
physical adsorption of gases is the use of adsorption isotherms to determine the
surface areas of porous and non-porous solids. The determination of the specific
surface area is generally considered for the specification of properties of adsorbents,
catalysts and other solids. The texture of solid materials is usually studied by means
of the Brunauer- Emmett-Teller (BET) equation.
The specific surface area of solids is calculated from their monolayer capacity
values which can be estimated from the adsorption isotherms. For this purpose the
BET equation is still the most frequently used adsorption equation. The reality of
monolayer capacity values of non-porous and mesoporous solids, determined from
the BET equation, is connected with the shape of adsorption isotherms.
The concept which is accepted for adsorption in micropores is that the initial
part of the type I isotherm represents micropore volume filling rather than surface
coverage. According to this view, the monolayer capacity of microporous adsorbents
determined from adsorption isotherm equations cannot be accepted as a real value.
The aim of the present work is to consider the reality of the monolayer
capacity values of microporous active carbons determined from the N-BET equation
in relation to the statistical number of adsorbed molecular layers, N. The monolayer
capacity is analyzed by the dependence of the amount of adsorption, n, on the
adsorption potential, . For this purpose, the adsorption isotherms of nitrogen
obtained at 77 K on three samples of active carbons have been interpreted by means
33
of the N-BET equation. The functional dependence of dn/d on , derived from the
equation, is used for the monolayer capacity and specific surface evaluation
Figure 2.7: One of Brunauer, Emmet And Teller (BET) Machine.
(Source: foord.chem.ox.ac.uk)
2.7.3 Scanning Electron Microscopy (SEM)
According to Patrick Echlin (2009), scanning electron microscopy (SEM) and
x-ray microanalysis can produce magnified images and in situ chemical information
from virtually any type of specimen. The two instruments generally operate in a high
vacuum and a very dry environment in order to produce the high energy beam of
electrons needed for imaging and analysis. With a few notable exceptions, most
specimens destined for study in the SEM are poor conductors and composed of beam
sensitive light elements containing variable amounts of water.
In the SEM, the imaging system depends on the specimen being sufficiently
electrically conductive to ensure that the bulk of the incoming electrons go to
ground. The formation of the image depends on collecting the different signals that
are scattered as a consequence of the high energy beam interacting with the sample.
34
Backscattered electrons and secondary electrons are generated within the
primary beam-sample interactive volume and are the two principal signals used to
form images. The backscattered electron coefficient () increases with increasing
atomic number of the specimen, whereas the secondary electron coefficient () is
relatively insensitive to atomic number. This fundamental difference in the two
signals can have an important effect on the way samples may need to be prepared.
The analytical system depends on collecting the x-ray photons that are generated
within the sample as a consequence of interaction with the same high energy beam of
primary electrons used to produce images.
There are two approaches to dealing with the frequent impasse that may exist
between the properties of the sample and the optimal operating conditions of the
SEM. Either modify the instruments so they employ less invasive procedures or can
modify the specimen to make it more robust to the withering beam of high energy
electrons. With a few exceptions, both approaches are a compromise. Sample
preparation is an absolute prerequisite for microscopy and analysis.
35
Figure 2.8: The basic premise of an SEM is that signal produced from a scanned
area of the specimen is displayed as an image with the exact same scan pattern on a
CRT. Source: (http://www.scribd.com/doc/15977565/Sem)
Figure 2.9:
SEM Photograph of Commercial Activated Carbon (AC)
Source: (lunalanun.blogspot.com)
36
CHAPTER 3
METHODOLOGY
1.1 INTRODUCTION
This work has been performed in laboratory scale in order to prepare
an activated carbon from raw material. Selections of raw material that use in this
final year project are waste of bamboo. Waste of raw material synthesis by using
chemical activation and physical activation method. The results from previous
studied have shown that degree of the impregnation of precursor, activation
temperature
and
activation
time are
important parameters that
govern the
development of porous structure of activated carbon.
For chemical activation, the activating agents used are ZnCl2. Activation
temperature is 500 C and 600 C. method that used in mixing of activating agent and
raw material is impregnation method, the ratio is one and two (weight of raw
material add with same weight of ZnCl2 and weight raw material add with weight
ZnCl2 two times of weight raw material ).Work had been carried out at Chemistry
lab, Faculty of Mechanical Engineering, University Technical of Malaysia Melaka.
The Iodine number of the resulting activated carbon was analyzed and used to
establish the appropriate operation condition including activation temperature
and activating agent impregnation ratio. Others characterized method, Bruneur,
Emmet, Teller (BET) and Scanning Electron Microscope (SEM).
37
Flow chart for project.
Start
Literature
review and
Research
materials
NO
Synthesis of bamboo
YES
Activated
Carbon
Characterization.
1. I2 number
2. BET
3. SEM/EDX
Data Analysis
Final report
3.1: Flow chart of project.
Waste of bamboo;
buluh lemang
(Schizostachyum
brachycladum)
Carbonization
Heated
Activation
Chemical /
physical
38
3.2 EXPERIMENTAL PROCEDURE
3.2.1
Procedure of Chemical activation.
The waste raw material was washed with water and dried in universal oven at
100C for 24 hours to remove the moisture. After raw material fully dried, the
sample was weighed, 50 grams used analytical balance and the raw material was put
inside the round bottom flask. 50 grams of dried raw material added with 200mL of
1M NaOH (The solution is already prepared). After that, setup the reflux apparatus.
Time for this process about 45 minutes. When this process completed done, take out
the raw material (bamboo waste) from the round bottom flask and washed with
plenty of distilled water. The raw material placed into beaker, then the sample placed
inside universal oven, dry at temperature 100C about 24 hours until the sample is
completely dry and weight again using analytical balance. The sample of dried
bamboo waste were impregnation with activating agent solution, zinc chloride
(ZnCl2) .
This was carried out by mixing activating agents. The method use is
impregnation method, impregnation ratio are g
raw material
:g
ZnCl2
, 1 : 2 ( weight of
raw material is equal with weight ZnCl2 ) and (weight of ZnCl2 is two times than
weight of raw material), than the mixer was stirred until completed homogenous and
dried in universal oven for 24 hours , the reagents are fully absorbed into the waste of
bamboo. The impregnation raw waste of bamboo samples was burn in furnace from
an initial room temperature 30 C to final temperature of 500C and 600C.
The final temperatures are a variable. Then, it was cooled down until constant
temperatures below 100 C. After the activation process, in order to remove the
remaining activating agent on the activated carbon, it was washed with hot distilled
water then filtered. Placed the sample into beaker, than, the sample was dried in
universal oven with temperatures, 100 C about 24hours. Lastly, change the sample
into powder activated carbon by using mortar. Now, activated carbon was fully
synthesis from raw waste materials (Buluh Lemang).
39
3.2.2
Procedure of physical activation.
30g of raw material was weighed. After that, placed the raw material inside
the steel tube in the reactor. The nitrogen gases inlet was adjusted and setting the
flow rate of gases about 2ml/min. This gases function for removes volatile organic
compounds during carbonizing. Then, switch on the power of carbon adsorbent
reactor, adjust and setting the temperatures programming, activation temperatures
500C or 600C as a variable. Setting mode of reactor (sp, stat, run) and wait this
process until the temperatures reached 500C or 600C Then, waiting about 5 10
minutes before switch CO2 gases inlet by used flow rate 0.2-0.3 ml/min, this process
call, activation process consists of selectively oxidizing part of the structure. Time
for activation process is 30 minutes. After 30 minutes, cooled down the reactor,
setting mode temperatures to 100C (stat, off). When the temperatures decrease until
100C, switch off all gases inlet to this reactor. Lastly, power supply to reactor was
switched off. The sample was bringing out from steel tube and weights the sample
using analytical balance. This waste raw material completed to synthesis as activated
carbon.
3.2.3 Iodine number analysis
Solutions used in the iodine number experiment were prepared as follows.
1. HCl, 70 ml of concentrated hydrochloric acid (36% concentrated) was
added to 550 ml of distilled water and mixed well.
2. Sodium thiosulfate. (0.1N)(commercial)
3. Standard iodine solution. (0.1N)(commercial)
4. Starch solution.
1.0g of starch was mixed with 10 ml of cold water to make a paste. 25
ml of water was added while stirring the starch paste. While stirring,
the mixture were poured into 1 liter of distill water and boiled until a
few boiling. The solution was made fresh daily.
3.2.3.1 Procedure
The determination of iodine number required an estimation of the three
necessary carbon dosages. This estimation had been carried out during a preliminary
experiment. The weights of carbon used in each flask were recorded.10 ml
40
hydrochloric acid (HCI) was pipetted into each flask. The mixture was swirled gently
until the carbon was completely wetted. Then, the flasks were placed on a hot plate
to boil the HCI for 30 seconds until a few boiled.
After the flasks were cooled at room temperature, 100 ml of 0.100 N iodine
was pipetted into each flask. The flasks were shaking vigorously for 30 second
before the contents were filtered through folded filter paper.
Afterwards, 50.0 ml of the each filtrate were pipetted into 250 ml elenmeyer
flasks. Each filtrate was titrated with 0.100 N sodium thiosulfate solution until the
solution resulted in a pale yellow color. Then, 2 ml of starch solution were added and
the titration was continued until one drop resulted in a colorless solution. The volume
of sodium thiosulfate used was recorded.
3.2.4 Nitrogen Adsorption
The activated carbons prepared were characterized by physical adsorption of
N2 at 77K using Quantachrome-Autosorb 6B. Prior to analysis, the sample was
degassed at 120C for 5 hour under vacuum. About 0.1 0.5g sample was used in
each adsorption experiment. Period for analysis about 6 12 hours. The BET
surface area is calculated from N2 adsorption isotherm using the BET method.
3.2.5 SEM-EDX
The surface morphology of the activated carbon were analyzed by using a
scanning electron microscope (SEM) Philips, Quanta FEG 200 model equipped with
an EDX spectrometer from Oxford instruments. XT microscope software was used
for data acquisition and analysis. The measurement was performed at high vacuum
mode with the accelerating voltage of 20kV. Microscope software was used for data
acquisition and analysis. Magnification that used are 400X - 50000X and the
zooming size, 100 Micro - 2 Micro.
41
CHAPTER 4
RESULT AND ANALYSIS
In this final year project, bamboo waste was utilized as the precursor for
activated carbon production using chemical activation and physical. Its adsorption
capacity as iodine number was evaluated. Other method that used for characterized
are Brunnete Emmet Teller method and scanning electron microscopy. Chemical
agents zinc chloride (ZnCl2) as a commercial activating agent that widely used in
industries.The effect of parameters to preparation condition i.e. the impregnation
ratio of raw material to activating agent and activation temperature were studied
in order to seek an optimal condition to produce activated carbon. The
resulting activated carbon was characterized the adsorption capacity as iodine
number due to it indicates the porosity of the activated carbon, and also the amount
of iodine that adsorbed to carbon as milligram per gram of carbon and was
commonly used in the industry as a rough estimation of the activated carbons
surface area. The experimental results are able to be explained as follows:
42
Following results were obtained:
Table 4.1: Sample activated carbon with chemical activation, (500C, impregnation
ratio, 1:1)
Run number
Weight of dry carbon used
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
23.1
1.9
22.6
2.0
20.9
Table 4.2: Sample activated carbon with chemical activation, (500C, impregnation
ratio, 1:2)
Run number
Weight of dry carbon use
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
23.9
1.9
23.4
2.0
22.4
Table 4.3: Sample activated carbon with chemical activation, (600C, impregnation
ratio, 1:1
Run number
Weight of dry carbon used
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
39.8
1.9
38.8
2.0
38.1
43
Table 4.4: Sample activated carbon with chemical activation, (600C impregnation
ratio, 1:2)
Run number
Weight of dry carbon use
Volume of Sodium
thiosulfate consumed
(mL)
1.8
42.0
1.9
41.3
2.0
38.5
Table 4.5: Sample activated carbon with physical activation, ( temperatures ,500C)
Run number
Weight of dry carbon used
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
72.3
1.9
68.5
2.0
62.5
Table 4.6 : Sample activated carbon with physical activation, ( temperatures, 600C)
Run number
Weight of dry carbon used
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
71.3
1.9
65.2
2.0
57.1
44
Table 4.7 : Sample activated carbon, commercial.
Run number
Weight of dry carbon used
Volume of Sodium
(g)
thiosulfate consumed
(mL)
1.8
11.5
1.9
10.9
2.0
9.2
Determination of iodine number:
X/M =
[A
( DF )( B)( S )]
M
Where:
S
= volume of sodium thiosulfate consumed, mL
= weight of carbon used, gram
and the normality of the residual filtrate (C) was calculated according to:
C =
( S .N 1 )
F
Where:
C = residual filtrate, N
N1 = sodium thiosulfate, N
F = filtrate, mL (50 mL of filtrate used from experiment)
S
= volume of sodium thiosulfate used
45
Calculation (Iodine Number)
1.
Sample activated carbon with chemical activation, 500C impregnation ratio,
1:1
For 1st run, where:
S
= 23.1 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
X/M
=
=
[A
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(23.1)]
1.8
= 346.80
with the value of C as follows:
(23.1)(0.1)
50
= 0.0462N
For 2nd run, where:
S
= 22.6 ml (volume of sodium thiosulfate solution consumed)
= 1.9 gram (weight of carbon used)
X/M
=
=
[A
( DF )( B)(S )]
M
[1269.3 (2.2)(12.693)(22.6)]
1.9
= 335.90
with the value of C as follows:
(22.6)(0.1)
50
= 0.0452N
46
For 3rd run, where:
S
= 20.9 ml (volume of sodium thiosulfate solution consumed)
= 2.0 gram (weight of carbon used)
X/M
[A
=
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(20.9)]
2.0
= 342.84
with the value of C as follows:
(20.9)(0.1)
50
C =
C = 0.0418N
2.
Sample activated carbon with chemical activation, 500C impregnation ratio,
1:2
For 1st run, where:
S
= 23.9 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(23.9)]
1.8
= 334.39
with the value of C as follows:
C =
(23.9)(0.1)
50
C = 0.047N
47
For 2nd run:
Where:
S
= 23.4 ml (volume of sodium thiosulfate solution consumed)
= 1.9 gram (weight of carbon used)
[A
X/M =
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(23.4)]
1.9
= 324.14
with the value of C as follows:
C =
(23.4)(0.1)
50
C = 0.0468N
For 3rd run:
Where:
S
= 22.4 ml (volume of sodium thiosulfate solution consumed)
= 2.0 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(22.4)]
2.0
= 321.89
with the value of C as follows:
C =
(22.4)(0.1)
50
C = 0.0448N
48
3.
Sample activated carbon with chemical activation, 600C impregnation ratio,
1:1
For 1st run, where:
S
= 39.8ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(39.8)]
1.8
= 87.72
with the value of C as follows:
(39.8)(0.1)
50
C =
C = 0.0796N
For 2nd run:
Where:
X/M
= 38.8 ml (volume of sodium thiosulfate solution consumed)
= 1.9 gram (weight of carbon used)
=
=
[A
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(38.8)]
1.9
= 97.80
with the value of C as follows:
C=
(38.8)(0.1)
50
C = 0.0776N
49
For 3rd run:
Where:
S
= 38.1 ml (volume of sodium thiosulfate solution consumed)
= 2.0 gram (weight of carbon used)
[A
X/M =
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(38.1)]
2.0
= 102.69
with the value of C as follows:
(38.1)(0.1)
50
C =
C = 0.0762N
4.
Sample activated carbon with chemical activation, 600C impregnation ratio,
1:2
For 1st run:
Where:
S
= 42.0 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(42.0)]
1.8
= 53.59
with the value of C as follows:
C =
(42.0)(0.1)
50
C = 0.084N
50
For 2nd run:
Where:
S
= 41.3 ml (volume of sodium thiosulfate solution consumed)
= 1.9 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(41.3)]
1.9
= 61.06
with the value of C as follows:
C =
(41.3)(0.1)
50
C = 0.0826N
For 3rd run:
Where:
X/M
= 38.5 ml (volume of sodium thiosulfate solution consumed)
= 2.0 gram (weight of carbon used)
=
=
[A
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(38.5)]
2.0
= 97.10
With the value of C as follows:
C =
(38.5)(0.1)
50
C = 0.077 N
51
5.
Sample activated carbon, commercial.
For 1st run:
Where:
S
= 11.5 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
[A
X/M =
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(11.5)]
1.8
= 526.76
with the value of C as follows:
C =
(11.5)(0.1)
50
C = 0.023N
For 2nd run:
Where:
S
= 10.9 ml (volume of sodium thiosulfate solution consumed)
= 1.9 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(10.9)]
1.9
= 507.85
with the value of C as follows:
C =
(10.9)(0.1)
50
C = 0.0218N
52
For 3rd run:
Where:
S
= 9.2 ml (volume of sodium thiosulfate solution consumed)
= 2.0 gram (weight of carbon used)
[A
X/M =
=
( DF )( B)( S )]
M
[1269.3 (2.2)(12.693)(9.2)]
2.0
= 506.20
with the value of C as follows:
C =
(9.2)(0.1)
50
C = 0.0184N
6.
Sample activated carbon with physical activation, 500C
st
For 1 run, where:
X/M
= 72.3 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
=
=
[A
( DF )( B)(S )]
M
[1269.3 (2.2)(12.693)(72.3)]
1.8
= - 416.47
7.
Sample activated carbon with physical activation, 600C
For 1st run, where:
X/M
= 72.3 ml (volume of sodium thiosulfate solution consumed)
= 1.8 gram (weight of carbon used)
[A
( DF )( B)(S )]
M
[1269.3 (2.2)(12.693)(71.3)]
1.8
=
= - 400.96
53
Table 4.8 : Result Iodine number analysis.
Sample activated carbon
Iodine number(mg/g)
Chemical, 500 C, 1:1
295
Chemical, 500 C, 1:2
270
Chemical, 600 C, 1:1
119
Chemical, 600 C, 1:2
96
Physical , 500 C
N/A
Physical , 600 C
N/A
Commercial
505
For iodine number graph, attach at appendices.
54
Table 4.9 : Result BET Analysis Data
Sample of
AC
Temperature
Surface
Area
(C)
(m2/g)
Average
pore
diameter
Total
Pore
Volume
Micropore
volume
(cc/g)
0.16850
0.1195
(cc/g)
Commercial
AC
500
235.50
(E)
28.61
Chemical,1:1
500
44.12
117.06
0.12970
0.0337
Chemical,1:2
500
53.10
38.42
0.05100
0.0276
Chemical,1:1
600
13.61
54.62
0.01859
0.0089
Chemical,1:2
600
306.00
77.75
0.05948
0.0310
Physical
500
339.10
46.49
1.04200
0.3223
Physical
600
79.63
30.69
0.06109
0.0438
55
Table 4.10 : Result SEM- EDX (percentage of atomic element) (%)
Sample Chemical Chemical Chemical Chemical Physical Physical Commercial
500C,
Element 1:1
500C,
600C,
600C,
1:2
1:1
1:2
500C
600C
AC
68.475
89.010
73.480
75.010
85.280
90.990
91.980
21.760
7.465
16.460
11.570
12.280
7.635
7.280
Si
0.105
Cl
2.340
0.740
2.000
4.310
8.770
0.085
Na
Ca
0.035
0.035
Fe
0.060
0.095
0.290
1.380
5.470
4.360
Zn
7.320
Al
P
Mo
0.245
0.230
1.760
0.880
0.145
0.185
0.290
0.065
0.050
0.080
56
Label.
Sample
AC, Chemical activation, temperature 500C, impregnation
ratio 1:1.
AC, Chemical activation, temperature 500C, impregnation
ratio 1:2.
AC, Chemical activation, temperature 600C, impregnation
ratio 1:1.
AC, Chemical activation, temperature 600C, impregnation
ratio 1:2.
AC, Physical activation, temperature 500C.
AC, Physical activation, temperature 600C.
AC, Commercial.
Table 4. 11 : Image AC ( SEM)
Sam
ple
1
Magnification
400X-1000X
Magnification
1500X- 5000X
Magnification
6000X-50000X
57
58
59
CHAPTER 5
DISCUSSION
This research follow by three method of characterize activated carbon. First,
use iodine test. Refer to ASTM, Designation: D4607- 94(Reapproved 1999)
Standard test Method for Determination of Iodine Number of Activated Carbon to
find the value of iodine number for synthesis activated carbon. Iodine test as a rough
estimate the surface area of the activated carbon. Iodine number for chemical
activation temperatures 500C better than 600C and physical activation. From data,
chemical activation for 500 C and impregnation ratio 1:1 is a best iodine number
among others activated carbon. This test also show, Commercial activated carbon
have good iodine number than synthesis AC . Iodine number for sample activated
carbon for physical activation are not available
.This are few factors that can
influence the result of iodine number Carbon raw material, processing condition and
pore volume distribution can influence the porosity for AC and affect measured
iodine number.
Second method, used BET method analysis. It is also known as physical
adsorption of N2 . The result for textural characteristic of sample prepared via
alternative method is summarized in Table 4.9. Data are tabulated for BET surface
area, average pore diameter, total pore volume and micropore volume. It is observed
that sample physical activation at temperature 500C exhibit the highest surface area
as well as total pore volume with the value of 339.1 m2/g and 1.042 cc/g
60
respectively. This BET analysis result shows the synthesis activated carbon have
good surface area and total pore volume than commercial AC. However, any
relationship between surface area and iodine number cannot be generalized.
Last method for characterized , used SEM-EDX . in generally this method to
see morphology and knows atomic percentage of element in each sample. During the
process of synthesis AC, many factors influence the existence of element. Refer to
table 4.10, this are element contain on activated carbon. Carbon, Oxygen, Silicon,
Chlorine, Potassium, Sodium, Calcium, Iron, Zinc, Aluminium, Phosphorus and
Molybdenum. The important element in activated carbon are carbon and oxygen.This
test shown, commercial activated carbon higher percentage atomic of carbon,
91.98%. the higher atomic percentage of element C among synthesis activated
carbon is sample physical activation ,temperature 600C is 90.99% and the lower
percentage atomic is sample chemical activation , temperature 500C, ratio 1:1,
68.475%. Refer to table 4.11 image SEM, we can see that physical activation are
more clearly shape type of pore than others sample AC.
61
CHAPTER 6
CONCLUSION
This research work has revealed some latent facts about the usefulness and
effectiveness of activated carbon produced from waste raw materials. From the
experimental information gathered, it has been shown in this study the result for
iodine number was different between each samples. It shows, temperatures,
impregnation ratio, method of activation have influence the iodine number and
adsorption capacity. From the characterized result, by using physical activation to
produce activated carbon is shows good result than chemical activation process it
have higher surface area, total pore volume and higher carbon content . However,
synthesis activated carbon from raw waste material still not achieve the appropriate
value refer to standard BET surface area and iodine number. That means, during
experimental maybe have some error that not qualified the standard procedure.
However, this problem can be improved in further investigate and study for waste of
bamboo to synthesis being activated carbon, such as assorted the variables that can
influence characteristic of activated carbon. Based on final year project, conclude
that waste of bamboo can produce and synthesis as activated carbon. Therefore, the
activated carbon produced and synthesis from waste bamboo (buluh lemang) been
established as commercial activated to apply in various industries The objective of
this project was successfully achieve.
62
REFERENCES
A.Linares-Solano, I. Martn-Gullon, C. Salinas-Martnez de Lecea, B. SerranoTalavera. (2000). Activated carbons from bituminous coal: effect of mineral matter
content. Fuel. 79(6). pp 635643.
Abd.Latif, M., W.A. W. Tarmeze, and A. Fauzidah. (1990). Anatomical features
and mechanical properties of three Malaysian bamboos. Journal Tropical Forest
Science. 2(3): 227-234.
Amada, S., Y. Ichikawa, T. Munekata, Y. Nagase, and K. Shimizu. (1997). Fiber
texture and mechanical graded structure of bamboo. Composite Part B. 28(B): 1320.
Ahmadpour A and Do D D.(1996). The preparation of active carbons from coal by
chemical and physical activation. Carbon,34(4), 471-479.
Austin Shepherd, P.E., C.I.H. (May 2001). Activated Carbon Adsorption for
Treatment of VOC Emissions Presented at the 13th Annual Environment Expo,
Boston Massachusetts.
ASTM International (1999), Standard Test Method for Determination of Iodine
Number of Activated Carbon Designation : D 4607, United States.
Bansal R C , Donnet J B, Stoeckli F. (1988). Active Carbon, New York.
Baksi, S., Biswas, S. and
Mahajan, S. (March 2003). Activated Carbon from
Bamboo Technology Development towards Commercialisation, Paper presented in
BAMTECH 2003, Guwahati.
63
Christopher A Toles, Wayne E Marshall , Mitchell M John, (1998) Phosphoric acid
activation of nutshells for metals and organic remediation process optimization
Journal Chemical technology and Biotechnology, 72, pp 255-263.
Chilton Ng, Jack N Losso, Wayne E, Marshall and Ramu M Rao. (2002). Physical
and chemical properties of selected agricultural by product-based activated carbons
and their ability to adsorb geomin. Bioresource technology .84. pp 177-185.
C. Srinivasakannan (12-14 February 2003), High Surface Area Activated Carbon
from Waste Biomass 2nd Regional Conference on Energy Technology Towards a
Clean Environment , Phuket, Thailand
Carolina R Loez, Mirta L Topalian and Alfredo Salibian. (1995) Effect of zinc on
the structure and growth dynamics of a natural freshwater phytoplankton assemblage
reared in the laboratory. Environmental pollution 88, pp 275-281.
D.Faulkner ,Mr.John E.Urbanic , Mr.Robert W.Ruckel (1987) . Activated Carbon
for precious metals recovery. Calgon Carbon Corporation Marketing.110th Annual
Meeting of the Society of Mining Engineers and the Metallurgical Society, Denver.
Devarly Prahas, Y. Kartika, N. Indraswati, S. Ismadji. (2008) Activated carbon
from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface
chemistry characterization. Chemical Engineering Journal.140. pp 32-42.
Don.o Burevski,(2004). Monolayer Capacity and Specific Surface of microporous
adsorbents. Bulletin of the Chemists and Technologists of Macedonia, Vol. 23, No.
1, pp.15.
Eric M. Suuberg, Indrek Aarna. (2007) Porosity development in carbons derived
from scrap automobile tires. Carbon. 45. pp 1719-1726.
FAO. 1997. Provisional outlook for global forest products consumption, production
and trade. Forestry Department, Policy and Planning Division, FAO, Rome.
64
F Rodriguez-Reinoso and M. Molina-Sabio. (1992). Activated carbons from
lignocellulosic materials by chemical and/or physical activation. An overview.
Carbon.30, pp 1111-1118.
Gielis, J. (2002). Future possibilities for bamboo in European agriculture. Oprins
Plant Sint-Lenaartsesteenweg . 91 B-2310 Rijkevorsel.
Hassler, J.W ,(1974). activated carbon, chemical publishing, New York, pp 169.
Hammett, A.L., R.L. Youngs, X.F. Sun, and M. Chandra.(2001). Non-wood fiber as
an alternative to wood fiber in Chinas pulp and paper industry. Holzforschung.
55(2):219-224.
IUPAC . (1972) Manual of symbols and terminology, app 2, pt 1, colloid and
surface chemistry. Pure applied chemistry, 31, pp 578.
Joanna Gorka, Aleksandra Zawislak, Jerzy Choma, Mietek Jaroniec. (2008) KOH
activation of mesoporous carbons obtained by soft-templating. Carbon. 46. pp
1159-1174.
Janssen, J.J.A. (1995). Building with bamboo. Intermediate Technology
Publication Limited, London. (2nd edition.) pp. 65.
J. Helena, S. Andrzej, and C. Jerzy. (1991). Active Carbon. Ellis Horwood Ltd.,
London.
Junichi Hayashi, Toshihide Horikawa, Katsuhiko Muroyama and Vincent G Gomes.
(2002), Activated Carbon from chickpea husk by chemical activation with K2CO3,
preparation and characterization .Microporous and Mesoporous Materials, 55, pp
63-68.
Khadija Qureshi, Inamullah Bhatti, Rafique Kazi , Abdul Khalique Ansari. (2008)
Physical and Chemical Analysis of Activated Carbon Prepared from Sugarcane
65
Bagasse and Use for Sugar Decolorisation. International Journal of Chemical and
Biological Engineering. 1:3
Kawamura, N., Katani, K.: The modification of culm epidermis. Proc. 40th Japan.
Timber Science. Conference. 1990,4.1
K.P Gadkaree, (1998) Carbon honeycomb structures for adsorption applications.
Carbon, pp 981-989,
L H Wartelle , WE Marshall , (2001) Nutshell as granular activated carbon
physical chemical and adsorptive properties, Journal of chemical technology and
biotechnology 76,pp 451-455.
Lakkad, S.C. and J.M. Patel. (1980.) Mechanical properties of bamboo, a natural
composite. Fiber Science Technologies. 14: pp 319-322.
Liese W. (1998). Anatomy of Bamboo Culms. International Network for Bamboo
and Rattan, Beijing.
Li, X. (2004). Physical, Chemical and Mechanical Properties of Bamboo and Its
Utilization Potential for Fiberboard Manufacturing. Thesis Master of Sciences.
M Ahmedna, WE Marshall, RM Rao. (2000) Surface Properties of granular
activated carbons from agricultural byproducts and their effects on raw sugar
decolorization, Bioresource technology , 71, 103-112.
M Kobya (2004) Adsorption kinetics and equlibrium studies of Cr(VI) by hazelnut
shell activated carbon. Adsorption science and technology , volume 22 number 1.
Minkova V, Razvigorova M, Goranova M, Ljutzkanov L and Angelova G. (2001)
Effect of water vapour on the pyrolysis of solid fuels Fuel, 70, pp 713-719.
McClure, F.A. 1967. The bamboos, A fresh perspective. Harvard University Press.
Cambridge, Massachusetts.
66
Nadhem K Hamadi , Xiao Dong Chen, Mohaqmmed M Farid , Max GH Lu (2001)
Adsorption kinetics for the removal of chromium (VI) from aqueous solution by
adsorbents derived from used tyres and saw dust . Journal of Chemical
Engineering,84, pp 95-105.
Nevin Yalem , Vadettin Sevine, (2000) Studies of the surface area and porosity of
actrivated carbon prepared from rice husk, Carbon vol 38 pp1943-1945.
Peter J F Harris1, Zheng Liu2 and Kazu Suenaga2 (2008) Imaging the atomic
structure of activated carbon Journal of physics, 20 362201 (5pp), Centre for
Advanced Microscopy, J J Thomson Physical Laboratory, University of Reading,
UK, and Research Centre for Advanced Carbon Materials, National Institute of
Advanced Industrial Science and Technology (AIST), Japan
Published by Deutsche Gesellschaft fr Technische Zusammenarbeit (GTZ) GmbH
(German Technical Cooperation), (July 2009). Selection and safe use of alternatives
to CTC Activated Carbon Testing, National CTC Phase-out Plan Edition 1,
Prof. Dr. Walter Liese, (Dec. 1992), BAMBOO AND ITS USE, International
Symposium on industrial use a bamboo, Beijing, China. International Tropical
Timber Organization.
Patrick Echlin (January 2009) . Handbook of Sample Preparation for Scanning
Electron Microscopy and X-Ray Microanalysis, Cambridge Analytical Microscopy,
United Kingdom .
Savova D, Apak E, Ekina E, Yardim F, Petrov N, Budinova T, Razvigorova M and
Minkovo V. (2001) Biomass conversion to carbon adsorbents and gas Biomass
and Bioenergy,21, 133-142.
T. Otowa, Y. Nojima T. Miyazaki. (1997) Development of KOH Activated High
Surface Area Carbon and Its Application to Drinking Water Purification. Carbon.
35(9). pp 13151319.
67
Tsutomu Suzuki, Kyoko Suzuki, Yukio Takahashi, Mitsuhiro Okimoto, Tetsuo
Yamada, Noriyasu Okazaki (2007). Nickel-catalyzed carbonization of wood for
coproduction of functional carbon and fluid fuels I: production of crystallized
mesoporous carbon. The Japan Wood Research Society, 53(1). pp 5456.
Wang, D., and S.J. Shen. (1987). Bamboos of China. Timber Press, Portland,
Oregon. pp. 428.4
Wang HW, Varmama RVRV & Tiansen X. (1998). Insect Pests of Bamboos in
Asia: An Illustrated Manual. International Network for Bamboo and Rattan,
Beijing.
68
APPENDICES
75
GANTT CHART PSM 1
Week
10
Task
Select the title
and
Confirmation.
Phase 1
Introduction
Literature
review
Selected material
E
R
M
Methodology
B
R
Report PSM 1
writing
E
A
Submit report
11
12
13
14
15
16
17
76
GANTT CHART PSM 2
Week
Task
PHASE 2
Physical
Activated Carbon
Development
Carbonization
Activation
Chemical Activation
Raw materials.
Reflux process.
Impregnation
Carbonization.
PHASE 3
Characterization
BET method
Nitrogen
adsorption
Iodine number
Final Report
10
11
12
13
14
15
16
17
18
77
Tulang belakang.
SHAFARUL MUSTAFA
BACHELOR OF MECHANICAL ENGINEERING (STRUCTURE AND MATERIALS)
2011 UTeM
You might also like
- GMP SsopDocument98 pagesGMP SsopNicoel100% (21)
- Concentration of Precious Metals During Their Recovery From Electronic WasteDocument11 pagesConcentration of Precious Metals During Their Recovery From Electronic WasteChrisNo ratings yet
- Cellulose: Structure, Properties and UsesDocument21 pagesCellulose: Structure, Properties and UsesabsuneelNo ratings yet
- Synthesis of Activated Carbon Using Orange and Lemon PeelDocument5 pagesSynthesis of Activated Carbon Using Orange and Lemon PeelAlessandra MoonNo ratings yet
- Activated CarbonDocument69 pagesActivated CarbonSaheed Adewale100% (2)
- A Four-Week Internship Report on PARCO's ETP and DHDS Assessment (39Document35 pagesA Four-Week Internship Report on PARCO's ETP and DHDS Assessment (39Aditya ChopraNo ratings yet
- Activated Carbon PDFDocument28 pagesActivated Carbon PDFCleverSeyramKetekuNo ratings yet
- Advantages and Disadvantages of The Hazardous Waste Treatment.Document4 pagesAdvantages and Disadvantages of The Hazardous Waste Treatment.Muslihah Mohd Razali100% (1)
- EIA Report (English Version)Document110 pagesEIA Report (English Version)sanji hNo ratings yet
- Biochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)Document35 pagesBiochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)wahyu hidayatNo ratings yet
- ENVIRONMENTAL SCIENCE: STUDY OF HUMAN AND NATURAL SYSTEMSDocument21 pagesENVIRONMENTAL SCIENCE: STUDY OF HUMAN AND NATURAL SYSTEMSprecious maningasNo ratings yet
- 4.2-1 Maintain Computer Systems PDFDocument16 pages4.2-1 Maintain Computer Systems PDFfaisal saghirNo ratings yet
- (III) PSSR PROCEDURE (Rev00) PDFDocument17 pages(III) PSSR PROCEDURE (Rev00) PDFDen Bagus Blitar100% (5)
- Develop Activated Carbon From Rice Husk by Using Acid Hydrochloric (HCL)Document5 pagesDevelop Activated Carbon From Rice Husk by Using Acid Hydrochloric (HCL)Chong Khai Lin100% (1)
- Waste Water TreatmentDocument3 pagesWaste Water TreatmentSana Saleem100% (1)
- Sciencedirect: Activated Carbon of Oil Palm Empty Fruit Bunch (Efb) Core and ShaggyDocument7 pagesSciencedirect: Activated Carbon of Oil Palm Empty Fruit Bunch (Efb) Core and ShaggyNierza AlfiannurNo ratings yet
- Review BiocharDocument10 pagesReview BiocharLeynard NatividadNo ratings yet
- Final Thesis 12 Co Iglesias Lomibao 1.0Document79 pagesFinal Thesis 12 Co Iglesias Lomibao 1.0coerick40No ratings yet
- Plasma Technology For Solid Waste ManagementDocument12 pagesPlasma Technology For Solid Waste Managementup4allNo ratings yet
- Home - Robert MuchamoreDocument160 pagesHome - Robert Muchamoredune125No ratings yet
- An Assignment On Design and Performance Evaluation of Effluent Treatment Plant (ETP) For Textile IndustryDocument28 pagesAn Assignment On Design and Performance Evaluation of Effluent Treatment Plant (ETP) For Textile IndustryRupiya Chakma100% (1)
- Production of Bio-Coal and Activated Carbon From BiomassDocument126 pagesProduction of Bio-Coal and Activated Carbon From BiomassChristi Naftali100% (1)
- Activated carbons from waste biomass for methylene blue adsorptionDocument9 pagesActivated carbons from waste biomass for methylene blue adsorptionyemresimsekNo ratings yet
- Martinez Vs Martinez, 1 Phil 182 Case DigestDocument1 pageMartinez Vs Martinez, 1 Phil 182 Case DigestJunjie Chalione0% (1)
- Report 2 Material and Energy Balance - Doc FinalDocument36 pagesReport 2 Material and Energy Balance - Doc Finalthembeka422100% (2)
- Activated Carbon From BambooDocument19 pagesActivated Carbon From BambooErik WeeksNo ratings yet
- Calgon CarbonDocument2 pagesCalgon CarbonHerik AziziNo ratings yet
- Msds Aplus RockwoolDocument6 pagesMsds Aplus Rockwoollucky mahadevaNo ratings yet
- Lecture 1 - Introduction - Biochemical EngineeringDocument10 pagesLecture 1 - Introduction - Biochemical EngineeringDarwin EugenioNo ratings yet
- 07 - Chapter 2 Adsorption Literature ReviewDocument59 pages07 - Chapter 2 Adsorption Literature ReviewMaheera MohamadNo ratings yet
- Municipal wastewater treatment using rice husk and kikar charcoalDocument4 pagesMunicipal wastewater treatment using rice husk and kikar charcoalIbrar ZahidNo ratings yet
- Waste Water Treatment in Chemical IndustriesDocument23 pagesWaste Water Treatment in Chemical IndustriesAzman Bin KadirNo ratings yet
- Removal of Methylene Blue Using Moss Grass and AlgaeDocument37 pagesRemoval of Methylene Blue Using Moss Grass and Algaesamar_biotech100% (2)
- Equilibrium, Kinetic and Thermodynamic Studies of Adsorption of Methylene Blue On Activated Carbon From Cocoa Pod ShellsDocument11 pagesEquilibrium, Kinetic and Thermodynamic Studies of Adsorption of Methylene Blue On Activated Carbon From Cocoa Pod ShellsIJAR JOURNALNo ratings yet
- RV College PACT ReportDocument14 pagesRV College PACT ReportHarish GowdaNo ratings yet
- Dye Removal From Textile Dye Wastewater Using RecycledDocument6 pagesDye Removal From Textile Dye Wastewater Using Recycledsabbir2029_310230403No ratings yet
- Biogas ProductionDocument7 pagesBiogas ProductionFagbohungbe MichaelNo ratings yet
- Biofilter DecisionsDocument23 pagesBiofilter DecisionsBokJrNo ratings yet
- Anaerobic DigestionDocument109 pagesAnaerobic DigestionAjaysingh BayasNo ratings yet
- Group 1 - Section 01G - Separation Project PDFDocument46 pagesGroup 1 - Section 01G - Separation Project PDFHatta AimanNo ratings yet
- Analysis of VOCs in Perfume Samples by Gas ChromatographyDocument14 pagesAnalysis of VOCs in Perfume Samples by Gas ChromatographyAninaNo ratings yet
- ECS 1002 Writing Session 1 - SKEDocument3 pagesECS 1002 Writing Session 1 - SKEWONG BAI SIONG A17KE0289No ratings yet
- Peroxo Compounds, InorganicDocument32 pagesPeroxo Compounds, InorganicKilsys AlvaradoNo ratings yet
- Biogas Production by Co-Digestion of Cassava Peels With UreaDocument3 pagesBiogas Production by Co-Digestion of Cassava Peels With UreaInnovative Research PublicationsNo ratings yet
- 6 CPMDocument70 pages6 CPMlakshminandan gogoiNo ratings yet
- Treatment and reuse of wastewater from beverage industryDocument7 pagesTreatment and reuse of wastewater from beverage industryChaeyoung YooNo ratings yet
- Project Report Mitesh & GRPDocument35 pagesProject Report Mitesh & GRPDishantNo ratings yet
- Biotreat Industrial EffluentsDocument9 pagesBiotreat Industrial EffluentsAmit ChristianNo ratings yet
- FGC Group LLC - Consulting & Engineering - Products - Thermal Processing Equipment - Activated Carbon Plant PDFDocument3 pagesFGC Group LLC - Consulting & Engineering - Products - Thermal Processing Equipment - Activated Carbon Plant PDFCleverSeyramKetekuNo ratings yet
- Caustic Recovery Tests ReportDocument17 pagesCaustic Recovery Tests ReportrashidafmNo ratings yet
- FYP Titles Chemical EngrDocument3 pagesFYP Titles Chemical EngrAsim MansoorNo ratings yet
- Plastic Waste (PET) To SyngasDocument9 pagesPlastic Waste (PET) To Syngaswaseemkhan49No ratings yet
- List of Tables List of Figures List of Abbreviations 1Document75 pagesList of Tables List of Figures List of Abbreviations 1AlohaaSwezzNo ratings yet
- Dairy Wastewater ETP Removes 94% of PollutantsDocument6 pagesDairy Wastewater ETP Removes 94% of PollutantsMortezaNo ratings yet
- Plant LocationDocument8 pagesPlant LocationEeHuey ChooNo ratings yet
- 2014 Cre IiDocument43 pages2014 Cre IiAnshul Agrawal100% (1)
- HumidificationDocument68 pagesHumidificationA AshokNo ratings yet
- Thickened Sludge Volume from Aeration WasteDocument3 pagesThickened Sludge Volume from Aeration WasteKai Faha LukumNo ratings yet
- Run Run Shaw Library: Users Must ComplyDocument123 pagesRun Run Shaw Library: Users Must ComplymusheermusheerNo ratings yet
- A Review: Heavy Metal Removal From Waste Water Using Tea and Coffee WasteDocument5 pagesA Review: Heavy Metal Removal From Waste Water Using Tea and Coffee WasteIJRASETPublicationsNo ratings yet
- Analysis of Enzyme Kinetics DataDocument4 pagesAnalysis of Enzyme Kinetics DataEureca Parra100% (2)
- Recent Developments of Textile Waste Water Treatment by Adsorption Process: A ReviewDocument14 pagesRecent Developments of Textile Waste Water Treatment by Adsorption Process: A ReviewAmin MojiriNo ratings yet
- BT 0413 Lab Methods Protein EstimationDocument21 pagesBT 0413 Lab Methods Protein EstimationvijaygovindarajNo ratings yet
- Indian Chlor-Alkali Industry Regional ReportDocument31 pagesIndian Chlor-Alkali Industry Regional ReportKu RatheeshNo ratings yet
- CPE680 Ethics EssayDocument2 pagesCPE680 Ethics EssayAeyrul KhairulNo ratings yet
- CNT 1Document11 pagesCNT 1Saba GheniNo ratings yet
- Synthesis of Activated Carbon and MCM-41 From BagaDocument6 pagesSynthesis of Activated Carbon and MCM-41 From BagaCarolina Restrepo LondoñoNo ratings yet
- Tensile Test WorksheetDocument5 pagesTensile Test WorksheetRaj Das100% (1)
- United States: (12) Patent Application Publication (10) Pub. N0.2 US 2013/0283854 A1Document16 pagesUnited States: (12) Patent Application Publication (10) Pub. N0.2 US 2013/0283854 A1wargajaya79No ratings yet
- Process Design and Costing of Bioethanol TechnologyDocument10 pagesProcess Design and Costing of Bioethanol Technologywargajaya79No ratings yet
- Tensile Test WorksheetDocument5 pagesTensile Test WorksheetRaj Das100% (1)
- Research On The Theory and Application of Adsorbed Natural Gas Used in New Energy Vehicles-A Review PDFDocument28 pagesResearch On The Theory and Application of Adsorbed Natural Gas Used in New Energy Vehicles-A Review PDFwargajaya79No ratings yet
- Na Blog - CZ: Pengalaman DiDocument6 pagesNa Blog - CZ: Pengalaman Diwargajaya79No ratings yet
- Specimen Preparation For Tensile Testing v.9.12.11Document2 pagesSpecimen Preparation For Tensile Testing v.9.12.11wargajaya79No ratings yet
- Tensile Test WorksheetDocument5 pagesTensile Test WorksheetRaj Das100% (1)
- 1bd6b02854ff2478affb41443bd18d2eDocument1 page1bd6b02854ff2478affb41443bd18d2ewargajaya79No ratings yet
- Guitar Necks and Fret BoardsDocument6 pagesGuitar Necks and Fret Boardswargajaya79No ratings yet
- Activated Carbon Properties of GACDocument2 pagesActivated Carbon Properties of GACwargajaya79No ratings yet
- How Well Do You Know Your Pipeline?Document4 pagesHow Well Do You Know Your Pipeline?wargajaya79No ratings yet
- Na Blog - CZ: Pengalaman DiDocument6 pagesNa Blog - CZ: Pengalaman Diwargajaya79No ratings yet
- Pik No MeterDocument7 pagesPik No Meterwargajaya79No ratings yet
- Investigation of Highpressure Selective AdsorptionDocument16 pagesInvestigation of Highpressure Selective Adsorptionwargajaya79No ratings yet
- What's New in Version 10Document43 pagesWhat's New in Version 10wargajaya79No ratings yet
- Card Operated Petrol BunkDocument6 pagesCard Operated Petrol BunksinxcosxNo ratings yet
- Responses of A LNG Receiving Terminal To Hurricane in Shallow WaterDocument1 pageResponses of A LNG Receiving Terminal To Hurricane in Shallow Waterwargajaya79No ratings yet
- Commercial and Technical Considerations in The Developments of Offshore Liquefaction PlantDocument12 pagesCommercial and Technical Considerations in The Developments of Offshore Liquefaction Plantwargajaya79No ratings yet
- Responses of A LNG Receiving Terminal To Hurricane in Shallow WaterDocument1 pageResponses of A LNG Receiving Terminal To Hurricane in Shallow Waterwargajaya79No ratings yet
- Juriah Mulyanti PDFDocument7 pagesJuriah Mulyanti PDFwargajaya79No ratings yet
- 10TPC 179firoozDocument1 page10TPC 179firoozwargajaya79No ratings yet
- Energy Focus Article April 2010Document3 pagesEnergy Focus Article April 2010wargajaya79No ratings yet
- Sadat HosseiniDocument4 pagesSadat Hosseiniwargajaya79No ratings yet
- 13 - 3 - New York - 09-69 - 0007Document3 pages13 - 3 - New York - 09-69 - 0007wargajaya79No ratings yet
- Petrol Rationing Smart Card SystemDocument12 pagesPetrol Rationing Smart Card Systemwargajaya79No ratings yet
- Pyrojet-Burner (34201)Document12 pagesPyrojet-Burner (34201)Long LêNo ratings yet
- ASL - (Worksheet)Document8 pagesASL - (Worksheet)gboy60210No ratings yet
- The Excretory System (The Urinary System) : Responsible For The Processing and Elimination of Metabolic Waste Consists ofDocument8 pagesThe Excretory System (The Urinary System) : Responsible For The Processing and Elimination of Metabolic Waste Consists ofDeeptanshu PrakashNo ratings yet
- Hempel Top Coat M.S.D.SDocument7 pagesHempel Top Coat M.S.D.Sfazeel mohammedNo ratings yet
- Week 2 - Assignment SolutionsDocument2 pagesWeek 2 - Assignment SolutionsSanyamNo ratings yet
- Ban On Plastic BagsDocument4 pagesBan On Plastic Bagsindian2011No ratings yet
- Defend SRG MSDS 1-1-2011Document1 pageDefend SRG MSDS 1-1-2011M. White DentalNo ratings yet
- Asda Stores LTD Is A British Super Market ChainDocument10 pagesAsda Stores LTD Is A British Super Market ChainSazzad Shahriar100% (1)
- Analytical Experiments ManualDocument132 pagesAnalytical Experiments ManualAnita Lim100% (1)
- Renewable Energy AssignmentDocument2 pagesRenewable Energy Assignmenttndud95No ratings yet
- Graunstadt v. USS-POSCO IndusDocument8 pagesGraunstadt v. USS-POSCO IndusNorthern District of California BlogNo ratings yet
- Sharma 2013Document9 pagesSharma 2013samandondonNo ratings yet
- Removal of Spilled Petroleum in Industrial Soils by Spent Compost of Mushroom Pleurotus PulmonariusDocument6 pagesRemoval of Spilled Petroleum in Industrial Soils by Spent Compost of Mushroom Pleurotus Pulmonarius8342921125No ratings yet
- NHDC Visit Report by Amit KumarDocument49 pagesNHDC Visit Report by Amit Kumaramit kumar67% (3)
- Epa 6020aDocument23 pagesEpa 6020acandyNo ratings yet
- Ojt LogbookDocument12 pagesOjt LogbookSandy UyNo ratings yet
- 370 PDFDocument2 pages370 PDFA MahmoodNo ratings yet
- IGBC Green Townships - Abridged Reference Guide (Pilot Version)Document63 pagesIGBC Green Townships - Abridged Reference Guide (Pilot Version)sarangmkulkarni0% (1)
- Toilet cleaning checklistDocument2 pagesToilet cleaning checklistGamer GirlNo ratings yet
- For Wording Letter of Sea Pollution and Its PreventionDocument4 pagesFor Wording Letter of Sea Pollution and Its PreventionnamemamunNo ratings yet
- Wall E OutlineDocument3 pagesWall E OutlineBallparkHippo99No ratings yet
- Uncertain Supply Chain ManagementDocument16 pagesUncertain Supply Chain ManagementAnonymous kTVBUxrNo ratings yet