Professional Documents
Culture Documents
BASF Patent On Double Contact Double Absorption
Uploaded by
AOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
BASF Patent On Double Contact Double Absorption
Uploaded by
ACopyright:
Available Formats
July 5, 1966
w. MULLER
3,259,459
PROCESS FOR THE PRODUCTION OF SULFUR TRIOXIDE
Filed Aug. 26, 1963
5\
4~
M8
L__
FIG]
,2
4/ 4/ f
~4
J
_I_NVENTOR.
W/LHELM MULLER
QWjWMIiYWQ M
ATTORNEQIS
United States Patent 0
CC
3,259,459
Patented July 5, 1966
3,259,459
dustrially in spite of the exceptional interest on the part
of the industry concerned with the production of S03 and
PROCESS FOR THE PRODUCTION OF
SULFUR TRIOXIDE
of the industrial control authorities in a substantial re
Wilhelm Miiller, Leverkusen, Germany, assignor to Far
duction of the S02 content in the waste gases from the
benfabriken Bayer Aktiengesellschaft, Leverkusen,
factories making sulfuric acid. This is obviously due to
the fact that when the above principle is followed, the
hot gas forming in the ?rst part of the catalysis is cooled
Germany, a corporation of Germany
Filed Aug. 26, 1963, Ser. No. 304,507
Claims priority, application Germany, Feb. 20, 1960,
F 30,595
11 Claims. (01. 23-176)
This application is a continuation-in-part of applica
tion Serial No. 89,137, ?led February 14, 1961, now
by the treatment with relatively cold sulfuric acid to the
temperature thereof and then has to be reheated to the
10 initiation temperature of the catalyst mass for carrying out
abandoned.
Sulfuric acid can be prepared catalytically by burning
the second catalysis. It is true that this heat requirement
is calculated to be recovered from the heat resulting from
the oxidation of S02 to S03, but as shown by experi
ments carried out on a large scale, it is exceptionally
industrially produced sulfur dioxide in admixture with 15 di?icult technically to maintain the necessary tempera
excess air while passing the gases at a suitable tempera
tures on account of the large heat exchanger surfaces and
ture, over catalysts (for example vanadium pentoxide
the unavoidable loss by radiation.
kieselguhr or platinum catalysts). It is only when the
The present invention is concerned with a process for
catalyst has predetermined minimum temperature, the
the production of SOS by the catalyst method in several
so-called initiation or ignition temperature (for example 20 stages, with interposition of an intermediate absorption,
450 C.) that the following reaction takes place:
the method comprising reacting the gas residue, which
remains after the removal of the S03 generated in the
forward part of the system, in another catalyst furnace
The value of the initiation temperature depends not
part at temperatures which are considerably lower, for
only on the catalysts, its composition and its method of 25 example 2060 C. or 4060 C. lower, than the initia
production, but also on the special furnace, wherein the
tion temperature obtaining prior to the dissolving out of
process is performed. Therefore, the initiation tempera
the S03. Contrary to former conceptions and experi
ture of a special catalyst determined in a laboratory equip
ment does not correspond to the value of the initiation
temperature necessary to start the reaction in a contact
furnace on a technical scale, with other words the initia
tion temperature is a value of some complexity.
The reaction heat which occurs on passing the gas
through the catalyst is proportional to the degree of con
version.
At a certain temperature which depends on the 35
ences, it has in fact been found that the gas which has
been freed from the S03 formed in the preliminary stages
surprisingly is oxidized further at temperatures which are
considerably below the expected minimum temperature,
i.e. the previously de?ned initiation temperature. This
fact could not be deduced or calculated from known
laws of physical chemistry.
Thus, the invention provides for removal of sulfur tri
initial composition of the gas, for example at 580 C.
oxide from partially converted gas between two of a suc
the reaction stops, because the speed of formation of the
cession of oxidation stages utilized for the conversion.
sulfur trioxide becomes equal to its speed 'of decomposi
The gas from which the sulfur trioxide is to be removed
tion. L1 order to produce a largest possible conversion
is cooled and the gas from which sulfur trioxide has been
in this catalyst portion, the temperature range should be 40 removed is heated to the initiation temperature of the
as large as possible, i.e. the initial temperature must be
oxidation stage to which it is passed following the re
kept as low as possible. In other words, the initiation
moval of sulfur trioxide. The process is characterized
temperature is chosen as the gas inlet temperature.
in that the heating to the initiation temperature of the
After this ?rst stage, the hot gas mixture is cooled in
subsequent oxidation stage is by indirect heat exchange
suitable manner to below the initiation temperature, for
with gas passed to the removal treatment. The gas
example by heat exchange or by direct or indirect cool
treated according to the invention for production of sul
ing, whereupon it is conducted through a second catalyst
fur trioxide can be a roasting gas in puri?ed condition
part, where heating again occurs. However, since con
and initially at less than about 70 C. The said gas can
siderable S03 is already present to inhibit the combustion,
be heated prior to contact with catalyst in an oxidation
the maximum temperature is now much lower, for ex 50 stage by indirect heat exchange with gas from at least
ample 500" C. After passing through this second cata
one of the oxidation stages. According to the invention,
lyst part, the mixture is again cooled to the initiation
the heating of gas for introduction into the various oxida
temperature and conducted through a third catalyst part
tion stages can be entirely by heat exchange with gas from
and possibly, after again being cooled, through a fourth
the oxidation stages. Thereby, use of an external supply
55 of heat is avoided.
catalyst part, and so on.
In this way, the combustion is carried a step further
The conversion can advantageously be carried out in
each time and theoretically to a degree of conversion
three stages, including an initial oxidation stage, a ?rst
which is dependent on the temperature of the gas leaving
oxidation stage from which the gas produced therein is
the last catalyst part and on the initial composition of
passed to the removal treatment, and a second oxidation
60 stage, wherein the ?nal conversion is carried out. The
the gas.
It is known (and is apparent from the law of mass ac
sulfur dioxide-containing gas serving as a feed gas for
tion) that higher conversions are obtained if gas which
the process can be heated from about 6070 C. to the
is already partially reacted is freed from the sulfur tri
temperature for the initial oxidation stage by indirect
heat exchange with product streams from various of the
furic acid, before it enters a further catalyst part. This 65 oxidation stages, and the product gas of the initial oxida
oxide already formed, for example by washing with sul
proposal is already fully explained in Handbuch der
Schwefelsaurefabrikation" by Bruno Waeser, volume III
(1930), pages 1492-1495, and is proved by numerical
examples. This principle, which theoretically should
tion stage can be cooled to the initiation temperature em
ployed in the ?rst oxidation stage by indirect heat ex
change with the feed gas to the initial oxidation stage.
The product gas of the ?rst oxidation stage is subjected
lead to a reduction of S02 concentration to below 0.05% 70 to absorption and is cooled and heated as has been de
of S02 in the waste gas, has not been fully exploited in
scribed above, and is then introduced into the second
3,259,459
3
oxidation stage at the initiation temperature obtaining
The new process has a number of advantages:
(1) The ultimate gases of the catalytical S02 combus
for that stage.
Apart from the technical simpli?cation produced by
this method, it also provides inter alia a considerable
lowering of the installation costs, namely to amounts
tion can be lead off to the atmosphere without highly
uneconomic puri?cation processes being necessary.
(2) As compared with processes which are carried out
with dilute S02 gases, the size of the gas-containing sys
tems in the entire apparatus can be kept small, since the
which can be borne economically, and also opens up the
possibility of carrying out the second oxidation stage in
temperature zones which permit a higher degree of con
version on the basis of the law of mass action.
The present invention is also concerned with a process 10
SO2-containing gases are not diluted during the entire
process by the admixture of air. This involve not
for lowering the initiation temperature of catalyst masses
for the oxidation of sulfur dioxide to sulfur trioxide, and
consists in that the sulfur trioxide formed in the partially
time and space.
only substantial economical advantages in the building
of the apparatus, but also increased yields per unit of
(3) Since the positive heat evolved in the reaction of
S02 to 50;, is substantially utilized in the plant, energy
reacted gas is wholly or partially extracted from the
15 need not be supplied from outside, even if cold SO2-con
latter.
The invention is further described in reference to the
accompanying drawings, wherein:
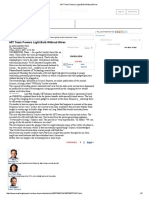
FIG. 1 is a ?ow sheet for a procedure according to the
invention; and
taining gases are used as starting products. Thus, the
heat energy obtained in the puri?cation of roasting gases
can be utilized for the production of steam. In the same
manner the heat of sulfur combustion gases which can
FIG. 2 is a ?ow sheet for another procedure accord 20 be supplied to the catalytical reaction without puri?cation
and without cooling, can be utilized for power generation.
ing to the invention.
The invention is further described in the following ex
In the drawings, like reference characters refer to
amples. In the examples, the initiation temperature of
corresponding parts.
a gas fed to a subsequent stage and from which 50;, has
Referring to FIG. 1, a sulfur dioxide containing gas
is introduced into a ?rst oxidation stage 4 and from this 25 been removed, is compared with the initiation tempera
ture of the gas fed to the preceding stage, as indicating the
oxidation stage, the product gas passes through an
signi?cance of the invention. The signi?cance of the in
absorber 6 wherein some of the sulfur trioxide present in
vention is at least this much, since the initiation tempera
the gas is absorbed by contacting the gas with sulfuric
ture of the gas before 80;, removal is as high or higher
acid. The product gas from the absorber is passed in
indirect heat exchange relation with the product gas 30 than the initiation temperature of the gas fed to the
preceding stage.
from the ?rst oxidation stage in the heat exchanger 5.
Example 1a
From the heat exchanger 5, the product gas of the
absorber passes through the second oxidation stage 8,
In an industrially produced roasting gas with 9.3% of
wherein the ?nal conversion is effected.
S02 and preheated to the initiation temperature of 450 C.
35
The operation of the process is reproduced diagram
matically in the accompanying drawing, FIG. 2. The
10% roasting gases arriving from the roasting furnace
pass, for the major part, through heat exchanger 1 and
to 460 C. about 85 to 90% of the $02 was converted to
S03 in a multistage kieselguhr-V2O5 catalytic furnace. By
introducing this gas mixture into an absorber of usual
design, all the S03 was dissolved out of the mixture with
2 and are heated therein with indirect heat exchange by
sulfuric acid. The residual gas cooled to 60 to 80 C.
40
means of hot catalyst gases to the starting temperature
was heated by a means of a heat exchanger to a temper
and then pass into a ?rst catalyst stage 3. For high S02
ature of only 390 to 410 C., this being in contrast to the
concentrations, such as 912%, the temperature rise with
preheating to 450 to 460 C. After having passed through
the oxidation to S03 is so large that the catalyst composi
the second part of the catalytic furnace, which contained
tion could suffer damage. In order to avoid this, cold
a single layer of catalyst, the total conversion was 99.6 to
roasting gas is supplied to the ?rst catalyst stage. The 45 99.8% of the sulfur dioxide which originally was present.
roasting gases react in the ?rst catalyst stage 3 and are
Example 1b
cooled in the heat exchanger 2 to the starting temperature
before they enter a second catalyst stage 4, which they
In an industrially produced roasting gas with 9.5%
leave with a conversion of about 8095%. The hot
of S02 and preheated to an initiation temperature of 360
catalyst gases of the second stage are cooled in a heat 50 C. about 95% of S02 was converted to S03 in a multi
exchanger 5 and then enter an intermediate absorber 6.
stage kieselguhr Kaoline platinum catalytic furnace. By
According to the invention, the height of the trickling
introducing this gas mixture into an absorber of usual de
sign all the SO;, was dissolved out of the mixture with
layer in this absorber can be only about 20-30% of that
of the ?nal absorber. The roasting gases leaving the
sulfuric acid. The residual gas cooled to 60 to 80 C.
intermediate absorber 6 are freed from entrained acid 55 was heated by means of a heat exchanger to a temper
by means of an asbestos ?lter 7 or any other suitable drop
ature of only 310 C., this being in contrast to the pre
separator. They are then preheated again to the starting
heating to 360 C. After having passed through the sec
or initiation temperature of the catalyst stage 8, in the
ond part of the catalytic furnace which contained a single
heat exchanger 5, and then are reacted once again in
layer of catalyst the total conversion was 99.5% to 99.6%
a catalyst stage 8 and, after passing through the heat 60 of the sulfur dioxide which originally was present.
exchanger 1, they are almost completely absorbed in the
Example 2
?nal absorber (not shown).
The total conversions are 99.5% and higher.
It is ad
A hurdle-type catalyst furnace of conventional design
with three vanadium catalyst stages, which produced a
hot absorption, and a hot absorption is the subject of 65 conversion of 97.5% when charged with approximately
7% roasting gas and with a loading of 22 tons S03 per
copending application Serial No. 137,779, ?led Septem
day, produced a conversion of only 94.5% with the same
ber 13, 1961. Whereas the residual gases which must
loading but with an iron pyritcs roasting gas with 10%
be heated again for further catalysis are cooled to 60
of S02 and
0f 02.
70 C. by absorption in the trickling towers with cold 70
An identical catalyst furnace, except that before the
98% sulfuric acid, they are not or only slightly cooled
last stage, the 50;, so far formed with absorbed by sulfuric
with the hot absorption. The hot absorption has the
acid, produced a conversion of 99.6% with the same
essential advantage that the heat exchange surfaces for
loading with pyritcs roasting gases (10% S02, 8% 02)
reheating the gas issuing from the intermediate absorp
without dilution with air. In this case, the input tem
tion can be kept correspondingly smaller.
perature of the ?rst catalyst stage was 451 C., the reac
vantageous to carry out the intermediate absorption as a
3,259,459
tion temperature prior to admixture of the cold roasting
gas was 590 C. and, after the mixing, 552 C.; the gas
initiation temperature which would .be required -by said
catalyst in said further catalytic oxidation stage without
prior sulfur trioxide separation.
6. Improvement according to claim 1 in which the same
type of oxidation catalyst is utilized in said ?rst and fur
ther catalytic oxidation stages and in which the gas is
left the ?rst catalyst stage 3 at 580 C. and a conversion
of 73.2%. The gas cooled by a heat exchanger to 450
C. entered the second stage. The discharging gas had a
temperature of 497 C. and a conversion of 90.6%.
After cooling to 175 C. using a heat exchanger, the gas
introduced into said further oxidation stage at a tempera
ture about 20 to 60 C. below the temperature of intro
entered the intermediate absorber, in which it was further
duction into said ?rst catalytic oxidation stage.
cooled to 50 C. The residue of gas freed from the 80;,
7. Improvement according to claim 1 in which the same
was preheated by way of the same heat exchanger to 428 10
type of oxidation catalyst is utilized in said ?rst and fur
C. In the last catalyst stage, the temperature rose to 450
ther catalytic oxidation stages and in which the gas is
C.; the total conversion was 99.6%.
Example 3
introduced into said further oxidation stage at a tem
perature about 40 to 60 C. below the temperature of
The same catalytic furnace as was used in Example 2 15 introduction into said ?rst catalytic oxidation stage.
with intermediate absorption produced a conversion of
8. Improvement according to claim 1 in which the
98.7% with a loading of 30 tons of S03 per day, when
oxidation catalyst in said ?rst and further catalytic oxida
using a gas containing 12.2% of S02 and 9% of 02. The
tion stages is a platinum catalyst and in which the gas is
input temperature into the ?rst catalyst stage was 440 C.;
introduced into said further stage at a temperature about
it was 610 C. before admixing the cold gas and 510 C.
40 to 60 C. below the temperature of introduction into
thereafter. The gas left the ?rst catalyst stage at 571 C.
the ?rst stage.
>
and with a conversion of 80%, was cooled by means of
9. Improvement according to claim 1 in which the oxi
a heat exchanger to 448 C. and introduced into the sec
dation catalyst in said ?rst and further catalytic oxidation
ond catalyst stage. In the latter, the temperature rose to
stages is a vanadium catalyst and in which the gas is
507 C. and the conversion to 92.7%. Thereafter, cool
introduced into said further stage at a temperature about
ing was carried out to 217 C. by means of a heat ex
40 to 60 C. below the temperature of introduction into
changer and in the intermediate absorber to 64 C.; the
the ?rst stage.
residual gas was heated again in the same heat ex
10. Improvement according to claim 1 in which said
changer to 412 C. and introduced into the third catalyst
sulfur trioxide-containin g gas is a puri?ed roasting gas hav
stage, in which the temperature rose to 439 C. and the 30 ing an initial temperature below about 70 C. and which
total conversion to 99.7%.
includes heating the gas prior to introduction into the
While the invention has been described with reference
?rst catalytic oxidation stage by indirect heat exchange
to particular embodiments thereof, these embodiments are
contact with the tail gas from at least one of the oxida
merely representative and do not serve to de?ne the limits
tion stages.
.
35
of the invention.
11. Improvement according to claim 1 in which the
What is claimed is:
1. In the process for the production of sulfur trioxide
by the catalytic oxidation of sulfur dioxideacontaining
gases the improvement which comprises introducing the
sulfur dioxide-containing gases into a ?rst catalytic oxi 40
dation stage containing an oxidation catalyst at the initia
tion-temperature for said catalyst to thereby partially
catalytically convert the sulfur dioxide to sulfur trioxide,
separating the sulfur trioxide from the tail gas from said
?rst catalytic oxidation stage and thereafter passing the 45
tail gas into at least one further catalytic oxidation stage,
containing an oxidation catalyst, at a temperature lower
than the initiation temperature of the ?rst stage and at
least about 20 C. below the initiation temperature which
would be required by said catalyst in said further cata 50
lytic oxidation stage without prior sulfur trioxide separa
tion.
2. Improvement according to claim 1 in which the
tail gas from said ?rst catalytic oxidation stage is cooled
for said sulfur trioxide separation and thereafter prior
to being passed to said further catalytic oxidation stage
is heated by indirect heat exchange contact with the tail
gas being passed for said sulfur trioxide separation.
sulfur dioxide~containing gas is passed through an initial
catalytic oxidation stage prior to being passed to said
?rst catalytic oxidation stage and is cooled prior to being
passed to said ?rst catalytic oxidation stage by indirect
heat exchange contact with the gas being introduced into
said initial oxidation stage.
References Cited by the Examiner
UNITED STATES PATENTS
719,332
1,930,125
1/1903
10/1933
Herreshoff _________ __ 23176
Fowler ___________ __ 23176
2,471,072
5/ 1949
Merrian __________ __ 23175
2,879,135
3/1959
Haltrneier _________ __ 23174
OTHER REFERENCES
Auden, H. A.: Sulfuric Acid and Its Manufacture,
London, Longmans, Green and Company.
Duecker et :al.: Manufacture of Sulfuric Acid, Rein
hold Publishing Company, New York (1959) pp. 163,
164.
Duecker, W. W., and West, J. R.: The Manufacture of
Sulfuric Acid, New York, Reinhold Publishing Co., 1959.
3. Improvement according to claim 1 in which the
Fairlie: Sulfuric Acid Manufacture, Reinhold Publish
oxidation catalyst in said ?rst and further catalytic oxida 60 ing Corp., New York (1949) pp. 377, 378.
tion stages is a platinum catalyst.
Miles, F. D.: The Manufacture of Sulfuric Acid, New
4. Improvement according to claim 1 in which the
York, D. Van Nostrand Co. (1925) pages 168-172.
oxidation catalyst in said ?rst and further catalytic oxi
dation stages is a vanadium catalyst.
OSCAR R. VERTIZ, Primary Examiner.
5. Improvement according to claim 1 in which the 65 MAURICE A. BRINDISI, Examiner.
tail gas is passed into said further catalytic oxidation
R. M. DAVIDSON, Assistant Examiner.
stage at a temperature about 40 to 60 0, below the
You might also like
- Saturated Steam Properties in a TableDocument68 pagesSaturated Steam Properties in a TableEirojram MarjorieNo ratings yet
- Flow Diagram Sulphuric Acid PlantDocument3 pagesFlow Diagram Sulphuric Acid PlantAnisa SudarmajiNo ratings yet
- Hargreaves ProcessDocument7 pagesHargreaves ProcessMuhammad BilalNo ratings yet
- Proposal KP HolcimDocument27 pagesProposal KP HolcimPutu Trisnayadhi DharmawanNo ratings yet
- Lampiran PerhitunganDocument15 pagesLampiran PerhitunganAchmadJa'farShodiqShahabNo ratings yet
- Heri Septya Kusuma: BiographyDocument13 pagesHeri Septya Kusuma: BiographyAstrolabeNo ratings yet
- Gambar 10. Diagram Alir Proses CD-IIDocument14 pagesGambar 10. Diagram Alir Proses CD-IIJulia Dwi Lestari0% (1)
- Analysis of The Adsorption Process and of Desiccant Cooling SystemsDocument155 pagesAnalysis of The Adsorption Process and of Desiccant Cooling SystemsRajesh VyasNo ratings yet
- Database CP Delta H Delta GDocument18 pagesDatabase CP Delta H Delta GsafinaNo ratings yet
- Reaction Kinetics of Ammonia & Nitric AcidDocument116 pagesReaction Kinetics of Ammonia & Nitric AcidMonica Garcia100% (1)
- Data Komposisi Gas AlamDocument23 pagesData Komposisi Gas AlamFakhri FachrezaNo ratings yet
- Tabel Antoine 1Document3 pagesTabel Antoine 1atikaindrnNo ratings yet
- Downterm ADocument2 pagesDownterm Awiyatmi0% (1)
- KAJIAN ANALISA KUALITAS AIR LIMBAH INFLUEN DAN EFFLUEN TERHADAP KINERJA WASTE WATER TREATMENT FACILITY DI PETROCHINA INTERNATIONAL JABUNG LTDDocument19 pagesKAJIAN ANALISA KUALITAS AIR LIMBAH INFLUEN DAN EFFLUEN TERHADAP KINERJA WASTE WATER TREATMENT FACILITY DI PETROCHINA INTERNATIONAL JABUNG LTDSuhendra, M.ScNo ratings yet
- Ocw Chapter 4Document48 pagesOcw Chapter 4Agam HanasichulaNo ratings yet
- RP44B TocDocument13 pagesRP44B TocPiespi PitwomNo ratings yet
- New agitated reactor and heat exchanger installation cost estimateDocument2 pagesNew agitated reactor and heat exchanger installation cost estimateDiego MoralesNo ratings yet
- Manufacture of Monobasic Potassium PhosphateDocument190 pagesManufacture of Monobasic Potassium PhosphateIndra Setio PujiNo ratings yet
- Fundamentals of Sulfur Recovery by Claus ProcessDocument45 pagesFundamentals of Sulfur Recovery by Claus Processl24n1rd4No ratings yet
- Biogas (Methane) EnglishDocument8 pagesBiogas (Methane) Englishveluthambi8888100% (1)
- Perancangan Proses Kimia 1Document15 pagesPerancangan Proses Kimia 1RantyNo ratings yet
- Tutorial Katalis BerporiDocument2 pagesTutorial Katalis BerporiMichael LevyNo ratings yet
- Ruzicka CP Estimation MethodDocument11 pagesRuzicka CP Estimation MethodAndreea Cristina PetcuNo ratings yet
- 1.3 Boiler OperationDocument23 pages1.3 Boiler OperationLydia RupidaraNo ratings yet
- Distillation and Hydrotreating ComplexDocument1 pageDistillation and Hydrotreating ComplexKatharina AjengNo ratings yet
- Lec 03 - Reactor 2020 ComparingDocument29 pagesLec 03 - Reactor 2020 ComparinggilangNo ratings yet
- Ethylene MsdsDocument9 pagesEthylene MsdsSyafiqa ZamriNo ratings yet
- Silica Plastic BlockDocument5 pagesSilica Plastic Blockdharshini deivasigamaniNo ratings yet
- AND Optimization OF Three Existing Ethylbenzene Dehydrogenation Reactors in SeriesDocument5 pagesAND Optimization OF Three Existing Ethylbenzene Dehydrogenation Reactors in SeriesMuhammad Ridwan TanjungNo ratings yet
- Butadiene SulfoneDocument58 pagesButadiene SulfoneChunchu AnilNo ratings yet
- Chemical Engineering Projects Can Be Divided Into Three TypesDocument25 pagesChemical Engineering Projects Can Be Divided Into Three Typestrungson1100% (1)
- PERHITUNGAN NERACA ENERGI BIOMASSADocument18 pagesPERHITUNGAN NERACA ENERGI BIOMASSARian Al-ayyubNo ratings yet
- Msds Palm Oil RBDDocument4 pagesMsds Palm Oil RBDSintaSofianaPutriNo ratings yet
- Patent Pabrik Phenyl Ethyl AlcoholDocument6 pagesPatent Pabrik Phenyl Ethyl AlcoholFaizhal DimazNo ratings yet
- Ionic Exchange HazopDocument64 pagesIonic Exchange Hazopjotas254No ratings yet
- Intro To Methyl Chloride Plant 1Document57 pagesIntro To Methyl Chloride Plant 1Kimberly ConleyNo ratings yet
- Kinetics and Mechanism of Urea FormaldehydeDocument5 pagesKinetics and Mechanism of Urea FormaldehydeDessy A. SariNo ratings yet
- Wo 2014185872 A 1Document11 pagesWo 2014185872 A 1Shahid AliNo ratings yet
- Presentasi TRK Topik 3 - Kelompok 8Document19 pagesPresentasi TRK Topik 3 - Kelompok 8Martha IvanaNo ratings yet
- LAMPIRAN Perhitungan Resin Urea FormaldehidDocument12 pagesLAMPIRAN Perhitungan Resin Urea FormaldehidAmiruddin KubikNo ratings yet
- Prediction of Deactivation Rates and Mechanisms of Reforming CatalystsDocument2 pagesPrediction of Deactivation Rates and Mechanisms of Reforming CatalystsSarahEkaPutriDarlismawantyaniNo ratings yet
- Heat Capacity of Liquids - Critical Review and Recommended ValuesDocument404 pagesHeat Capacity of Liquids - Critical Review and Recommended ValuesDoris AngNo ratings yet
- AfdhalDocument11 pagesAfdhalRiky Mario YuluciNo ratings yet
- Kinetics and Modelling of The S02 To The SO3 ProcessDocument23 pagesKinetics and Modelling of The S02 To The SO3 ProcessAbdullah KhanNo ratings yet
- Risky Septian Proposal KP PT Salim Ivomas Pratama TBKDocument12 pagesRisky Septian Proposal KP PT Salim Ivomas Pratama TBKRisky SeptianNo ratings yet
- Pemanfaatan Cangkang Biji Pala Sebagai Briket Dengan Proses PirolisisDocument7 pagesPemanfaatan Cangkang Biji Pala Sebagai Briket Dengan Proses PirolisisenvistNo ratings yet
- Daftar PustakaDocument2 pagesDaftar PustakaHammany Nur ZulkyNo ratings yet
- Erza Novarindra PT Tribhakti Inspektama English Test, Form Aplikasi Lamaran, Test DiscDocument5 pagesErza Novarindra PT Tribhakti Inspektama English Test, Form Aplikasi Lamaran, Test DiscMuhammad Meldi Oktariandi100% (1)
- Sizing StrippingDocument14 pagesSizing StrippingEka trisnawatiNo ratings yet
- Daftar Konstanta AntoineDocument3 pagesDaftar Konstanta AntoineEllen Novian MufidahNo ratings yet
- Lamp IranDocument8 pagesLamp IranWint RukmanaNo ratings yet
- Galuh Cakra Panigas - Yayuk Deviyanti - 2bd4 - PFD Pt. Samator Gas - Referensi PFDDocument1 pageGaluh Cakra Panigas - Yayuk Deviyanti - 2bd4 - PFD Pt. Samator Gas - Referensi PFDlebay cokNo ratings yet
- Rustic Wood Toy Manufacturing Cash Flow AnalysisDocument2 pagesRustic Wood Toy Manufacturing Cash Flow AnalysispratitatriasalinNo ratings yet
- MIXER DESIGN FOR BIODIESEL AND TOCOPHEROL MIXTUREDocument13 pagesMIXER DESIGN FOR BIODIESEL AND TOCOPHEROL MIXTUREkurniawanNo ratings yet
- TPL P&id PDFDocument1 pageTPL P&id PDFbinay kumarNo ratings yet
- Double Conversion Sulfuric Acid Process Using Cold Feed GasDocument11 pagesDouble Conversion Sulfuric Acid Process Using Cold Feed GasOji Luthpiansyah FazrinNo ratings yet
- SAPDocument16 pagesSAPsourav84No ratings yet
- Effect of Reaction Furnace and Converter Temperatures On Performance of Sulfur Recovery Units (SRUs)Document3 pagesEffect of Reaction Furnace and Converter Temperatures On Performance of Sulfur Recovery Units (SRUs)SEP-PublisherNo ratings yet
- Claus process converts H2S to sulfurDocument5 pagesClaus process converts H2S to sulfurAshish SutariyaNo ratings yet
- The Chemical Industry - FeedstockDocument12 pagesThe Chemical Industry - FeedstockANo ratings yet
- 8 2 7Document19 pages8 2 7Hussain AbouelkhairNo ratings yet
- MIT Team Powers Light Bulb Without WiresDocument2 pagesMIT Team Powers Light Bulb Without WiresANo ratings yet
- Chapter 16 Lime SofteningDocument10 pagesChapter 16 Lime SofteningPankaj SinghNo ratings yet
- The Chemical Industry - FeedstockDocument12 pagesThe Chemical Industry - FeedstockANo ratings yet
- Improve Salt Purity With New Washery UnitDocument7 pagesImprove Salt Purity With New Washery UnitANo ratings yet
- 8 2 7Document19 pages8 2 7Hussain AbouelkhairNo ratings yet
- Pharmaceutical Inorgcanic Chemistry: CHAPTER 1: Group Properties of ElementsDocument13 pagesPharmaceutical Inorgcanic Chemistry: CHAPTER 1: Group Properties of Elementsren100% (1)
- 9701 w09 QP 12Document6 pages9701 w09 QP 12FahmiAlziePutraNo ratings yet
- Biological Corrosion of MetalsDocument12 pagesBiological Corrosion of MetalsStanley KitowskiNo ratings yet
- Atmos Pollution Exam QuestionsDocument7 pagesAtmos Pollution Exam QuestionslaxsannithiyakumarNo ratings yet
- MSDS Full1Document92 pagesMSDS Full1Sunthron SomchaiNo ratings yet
- ChE 140 - Sulfur and Sulfuric AcidDocument29 pagesChE 140 - Sulfur and Sulfuric AcidMarialie EnecioNo ratings yet
- Top 10 World'S Air Polluted Countries: 1-ChinaDocument5 pagesTop 10 World'S Air Polluted Countries: 1-ChinaPRINTDESK by DanNo ratings yet
- Biodiesel Standards ASTM D6751 EN14214Document3 pagesBiodiesel Standards ASTM D6751 EN14214Gregoritha RomanNo ratings yet
- 0620 Chemistry Example Candidate Responses Booklet 2012Document158 pages0620 Chemistry Example Candidate Responses Booklet 2012sookchin100% (2)
- Biogeochemical ProcessDocument7 pagesBiogeochemical ProcessDarling AprilNo ratings yet
- HPCL IT DHDS Block OverviewDocument37 pagesHPCL IT DHDS Block OverviewSrija Mummidi100% (1)
- 9E Reactions of Metals andDocument18 pages9E Reactions of Metals and陳信羽No ratings yet
- Acid Rain: Acid Rain Is Basically Rain That Has A Higher Than Normal Acid Level (Low PH)Document36 pagesAcid Rain: Acid Rain Is Basically Rain That Has A Higher Than Normal Acid Level (Low PH)Arjit GoswamiNo ratings yet
- Bryers1996 PDFDocument92 pagesBryers1996 PDFBayu LukmanaNo ratings yet
- CH 01Document14 pagesCH 01jessicasjsNo ratings yet
- Short Notes On Carbon Cycle, Nitrogen Cycle and Sulphur CycleDocument16 pagesShort Notes On Carbon Cycle, Nitrogen Cycle and Sulphur Cyclesivaaero41No ratings yet
- 10 Soil MicrobiologyDocument45 pages10 Soil Microbiologydrahmad100% (4)
- Chemistry Exam On Bonding and Moles Total Mark: 45 Duration: 1 Hour 30 MinutesDocument5 pagesChemistry Exam On Bonding and Moles Total Mark: 45 Duration: 1 Hour 30 MinutesMusic BatNo ratings yet
- CSR Catalog ColorDocument48 pagesCSR Catalog ColorMaritza LopezNo ratings yet
- Warning and DisclaimerDocument661 pagesWarning and DisclaimerA AhmedNo ratings yet
- 545 Uce Paper 1 OkDocument175 pages545 Uce Paper 1 OkAnonymous M7aBZlNo ratings yet
- Chapter 9 Manufactured Substances in IndustryDocument10 pagesChapter 9 Manufactured Substances in IndustryEric ChewNo ratings yet
- CHECKING WATER QUALITYDocument20 pagesCHECKING WATER QUALITYVaibhav AgarwalNo ratings yet
- Common Names of ChemicalsDocument6 pagesCommon Names of ChemicalstpplantNo ratings yet
- Industrial Chemistry Modules Cover Unit Operations ProcessesDocument194 pagesIndustrial Chemistry Modules Cover Unit Operations ProcessesSiti Mastura Abdul Rahman50% (2)
- Best Practices For Solid Sulphur Handling Dust Mitigation and Control PDFDocument25 pagesBest Practices For Solid Sulphur Handling Dust Mitigation and Control PDFpanoram bou20No ratings yet
- Ma Chemical SuicideDocument26 pagesMa Chemical SuicideRahul VermaNo ratings yet
- Naming Chemical Compounds WorksheetDocument4 pagesNaming Chemical Compounds WorksheetChii YenNo ratings yet
- Abbott 1925Document3 pagesAbbott 1925Nguyễn Thị Lan PhươngNo ratings yet
- Resourceful Mock Ii Examinations 2017Document8 pagesResourceful Mock Ii Examinations 2017Baguma MichaelNo ratings yet