Professional Documents
Culture Documents
Simulation of Manufacturing Process of Nitrobenzene
Uploaded by
Rashid FikriCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Simulation of Manufacturing Process of Nitrobenzene
Uploaded by
Rashid FikriCopyright:
Available Formats
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
INDEX
SR.NO.
CONTENTS
PAGE NO.
Chapter 1
Introduction
Chapter 2
Literature Review
2.1. Process For Production Of Nitrobenzene
2.2. Selection Of Process
2.3. Manufacturing Process Of Nitrobenzene
2.4. Chemical And Physical Properties
3
Chapter 3
15
Thermodynamic Feasibility
3.1. Reaction Data For Formation Nitrobenzene
3.2. Calculations
Chapter 4
23
Design Of Distillation Column
Chapter 5
29
Simulation Using Aspen
5.1 Introduction to Aspen
5.2 Starting With Process Simulation
6
Chapter 6
49
Result summary
6.1 Material Balance Over Reactor
6.2 Material Balance Over Decanter
6.3 Material Balance Over Distillation Column
6.4 Overall Material Balance
7
Chapter 7
53
Conclusion
Chapter 8
55
References
APPENDIX A
58
FAMT ,Ratnagiri
Page 1
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
FIGURE INDEX
FIGURE
FIGURE NAME
PAGE NO.
NO.
1
2.1 Manufacturing Process Of Nitrobenzene
11
4.1 Rectification section
27
4.2 Stripping Section
28
5.1 Flowsheeting
34
5.2 Title Page
35
5.3 Component Entry
36
5.4 Selection Of Property Method
37
5.5 Mixer
38
5.6 Reactor
39
10
5.7 Reaction Input
40
11
5.8 Decanter
41
12
5.9 Distillation
42
13
5.10 Result Summary
43
14
5.11 Strem Result Over Mixer
44
15
5.12 Strem Result Over Reactor
45
16
5.13 Strem Result Over Decanter
46
17
5.14 Strem Result Over Distilation Column
47
FAMT ,Ratnagiri
Page 2
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
TABLE INDEX
TABLE NO.
TABLE NAME
PAGE NO
2.1 Properties Of Benzene
11
2.2 Propetries Of Suphuric Acid
12
2.3 Properties Of Nitric Acid
13
2.4 Properties Of Nitrobenzene
14
2.5 Enthalpy Data At Standard State
16
2.5 Entropy Data At Standard State
16
2.5 Specific Heat Data At Standard State
17
5.1 Stream Result Overall
48
6.1 Material Balance Over Reactor
50
10
6.2 Material Balance Over Decanter
50
11
6.3 Material Balance Over Distillation Column
51
12
6.4 Overall Material Balance
52
FAMT ,Ratnagiri
Page 3
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER-I
INTRODUCTION
FAMT ,Ratnagiri
Page 4
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
INTRODUCTION
Nitrobenzene (some time called the oil of Mira-bane) C6H5NO2 is pale yellow liquid
with an odour that resembles bitter almonds, Depending upon the compounds purity. Its
colour various from pale yellow to yellowish brown liquid boiling at 483 K (101 KPa) and
freezing at 287.7 K as bright yellow crystals. It is quite toxic to human system.
Nitrobenzene was first synthesized in 1834 by treating benzene with fuming nitric
acid. And it was first produced commercially in England in 1856. The electives ease of
aromatic nitration has contributed significantly to the large and varied industrial application
of nitrobenzene, other aromatic nitro- compounds and their derivatives
A continues process for the production for the production has been developed by
M/S.Biazzi of Switzerland. The advantages of this process are lower concentration of mixed
said used and higher reaction rate. The reactants are kept mixed under high speed agitation
(600 rpm) and cooling due to control feed rate and rapid agitation. The reaction time is about
15 20 minutes, where the yield is higher than 99% of theoretical
FAMT ,Ratnagiri
.[4][5]
Page 5
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER-II
LITERATURE REVIEW
2.1. Process For Production Of Nitrobenzene
2.2. Selection Of Process
2.3. Manufacturing Process Of Nitrobenzene
2.4. Chemical And Physical Properties
FAMT ,Ratnagiri
Page 6
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
LITERATURE REVIEW
2.1 PROCESS FOR PRODUCTION OF NITROBENZENE
Nitrobenzene is manufactured by nitration of benzene using mixture of Nitric and sulphuric
acid.
Nitration can be done by two processes. Via.
[1]
Batch Process.
[2]
Continuous process.
2.1.1 BATCH PROCESS
In batch process the nitrator is charged with benzene and mixed acid (HNO 3 32 39
%, H2SO4 60 -53 %, H2O 8%) is added slowly below surface of benzene. The rate of
agitation is such that both the acid & benzene phases are in intimate contact. The feed rate of
mixed acid and the rate of cooling are such that during the entire period of acid addition, the
temperature of nitrator is maintained at 323 -328 K. after complete addition of acid, The acid
and organic layers are drained into separate vessel from where spent acid is drawn off for
reconcentration. This crude product is washed with water to remove contamination in the
nitrobenzene and the aqueous sodium carbonate solution to remove small traces of nitro
phenols formed during nitration. Particularly when the product is to be further nitrated,
removal of nitrophenolic impurities is important, since they way undergo unwanted side
reaction during subsequent nitration. The product is further purified by distillation and the
yield is 95 98% of the theoretical.
FAMT ,Ratnagiri
[4][5]
Page 7
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
2.1.2 CONTINUOUS PROCESS
A continuous process for the production of nitrobenzene has been developed by M /
S.Biazzi of Switzerland. The advantages of this process are the lower concentration of mixed
acid is used and higher reaction, rates though the sequence of operations is the same as in
bath process. Continuous nitrator with capacity of 150 lit. Can produce as a 7500 capacity
batch nitrator, but at the same time of quantity a reactants in nitrator is considerably small,
unlike the batch process.
Mixed acid and benzene are fed to nitrator in such that all nitric acid is utilized for nitraton of
benzene. The reactants are kept mixed under high speed agitation (600 rpm) and cooling.
Due to the controlled feed rate and rapid agitation, the reaction time is 15 to 20 minutes only
at reaction mixture is drawn off side of nitrator. The mixture is sent to decanter, where the,
product is separated from spend acid for further processing.
[4][5]
2.2 SELECTION OF PROCESS
Continuous process, in general, will be found to have the following to have the
following advantages over batch process.
[1]
Lower Capital Cost.
[2]
Safety
[3]
Labour Usage.
2.2.1 LOWER CAPITAL COST
For a given rate of production, the equipment needed for a continuous process is
smaller than for a batch process. This is usually the striking different between the two types
of process. The reason for that is obvious since, it is not necessary to accumulate material in
a continuous process anywhere; the vessel is designed with capacity dictated by the rate of
reaction process step which they must accommodate. Alternatively, because of relatively
FAMT ,Ratnagiri
Page 8
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
small size of continuous process equipment, it is often possible and excessively high in cost
for batch scale equipment. Thus for example Corrosion resistance alloys such as appropriate
S.S. may be detected for a batch plant because of cost. In case of S.S. corrosion problems are
completely eliminated.
2.2.2 SAFETY
Because of relatively small size of continuous process equipment, there is less
material in process at any time than at certain in a comparable batch process. At the
completion of batch process nitration and during its normal separation of product from spent
nitrating acid, the entire batch of an often hazardous compound will be present in the
equipment.
In the continuous process, only as much material need be present in hazardous
conditions as needed to again sufficient reaction of process time. In case of high explosive
made by nitration, this process has inherent safety factor is very attractive
[3].
2.2.3 LABOUR USAGE
In the nitration filed the continuous process is usually more efficient labour usage
than a batch process. This is particularly true for small or medium scale production and for
hazardous products, since continuous processing
minimizes the amount the material in
process on average. It is often possible to handle operations at one place that efficiency tends
to disappear as the scale of operations increases.
2.3 MANUFACTURING PROCESS OF NITROBENZENE
Nitrobenzene is manufactured commercially by direct nitration of benzene using a
mixture of nitric acid and sulphuric acid, which is commonly referred to as mixed acid for
nitrating acid. The reaction is conducted is specially build cast iron are S.S. reaction vessel
FAMT ,Ratnagiri
Page 9
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
provided with agitator, external jacket and internal coils. Since two phases ate formed in
reaction mixture and reactant ate distributed between them. Rate of nitration is controlled by
transfer between the phases as well as by chemical kinetics.
Benzene used is of commercial quality. Mixed acid contain of 56 60 wt % H2SO4, 20 26
wt% nitric acid and 15 18% water. Sulphuric acid used is of 94% - 98% concentration and
nitric acid commercial grade of 55% - 60% concentration.
Benzene is charged to the nitrator. Mixed acid is slowly added on surface of benzene from
dosing tank with stirring. The ratio of mixed acid to benzene is kept around 2.5 : 1.0. The
temperature mass is maintain initially at 25 30C. So by high speed agitator and proper
cooling coils reaction temperature can maintained upto 50 55C. By obvious agitation, the
interfacial area, of the reaction mixture is maintained as high as possible, thereby enhancing
the mass transfer of reactants and cooling coils, which control the temperature of highly
.[4]
exothermic reaction
A slight excess of benzene usually is fed into the nitrator of ensure that the nitric acid in
mixed acid is formation of denitrobenzene. Reaction time is only 15 20 minutes because of
rapid and efficient agitation.
Nitrobenzene and spent acid are removed from the side reactor and send to decanter unit.
Organic and aqueous layers are formed, where two layers are separate in 10 to 20 minutes.
The aqueous phase or spent acid is drawn from the bottom and is concentrated in a sulphuric
acid is drawn from the bottom and is concentrated in a sulphuric acid reconcentration step or
is recycled to the nitrator, where it is mixed nitric acid and sulphuric acid immediately prior
to being fed into nitrator.
The crude Nitrobenzene can used directly for production of aniline if required, otherwise the
crude nitrobenzene flows through a series of washer separators, where residual acid is
removed by washing with a dilute sodium carbonate solution followed by final washing with
water.The product is then distilled to remove benzene and the nitrobenzene can be refined by
vacuum distillation. Theoretical yields are 96 99 %. The nitration process is unavoidably
associated with the disposal of waste water from washing step. This water principally
contains Nitrobenzene, some sodium carbonate and inorganic salts from the neutralized spent
FAMT ,Ratnagiri
Page 10
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
acid which was present in the product. Generally, the waste water is extracted with benzene
to remove the nitrobenzene and the benzene that is dissolved in the water is stripped from
water prior to the final waste treatment.
[6]
Fig No-2.1 Manufacturing Process Of Nitrobenzene
2.4 CHEMICAL AND PHYSICAL PROPETRIES
[7]
2.4.1 PROPERTIES OF BENZENE
PHYSICAL PROPERTYPROPERTY
VALUE
Molecular Weight
78.11
Melting Point, C
5-533
Boiling Point, C
80.1
Density, Kg/cum
873.7
FAMT ,Ratnagiri
Page 11
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Refractive index
1.49792
Viscosity (absolute, at 20C)
0.6468
Flash point, C
-11.1
Heat of fusion, kJ/kmole
9.847
Table No-2.1 Properties Of Benzene
CHEMICAL PROPERTY
[14][15][16]
REACTION WITH WATER:Water and benzene are non-reactive unless high and pressure are applied.
2.4.2 PROPETRIES OF SUPHURIC ACID
PHYSICAL PROPERTY-
PROPERTY
VALUE
Molecular Weight
98.08
Boiling Point, c
330.0
Density, at 20C, gm/cc
1.834
Flash Point
None
Vapour pressure at 145C mmHg
1.0
TLV, mg/cum.
1.0
Freezing Point, C
10.48
Table No-2.2 Propetries Of Suphuric Acid
FAMT ,Ratnagiri
Page 12
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHEMICAL PROPERTY
[14][15][16]
REACTION WITH WATER:Has great affinity for water, absorbs atmospheric moisture and absorbs water from organic
material causing charring. Sulphuric Acid reacts with water vigorously liberating large
amount of heat.
REACTION WITH METAL AND OTHER ELEMENTS:When cold, it reacts with metal including platinum when not, reactivity is intensified.
Sulphuric acid on reaction with metals causes liberations of flammable hydrogen.
Cu + H2SO4
CuSO4 + H2
Zn + H2SO4
ZnSO4 + H2
2.4.3 PROPERTIES OF NITRIC ACID
PHYSICAL PROPERTYPROPERTY
VALUE
Molecular Weight
63.02
Boiling Point
86.0
Melting point C
-42.0
Density, at 20C,gm/cc
1.502
Flash point
None
Solubility in water
Soluble in water
TLV, mg/cum.
2-5
Freezing point, C
10.48
Table No. 2.3 Properties Of Nitric Acid
FAMT ,Ratnagiri
Page 13
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHEMICAL PROPERTIES :REACTION WITH WATER :Nitric Acid reacts with water to produce heat, toxic and corrosive fumes.
REACTION WITH METALS AND OTHER ELEMENTS :Nitric acid is corrosive to most of metals like zinc to form nitrate with evolution of hydrogen.
Cu + 2HNO3 Cu (NO3)2 + H2
Zn + 2HNO3 Zn (NO3)2 + H2
2.4.4 PROPERTIES OF NITROBENZENE
PHYSICAL PROPERTYPROPERTY
VALUE
Molecular Weight
123.0
Boiling Point, C
201.9
Melting point, C
5.85
Density, at 20C, gm/cc
1.344
Flash point
88.0
Auto ignition temp., C
482.0
Explosive limit (at 93)
1.8 Vol % in air
Vapour density
4.1
Table No. 2.4 Properties Of Nitrobenzene
FAMT ,Ratnagiri
Page 14
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER III
THERMODYNAMIC FEASIBILITY
3.1. Reaction Data For Formation Nitrobenzene
3.2. Calculations
FAMT ,Ratnagiri
Page 15
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
THERMODYNAMIC FEASIBILITY
3.1 REACTION DATA FOR FORMATION NITROBENZENE[7]
REACTION:C6H6
HNO3
<===============>
C6 H5 NO2 +
H2 O
DATA :HEAT OF FORMATION
( kcal/gmole)
Benzene (liquid)
11.71
Nitrobenzen (liquid)
13.76
Nitric acid (liquid)
-41-61
Water (liquid)
-68.315
Table No. 2.5 Enthalpy Data At Standard State
ENTHROPY
kJ/(kmol.K)
Benzene (liquid)
172.915
Nitrobenzene (Liquid)
364.61
Nitric acid (liquid)
110.113
Water (liquid)
69.92
Table No. 2.6 Entropy Data At Standard State
FAMT ,Ratnagiri
Page 16
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
SPECIFIC HEAT AT 25 C
kJ/(kmol.K)
Benzene (liquid)
91.73
Nitrobenzene (liquid)
185.361
Nitric acid (liquid)
111.113
Water (liquid)
75.362
Table No. 2.7 Specific Heat Data At Standard State
3.2 CALCULATIONS[11]
From heat of formation data:
HR = HPRODUCTS - HREACTANTS
= ( HNB + HWATER ) - ( HBENZENE + HNITRIC ACID )
= ( 13.76 68.315 )
- (11.71 41.61)
HR = -24.655 kcal/gmmole
HR = -103157 kJ/(kmol)
From specific heat data:
Cpavg = CpPRODUCT - CpREACTANT
= ( CpNB + CpWATER ) - ( CpBENZENE + CpNITRIC ACID )
FAMT ,Ratnagiri
Page 17
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
= ( 185.361 + 75.362 ) - ( 91.73 + 111.113 )
Cpavg =
57.88 kJ/(kmol.K)
From entropy data:
= SPRODUCTS - SREACTANTS
= ( SNB + SWATER ) - ( SBENZENE + SNITRIC ACID )
= ( 364.61 + 69.92 ) - ( 172.91 + 110.113 )
= 151.507 kJ/(kmol.K)
For HR At Reaction Temperature:
HR = H - Cp.T
H = HR + Cp.T
= -103157 + 57.88 298
= -85908.76 kJ/(kmol)
Therefore, HR at 323 K,
HR = -85908.76 ( 57.88 323 )
= -104604 kJ/(kmol)
Similarly, for S At Reaction Temperature:
S = S + CplnT
FAMT ,Ratnagiri
Page 18
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
S= S - CplnT
= 157.507 - 57.88 In (298)
= -178.24 kJ/(kmol.K)
Therefore, S at 323 K,
S = -178.507 + 57.88 In (323)
= 156.17 kJ/(kmol.K)
Now using Standard free energy change relation,
G = HR - TS
= -104604 (323156.17)
= -155046.91 kJ/(kmol)
Since G is negative it can thermodynamically feasible Reaction
By using Vant Hoff Isotherm,
G = -RT lnKp
lnKp =
=
= 57.73
Kp = 1.18 1025
Since Kp = KxPn
For our reaction,
n = (1+1)-(1-1)
FAMT ,Ratnagiri
Page 19
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
= 0
Kp =
P0
Kp =
= Kx
Now,taking material balance,
Composition of mixed acid(Weight basis):
25%
Nitric acid
58%
Sulphuric acid
17%
Water
Consider 1000 kg of mixed acid.
Nitric acid 250 kg
3.97 kmole
Water 170kg
9.44 kmole
Sulphuric acid 580 kg
5.92 kmole
----------------------------
Total moles
FAMT ,Ratnagiri
19.33 kmole
Page 20
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Mole % of
Nitric acid
20.5 %
Water
48.8 %
Sulphuric acid
30.7 %
But benzene mixed acid
1---------------------------->
400kg <-------------------------------
2.5
1000kg
Moles of benzene
5.128 -------------------->
19.314
Moles of benzene
---------------->
3.766 moles of
Moles of acid
3.766
0.205
= 0.772 moles
Reaction of nitrobenzene
C6H6
FAMT ,Ratnagiri
HNO3
C6 H5 NO2 +
H2 O
Page 21
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Initially
0.772
Reacted
At. equilibrium
Kx
(1-X)
(0.772-X)
X2
----------------------(1 - X) (0.772 - X)
X2
1.18 1025 =
---------------------------------------
X2 - 1.73 X + 0.73
X2 - 1.772 X + 0.772
X
0.772
Extent of reaction = 0.772
FAMT ,Ratnagiri
Page 22
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER IV
DESIGN OF DISTILLATION COLUMN
FAMT ,Ratnagiri
Page 23
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
DESIGN OF DISTILLATION COLUMN
[10]
Basis ; 1 hour of operation.
Mass flow rate of feed = 740.75 kg/hr.
Mass flow rate of distillate = 32.3 kg/hr.
Mass flow rate of bottom = 708.38 kg/hr.
Xf =
= 0.317/1.401
= 0.226
Xd = 2.8075/3.048
= 0.92
Xw = 0.0036/1.08667
= 0.003
Average Molecular weight of feed = 110.556
Feed rate = 593.568 kg/hr
Slope of q-line ;
We know that q = Hg-Hf / Hg-Hl
q=1
slope of q-line:
slope of q-line = q/q-1
= 1/1-1
Tan-1() = 0
FAMT ,Ratnagiri
Page 24
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
q line is st.line
Xd / Rm+1 = 0.05
Rm+1 = 1/0.05
Rm+1 = 20
Rm = 19
R = 1.2 Rm
R = 22.8 23
Xd = 1 = 0.042
Rm+1= 23+1 =24
From Mc-cabe Thile Graph
X 0 0.01 0.02 0.03 0.045 0.07 0.10 0.155 0.20 0.30
Y 0 0.03 0.485 0.63 0.74 0.82 0.88 0.92 0.94 0.964
Ideal Plate = 16 (From Graph)
Actual Plate = Ideal/n = 16/0.6
Actual Plate = 26.66
Height:
Plate Spacing = 450 mm = 0.45m
Ht = (Actual Plate-1)0.45 + 2(0.45)
= 12.45m
FAMT ,Ratnagiri
Page 25
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Diameter :
Vap rate = v = D(R+1)
= 0.0087(23+1)
n = 0.21 kmole/hr
Top Column :
Vol.rate = nRT/P
= 0.218.314103(82+273)/ 1.01325105 = 6.1170 m3/hr
Vol rate = 1.710-3 m3/sec
Velocity = 1 m/sec
Area = Vol rate / Velocity
= 1.710-3 /1 = 1.710-3 m2
Area = D2 /4
D2 = 4A /
D = 0.047 m
Bottom column:
Vol.rate = nRT/P
= 0.218.314103(210+273)/ 1.01325105 = 8.32 m3/hr
Area = Vol .rate / Velocity
Velocity = 1 m/sec
Area = 2.3110-3 m2
2
A=D /4
FAMT ,Ratnagiri
Page 26
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
D2 = 4A /
D = 0.054 m
Both diameters are approximately same ,
we choose the larger diameter (i.e) bottom diameter
Bottom diameter D= 0.054 m
DESIGN SUMMARY
Ideal plate = 16.00
Actual Plates = 26.66
Column Height = 12.45 m
Column Diameter = 0.054 m
Fig No. 4.1 Rectification Section
FAMT ,Ratnagiri
Page 27
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Fig No-4.2 Stripping Section
FAMT ,Ratnagiri
Page 28
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER V
SIMULATION USING ASPEN
FAMT ,Ratnagiri
Page 29
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
SIMULATION USING ASPEN
5.1 INTRODUCTION TO ASPEN[8]
5.1.1
What is a Process Flowsheet?
Process flowsheet can simply be defined as a blue print of a plant or part of it. It
identifies all feed streams, unit operations, streams that inter-connect the unit Operations and
finally the product streams. Operating conditions and other technical Details are included
depending on the detail level of the flowsheet. The level can vary from a rough sketch to a
very detailed design specification of a complex plant. For steady-state operation, any process
flowsheet leads to a finite set of algebraic equations. For a case where we have only one
reactor with appropriate feed and Product streams the number of equations may be
manageable by manual hand calculations or simple computer applications. However, as the
complexity of a flowsheet Increases and when distillation columns, heat exchangers,
absorbers with many purge and recycle streams come into the picture the number of
equations easily approach many ten thousands. In these cases, solving the set of algebraic
equations becomes a Challenge in it. However, there are computer applications called
process flowsheet simulators specialized in solving these kinds of large equation sets. Some
well-known process flowsheet simulators are Aspen Plus, ChemCad and PRO/II.These
products have highly refined user interfaces and on-line component databases. They are used
in real world applications from interpreting laboratory scale data to monitoring a full scale
plant.
5.1.2 Advantages of using a process flowsheet simulator
The use of a process flowsheet simulator is beneficial in all the three stages of aPlant:
research & development, design and production. In research & development they help to cut
down on laboratory experiments and pilot plant runs. In design stage they enable a speedier
development with simpler comparisons of various alternatives.
FAMT ,Ratnagiri
Page 30
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
Finally, in the production stage they can be used for risk-free analysis of various what-if
scenarios
5.1.3 Disadvantages of using a process flowsheet simulator
Disadvantages of using a process flowsheet simulatorManual solution of a problem
usually forces someone to think deeper on theProblem, find novel approaches to solve it, and
evaluate and re-evaluate the Assumptions closer. A drawback of process flowsheet
simulators may be cited as the Lack of this detailed interaction with the problem. This might
act as a double edged Sword. On one side it hides the complexities of a problem so you can
concentrate on the real issues at hand. On the other side this hiding may also hide some
important Understanding of the problem as well,
[8]
5.1.4 History
In 1970s the researchers at MITs Energy Laboratory developed a prototype
forProcess simulation. They called it Advanced System for Process Engineering
(ASPEN).This software has been commercialized in 1980s by the foundation of a
companyNamed AspenTech. AspenTech is now a publicly traded company that employs
1800People worldwide and offers a complete
integrated solution to
chemical
processIndustries.This sophisticated software package can be used in almost every aspect of
processengineering from design stage to cost and profitability analysis. It has a built-in
modelLibrary for distillation columns, separators, heat exchangers, reactors, etc. Custom
orPropriety models can extend its model library. These user models are created with
FORTRAN subroutines or Excel worksheets and added to its model library. Using
VisualBasic to add input forms for the user models makes them indistinguishable from
theBuilt-in ones. It
has a
built-in property databank for thermodynamic and
physicalParameters. During the calculation of the flow sheet any missing parameter can
beestimated automatically by various group contribution methods.In this workshop we will
only scratch the surface of this tool. We will discuss itsAdvantages and disadvantages. Our
focus will be on reactors and our goal is to provideyou with a smooth and simple introduction
FAMT ,Ratnagiri
Page 31
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
to Aspen Plus. Lets start our workshop bylearning how to access Aspen Plus at the
University of Michigan.
5.1.5 What is an Aspen plus Process Simulation Model?
A process consists of components being mixed, separated, heated, cooled a Converted
by unit operations. These components are transferred from unit to unitthrough process stream
you can translate a process into an Aspen plus process simulation model bydoing the
following steps:
1. Define the process flowsheet configuration. To do this step, you:
Define the unit operations in the process
Define the process streams that flow between these unit operations
Select unit operation models from the Aspen Plus model library to
Describe each unit operation
2. Specify the chemical components in the process. You can take these
Components from the Aspen Plus databanks, or you can define them.
3. Choose appropriate thermodynamic models from those available in Aspen
Plus, to represent the physical properties of the components and mixtures in
The process.
4. Specify the component flow rates and the thermodynamic conditions (for
Example, temperature and pressure) of feed streams to the process.
5. Specify the operating conditions for the unit operations in the flowsheet.
When you have specified this information, you have defined an Aspen Plus
Process simulation model of your process. You can use the Aspen plus processSimulation
model to predict process behaviour.
FAMT ,Ratnagiri
Page 32
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
With
Aspen
Plus
you
can
interactively
change
specifications,
such
as
flowsheetConfiguration, operating conditions, and feed compositions, to run new cases
andAnalyse alternatives. In addition to process simulation, Aspen Plus allows you to perform
a wide rangeof other tasks such as estimating and regressing physical properties,
generatingCustom graphical and tabular output results, data-fitting plant data toSimulation
models, costing your plant, optimizing your process, and interfacingResults to spread sheets.
5.1.6 Why Use Process Simulation?
Process simulation allows you to predict the behaviour of approves by using
basicEngineering relationships, such as mass and energy balances, and phase and Chemical
equilibrium. Given reliable thermodynamic data, realistic operating Conditions, and rigorous
equipment models, you can simulate actual plant Behaviours. Process simulation enables you
to run many cases conduct "what if" Analyses, and perform sensitivity studies and
optimization runs. With simulation, you can design better plant and increase profitability in
existing plants.
FAMT ,Ratnagiri
Page 33
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
5.2 STARTING WITH PROCESS SIMULATION
1] First stating with Blank Simulation we must design our required flowsheet with proper
stream names & block names .each stream is properly connect to the proper unit.After doing
this we click Next to the required input step by step.
Fig No 5.1-Flowsheeting
FAMT ,Ratnagiri
Page 34
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
2] we input Title of our simulation with all units are in SI units.
Fig No 5.2-Title Page
FAMT ,Ratnagiri
Page 35
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
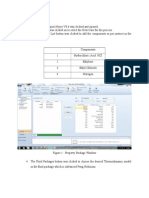
3] We input our components that takes part in process operation,all conventional types
It involves nitrobenzene,benzene,water,sulphuric acid,nitric acid.
Fig No 5.3 Component Entry
FAMT ,Ratnagiri
Page 36
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
4] This is the step where you put property method.From our investigation in aspen running
plant we know that NRTL is the best property method applied where large water usage in
operation or process.
Fig No 5.4- Selection Of Property Method
FAMT ,Ratnagiri
Page 37
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
5] Then we come at Block of Mixer where we fed H2SO4, H2O, HNO3 in desired proportion
to make Mixed acid.In mixer we operate at normal temperature & pressure.
Fig No 5.5-Mixer
FAMT ,Ratnagiri
Page 38
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
6] Next to we selected stoichiometric reactor since we know only the extent of reaction &
stoichiometric reaction coefficients operating at 50 C
Fig No 5.6-Reactor
FAMT ,Ratnagiri
Page 39
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
7] Insert our reaction in new option with correct coefficient
Fig No 5.7 Reaction Input
FAMT ,Ratnagiri
Page 40
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
8] Moving on to decanter we fed extra water to this unit in order to remove sulphuric acid
effectively.we select nitrobenzene is our key component
Fig No 5.8-Decanter
FAMT ,Ratnagiri
Page 41
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
9] Distillation column is where we obtained our desired product in Bottom stream from data
we find out optimum feed ratio
Fig No 5.9- Distilation
FAMT ,Ratnagiri
Page 42
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
10] Final next to Run the simulation
Summary obtained,
Fig No 5.10-Result Summary
FAMT ,Ratnagiri
Page 43
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
11 Now we take stream result over each block
First is Mixer which has 3 inlet stream & 1 outlet stream
Fig No 5.11-Strem Result Over Mixer
FAMT ,Ratnagiri
Page 44
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
12] Second block is stoichiometric reactor where we provide benzene with mixed acid in
1:2.5 proportion.Crude nitrobenzene is obtained .
Fig No 5.12-Strem Result Over Reactor
FAMT ,Ratnagiri
Page 45
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
13] Third stream result over Decanter
Fig No 5.13 -Strem Result Over Decanter
FAMT ,Ratnagiri
Page 46
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
14] Last stream result over a distillation column in the bottom stream we get our final
product
Fig No 5.14 -Strem Result Over Distilation Column
FAMT ,Ratnagiri
Page 47
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
15] Steam result obtained from overall result
Table No 5.1-Strem Result Overall
FAMT ,Ratnagiri
Page 48
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTERVI
RESULT SUMMARY
FAMT ,Ratnagiri
Page 49
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
RESULT SUMMARY
6.1 MATERIAL BALANCE OVER REACTOR
SR.
NO
COMPONENTS
INPUT
(kg/hr)
OUTPUT
(kg/hr)
BENZENE
400
91.07
NITROBENZENE
486.2
WATER
241.84
1000
NITRIC ACID
SULPHURIC ACID
0.89
580
TOTAL
1400
1400
Table No.6.1 Material Balance Over Reactor
6.2 MATERIAL BALANCE OVER DEACNTER
INPUT
(kg/hr)
SR.
NO
COMPONENTS
BENZENE
OUTPUT
(kg/hr)
SPENT ACID
STREAM
ORGANIC PHASE
91.07
2.09
88.98
NITROBENZENE 486.2
9.88
476.32
WATER
241.84+2000
2241.43
0.41
NITRIC ACID
0.89
0.89
SULPHURIC
ACID
580
553.51
26.49
3400
3400
TOTAL
Table No.6.2 Material Balance Over Decanter
FAMT ,Ratnagiri
Page 50
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
6.3 MATERIAL BALANCE OVER DISTILLATION COLUMN
INPUT
(kg/hr)
SR. COMPONENTS
NO
OUTPUT
(kg/hr)
TOP PRODUCT
BOTTOM PRODUCT
BENZENE
88.98
78.6
10.38
NITROBENZENE
476.32
476.32
WATER
0.41
0.41
NITRIC ACID
SULPHURIC ACID
26.49
26.49
592.2
592.2
TOTAL
Table No.6.3 Material Balance Over Distillation Column
FAMT ,Ratnagiri
Page 51
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
6.4 OVERALL MATERIAL BALANCE
SR.
COMPONENTS
INPUT
OUTPUT
(kg/hr)
(kg/hr)
TOTAL
NO
SPENT
TOP PDT
BOTTOM
ACID
STREAM
PDT
STREAM
STREAM
BENZENE
400
2.09
78.6
10.38
NITRIC ACID
250
0.89
SULPHURIC
580
553.51
26.49
ACID
4
WATER
170 + 2000
2241.43
0.41
NITROBENZENE
9.88
476.32
TOTAL(kg/hr)
3400
3400
Table No. 6.4 Overall Material Balance
Conversion of benzene is 77 %
Purity of Nitrobenzene in bottom product is 92.8 %.
FAMT ,Ratnagiri
Page 52
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER VII
CONCLUSION
FAMT ,Ratnagiri
Page 53
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CONCLUSION
It is very important for any process to kow that parameters like composition, streams,
temperature, pressure etc may affect the production rate.One must have perform pilot plant in
order to know this, so each time we need manual calculation to get desired results,this is so
time consuming. So the use of simulaters like ASPEN, CHEMCAD are helpful.Simulation &
modeling useful in doing risk analysis in production process.
In our project we simulate continuous process for nitrobenzene production using
benzene nitration.In that we know about how actually parameters mention above may affect
each stream.For example we first added calculated amount of extra water to decanter,but
from that action we know that how much extent it affect the each stream,so we are finaly able
to find the optimum amount of water required for operation.
Generally it is difficult to obtain desired result manually that is why we simulate it
using ASPEN PLUS .And we searching new techniques as possible in order to get the
optimum production. Also we can check where is the opportunity to increase the conversion
& reduce the losses as well as maintenance cost.
FAMT ,Ratnagiri
Page 54
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
CHAPTER VIII
REFERENCES
FAMT ,Ratnagiri
Page 55
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
REFERENCES
Books,
[1]B.I. Bhatt & S.M. Vora. Stoichiometry, Tata Mcgraw Hill Publishing Co. Ltd.
[2]Dryden C. E., Drydens Outline Of Chemical Technology. East West Press Pvt.
Ltd;(536)
[3]G. D. Muir, Hazardous In Chemical Laboratory The Chemical Society, London.
[4]Kirk Othmer Encyclopedia Of Chemical Technology.Vol. 15. Wiley
Intenscience Publications, 1979.(138-139)
[5]P.H.Groggins .Unit Process In Porganic Synthesis. Mcgraw Hill International
Book Co.
[6]R.Norris Shreve & Joseph A. Brink Jr.Chemical Process Industries.Mcgraw Hill
International Publications.(776-778)
[7]Robert H. Perry Perrys Chemical Engineering Handbook.Mcgraw Hill
International Publications.(642-644)
[8]Amiya K. Jana. Process Simulation And Controle Using Aspen.PHI Learning Private
Limited ,Second Edition ,2012
[9]Bhattacharya A., Purohit V. C., Suarez, V.; Tichkule, R; Parmer, G.; Rinaldi, F.
(2006). "One-step reductive amidation of nitro arenes: application in the synthesis of
Acetaminophen" Volume 47, Issue 11, 13 March 2006, Pages (18611864)
[10]M.V.Joshi,Mahajani, Joshi's Process Equipment Design, Macmillan, 2009
[11]K.A.Gavane,Chemical Reaction Engineering-I,Nirali Publication,2012, Chapter 6
(6.1-6.15)
Journal Papers,
[12]R. D. BIGGS and R. R. WHITE Rate of Nitration of Benzene with Mixed Acid
University of Michigan, Ann Arbor, Michigan 2000
[13]J. Chil. Chem. Soc. vol.57 no.2 Concepcin 2012, pgs: 1194-1198.
FAMT ,Ratnagiri
Page 56
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
[14]V. Dubois, G. James, J.L. Dallons, A. Van Geysel, In Catalysis of Organic Reactions,
M. Ford, Ed; Marcel Dekker, New York, 1994, Vol.82, p. 1.
[15]Laali, Kenneth K., and Volkar J. Gettwert. Electrophilic Nitration of Aromatics in
Ionic Liquid Solvents. The Journal of Organic Chemistry 66 (Dec. 2000): 35-40.
American Chemical Society.
[16]Sauls, Thomas W., Walter H. Rueggeberg, and Samuel L. Norwood. On the
Mechanism of Sulfonation of the Aromatic Nucleus and Sulfone Formation. The
Journal of Organic Chemistry 66 (1955): 455-465. American Chemical Society.
FAMT ,Ratnagiri
Page 57
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
APPENDIX A
FAMT ,Ratnagiri
Page 58
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
SIMULATION REPORT
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 1
MANUFCTURING OF NITROBENZENE
RUN CONTROL SECTION
RUN CONTROL INFORMATION
-----------------------
THIS COPY OF ASPEN PLUS LICENSED TO
TYPE OF RUN: NEW
INPUT FILE NAME: _0812ogh.inm
OUTPUT PROBLEM DATA FILE NAME: _0335nde VERSION NO. 1
LOCATED IN:
FAMT ,Ratnagiri
Page 59
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
PDF SIZE USED FOR INPUT TRANSLATION:
NUMBER OF FILE RECORDS (PSIZE) =
NUMBER OF IN-CORE RECORDS
= 256
PSIZE NEEDED FOR SIMULATION
CALLING PROGRAM NAME:
LOCATED IN:
apmain
C:\PROGRA~2\ASPENT~1\ASPENP~1.2\Engine\xeq
SIMULATION REQUESTED FOR ENTIRE FLOWSHEET
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 2
MANUFCTURING OF NITROBENZENE
INPUT SECTION
INPUT FILE(S)
-------------
;
;Input Summary created by Aspen Plus Rel. 10.2.1 at 19:39:35 Sun Apr 27, 2014
;Directory G:\Aspen new\aspen save Filename _0812ogh.dan
;
FAMT ,Ratnagiri
Page 60
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
TITLE 'MANUFCTURING OF NITROBENZENE'
IN-UNITS SI
DEF-STREAMS CONVEN ALL
SIM-OPTIONS
IN-UNITS ENG
SIM-OPTIONS NPHASE=1 PHASE=L ATM-PRES=101325. <N/sqm>
DATABANKS PURE10 / AQUEOUS / SOLIDS / INORGANIC / &
NOASPENPCD
PROP-SOURCES PURE10 / AQUEOUS / SOLIDS / INORGANIC
COMPONENTS
C6H5NO2 C6H5NO2 /
H2SO4 H2SO4 /
H2O H2O /
HNO3 HNO3 /
C6H6 C6H6
FAMT ,Ratnagiri
Page 61
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
FLOWSHEET NBMFG
BLOCK RSTO IN=C6H6 MIXACID OUT=CNB
BLOCK DECANTER IN=CNB OUT=SPA ORGANIC
BLOCK DIST IN=ORGANIC OUT=TOP BOTTOM
BLOCK MIXER IN=HNO3 H2O H2SO4 OUT=MIXACID
DEF-STREAMS CONVEN NBMFG
PROPERTIES NRTL
PROP-DATA NRTL-1
IN-UNITS SI
PROP-LIST NRTL
BPVAL C6H5NO2 H2O -5.154900000 2270.617200 .2000000000 0.0 &
0.0 0.0 273.1500000 379.7500000
BPVAL H2O C6H5NO2 5.854700000 229.4967000 .2000000000 0.0 &
0.0 0.0 273.1500000 379.7500000
BPVAL C6H5NO2 C6H6 -.8730000000 630.1689000 .3000000000 0.0 &
0.0 0.0 343.1500000 484.1500000
BPVAL C6H6 C6H5NO2 -1.289300000 98.83280000 .3000000000 0.0 &
0.0 0.0 343.1500000 484.1500000
FAMT ,Ratnagiri
Page 62
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
BPVAL H2O C6H6 140.0874000 -5954.307100 .2000000000 0.0 &
-20.02540000 0.0 273.9500000 350.1500000
BPVAL C6H6 H2O 45.19050000 591.3676000 .2000000000 0.0 &
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 3
MANUFCTURING OF NITROBENZENE
INPUT SECTION
INPUT FILE(S) (CONTINUED)
-7.562900000 0.0 273.9500000 350.1500000
STREAM C6H6
SUBSTREAM MIXED TEMP=298. PRES=101325. MASS-FLOW=400. <kg/hr>
MASS-FRAC C6H6 1.
STREAM H2O
SUBSTREAM MIXED TEMP=298. PRES=101325. MASS-FLOW=170. <kg/hr>
MASS-FRAC H2O 1.
STREAM H2SO4
FAMT ,Ratnagiri
Page 63
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
SUBSTREAM MIXED TEMP=298. PRES=101325. MASS-FLOW=580. <kg/hr>
MASS-FRAC H2SO4 0.98
STREAM HNO3
SUBSTREAM MIXED TEMP=298. PRES=101325. MASS-FLOW=250. <kg/hr>
MASS-FRAC HNO3 0.6
BLOCK MIXER MIXER
PARAM PRES=101325. T-EST=298.
BLOCK DECANTER DECANTER
PARAM TEMP=298. PRES=101325. L2-COMPS=C6H5NO2
;
;Input file created by Aspen Plus Rel. 10.2.1 at 00:20:55 Mon Apr 28, 2014
;Directory G:\Aspen new\aspen save Runid simu1
;
BLOCK DIST DISTL
PARAM NSTAGE=26 FEED-LOC=16 RR=0.45 PTOP=101325. &
FAMT ,Ratnagiri
Page 64
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
PBOT=101325. D:F=0.205
BLOCK RSTO RSTOIC
PARAM TEMP=323. PRES=101325.
STOIC 1 MIXED C6H6 -1. / HNO3 -1. / C6H5NO2 1. / H2O &
1.
CONV 1 MIXED C6H6 0.772
REPORT INPUT
;
;
;
;
;
;
;Input file created by Aspen Plus Rel. 10.2.1 at 00:16:43 Mon Apr 28, 2014
;Directory G:\Aspen new\aspen save Runid simu1
;
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 4
MANUFCTURING OF NITROBENZENE
FAMT ,Ratnagiri
Page 65
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
INPUT SECTION
INPUT FILE(S) (CONTINUED)
STREAM EXH2O
SUBSTREAM MIXED TEMP=298. PRES=101325. MOLE-FLOW=0.0309
MOLE-FRAC H2O 1.
;
;Input file created by Aspen Plus Rel. 10.2.1 at 00:05:56 Mon Apr 28, 2014
;Directory G:\Aspen new\aspen save Runid SIMU1
;
FLOWSHEET NBMFG
BLOCK RSTO IN=C6H6 MIXACID OUT=CNB
BLOCK DECANTER IN=CNB EXH2O OUT=SPA ORGANIC
BLOCK DIST IN=ORGANIC OUT=TOP BOTTOM
BLOCK MIXER IN=HNO3 H2O H2SO4 OUT=MIXACID
;
;Input file created by Aspen Plus Rel. 10.2.1 at 00:26:14 Mon Apr 28, 2014
;Directory G:\Aspen new\aspen save Runid simu1
FAMT ,Ratnagiri
Page 66
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
FLOWSHEET NBMFG
BLOCK RSTO IN=C6H6 MIXACID OUT=CNB
BLOCK DECANTER IN=CNB EXH2O OUT=SPA ORGANIC
BLOCK DIST IN=2 OUT=TOP BOTTOM
BLOCK MIXER IN=HNO3 H2O H2SO4 OUT=MIXACID
BLOCK B1 IN=ORGANIC OUT=2
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 5
MANUFCTURING OF NITROBENZENE
FLOWSHEET SECTION
FLOWSHEET CONNECTIVITY BY STREAMS
---------------------------------
STREAM
SOURCE
DEST
STREAM
EXH2O
----
DECANTER
H2SO4
----
MIXER
H2O
----
HNO3
----
MIXER
CNB
RSTO
SPA
DECANTER ----
FAMT ,Ratnagiri
C6H6
ORGANIC
SOURCE
----
DEST
RSTO
MIXER
DECANTER
DECANTER DIST
Page 67
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
TOP
DIST
MIXACID
----
MIXER
BOTTOM
DIST
----
RSTO
FLOWSHEET CONNECTIVITY BY BLOCKS
--------------------------------
BLOCK
RSTO
INLETS
C6H6 MIXACID
DECANTER
DIST
MIXER
OUTLETS
CNB
CNB EXH2O
ORGANIC
SPA ORGANIC
TOP BOTTOM
HNO3 H2O H2SO4
MIXACID
COMPUTATIONAL SEQUENCE
----------------------
SEQUENCE USED WAS:
MIXER RSTO DECANTER DIST
OVERALL FLOWSHEET BALANCE
-------------------------
*** MASS AND ENERGY BALANCE ***
FAMT ,Ratnagiri
Page 68
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
IN
OUT
GENERATION RELATIVE DIFF.
CONVENTIONAL COMPONENTS
(KMOL/SEC)
C6H5NO2
0.000000E+00 0.109812E-02 0.109812E-02 -0.336175E-06
H2SO4
0.164266E-02 0.164266E-02 0.000000E+00 -0.189866E-08
H2O
0.335212E-01 0.346193E-01 0.109812E-02 0.138954E-07
HNO3
0.110207E-02 0.395223E-05 -0.109812E-02 -0.680900E-11
C6H6
0.142243E-02 0.324314E-03 -0.109812E-02 -0.764627E-07
TOTAL BALANCE
MOLE(KMOL/SEC)
0.376884E-01 0.376884E-01 0.000000E+00 0.000000E+00
MASS(KG/SEC )
0.945561
ENTHALPY(WATT
) -0.110013E+08 -0.111195E+08
ASPEN PLUS PLAT: WIN32
0.945561
VER: 10.2.1
-0.482081E-07
0.106313E-01
04/28/2014 PAGE 6
MANUFCTURING OF NITROBENZENE
PHYSICAL PROPERTIES SECTION
COMPONENTS
----------
ID
TYPE FORMULA
C6H5NO2 C
H2SO4 C
FAMT ,Ratnagiri
C6H5NO2
H2SO4
NAME OR ALIAS
C6H5NO2
H2SO4
REPORT NAME
C6H5NO2
H2SO4
Page 69
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
H2O
H2O
H2O
HNO3
HNO3
C6H6
C6H6
H2O
HNO3
HNO3
C6H6
ASPEN PLUS PLAT: WIN32
C6H6
VER: 10.2.1
04/28/2014 PAGE 7
MANUFCTURING OF NITROBENZENE
U-O-S BLOCK SECTION
BLOCK: DECANTER MODEL: DECANTER
-------------------------------INLET STREAMS:
CNB
EXH2O
FIRST LIQUID OUTLET: SPA
SECOND LIQUID OUTLET: ORGANIC
PROPERTY OPTION SET: NRTL
RENON (NRTL) / IDEAL GAS
*** MASS AND ENERGY BALANCE ***
IN
OUT
RELATIVE DIFF.
TOTAL BALANCE
MOLE(KMOL/SEC)
0.376884E-01 0.376884E-01 0.000000E+00
MASS(KG/SEC )
ENTHALPY(WATT
FAMT ,Ratnagiri
0.945561
)
0.945561
-0.482081E-07
-0.111411E+08 -0.111630E+08 0.196334E-02
Page 70
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
*** INPUT DATA ***
LIQUID-LIQUID SPLIT, TP SPECIFICATION
SPECIFIED TEMPERATURE
SPECIFIED PRESSURE
298.000
N/SQM
101,325.
CONVERGENCE TOLERANCE ON EQUILIBRIUM
0.10000E-03
MAXIMUM NO ITERATIONS ON EQUILIBRIUM
30
EQUILIBRIUM METHOD
EQUATION-SOLVING
KLL COEFFICIENTS FROM
OPTION SET OR EOS
KLL BASIS
MOLE
KEY COMPONENT(S):
C6H5NO2
*** RESULTS ***
OUTLET TEMPERATURE
OUTLET PRESSURE
N/SQM
CALCULATED HEAT DUTY
WATT
MOLAR RATIO 1ST LIQUID / TOTAL LIQUID
FAMT ,Ratnagiri
298.00
0.10132E+06
-21917.
0.96043
Page 71
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
L1-L2 PHASE EQUILIBRIUM :
COMP
C6H5NO2
0.029137
H2SO4
H2O
X1
0.00061841 0.72129
0.043585
0.91857
X2
0.043344
0.95573
0.049433
0.016631
1,166.36
1.14047
0.017401
HNO3
0.00010487 0.00010429 0.00011894
C6H6
0.0086051
0.00020300 0.21253
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
1.14047
1,046.96
04/28/2014 PAGE 8
MANUFCTURING OF NITROBENZENE
U-O-S BLOCK SECTION
BLOCK: DIST
MODEL: DISTL
----------------------------INLET STREAM:
ORGANIC
CONDENSER OUTLET:
REBOILER OUTLET:
TOP
BOTTOM
PROPERTY OPTION SET: NRTL
RENON (NRTL) / IDEAL GAS
*** MASS AND ENERGY BALANCE ***
IN
OUT
RELATIVE DIFF.
TOTAL BALANCE
FAMT ,Ratnagiri
Page 72
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
MOLE(KMOL/SEC)
0.149140E-02 0.149140E-02 0.000000E+00
MASS(KG/SEC )
ENTHALPY(WATT
0.164883
)
0.164883
-34983.6
0.338098E-08
8548.66
-1.24436
*** INPUT DATA ***
THEORETICAL STAGES
26
FEED STAGE NO. FROM TOP
16
REFLUX RATIO
0.45000
TOP STAGE PRESSURE (N/SQM )
101,325.
BOTTOM STAGE PRESSURE (N/SQM )
101,325.
DISTILLATE TO FEED RATIO
0.20500
CONDENSER TYPE: TOTAL CONDENSER
*** RESULTS ***
FEED-QUALITY
-0.31849
FEED STAGE TEMPERATURE (K
TOP STAGE TEMPERATURE (K
365.058
BOTTOM STAGE TEMPERATURE (K
324.418
)
478.860
CONDENSER COOLING REQUIRED (WATT
NET CONDENSER DUTY (WATT
REBOILER HEATING REQUIRED (WATT
NET REBOILER DUTY (WATT
FAMT ,Ratnagiri
14,284.2
-14,284.2
57,816.5
57,816.5
Page 73
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
BLOCK: MIXER
MODEL: MIXER
----------------------------INLET STREAMS:
HNO3
OUTLET STREAM:
H2O
H2SO4
MIXACID
PROPERTY OPTION SET: NRTL
ASPEN PLUS PLAT: WIN32
RENON (NRTL) / IDEAL GAS
VER: 10.2.1
04/28/2014 PAGE 9
MANUFCTURING OF NITROBENZENE
U-O-S BLOCK SECTION
BLOCK: MIXER
MODEL: MIXER (CONTINUED)
*** MASS AND ENERGY BALANCE ***
IN
OUT
RELATIVE DIFF.
TOTAL BALANCE
MOLE(KMOL/SEC)
0.536596E-02 0.536596E-02 0.000000E+00
MASS(KG/SEC )
ENTHALPY(WATT
0.277778
)
0.277778
-0.199840E-15
-0.224319E+07 -0.224319E+07 0.415178E-15
*** INPUT DATA ***
ONE
PHASE
FAMT ,Ratnagiri
FLASH SPECIFIED PHASE IS LIQUID
Page 74
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
MAXIMUM NO. ITERATIONS
30
CONVERGENCE TOLERANCE
0.00010000
OUTLET PRESSURE N/SQM
BLOCK: RSTO
101,325.
MODEL: RSTOIC
-----------------------------INLET STREAMS:
C6H6
OUTLET STREAM:
CNB
MIXACID
PROPERTY OPTION SET: NRTL
RENON (NRTL) / IDEAL GAS
*** MASS AND ENERGY BALANCE ***
IN
OUT
GENERATION RELATIVE DIFF.
TOTAL BALANCE
MOLE(KMOL/SEC)
0.678839E-02 0.678839E-02 0.000000E+00 0.000000E+00
MASS(KG/SEC )
0.388889
0.388889
ENTHALPY(WATT
) -0.217334E+07 -0.231317E+07
0.000000E+00
0.604497E-01
*** INPUT DATA ***
SIMULTANEOUS REACTIONS
STOICHIOMETRY MATRIX:
FAMT ,Ratnagiri
Page 75
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
REACTION # 1:
SUBSTREAM MIXED :
C6H5NO2
1.00 H2O
1.00 HNO3
-1.00 C6H6
REACTION CONVERSION SPECS: NUMBER=
-1.00
REACTION # 1:
SUBSTREAM:MIXED
KEY COMP:C6H6
ASPEN PLUS PLAT: WIN32
CONV FRAC: 0.7720
VER: 10.2.1
04/28/2014 PAGE 10
MANUFCTURING OF NITROBENZENE
U-O-S BLOCK SECTION
BLOCK: RSTO
ONE
MODEL: RSTOIC (CONTINUED)
PHASE TP FLASH SPECIFIED PHASE IS LIQUID
SPECIFIED TEMPERATURE K
SPECIFIED PRESSURE
N/SQM
MAXIMUM NO. ITERATIONS
CONVERGENCE TOLERANCE
FAMT ,Ratnagiri
323.000
101,325.
30
0.00010000
Page 76
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
*** RESULTS ***
OUTLET TEMPERATURE
OUTLET PRESSURE
HEAT DUTY
323.00
N/SQM
WATT
0.10132E+06
-0.13983E+06
REACTION EXTENTS:
REACTION
REACTION
NUMBER
EXTENT
KMOL/SEC
0.10981E-02
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 11
MANUFCTURING OF NITROBENZENE
STREAM SECTION
BOTTOM C6H6 CNB EXH2O H2O
------------------------FAMT ,Ratnagiri
Page 77
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
STREAM ID
BOTTOM
FROM :
DIST
TO :
----
C6H6
----
CNB
RSTO
RSTO
EXH2O
----
H2O
----
DECANTER DECANTER MIXER
SUBSTREAM: MIXED
PHASE:
LIQUID
LIQUID
LIQUID
LIQUID
LIQUID
COMPONENTS: KMOL/SEC
C6H5NO2
1.0757-03
H2SO4
H2O
7.3724-05
3.1510-18
0.0
1.0981-03
0.0
0.0
1.6427-03
0.0
0.0
0.0
0.0
3.7193-03 3.0900-02 2.6212-03
HNO3
1.9992-11
0.0
3.9522-06
C6H6
3.6209-05 1.4224-03 3.2431-04
0.0
0.0
0.0
0.0
TOTAL FLOW:
KMOL/SEC
1.1857-03 1.4224-03 6.7884-03 3.0900-02 2.6212-03
KG/SEC
0.1424
CUM/SEC
1.4003-04 1.2713-04 3.2101-04 5.6022-04 4.7524-05
0.1111
0.3888
0.5566 4.7222-02
STATE VARIABLES:
TEMP K
PRES N/SQM
478.8604 298.0000 323.0000 298.0000 298.0000
1.0133+05 1.0133+05 1.0133+05 1.0133+05 1.0133+05
VFRAC
0.0
LFRAC
1.0000
FAMT ,Ratnagiri
0.0
0.0
1.0000
0.0
1.0000
0.0
1.0000
1.0000
Page 78
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
SFRAC
0.0
0.0
0.0
0.0
0.0
ENTHALPY:
J/KMOL
2.7788+05 4.9107+07 -3.4075+08 -2.8569+08 -2.8569+08
J/KG
2312.1927 6.2866+05 -5.9481+06 -1.5858+07 -1.5858+07
WATT
329.4732 6.9851+04 -2.3132+06 -8.8279+06 -7.4887+05
ENTROPY:
J/KMOL-K
-3.3127+05 -2.5267+05 -2.3857+05 -1.6272+05 -1.6272+05
J/KG-K
-2756.4033 -3234.6200 -4164.5254 -9032.4484 -9032.4484
DENSITY:
KMOL/CUM
8.4673 11.1885 21.1467
KG/CUM
55.1564 55.1564
1017.6101 873.9777 1211.4430 993.6590 993.6590
AVG MW
120.1805 78.1136 57.2873
ASPEN PLUS PLAT: WIN32
18.0152 18.0152
VER: 10.2.1
04/28/2014 PAGE 12
MANUFCTURING OF NITROBENZENE
STREAM SECTION
H2SO4 HNO3 MIXACID ORGANIC SPA
------------------------------
STREAM ID
FAMT ,Ratnagiri
H2SO4
HNO3
MIXACID
ORGANIC
SPA
Page 79
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
FROM :
TO :
----
----
MIXER
MIXER
MIXER
DECANTER DECANTER
RSTO
DIST
----
SUBSTREAM: MIXED
PHASE:
LIQUID
LIQUID
LIQUID
LIQUID
LIQUID
COMPONENTS: KMOL/SEC
C6H5NO2
0.0
0.0
H2SO4
1.6427-03
H2O
0.0
0.0
0.0
HNO3
0.0
C6H6
0.0
0.0
1.0757-03 2.2385-05
1.6427-03 7.3724-05 1.5689-03
2.6212-03 2.4803-05 3.4595-02
1.1021-03 1.1021-03 1.7738-07 3.7748-06
0.0
0.0
3.1697-04 7.3479-06
TOTAL FLOW:
KMOL/SEC
1.6427-03 1.1021-03 5.3660-03 1.4914-03 3.6197-02
KG/SEC
0.1611 6.9444-02
CUM/SEC
8.8976-05 4.5735-05 2.0328-04 1.4334-04 7.5076-04
0.2777
0.1648
0.7806
STATE VARIABLES:
TEMP K
PRES N/SQM
298.0000 298.0000 298.0000 298.0000 298.0000
1.0133+05 1.0133+05 1.0133+05 1.0133+05 1.0133+05
VFRAC
0.0
LFRAC
1.0000
SFRAC
0.0
0.0
0.0
1.0000
0.0
0.0
0.0
1.0000
0.0
0.0
1.0000
1.0000
0.0
ENTHALPY:
FAMT ,Ratnagiri
Page 80
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
J/KMOL
-7.9337+08 -1.7338+08 -4.1804+08 -2.3457+07 -3.0743+08
J/KG
-8.0891+06 -2.7516+06 -8.0755+06 -2.1217+05 -1.4254+07
WATT
-1.3032+06 -1.9108+05 -2.2432+06 -3.4984+04 -1.1128+07
ENTROPY:
J/KMOL-K
-3.3300+05 -3.1260+05 -2.3701+05 -3.7927+05 -1.6879+05
J/KG-K
-3395.2154 -4960.9513 -4578.3531 -3430.5411 -7826.0507
DENSITY:
KMOL/CUM
18.4618 24.0968 26.3970
KG/CUM
10.4047 48.2138
1810.7264 1518.4146 1366.4855 1150.2998 1039.8510
AVG MW
98.0794 63.0128
ASPEN PLUS PLAT: WIN32
51.7666 110.5555 21.5674
VER: 10.2.1
04/28/2014 PAGE 13
MANUFCTURING OF NITROBENZENE
STREAM SECTION
TOP
---
STREAM ID
FROM :
TO :
TOP
DIST
----
SUBSTREAM: MIXED
FAMT ,Ratnagiri
Page 81
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
PHASE:
LIQUID
COMPONENTS: KMOL/SEC
C6H5NO2
0.0
H2SO4
H2O
0.0
2.4803-05
HNO3
1.7736-07
C6H6
2.8076-04
TOTAL FLOW:
KMOL/SEC
3.0574-04
KG/SEC
2.2389-02
CUM/SEC
2.6121-05
STATE VARIABLES:
TEMP K
324.4181
PRES N/SQM
1.0133+05
VFRAC
0.0
LFRAC
1.0000
SFRAC
0.0
ENTHALPY:
J/KMOL
J/KG
WATT
2.6883+07
3.6711+05
8219.1914
ENTROPY:
FAMT ,Ratnagiri
Page 82
DESIGN & SIMULATION OF NITROBENZENE MANUFACTURING PROCESS
J/KMOL-K
-2.3016+05
J/KG-K
-3142.9623
DENSITY:
KMOL/CUM
11.7048
KG/CUM
857.1411
AVG MW
73.2293
ASPEN PLUS PLAT: WIN32
VER: 10.2.1
04/28/2014 PAGE 14
MANUFCTURING OF NITROBENZENE
PROBLEM STATUS SECTION
BLOCK STATUS
-----------***************************************************************************
*
*
* Calculations were completed normally
*
*
*
* All Unit Operation blocks were completed normally
*
* All streams were flashed normally
*
*
*
***********************************************************
FAMT ,Ratnagiri
Page 83
You might also like
- Qualitative Analysis Tests for Organic CompoundsDocument20 pagesQualitative Analysis Tests for Organic CompoundsNazrene LeysaNo ratings yet
- Exp. 5 Heat Transfer Study On Plate Heat ExchangerDocument6 pagesExp. 5 Heat Transfer Study On Plate Heat ExchangerElaine PuiNo ratings yet
- Minitab DOE Tutorial PDFDocument32 pagesMinitab DOE Tutorial PDFnmukherjee20100% (3)
- Minitab DOE Tutorial PDFDocument32 pagesMinitab DOE Tutorial PDFnmukherjee20100% (3)
- Lab Report Aspen Hysis UiTMDocument12 pagesLab Report Aspen Hysis UiTMAhmad SiddiqNo ratings yet
- Chemistry and Technology of Explosives - Vol. IVDocument704 pagesChemistry and Technology of Explosives - Vol. IVUSMC2RecCharlieMike100% (3)
- Cre Una PDFDocument164 pagesCre Una PDFChetana PatilNo ratings yet
- Interview Questions For Chemical EngineeringDocument6 pagesInterview Questions For Chemical EngineeringNikunj PatelNo ratings yet
- HIRARC GuideLine From DOSHDocument34 pagesHIRARC GuideLine From DOSHMohd Hadri50% (2)
- UntitledDocument12 pagesUntitledapi-256504985No ratings yet
- Project Report PDFDocument45 pagesProject Report PDFvyankatesh vispute100% (1)
- Allyl Chloride Production PDFDocument4 pagesAllyl Chloride Production PDFmarisolNo ratings yet
- Distillation Column Report PDFDocument81 pagesDistillation Column Report PDFpavan kumarNo ratings yet
- Allyl Chloride Production A Case Study in Debottlenecking Retrofitting and DesignDocument7 pagesAllyl Chloride Production A Case Study in Debottlenecking Retrofitting and DesignPaola PorrasNo ratings yet
- Arenes Tutorial With AnswersDocument16 pagesArenes Tutorial With AnswersCorvo Haosen Al-Han100% (1)
- Hydrogenation of Nitrobenzene To AnilineDocument8 pagesHydrogenation of Nitrobenzene To AnilineYu HuiNo ratings yet
- Organic Chemistry Board Exam QuestionsDocument10 pagesOrganic Chemistry Board Exam QuestionsRiza Joie Versales100% (1)
- Distillation Design and Control Using Aspen SimulationFrom EverandDistillation Design and Control Using Aspen SimulationRating: 5 out of 5 stars5/5 (2)
- Petrochem 10 - SEM 1 12-13Document40 pagesPetrochem 10 - SEM 1 12-13Saifuddin AzizNo ratings yet
- Chemical Design of Heat Exchanger TerdesakDocument22 pagesChemical Design of Heat Exchanger TerdesakNor Ain100% (4)
- AQUAPONICDocument40 pagesAQUAPONICRashid FikriNo ratings yet
- Handbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4From EverandHandbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Rating: 5 out of 5 stars5/5 (1)
- CE 3003 Advanced Process Design - Individual Project: Executive SummaryDocument88 pagesCE 3003 Advanced Process Design - Individual Project: Executive SummaryLee Junming100% (1)
- Final ProjectDocument73 pagesFinal ProjectKedar Yadav100% (2)
- Production of N Octane From Ethylene and I ButaneDocument2 pagesProduction of N Octane From Ethylene and I ButaneRamyaNo ratings yet
- Distillation Column ReportDocument27 pagesDistillation Column Reportvaqif100% (1)
- Unit ProcessDocument35 pagesUnit ProcessAnonymous 4CsRmD100% (1)
- Project On NitrobenzeneDocument65 pagesProject On NitrobenzeneAmit Khosla80% (10)
- Cooling Tower TheoryDocument23 pagesCooling Tower Theorysimodo1No ratings yet
- Hysys - Multiple Reactions - StyreneDocument10 pagesHysys - Multiple Reactions - Styrenejenny2409No ratings yet
- Unit-10 Methanol To OlefinDocument19 pagesUnit-10 Methanol To OlefinDurgesh Dev TripathiNo ratings yet
- Module#3-Heat ExchangersDocument19 pagesModule#3-Heat ExchangersLa Casa JordanNo ratings yet
- Distillation Tower DesignDocument65 pagesDistillation Tower DesignAntonio SilvaNo ratings yet
- Design of Allyl TowerDocument11 pagesDesign of Allyl TowerMohammad OmarNo ratings yet
- Hydrodealkylation SimulationDocument8 pagesHydrodealkylation SimulationSchaieraNo ratings yet
- Simulation of Ammonia Production From Synthesis GaDocument12 pagesSimulation of Ammonia Production From Synthesis Gasagar dasguptaNo ratings yet
- Nitro Benzene Sai PDFDocument65 pagesNitro Benzene Sai PDFDeepak Pandey100% (1)
- Plate Column Distillation EfficiencyDocument7 pagesPlate Column Distillation EfficiencyVijay PrasadNo ratings yet
- Brian JR Geoffroy CHNG 3012 Part II PDFDocument103 pagesBrian JR Geoffroy CHNG 3012 Part II PDFjanelle ramdahinNo ratings yet
- Kuwait University Chemical Engineering Plant Design Hysys ReportDocument20 pagesKuwait University Chemical Engineering Plant Design Hysys ReportCrazy HelloNo ratings yet
- Production of Aniline by Hydrogenation of NitrobenzeneDocument4 pagesProduction of Aniline by Hydrogenation of Nitrobenzenezainab zebNo ratings yet
- Gas Absorption LabDocument8 pagesGas Absorption Labsolehah misni100% (1)
- Distillation Column Design for Levulinic Acid SeparationDocument35 pagesDistillation Column Design for Levulinic Acid SeparationKirstie ImeldaNo ratings yet
- Cumene BDocument6 pagesCumene BimanchenNo ratings yet
- Overall Flowsheet Simulation Benzene Cyclohexane TW6Document7 pagesOverall Flowsheet Simulation Benzene Cyclohexane TW6Mitesh ParmarNo ratings yet
- Instrumentation of Distillation ColumnDocument6 pagesInstrumentation of Distillation ColumnHashir Ali0% (1)
- FYDP Final Report G13 PDFDocument30 pagesFYDP Final Report G13 PDFJeanette Hong May Hurn0% (1)
- Economic Aspects of Setting Up Purge Gas Recovery Unit (PGRU) With Ammonia Production ProcessDocument7 pagesEconomic Aspects of Setting Up Purge Gas Recovery Unit (PGRU) With Ammonia Production ProcessWilly ChandraNo ratings yet
- Experiment 4a – Pressure Drop in Packed ColumnsDocument21 pagesExperiment 4a – Pressure Drop in Packed ColumnsMohamad Samer KansouNo ratings yet
- Allyl BDocument9 pagesAllyl BDemet AcargilNo ratings yet
- Energy Balance For Distillation ColumnDocument2 pagesEnergy Balance For Distillation ColumnKarar AlalihNo ratings yet
- 2 5188584049941152568Document24 pages2 5188584049941152568Slem Hamed100% (1)
- CSTRDocument11 pagesCSTRfarahanisiliasNo ratings yet
- Production of Acrylonitrile by Ammoxidation of PropyleneDocument33 pagesProduction of Acrylonitrile by Ammoxidation of PropyleneJ José B VelasquezNo ratings yet
- Hidratação Direta PropenoDocument53 pagesHidratação Direta Propenossargo100% (2)
- A Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabDocument51 pagesA Major Project Report On Design of Multicomponent Distillation Column by Approximate and Rigorous Method Using MatlabMasood HassanNo ratings yet
- GROUP 14 PRODUCTION OF 21,000 MTPA OF ETHYLENE: COMPUTER SIMULATION, HEAT INTEGRATION, AND PROCESS EQUIPMENT SIZINGDocument54 pagesGROUP 14 PRODUCTION OF 21,000 MTPA OF ETHYLENE: COMPUTER SIMULATION, HEAT INTEGRATION, AND PROCESS EQUIPMENT SIZINGAtika Mohd Yatim100% (1)
- Mini Project Full PDFDocument37 pagesMini Project Full PDFMohamad El KheirNo ratings yet
- Assignment 1 QDocument2 pagesAssignment 1 Qlastlanding100% (2)
- Material and Balance For Sohio Process That Produce AcrytonitrileDocument2 pagesMaterial and Balance For Sohio Process That Produce Acrytonitrileafnan_lion940% (1)
- PFD Diagram of Benzoic Acid FormationDocument5 pagesPFD Diagram of Benzoic Acid FormationShailesh LahotiNo ratings yet
- Distillation - Written ReportDocument17 pagesDistillation - Written ReportmichsantosNo ratings yet
- RI Vs Composition Methanol-Water MixtureDocument12 pagesRI Vs Composition Methanol-Water MixtureAnonymous VeJYFSMWLINo ratings yet
- MATERIAL BALANCE TITLEDocument46 pagesMATERIAL BALANCE TITLEG Vamsee KrishnaNo ratings yet
- Heat Transfer Process TUT (After Mid Semester)Document11 pagesHeat Transfer Process TUT (After Mid Semester)vaishnavi singh100% (1)
- Distillation ColumnDocument32 pagesDistillation ColumnTatiana RosarioNo ratings yet
- Continuous Regenerative (Moving Bed) CCR PlatformingDocument4 pagesContinuous Regenerative (Moving Bed) CCR PlatformingAnnissa Nur HidayatiNo ratings yet
- Process SelectionDocument4 pagesProcess SelectionZahid HissamNo ratings yet
- Project ON NitrobenzeneDocument65 pagesProject ON NitrobenzeneRomil GandhiNo ratings yet
- Project ON NitrobenzeneDocument65 pagesProject ON NitrobenzeneRomil GandhiNo ratings yet
- OSHMS Guideline-DOSHDocument77 pagesOSHMS Guideline-DOSHMohd ZulhaidyNo ratings yet
- OSHMS Guideline-DOSHDocument77 pagesOSHMS Guideline-DOSHMohd ZulhaidyNo ratings yet
- Telemetry and Geographical Information System To Assess Urban AreaDocument12 pagesTelemetry and Geographical Information System To Assess Urban AreaRashid FikriNo ratings yet
- Cooling Towers Permit - 0Document1 pageCooling Towers Permit - 0Rashid FikriNo ratings yet
- Vegas Pro 1.0Document312 pagesVegas Pro 1.0Rashid FikriNo ratings yet
- CHAPTER 3 (Water&Air)Document31 pagesCHAPTER 3 (Water&Air)Rashid FikriNo ratings yet
- Chapter 4 Issues in Engineering TechnologyDocument45 pagesChapter 4 Issues in Engineering TechnologyRashid FikriNo ratings yet
- Dna Replication 2Document28 pagesDna Replication 2Rashid FikriNo ratings yet
- DR 2000 Spectrophotometer Procedures Manual, O-ZDocument135 pagesDR 2000 Spectrophotometer Procedures Manual, O-ZRashid FikriNo ratings yet
- Lecture 4 CloningDocument44 pagesLecture 4 CloningRashid FikriNo ratings yet
- Chapter 16 SolutionsDocument10 pagesChapter 16 SolutionsShaoxin LuNo ratings yet
- Jiping Liu - Nitrate Esters Chemistry and Technology-Springer (2019) PDFDocument694 pagesJiping Liu - Nitrate Esters Chemistry and Technology-Springer (2019) PDFClaudia Patricia Tapia RiveraNo ratings yet
- MNT Design 2520of 2520equipmentsDocument32 pagesMNT Design 2520of 2520equipmentsshamsabbasNo ratings yet
- A02 303Document15 pagesA02 303jaimeNo ratings yet
- Chem A A2 Task 3 EVAL Task Jun12 PDFDocument7 pagesChem A A2 Task 3 EVAL Task Jun12 PDFSophie PriorNo ratings yet
- Essay On StalinDocument8 pagesEssay On Stalinfz6zke9m100% (2)
- BOW Guru Antra Goal: Anand PrakashDocument35 pagesBOW Guru Antra Goal: Anand PrakashCube WorldNo ratings yet
- Practice Exam OC1.1Document9 pagesPractice Exam OC1.1Stella CheaNo ratings yet
- AROMATIC SUBSTITUTION REACTIONSDocument54 pagesAROMATIC SUBSTITUTION REACTIONSTariq ZiaNo ratings yet
- 6.02 Pyridines and Their Benzo Derivatives: Reactivity at The RingDocument59 pages6.02 Pyridines and Their Benzo Derivatives: Reactivity at The RingbhawanisrNo ratings yet
- Xanthoproteic TestDocument2 pagesXanthoproteic TestLou Andrae G. Santos0% (1)
- Exersice PDFDocument24 pagesExersice PDFharsh mishraNo ratings yet
- Propellant Chemistry JWDocument9 pagesPropellant Chemistry JWmydaddy123100% (1)
- 12th Chem ch#9. MCQDocument2 pages12th Chem ch#9. MCQAli MalikNo ratings yet
- Test 2 Organic Chemistry AnswersDocument6 pagesTest 2 Organic Chemistry AnswersPawan BabelNo ratings yet
- Tests For FlavonoidsDocument9 pagesTests For FlavonoidsPiryaNo ratings yet
- Colour Reactions of Chalcones and Their Mechanism (A Review)Document6 pagesColour Reactions of Chalcones and Their Mechanism (A Review)Walid EbaiedNo ratings yet
- Regioselective Nitration of Phenol Over Solid Acid CatalystsDocument4 pagesRegioselective Nitration of Phenol Over Solid Acid CatalystsAlvin Renard SusantoNo ratings yet
- Nitro CompoundDocument7 pagesNitro CompoundEng. Hunter100% (1)
- Final FileDocument209 pagesFinal Filenavneetkaur77No ratings yet
- Test No 3 AROMATIC HYDROCARBONSDocument11 pagesTest No 3 AROMATIC HYDROCARBONSMuhammad. Hafeez.MughalNo ratings yet
- Synthesis of Nitrobenzene ReportDocument6 pagesSynthesis of Nitrobenzene ReportHasrilia BeskaraNo ratings yet
- Mononitration of TolueneDocument4 pagesMononitration of TolueneNur Syafiqah Izzuddin100% (1)