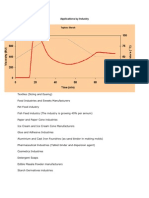
Professional Documents
Culture Documents
Modified Starch
Uploaded by
rajeevtyagi41Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Modified Starch
Uploaded by
rajeevtyagi41Copyright:
Available Formats
2.
Review of Literature
Chapter 2
REVIEW OF LITERATURE
2.1
Starch-chemistry.
Starch is the predominant carbohydrate reserve in many plants; Starch is semicrystalline in
nature with varying levels of crystallinity. The packaging of amylose and amylopectin within
the granules has been reported to vary among the starches from different species. The activity
of the enzymes involved in starch biosynthesis may be responsible for the variation in
amylose content among the various starches (Krossmann and Lloyd, 2000). Starch granule
differences amongst various plant species are accounted for, not only by the ratio of
constituent molecules, but also by their location and interaction and it is probably the most
commonly used hydrocolloid. Starch is a morphological complex polymer substance, (Fig.
2.1). The crystalline composition consists of around 15-45% of the starch granules. The
crystallinity is exclusively associated with the amylopectin component, while the amorphous
regions mainly represent amylose (Zobel, 1988a, 1988b).
Fig 2.1: Backbone of starch molecule
Amylose: Amylose is defined as a linear molecule of D-glucopyranosyl units joined by (1
4) linkage, but it is today well established that some molecules are slightly branched by (1
6) linkages (Fig 2.2). Amylose solutions can be easily characterized by size-exclusion
chromatography coupled on-line to multi-angle laser light scattering (SECMALLS). It is the
8
2. Review of Literature
smaller of the two polysaccharides making up starch molecule. The amylose is essentially
linear but not purely and its solution properties are generally regarded as typical for those of
a linear polymer (Biliaderis, 1991). The inside of the helix is lipophilic where there are only
hydrogen atoms. On the outside, there are hydrophilic hydroxyl groups. The exact position of
amylose in the granules is uncertain, but it is generally believed that it acts as an amorphous
space filler in the granules, whereas the amylopectin is highly branched with shorter chains
arranged as double helices in clusters of a partially crystalline character (French, 1984,
Zobel, 1988). Amylose is located in the granule as bundles between amylopectin clusters and
or randomly dispersed. They could be located therefore among the amorphous and crystalline
regions of the amylopectin clusters (Robin et al., 1974). In starch granules, the amylose chain
displays a natural twist in a helical conformation with six anhydroglucose units per turn
(Zobel, 1988a). Amylose is probably the first biopolymer for which a helical structure was
proposed. The ability of amylose to form complexes with butanol provides a method for
separating amylose from amylopectin by selective precipitation (Schoch, 1968).
Amylopectin- Amylopectin is the highly branched component of starch and it is formed
through chains of -D glucopyranosyl residues linked together mainly by (1.4) linkages
but with 56% of (1,6) bonds at the branch points (Fig 2.2).
2. Review of Literature
Fig 2.2: Structure of amylose and amylopectin molecules
The multiplicity in branching is a common feature of both amylopectin and glycogen. The
basic organization of the chains is described in terms of the A, B and C chains as defined by
Peat et al (1952). Thus, the outer chains (A) are glycosidically linked at their potential
reducing group through C6 of a glucose residue to an inner chain (B); such chains are in turn
defined as chains bearing other chains as branches. The single C chain per molecule likewise
carries other chains as branches but contains the sole reducing terminal residue. The ratio of
A-chains to B-chains is an important parameter which is also referred to as the degree of
multiple branching.
Minor components- Minor components associated with starches correspond to three
categories of materials: (i) particulate material, composed mainly of cell-wall fragments; (ii)
surface components, removable by extraction procedures; and (iii) internal components.
Lipids represent the most important fraction associated with the starch granules. Starch
quality is also influenced by the presence of lipids, proteins and phosphorous. Lipid levels
are lower in tuber than in cereal starches. In tuber starches, lipids are only found on the
10
2. Review of Literature
granule surface, while starches from cereal endosperm have surface and integral lipids
(Morrison, 1988) Starch also contains several different minerals in small amounts, and the
most important mineral is phosphorus (Bulen et al., 1998).
2.2 Structural properties of starch
The extent of crystallinity of native starch granules ranges from about 15% for high-amylose
starches to about 4550% for waxy starches. The granules have a hierarchical structure that
can be observed readily by light and electron microscopy. The morphology of starch granules
depends on the biochemistry of the chloroplast or amyloplast, as well as physiology of the
plant (Badenhuizen, 1969). The granule is a partially crystalline material, i.e., within it, there
are amorphous and crystalline regions and the degree of crystallinity is reported to be in the
range 15-35% (French, 1984). The long range molecular order in the starch granules can be
studied by the X-ray powder diffraction technique. Depending on the plant origin, native
starch exhibits three different X-ray diffraction patterns. A- pattern, characteristic of cereal
starches like wheat, barley, rye, oat, maize, rice etc with characteristic d-spacing at 5.8, 5.2
and 3.8 , B- pattern characteristic of certain tuber and stem starches like potato which have
characteristic d-spacing at 15.8, 5.9, 5.2, 4 and 3.7 A0
and retrograded starch (Ring et al.,
1987), and C-pattern, intermediate between A- and B-types which is found in legume
starches and some tuber and seed starches (French, 1984). It has the characteristic d-spacing
found in the A-pattern and the 15.8 A0 d-spacing of the B-pattern (Zobel, 1988a). The sharp
diffraction patterns in the XRD are usually associated with crystalline material and the nonsharp areas with amorphous regions.
The crystallinity of starch has been assigned to the well-ordered structure of the
amylopectin molecules inside the granules. The absolute crystallinity of starch from four
varieties of cassava was found to lie between 8-14%. (Moorthy et al., 2002). Cassava starch
possesses A, C or a mixed pattern with three major peaks at 2=15.3, 17.1 and
23.5(Rickard et al 1991/14) ; Sweet potato starch also has A (Takeda et al., 1986 ,Gallant
et al., 1982) C (Zobel, 1988, Chiang and Chen, 1988) or intermediate pattern (Tian et al.,
1991). Takeda et al., observed A pattern for two varieties of sweet potato while it was CA
for another variety with absolute crystallinity of 38%. Colocasia, Xanthosoma, Pachyrrhizus,
11
2. Review of Literature
Arrowroot, Amorphophallus and D dumetorum starches possess A pattern (Moorthy,2001,
Gallant et al., 1982) and Dioscorea alata, Dioscorea esculenta, Dioscorea rotundata,
Dioscorea abysinica and Dioscorea cayensis starch possess B patterns It was found that the
XRD pattern of extracted starch is the same throughout the growth period of Diosocrea
rotundata. Canna edulis and Curcuma sp. starches exhibit B XRD pattern. The absolute
crystallinity of Canna starch was 26% (Zoebel, 1988a).
Tuber crops
Starchy tubers and root crops are important subsidiary or subsistence food in tropical and sub
tropical countries. Although a wide range of tuber crops are grown worldwide, only five
species account for almost 99% of the total world production. These are potato (Solanum
tuberosum, 46%), cassava (Manihot esculenta, 28%), sweet potato (Ipomea batatas, 18%),
yams (Dioscorea spp., 6%) and taro (Colocassia, Cytosperma, Xanthosoma spp., 1%). Root
and tuber crops are grown worldwide and usually have low commercial value for direct
consumption. The starch of such crops would be a good source for different food industries
(Alves et al., 1999, Amani et al., 2004, Brunnschweiler et al., 2005, Moorthy et al., 1993).
The tropical root starches have widely varying physicochemical and functional properties
unlike the cereal starches which possess almost similar characteristics (Moorthy, 1994). The
large variability in the starch properties can be attributed to the differences in the
morphological and structural features of the starches
2.3 Functional properties of starch
Applications of starch in food and industry depend on various functional properties like
viscosity, swelling, retrogradation etc., which in turn depend on the source of starch,
presence of various ingredients and processing conditions. These properties are discussed
below.
Swelling volume
The swelling power (SP) is the ratio of the wet weight of the sedimented gel to its dry weight
of starch (Crosbie, 1991). Swelling factor (SF) is the ratio of the volume of sedimented gel to
12
2. Review of Literature
the volume of dry starch granules with a density of 1.4 g/ml (Tester and Morrison, 1990).
Swelling apparently is a property of amylopectin. High proportions of long chain (degree of
polymerization >35) molecules in amylopectin contributed to the increase in swelling (Sasaki
and Matsuki, 1998). When swollen granules are the dominant structural feature in aqueous
starch systems, starch concentration is an important factor. In the dilute regime, the viscosity
is governed by the volume fraction of swollen granules (Steeneken, 1989). In the
concentrated regime, the viscosity is governed by particle rigidity. The swelling power and
solubility provide evidence of the magnitude of interaction between starch chains within the
amorphous and crystalline domains. The extent of this interaction is influenced by the
amylose to amylopectin ratio, and by the characteristics of amylose and amylopectin in terms
of molecular weight/distribution, degree and length of branching and conformation (Hoover,
2001).
Gelatinization properties
Gelatinization is a major step which exhibits featured characteristics of starch. The granules
absorb water and swell, and the crystalline organization is irreversibly disrupted (Fig 2.3).
The gelatinization temperature of most starches is between 60 and 800C. In general, there is a
negative relationship between the amylose content of starch and the gelatinization
temperature. Collapse of crystalline order within the starch granules manifests itself as
irreversible changes in properties, such as granule swelling, pasting, loss of optical
birefringence, loss of crystalline order, uncoiling and dissociation of the double helices, and
starch solubility (Atwell et al., 1988, Hoover, 2001, Stevens and Elton, 1981). The orderdisorder transitions that occur on heating an aqueous suspension of starch granules have been
extensively investigated using DSC (Donovan, 1979, Jenkins, 1994). Starch transition
temperatures and gelatinization enthalpies by DSC may be related to characteristics of the
starch granule such as degree of crystallinity. Gelatinization occurs initially in the amorphous
regions, as opposed to the crystalline regions, of the granule, because hydrogen bonding is
weaker in these areas. The differences in transition temperatures between the different
starches may be attributed to the differences in the degree of crystallinity. The gelatinization
and swelling properties are controlled in part by the molecular structure of amylopectin (unit
chain length, extent of branching, molecular weight, and polydispersity). Differential
13
2. Review of Literature
Scanning Calorimetry (DSC) is the most common technique used to study the thermal
properties of starches. It measures first-order (melting) and second-order (glass transition)
transition temperatures and heat flow changes in polymeric materials and gives information
on order-disorder phenomena of starch granules (Biliaderis et al., 1986a). Gelatinization is an
endothermic process.
Fig 2.3 Swelling of starch granule during heating process in the presence of water
(Mechanism for Starch Gelatinisation. (Harper, 1981b)
Retrogradarion
The molecular interactions (hydrogen bonding between starch chains) after cooling of the
gelatinized starch paste have been called retrogradation (Hoover, 2001). During
retrogradation, amylose forms double-helical associations of 40-70 glucose units (Jane and
Robyt, 1984) whereas amylopectin crystallization occurs by association of the outermost
short branches. In the case of retrograded starch, the value of enthalpy provides a quantitative
measure of the energy. Starch retrogradation enthalpies are usually 60-80% lower than
gelatinization enthalpies and transition temperatures are 10-26 C lower than those for
14
2. Review of Literature
gelatinization of starch granules. The crystalline forms are different in nature from those
present in the native starch granules (Karim et al., 2000). Fig 2.4 summarizes the changes
during heating and cooling of starch suspensions.
Fig 2.4:
Terms to describe changes induced by heating and cooling (b) Physico-
chemical changes that take place during heating and cooling. (Svegmark 1993)
Retrograded starches show lower gelatinization temperatures and enthalpy than native
starches because they have weaker starch crystallinity (Sasaki et al., 2000). The crystalline
forms are different in nature from those present in the native starch granules (Karim et al.,
2000). Both amylose and amylopectin fractions are important in the retrogradation process.
Amylose undergoes rapid crystallization as soon as cooling begins and retrogradation
depends on the amylose content in the sample, the amount that is free and uncomplexed with
lipids, and its molecular weight distribution. Amylopectin, on the other hand, recrystallizes
slowly and the degree of retrogradation depends on the chain length distribution of
15
2. Review of Literature
amylopectin. Recrystallization and retrogradation of amylopectin is dominant at a higher
concentration of solids and the polymer formed is more loosely bound than retrograded
amylose and hence, highly susceptible to amylolysis (Ring et al., 1988).
Pasting properties
Continued heating of starch in excess water with stirring causes the granules to further swell,
the amylose to leach more, and the granules to disintegrate, forming a viscous material called
paste (BeMiller, 2007). Pasting occurs after or simultaneously with gelatinization. Pasting
properties of starch are important indicators of how the starch will behave during processing
and are commonly measured using various viscometers like Brabender Viscometer and Rapid
Visco Analyzer (RVA). Initially heating starch suspension results in swelling of starch
granules. As heating continues, an increase in viscosity can be observed, which reflects the
process of pasting. The temperature at the onset of viscosity increase is termed pasting
temperature. Viscosity increases with continued heating, until the rate of granule swelling
equals the rate of granule collapse, which is referred to as the peak viscosity (PV). PV
reflects the swelling extent or water-binding capacity of starch and often correlates with final
product quality since the swollen and collapsed granules relate to texture of cooked starch.
Once PV is achieved, a drop in viscosity, or breakdown, is observed as a result of
disintegration of granules. Break-down is a measure of the ease of disrupting swollen starch
granules and suggests the degree of stability during cooking (Adebowale and Lawal, 2003).
Minimum viscosity, also called hot paste viscosity, holding strength, or trough, marks the end
of the holding stage at the maximum temperature of the RVA. Cooling stage begins and
viscosity again rises (setback) which is caused by retrogradation of starch, particularly
amylose. Setback is an indicator of final product texture and is linked to syneresis or weeping
during freeze-thaw cycles.
16
2. Review of Literature
Fig 2.5: Starch granules during cooking. (Fapet Oy, Helsinki Finland from Pigment Coating
and Surface)
Viscosity normally stabilizes at a final viscosity or cold paste viscosity, which is related to
the capacity of starch to form viscous paste or gel after cooking and cooling (Batey, 2007,
Newport Scientific, 1998). Other components naturally present in the starchy material or
additives interact with starch and influence pasting behavior.
Rheology
Rheology is the study of the flow and deformation of materials (Barnes et al., 1989) and
associate the physical flow behavior with the materials internal structure and a distinction is
made between liquid, solid and viscoelastic materials. Which property dominates, and what
the values of the parameters are depend on the stress and the duration of stress application.
Thus, a given material can behave like a solid or like a liquid, depending on the time scale of
the deformation process. If the experiment is performed relatively slowly, the sample appears
to be viscous rather than elastic; if the experiment is performed relatively fast, it appears to
be elastic rather than viscous (Barnes et al., 1989). Shear stress, shear rate and viscosity are
the building blocks of understanding rheology. Fig 2.6 is a depiction of the velocity gradient
created in a liquid between two parallel plates of area A. One plate is positioned at y = 0, and
the other is positioned at y = d. The plate at y = d is moved at a relative velocity U while the
17
2. Review of Literature
plate at position y = 0 remains stationery. The force F exerted on the liquid creates a velocity
gradient where the small arrows are proportional to the local velocity. This motion creates
internal friction. The resistance to this force is the shear viscosity. Shear strain is described
as "the movement of a layer of material relative to parallel adjacent layers, and is generally
referred to as shear. The change in shear strain per unit time, known as the shear rate y,
creates the velocity gradient. The force parallel to the plate at y = d is known as the shear
stress. Steady shear measurements are traditionally regarded as the most important material
properties that result in the knowledge of the material response.
Fig 2.6: Shear deformation of a material
There are generally two types of materials, Newtonian and non-Newtonian. Newtonian
materials are characterized by constant viscosity over a large range of stress. Examples of
Newtonian materials include water, alcohol, and most oils. Non-Newtonian materials are
defined by remarkable changes in the viscosity of suspensions with changes in stress. NonNewtonian suspensions exhibit elastic properties as well as viscous properties. Some exhibit
properties of a viscous fluid and an elastic solid. These particular characteristics are known as
viscoelasticity. Elasticity describes the ability to store mechanical energy reversibly during
deformation. Viscoelastic properties are attributed to the breakdown and reformation of the
network structure of applied shear stress. Linear viscoelasticity is based on the superposition
principle that implies that the strain at any time is directly proportional to the stress.
18
2. Review of Literature
The general aims of rheological measurements are:
to obtain a quantitative description of the materials mechanical properties
to obtain information related to the molecular structure and composition of the to
material
characterize and simulate the materials performance during processing and for quality
control
Several methods are available for measuring the rheological properties of a solution, but the
geometry of the measurement device is of great importance. Several different measurement
geometries exist, like spindle type, concentric cylinders, cone and plate etc. (Fig.2.7).
The most common types of fundamental rheological tests used in cereal testing are: (i) flow
viscometry (ii) small and large deformation shear creep and stress relaxation; (iii) large
deformation extensional measurements; and (iv) small deformation dynamic shear
oscillation. Here we discussing about the two major rheological analyses, the flow curve and
the dynamic shear oscillation tests.
Fig 2.7: Different measuring systems used for rheological analysis.
19
2. Review of Literature
Steady shear flow curves
When shear is slowly imparted to such a system, it becomes progressively easier as
successive increments of force are applied. The nature of the interlocking or interwoven
structure dictates whether initial flow occurs with difficulty until sufficient structure is lost or
whether a sufficient initial force is required to initiate motion, that is, whether a yield value
has to be exceeded. In any case, continued shearing breaks further linkages, so that the
apparent viscosity drops with increasing shear. A flow curve, viscosity (h) versus shear rate
(g), across a wide range of shear rates can provide important information about storage
stability; optimal conditions for mixing, pumping, and transferring; and end-user
applications. It also provides important information regarding the ways in which the structure
changes to comply with the applied shear in different conditions, such as storage, processing,
and application. If the shear rate changes during an application, the internal structure of the
sample will change and the change in stress or viscosity can then be seen.
Yield stress phenomena
The yield stress measurement is crucial for modified starch. Yield stress (y) is defined as the
minimum shear stress required for initiating flow. Yield stress can be measured using a stress
ramp experiment. Yield stress can also be defined as the stress below which a material will
not exhibit a fluid like behavior. This means that subjecting a material to stresses less than
the yield stress will lead to a non permanent deformation or a slow creeping motion over the
time scale of the experiment.
Thixotropy
Time-dependent flow measures the increase or decrease in viscosity with time, while a
constant shear is applied. The flow is called thixotropic if viscosity decreases with time or
rheopetic if it increases. Thixotropic behavior describes a degradation of the structure during
the loaded phase; thus, a reduction in viscosity with time occurs when shear is applied.
During the relieved phase, the original structure is recoverable. The extent of structural
recovery is dependent on the time allowed for the recovery. Therefore, a thixotropic material
will have a shear thinning behavior when a gradually increasing shear is applied. This is
20
2. Review of Literature
because the orientation of the structures molecules or particles will change to align with the
flow direction. However, its original orientation can be restored over a period of time after
the external force is removed. There is a delay in time for the structure to recover completely.
Viscoelastic test methods (dynamic shear oscillation)
Two different types of methods are available to determine the linear viscoelastic behaviour of
a material: dynamic and static methods (Barnes et al., 1989). The dynamic methods involve
the application of harmonically varying stress or strain. The static methods involve the
imposition of a step change in the stress or strain and the observation of the subsequent
development of the strain or stress as a function of time. Analyses of the viscoelastic
materials are designed not to destroy the structures, so that the measurements can provide
information about the intermolecular and inter-particle forces of the materials (Martin, 1993).
Oscillatory measurements provide information about the structure and elasticity of a material.
They can, for example, be used to determine the storage stability. The oscillation strain
sweep measurement is used to find a range of strain at which the rheological properties of the
samples are independent of the applied strain. These strain values should not be exceeded in
further measurements. A strain amplitude sweep is utilized to determine the linear
viscoelastic region of material response, which is used to establish the correct parameters for
subsequent dynamic testing. The maximum strain at which G remains constant is called the
critical strain and defines the limit of the linear viscoelastic region (LVE). In general, the
material can respond to this type of deformation through two mechanisms: elastic energy
storage and viscous energy dissipation. Quantitatively, these responses can be represented as
storage modulus (G), energy stored per unit volume, and loss modulus (G), energy
dissipated per unit deformation rate per unit volume. Storage modulus (G) is proportional to
the extent of the elastic component (contributed by crosslinking, entanglement, and/or
aggregation) of the system, and loss modulus (G) is proportional to the extent of the viscous
component (contributed by the liquid like portion) of the system (Larson 1999).
Typically, the strength of interaction or internal structure in an emulsion is measured by the
magnitude ofthe ratio G/G = tan , which is called damping factor ( is phase angle). The
21
2. Review of Literature
smaller the tan (or the greater G), the stronger the interaction (Radebaugh and Simonelli,
1983).
Phase angle tan d is associated with the degree of viscoelsticity of the sample. A low value in
tan d or d indicates a higher degree of viscoelasticity (more solid like). The phase angle d can
be used to describe the properties of a sample.
d = 90 G*= G and G= 0 viscous sample
d = 0 G*= G and G= 0 elastic sample
0 < d < 90 viscoelastic sample
d > 45 G> G semi liquid sample
d < 45 G> G semi solid sample
Complex viscosity - h*
Complex viscosity describes the flow resistance of the sample in the structured state,
originating as viscous or elastic flow resistance to the oscillating movement.
Static methods
Static methods are either creep tests at constant stress or relaxation tests at constant strain.
The creep test is used far more often than the relaxation test. In the creep test, a constant
stress is applied and the strain of a sample is determined as a function of time. In the
relaxation test the sample is subjected to a predetermined strain, and the stress required
maintaining this strain is measured as a function of time (Marriott, 1988). The creep/recovery
test is therefore an alternative for obtaining the relaxation time and viscoelastic properties of
a material.
2.4 Starch modification.
The industrial applications of starch are often limited because it is used mainly in its
unmodified form. Very often the viscosity of cooked native starch is so high that it precludes
22
2. Review of Literature
its use in specific applications. For example, tuber starches on cooking give high peak
viscosity, which upon continued cooking and cooling drops, contrary to cereal starches,
which show moderate host paste viscosity, but result in substantial increase in setback
viscosity upon cooling; some starch dispersions are gummy and not palatable; amylose-rich
starches form rigid, opaque gels on cooling (due to retrogradation), which on storage lose
water (syneresis), whereas amylopectin-rich starches (waxy-type) form soft gels.
To meet the demanding technological needs of today, the properties of starch are modified by
a variety of modification methods. Starch modification is aimed at correcting one or some of
the short comings, which will enhance its versatility and satisfy consumer demand. Thus, the
various chemically or otherwise modified starch derivatives offer significant value addition
and give scope to develop a variety of fabricated food products having varied texture and
mouth feel. These modifications are aimed at introducing desirable alterations in the starch
structure so that its behavior is predictable and controllable (Fig 2.8).
Fig2.8: Modification of starch
23
2. Review of Literature
Therefore, the modified starch derivatives are the products of either glucosidic bond cleavage
(acid modification to dextrins) or forming new functional groups (carbonyl group formation
during oxidation), or substitution of free available hydroxyl groups (by etherification or
etherification) or bridging of molecular chains by cross-linking reactions. The various
modifications employed are physical modification, chemical and enzymatic modifications.
Physical Modification
The common physical modification methods of starch include pregelatinisation, heatmoisture treatment (HMT), annealing, steam treatment, extrusion and gamma irradiation
(Eliasson and Gudmundsson,1996, Sair, 1967, Raja, 2000, Bao and Corke, 2003). Physical
modification involves the simultaneous action of several conditions such as temperature,
pressure, moisture and shear. Temperature and moisture contents during processing of starch
alter its functional properties.
Pre-gelatinization: Pre-gelatinization is the simplest of all starch modifications. It is
effected by the cooking of aqueous starch slurry and subsequent drying. These starches are
very useful in the preparation of ready-to-eat convenience foods; they give a palatable texture
and help to hold other components in a uniform suspension. The market for such starches is
steadily expanding. They are also useful as wall paper adhesives. The process for the
production of pregelatinised starch involves drying of 30-40 % (dry solid) starch slurry on a
roller drum drier heated to 160-1700C by direct steam. The product exhibits high
transparency, high viscosity and good color carrier properties Drum drying is the most
common method of producing prelatinised starch. In general there are two types of drum
dryers, the single and double drum dryer and are used in large scale manufacture of
pregelatinised starch.
Heat moisture treated starch (HMT): Heat moisture treatment of starches is defined as the
physical modification that involves the treatment of starch granules at low moisture levels for
a certain period at a temperature above glass transition points. The studies on HMT of tuber
and root starches indicated that the extent of starch chain association within the amorphous
region and the degree of crystalline order of the starch granules is altered during HMT
(Gunaratne and Hoover, 2001). Structural changes within the amorphous region and
24
2. Review of Literature
crystalline region of starch granules such as starch chain interaction within the amorphous
region and disruption and reorientation of starch crystallites are caused by heat-moisture
treatment. (Hoover and Manuel, 1996b). It seems that disruption of starch crystallites and
reorientation of amylopectin double helices by heat-moisture treatment allow more reaction
reagents to access the crystalline region increasing derivatization in that region. Heat
moisture treatment makes significant variations in the XRD pattern of starches.
Transformations from B to A or B+A type by HMT have been reported for potato starch by,
Gunaratne and Hoover (2002), Hoover and Vasanthan (1994). As stated by Genkina, et al.,
(2004), HMT often results in transformation of the less thermodynamically stable Bpolymorphic structure (with hexagonal packing of double helices and about 36 water
molecules inside every cell) to a more stable monoclinic structure of A-type polymorphs
(with about six water molecules inside the helices)
Extrusion: Extrusion cooking, because of its low cost and continuous processing capability
is a popular means of modifying the functional characteristics of starches. Numerous studies
have reported on the complexities of extrusion process and modeling of the process.
Extrusion cooking can be described as a process whereby the moistened materials are cooked
and worked into viscous, plastic like dough. Extrusion cooking represents a more modern and
versatile process, but depending on the specific mechanical energy input and product
temperature, solubility is the pronounced functional characteristic. The improved functional
characteristics favour application in various fields (Colonna et al., 1989).
Annealing: Annealing refers to treatment of starch in excess water (<65%, w/w) or at
intermediate water contents (4050%, w/w) at temperatures below the onset temperature of
gelatinization. The physical aim of annealing is to approach the glass transition temperature
which enhances molecular mobility without triggering gelatinization. (Knutson, 1990,
Hoover and Vasanthan, 1994, Larsson and Eliasson, 1991). Annealing of lentil, smooth pea
and wrinkled pea starch has also been shown to decrease granular swelling and amylose
leaching and to increase gelatinization temperatures, thermal stability, and susceptibility
towards -amylase. These changes have been attributed to an increase in crystalline
perfection and increased interaction between amyloseamylose and amyloseamylopectin
chains. Lorenz and Kulp (1984) have reported an increase in the V-pattern due to annealing
25
2. Review of Literature
for normal and high amylose starches. Amylopectin regions in the starch are perfected by
annealing process (Knutson, 1990). The swelling power and solubility of the starches are
reported to be lowered by annealing (Lorenz and Kulp, 1978, Lorenz and Kulp, 1984)
The pregelatinised and heat-moisture treated starches are the major physically modified
products from cassava starch (Sriroth, 2002). The process for the production of pregelatinised
starch involves drying of 30-40 % (dry solid) starch slurry on a roller drum drier heated to
160-1700C by direct steam. The product exhibits high transparency, high viscosity and good
colour carrier properties.
Pyrodextrins: Pyrodextrins are starch derivatives obtained by either dry heating or heating
of the aqueous starch slurry with or without pH change (Fig 2.9)
Fig 2.9: Pyrodextrinisation of starch
The three major reactions taking place during dextrinization are glycosidic bond cleavage (by
hydrolysis), glycosidic bond formation (transglycosidation), and repolymerization. They are
commercially very useful products for various applications
Photooxidation: Photooxidation of starch in the presence of atmospheric oxygen gives rise
to gluconic and glucuronic acids. The former further degrades to yield D-arabinose. The
26
2. Review of Literature
reaction is of free radical type involving glycosidic bond cleavage, as well as the cleavage of
C-5 and C-1 bond of glucose residues (Fiedorowicz et al., 2001)
Micronization: The micronization (a physical damage induced by McCrone micronizing
mill) of barley starch showed amylopectin of low molecular weight being preferentially
solubilized in cold water. Depending upon the conditions, more amylose is extracted into
cold water (Tomasik and Zaranyika 1995).
Polarized light treatment: Some studies have been made on the degradation of starch by
polarized light. Moonlight is assumed to be a source of polarized light (Hoover, 1998).
Preliminary exposure to polarized light did not affect the crystalline structure of starch,
although some changes in melting temperature and transition enthalpy were seen. Prolonging
the exposure led to some degree of cross-linking, as shown by increased molecular weight.
This has been attributed to activation of enzymes adhering to starch granule surface.
Sensitivity of amylopectin to illumination exceeds that of amylose (Whistler 1998).
UV light induced starch degradation: In the case of UV light induced starch degradation,
the radiation is first absorbed by acetal chromophore at C-1 of glucose unit followed by
further photoreaction. Formation of peroxide ion at C-1 leads to gradual chain scission and
reduced molecular weight, paste clarity/viscosity, and melting enthalpy. Prolonged
irradiation leads to cross-linking with increases in molecular weight (Tharanathan, 1995).
The surface derivatization of starch: The surface derivatization of starch granules is
another approach for bringing in desirable changes. The complexing of amylose by lipid
molecules influences both thermal and rheological properties of wheat starch, whereby the
leaching of amylose molecules from the granules to the water is restricted (Setser and
Racette, 1992, Radhika et al., 2008)
Chemical modifications
Chemical modification of starch generally occurs via the introduction of functional groups
that change starch properties. Thus the aim of chemical modification is to modify cooking
characteristics, decrease retrogradation, decrease the gelling tendencies of pastes, increase
27
2. Review of Literature
freeze-thaw stability, decrease gel syneresis, improve film formation, improve adhesion and
improve emulsion stability.
Acid thinned starch: This is one of the earliest methods of starch modification, and the
derived degradation products have a vast application potential. In its simplest methodology,
the native granular starches are subjected to treatment with acids, either at room temperature
(for a period of several days) or at elevated temperature (for several hours). The extent of
degradation is measured by the release of reducing sugar (called dextrose equivalent). Acid
modification is widely used in the starch industry to prepare thin boiling starches for use in
food, paper, textile and other industries (Rohwer and Klem, 1984). The amorphous regions of
starch are more rapidly hydrolyzed than are the crystalline regions during acid hydrolysis at
temperatures below the gelatinization temperature (BeMiller, 1965). Kerr (1952)
demonstrated that in early stages of acid modification, the amount of amylose or linear
fraction in starch increased and amylopectin was preferentially hydrolyzed, inferring amylose
was protected by forming a resistant complex with particles of amylopectin. The
retrogradation rate of acid-thinned starch gels increased as hydrolysis proceeded (Kang etal
1997). Acid modification also increased solubility and gel strength and decreased viscosity of
starches (Kim and Ahn, 1996, Osunsam et al., 1989). The viscoelastic properties of starches
are also affected by acid hydrolysis. Virtanen, et al, (1993) reported that the gel of acid
modified oat starch, although less rigid, was more elastic than the corresponding native starch
gel. Dynamic rheological tests showed that the dispersions of acid modifiedwaxy corn starch
behaved as Newtonian liquid-like solution, while the unmodified counterpart behaved like
weak gels (Chamberlain and Rao, 1999).
Oxidation-Oxidation is an important modification method for bringing about changes in
physicochemical properties of starch. Oxidative agents modify starch by forming new
functional groups in the molecule. Oxidation with hypochlorite or more rarely with
potassium permanganate is an old method but still used. It involves conversion of primary
hydroxyl group to carboxyl group. Oxidized starches (Wurzburg, 1986) are mainly prepared
by treating starch with sodium hypochlorite, the rate of the reaction being influenced by the
acidity of the reaction medium. Whistler and Schweiger (1965) demonstrated that
hypochlorite oxidation of corn amylopectin was most rapid at neutral pH while the reaction
28
2. Review of Literature
rate decreased with increasing acidity and alkalinity. Similar results were observed on wheat
and corn starches. The type and amount of functional groups formed in the starch molecule
depend on the reaction pH as well. The formation of carbonyl group was found to be higher
under acidic conditions while the amount of carboxyl group increased with increasing pH
(Schmorak et al., 1963).
Substitution:
Substituted starches are prepared by treating starches with various
chemicals under controlled conditions. Studies have shown that substitution decreases the
extent of syneresis (exudation of water during frozen storage), gelatinization transition
temperatures, and pasting temperatures Esterification is an important modification method of
starch. The commonly used reagents for esterification are acetic anhydride, acetic acid, vinyl
acetate, succinic anhydride, alkenyl succinic anhydrides, citric acid and formic acid.
Fig: 2.10: Starch modification reactions ( Rudrapatnam N. Tharanathan 2002)
29
2. Review of Literature
Starch acetate: A common starch modification is acetylation, which is the esterification of
starch polymers with acetyl groups to form starch acetates (Jarowenko, 1986). Acetylation
has been reported to increase the water absorption and to lower the pasting temperature and
set back of rice starch (Gonzalez and Perez, 2002). Gelatinisation temperature was
significantly reduced by the introduction of acetyl groups. Factors such as amylose to
amylopectin ratio, intragranular packing and the presence of lipids mainly govern the degree
of substitution during acetylation of starches from different sources (Singh, Kaur et al.,
2004a and Singh, Chawla et al., 2004). Starches with low amylose content have been
observed to exhibit a higher degree of substitution after acetylation. Rutenberg and Solarek
(1984) reported that the introduction of acetyl groups upon acetylation reduces the bond
strength between starch molecules and thereby increases the swelling power and solubility of
the starch granule, decreases the coagulation of the starch, and provides improved freezethaw stability. The extent of physico-chemical property changes in the acetylated starch
compared to the native starch is proportional to the degree of acetylation or degree of C = O
substitution incorporated into the starch molecules. The C = O bond of the acetyl group
experiences a different molecular environment depending on whether it is a substituent on
amylose or on amylopectin. (Phillips et al., 1999). Equation depicting the reaction of starch
with acetic anhydride is given below (Fig 2.11).
Fig 2.11: Formation and structure starch acetate.
Starch succinates : Starch succinates are prepared by a base catalyzed reaction of succinic
anhydride in aqueous medium. Succinylation increases hydrophilicity of the starches. The
30
2. Review of Literature
starch side chain carboxylic groups in succinates provide useful properties such as metal
chelation (Jeon et al., 1999). Low DS starch succinates could be obtained by refluxing starch
with succinic anhydride in pyridine at 115C for varying reaction times without prior
gelatinization (Lohmer and Rist, 1950, Rutenberg and Solarek, 1984, Bilmers and Tessler,
1995). Bhandari and Singhal (2002) have optimized the reaction conditions for the
preparation of succinate derivatives from corn and amaranth starches in non-aqueous medium
and a starch: pyridine ratio of 2:1 was found to be vital for the reaction to take place.
Bhandari et al., (2002) have studied the rheological properties of succinylated corn and
amaranth starches. The effect of various reaction conditions (pH, time, temperature and
reagent concentration) on the succinylation of Canavalia ensiformis starch was studied by
Betancur et al., (2002). Typical equation depicting the reaction of starch with succinic
anhydride is given below (Fig 2.12).
Fig 2.12: Formation and structure of starch succinate
Starch Octenyl succinate : Starch octenyl succinates can be prepared by the esterification of
starch with a substituted dicarboxylic acid anhydride, 1-octenylsuccinic anhydride (OSA) at
pH 7-9 (Thomas and Atwell, 1997). They are used as emulsifiers and emulsion stabilisers in
salad dressings, in beverages etc and as clouding agents used to stabilise the oil-water
interface of an emulsion. One key application of OSA treated starch is the replacement of
gum
arabic
in
systems
that
require
emulsion
stabilisation
or
encapsulation.
Octenylsuccinylation of starch lowers the gelatinisation temperature, improves paste clarity,
provides stability to retrogradation and modifies the texture of the starch (Trubiano, 1986).
Modification with octenyl succinic anhydride has been reported to increase the paste
viscosity and swelling volume and reduce the gelatinisation temperature of rice starch (Bao et
al., 2003, Shih and Daigle, 2003). High DS OSA derivatives produced firmer starch gels.
31
2. Review of Literature
Park et al., (2004) have studied the rheological properties of corn starch octenyl succinates.
The OSA starch pastes exhibited high shear-thinning behavior. Equation depicting the
reaction of starch with octenyl succinic anhydride is given below (Fig 2.13).
Fig 2.13: Formation and structure of starch octenyl succinates
Starch citrates -Citric acid is considered harmless in food applications compared to other
substances used for starch derivatisation (Klaushofer et al., 1978a). Starch citrates are used in
various food products to increase the dietary fiber contents in the form of resistant starch
(RS4). Weppner et al., (1999) have reported the synthesis of citric acid esters of corn, pea,
potato and wheat starches and they have obtained derivatives with resistant starch content up
to 57.5 %. They have also observed that the resistant starch content increased with increase in
the DS. The effect of various reaction conditions on resistant content in the corn starch citrate
was investigated by Xie and Liu (2004). When the reaction was carried out at 140C for 7 h,
the highest RS content of 87.5 % was obtained in the waxy corn starch citrate having a DS of
0.16. When heated, citric acid is dehydrated to yield the citric anhydride which can react with
starch present in the reaction medium to form the starch citrate. On further heating, additional
dehydration occurs which results in the formation of cross-links between starch chains
(Wing, 1996). Equation depicting the reaction of starch with citric acid anhydride is given
below (Fig 2.14).
32
2. Review of Literature
Fig 2.14: Formation and structure of starch cirates
Cross-linked starch-Cross-linked starches constitute a major class of modified starches.
Cross-linking reinforces the H-bonds in the granules with chemical bonds. Therefore, crosslinked starches are more resistant to acid, heat and shearing than the native starch. The most
widely used cross-linking agents include STMP, STPP, phosphorus oxychloride,
epichlorohydrin (EPI) and adipic acetic mixed anhydride (Wu and Seib, 1990, Yeh and Yeh,
1993, Yook et al., 1993, Wurzburg, 1986c and Bergthaller, 2004). Cross-linking using
multifunctional reagents introduces intermolecular bridges which result in restricted swelling
of the granules during gelatinisation and minimize granule rupture (Eliasson and
Gudmundsson, 1996, Woo and Seib, 2002, Reddy and Seib, 1999). Reagents such as
epichlorohydrin, phosphorous oxychloride, metaphosphate, citric, or adipic acids, react with
starch forming intermolecular cross-linking of molecules. Langan (1986) has reported that
pastes from cross-linked starches are more viscous, heavily bodied and are more stable to
heat, shear and low pH. Cross-linking reduces loss of viscosity and formation of stringy paste
during cooking (Woo and Seib, 2002). According to Liu et al., (1999) the effect of crosslinking is different for the waxy and non-waxy starches. Cross-linking minimizes granule
rupture, loss of viscosity and the formation stringy paste during cooking (Woo and Seib,
1997), yielding starch that is suitable for canned foods and other food applications (Hirsch
and Kokini, 2002, Rutenberg and Solarek, 1984). Jane, Radosavljevic, and Seib (1992) found
that cross-linking of starch chains occurred mainly in amylopectin region of the starch
Another factor that may influence the extent of cross-linking is the size distribution of starch
granule population (Hung and Morita, 2005). During cross-linking, small size granules have
been reported to be derivatized to a greater extent than the large size granules (Bertolini et
33
2. Review of Literature
al., 2003). Equation depicting the reaction of starch with cross linking agent is given below
(Fig 2.15).
Fig 2.15: Formation of crosslinked starch
Hydroxypropyl Starch (HP): Modification by hydroxypropylation is especially interesting
because of its toxicological safety and accommodative properties (Wurzburg, 1986c).
Hydroxypropylation is a form of starch etherification. Hydroxypropyl starch (HPS)
derivatives are prepared by reacting starch with propylene oxide as etherifying reagent,
leading to introduction of hydroxypropyl groups onto the polymeric chain of starch.
Propylene oxide is reactive as a result of its highly strained three-membered epoxide ring.. In
forming HPS, part of the hydroxyl groups of the anhydroglucose unit (AGU) would be
converted into O-(-2-hydroxypropyl) groups (Bergthaller, 2004). Though all the three
hydroxyl groups (at O-2, O-3 and O-6) are amenable for substitution, the derivation mainly
takes place at O-2. The substitution of HP groups takes place preferentially in the amorphous
domains of amylopectin, with most of it at the C-2 hydroxyl group (67-78%), whereas at O-3
and O-6 it is 15-29% and 2-17%, respectively. Bulky HP groups prevent the alignment of
starch chains (due to steric effects) and reduce starch retrogradation characteristics. The
substituent disturbs the association of the polysaccharide chains preventing retrogradation
due to the hydrogen bonds. Improved functional properties of hydroxypropylated starches
such as extended shelf life of cold storage products (freeze-thaw stability), higher peak
viscosity and paste clarity, and decreased gelatinization temperatures are well documented
(Hoover etal 1988 , Kim and Eliasson, 1993, Liu et al, 1999, Pal et al., 2002, Perera et al.,
1997). The disruption of H-bonds due to the introduction of hydroxypropyl groups weakens
the granular structure of starch and this effect alters its pasting properties (Seow and
Thevamalar, 1993, Wootton and Mantsathi, 1983, Choi and Kerr, 2003, Yeh and Yeh, 1993,
34
2. Review of Literature
Kim et al., 1992, Liu et al., 1999b). The degree of syneresis has been observed to decrease
with increasing MS of hydroxypropyl potato starch (Eliasson and Kim, 1992). HP starches
have excellent film forming properties that are of use in biodegradable films. In general,
starch ethers are more stable to cleavage by acids. Equation depicting the reaction of starch
with propylene oxide is given below (Fig 2.16).
Fig 2.16: Formation and structure of hydroxypropyl starch
Hydroxypropyl (HP) starches are designed to withstand vagaries of cooking, such as high
temperatures, shear forces, and extremes of pH. HP starches offer pastes of increased clarity
and freeze-thaw and cold storage stabilities. The substituent disturbs the association of the
polysaccharide chains preventing retrogradation due to the hydrogen bonds. Improved
functional properties of hydroxypropylated starches such as extended shelf life of cold
storage products (freeze-thaw stability), higher peak viscosity and paste clarity, and
decreased gelatinization temperatures are well documented (Hoover et al., 1988, Pal et al.,
2002,
Perera et al., 1997). Kim, et al., (1992) have used light microscopy for
hydroxypropylated potato starches and suggested that hydroxypropylation mainly takes place
at the central region of the granules. It has been reported that granule swelling is essential
for the substitution reaction to take place in granular starch (Hauber et al., 1992).
35
2. Review of Literature
Starch phosphorylation: Starch phosphorylation is the earliest method of starch
modification. The reaction gives rise to either monostarch phosphate or distarch phosphate.
Phosphorylated starch is produced through esterification of the starch with phosphorylating
agents such as sodium tripolyphosphate (STPP), sodium trimetaphosphate (STMP), sodium
orthophosphate, phosphorus oxychloride or sodium hexametaphosphate (Kerr and Cleveland,
1962). Monosodium hydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate
(Na2HPO4) can produce derivatives with DS up to 0.2 (Bergthaller, 2004). Phosphorylation is
usually done by a dry heat reaction in the temperature range 140-1600C. The phosphate
diester starches have the phosphate esterified with two hydroxyl groups, very often from two
neighboring starch molecules. This leads to the formation of a covalent bridge or crosslinking. Phosphate cross-linked starches show resistance to high temperature, low pH, high
shear and leads to increased stability of the swollen starch granule. They improve viscosity
and textural properties of the starch. As a thickener and stabilizer, starch phosphate diesters
are superior to unmodified starches. They also provide resistance to gelling and
retrogradation, and do not synerise on storage. Starch phosphate can substitute gum arabic (at
~ 0.5% level) in sugar syrups, ice cream mixes, salad dressing, and pudding. Waly et al.,
(1994) have studied the effect of various reaction conditions on the phosphorylation of starch
with urea, phosphoric acid and tetra sodium phosphate decahydrate. The effect of pH on the
phosphorylation of sago starch with STMP and STP was studied by Muhammad et al.,
(2000). Efforts have been made to replace the conventional processes for the production of
starch phosphates with an extrusion cooking process Phosphorylation of rice starch by
extrusion cooking showed that increased barrel temperature (120 180 0C) resulted in greater
phosphorus incorporation into the starch. Phosphorylation at low levels of substitution
resulted in greater solubility, swelling power, paste viscosity and clarity for rice starch.
Starch granule size has impact on the effect of phosphorylation on the properties of rice
starch.
Phosphorylated rice starch has been reported to exhibit a reduced degree of
hydrolysis by acid or amylase. Equation showing the reaction of starch with phosphorylating
agents is given below (Fig 2.17).
36
2. Review of Literature
Fig 2.17: Formation and structure of starch phosphate.
Carboxymethyl starch- Here the hydroxyl groups of starch are partly substituted with
sodium
monochloroacetate
(SMCA)
to
give carboxymethyl
starch (CMS).
The
carboxymethyl substitution of starch hydroxyl groups gives rise to derivatives that are
coldwater-soluble. This modification procedure has a positive effect on the applicability in
the
areas like in the textile, papermaking and pharmaceutical industries. To prevent starch
gelatinization, the reaction has to be carried out in an organic medium. Carboxymethyl
starch, under the name sodium starch glycolate, is used in the pharmaceutical industry as a
disintegrant and as sizing and printing agent in the textile industry. Highly substituted
derivatives are possible. Ohtani et al., (1977) have studied the carboxymethylation of
hydrolyzed hemicellulose and starch, which are useful as builders for detergents. Stojanovic
et al., (2000) have reported the synthesis of carboxymethyl starches in an ethanol/water
medium under different experimental conditions. The starch type was found to influence the
DS values of the products in ethanol medium. CMS is reported to have increased water
solubility and with increase in DS, the solubility also increased. The effects of various
reaction conditions on the carboxymethylation of arrowroot starch in isopropanol water
media were investigated by Kooijman et al., (2003). Ragheb et al., (1997) have synthesized
carboxymethylated derivatives of native and oxidized starches of different molecular sizes
and studied their application in textile printing.
37
2. Review of Literature
Resistant Starch
The greater awareness on the part of consumers of the relationship between a nutritious diet
and health and well-being has been one of the reasons for the increase in popularity of novel
foods with good nutritional properties (Prez-Alvarez, 2008b, Sanz, et al., 2008a). Resistant
starch refers to the portion of starch and starch products that resist digestion as they pass
through the gastrointestinal tract. RS is an extremely broad and diverse range of materials
and a number of different types exist (RS1 4). At present, these are mostly defined
according to physical and chemical characteristics (Nugent, 2005). They provide the mouthfeel of high fat emulsions in low fat or fat free products, lend a glossy, fat-like appearance,
allow less fat pickup in some fried products, and are of use in the formulation of dietetic
foods. They are either partially or totally undigested, thus contributing zero calories to the
food on consumption. The four distinct classes (Fig 2.18) of RS in foods are: (1) RS1
physically inaccessible starch, which is entrapped within whole or partlymilled grains or
seeds; (2) RS2 some types of raw starch granules (such as banana and potato) and highamylose (high-amylose corn) starches; (3) RS3 retrograded starch (either processed from
unmodified starch or resulting from food processing applications); (4) RS4 starches that are
chemically modified to obtain resistance to enzymatic digestion (such as some starch ethers,
starch esters, and cross-linked starches) (Ratnayake and Jackson, 2008, Sanz et al., 2009)
Fig 2.18: Different type of resistant starch
38
2. Review of Literature
Dual modified (substituted and cross-linked) normal maize, waxy maize, tapioca, potato and
normal
wheat
starches
are
available
commercially
with
varying
degrees
of
hydroxypropylation and cross-linking. The temperatures and enthalpies of gelatinization of
the dual modified (hydroxypropylated/cross-linked or acetylated/cross-linked) waxy wheat
and maize starches are generally lower than those of the unmodified starches. Gelatinization
temperatures (To; Tp; Tc) of cross-linked waxy wheat starch and its hydroxypropylated/crosslinked and acetylated/cross-linked forms are 5-7 C below than those of unmodified forms of
waxy maize starch when measured in excess water (Reddy and
Seib, 2000).
Dual-
modification, hydroxypropylation and crosslinking, is commercially carried out (Lopez,
1987, Tessler, 1975, Tuschhoff, 1986, Wurzburg, 1986, Yeh and Yeh, 1993). Starches with
high amylose content can be stabilized by initially reacting them with propylene oxide and
this reaction is inhibited by adding cross linking agents to yield modified starches having
outstanding high temperature and short time retort properties (Tessler, 1975). Phosphorus
oxychloride, sodium trimetaphosphate, and epichlorohydrin are generally used as cross
linking reagents by several authors (Luallen, 1985, Smolka and Alexander, 1985, Takahashi
et al., 1989, Tessler, 1975, Valle, et al., 1978, Wu and Seib, 1990, Yeh and Yeh, 1993,
Yook et al., 1993).
Enzyme modified starch:
Enzymatic modification of native starch may be considered
as one of the techniques to modify native starch by decreasing the molecular weight .The
Enzyme conversion of starch
(Fig 2.19) is used to produce derivatives with varying
viscosity, gel strength, thermo reversibility and sweetness. In enzymatic modification
techniques, the gelatinised starch is subjected to degradation by enzymes resulting in various
products (Alexander, 1992).
39
2. Review of Literature
Fig 2.19: Enzyme hydrolysis of starch
Selective enzyme hydrolysis of starch produces a range of products like glucose, maltose,
oligosaccharides and polysaccharides with varying chain length and dextrose equivalent (DE)
(Taggart, 2004). The major enzyme modified products are linear dextrins of varying DE,
high fructose syrups, glucose syrups, dextrose, maltodextrins and cyclodextrins (Raja, 1995,
Blanchard and Katz, 1995). The commonly used enzymes are and amylases, iso amylase
and pullulanase. The amylase selectively attacks the (1, 4)-linkages of the starch and
produces maltodextrins and low DE dextrins. However, amylase hydrolyzes every other 1,
4- linkages to give lower molecular fragments and higher DE syrups like maltose.
Isoamylases and pullulanase give high DE syrups through hydrolytic attack at specific sites
such as 1, 6-linkages in the starch. Cyclodextrins are produced by the enzyme hydrolysis of
starch using Cyclodextrin glycosyltransferases (Kumar, 1995). Cyclodextrins find application
in food processing, pharmaceutical and agrochemical industries for preparation, separation,
purification and protection of pharmaceuticals, fragrance, flavors and steroids.
40
2. Review of Literature
2.5 Applications of modified starches
Starch is a staple in the diet of much of the worlds population and is also widely used in the
Western world in the food and beverage industries as a thickener and a sweetener, as well as
having some manufacturing applications in the paper and textile industries. The more
prevalent use of starch for industrial purposes will become economically viable when its use
as a raw material rivals those derived from petroleum-based products. Starch based products
have traditionally been used by the water treatment industry as a coagulant or flocculant aid.
Potato starch is associated with better performance than other types because of its high
potassium content. Starch based products have been displaced to a large extent by synthetic
poly electrolytes because of the superior performance and lower dosage rates required of the
latter. The textiles industry is an important market for starch. There are three main
applications of starch: sizing printing, and finishing. Adhesives are a traditional application
for starch. Starch based adhesives are primarily used for paper bonds with the most important
sector corrugated board production. The binding and bonding properties of starches with high
levels of amylopectin make a good addition to adhesives, especially on bottle labels, which
are often subject to water and high humidity. These all are the traditional and declining
markets of starch (Garth Entwistle, 1997), Some of the new and developing markets for
starch are,
Mineral oil drilling - Starch can be used in the oil industry as a drilling aid included in
water-based fluids. Drilling fluids, in the form of circulating aqueous clay suspensions are
used to stabilize bore hole walls and enable drilled solids to be transported to the surface.
Starch products are incorporated into water-based drilling fluids to control fluid loss. These
products are degradable and reduce environmental damage when they replace oil-based
fluids.
Agrochemicals-Starch is of interest in the agrochemical industry as an encapsulation agent
for pesticides, and for the production of aqueous base pesticide formulations. Starch
encapsulation leads to the safer handling of pesticides and can improve the efficiency of the
active ingredient with an improved delivery to the target pest and a reduction in losses due to
evaporation, leaching and light decomposition.
41
2. Review of Literature
Biodegradable plastic-films-Growing interest has been shown in the incorporation of starch
into plastics to make them biodegradable. The use of starch as a thermoplastic material is of
recent origin. Compared to starch films, low dextrose equivalent dextrins and corn syrup
were more resistant to water vapor transport. Films of starch hydrolysates have shown some
resistance to oxygen transmission.
Cosmetics and toiletries-Starch and starch derivatives, could potentially be used for the
production of a wide range of cosmetics and toiletry products. The use of sorbitol in
toothpaste and cosmetic creams is an example of a well established use of a starch derivative
in this sector. Starch grafted co-polymers after saponification, find important applications as
absorbents in disposable soft goods designed to absorb body fluidsnappies, incontinence
pads, female sanitary products. Enzyme catalyzed esterification of n-butylglucosidea
process patented by Cerestar, gives a product with a number of equally interesting features
that are useful within cosmetic formulations.
Pharmaceuticals : Starch considered as the most used
EXCIPIENT
in pharmaceutical
formulations. It has many pharmaceutical applications and it is used mainly in tablets as a
filler, binder or disintegrant
Excipients are increasingly being recognized for the critical role they play in pharmaceutical
products. Providing specific special functionalities in formulations, pharmaceutical
excipients contribute enormously to the efficacy of a product. They bind tablets together
under the stress of direct compression, control the release of active ingredients, help tablets
disintegrate and dissolve efficiently and influence absorption. In a tablet formulation, a range
of excipient materials is normally required along with the active ingredient in order to give
the tablet the desired properties
Filler: Fillers are used to make tablets of sufficient size for easy handling by the patient and
to facilitate production. Tablets containing a very potent active substance would be very
small without additional excipients. Good filler will have good compactability and flow
properties, acceptable taste, will be non-hygroscopic and preferably chemically inert. It may
also be advantageous to have a filler that fragments easily, since this counteracts the negative
effects of lubricant additions to the formula (de Boer et al., 1978.).
42
2. Review of Literature
Binder: A material with a high bonding ability can be used as a binder to increase the
mechanical strength of the tablet. A binder is usually a ductile material prone to undergo
plastic (irreversible) deformation. Typically, binders are polymeric materials, often with
disordered solid state structures. Of special importance is the deformability of the peripheral
parts (asperities and protrusions) of the binder particles (Nystrm et al., 1993.) The effect of
the binder depends on both its own properties and those of the other compounds within the
tablet. A binder is often added to the granulation liquid during wet granulation to improve the
cohesiveness and compactability of the powder particles, which assists formation of
agglomerates or granules.
Disintegrating agent: A disintegrant is normally added to facilitate the rupture of bonds and
subsequent disintegration of the tablets. This increases the surface area of the drug exposed to
the gastrointestinal fluid; There are several types of disintegrants, acting with different
mechanisms: (a) promotion of the uptake of aqueous liquids by capillary forces, (b) swelling
in contact with water, (c) release of gases when in contact with water and (d) destruction of
the binder by enzymatic action (Rudnic and Kottke, 1999). Starch is a traditional
disintegrant; the concentration of starch in a conventional tablet formulation is normally up to
10% w/w. The starch particles swell moderately in contact with water, and the tablet disrupts.
So-called superdisintegrants are now commonly used;
Glidant, antiadherent and lubricant: Glidants are added to increase the flowability of the
powder mass, reduce interparticular friction and improve powder flow in the hopper shoe and
die of the tabletting machine. An anti adherent can be added to decrease sticking of the
powder to the faces of the punches and the die walls during compaction, and a lubricant is
added to decrease friction between powder and die, facilitating ejection of the tablet from the
die. However, addition of lubricants (here used as a collective term, also including glidants
and antiadherents) can have negative effects on tablet strength, since the lubricant often
reduces the creation of inter-particular bonds (de Boer et al., 1978)
Flavours, sweetener and colourant: Flavour and sweeteners are primarily used to improve
or mask the taste of the drug, with subsequent substantial improvement in patient
43
2. Review of Literature
compliance. Colouring tablets also has aesthetic value, and can improve tablet identification,
especially when patients are taking a number of different tablets (Susanne bredenberg, 2003)
Tablets account for more than 80% of all pharmaceutical dosage forms administered to
people Pharmaceutical formulation is the process by which active ingredients of drugs are
converted into preparations which are safe, effective and convenient to use.Of the two oral
solid dosage forms commonly employed in this country, the tablet and the capsule, the tablet
has a number of advantages. One of the major advantages of tablets over capsules, which has
recently proved significant, is that the tablet is an essentially tamperproof dosage form.
In consideration of the following may be cited as primary potential advantages of tablets.
1. They are a unit dose form, and they offer the greatest capabilities of all oral dosage
forms for the greatest dose precision and the least content variability.
2. Their cost is lowest of all oral dosage forms.
3. They are the lightest and most compact of all oral dosage forms.
4. They are in general the easier and cheapest to package and ship of all oral dosage
forms.
5. Product identification is potentially the simplest and cheapest, requiring no additional
processing steps when employing an embossed or monogrammed punch face.
6. They may provide the greatest ease of swallowing with the least tendency for hangup above the stomach, especially when coated, provided that tablet disintegration is
not excessively rapid. They lend themselves to certain special release profile
products, such as enteric or
delayed-release products
7. They are better suited to large-scale production than other unit oral forms. They have
the best combined properties of chemical, mechanical and microbiologic stability of all
the oral forms.
2 .6
Tablet Formulation
A tablet formulation typically consists of an active pharmaceutical ingredient (API) together
with nonactive ingredients, or excipients, such as fillers or diluents, binders or adhesives,
44
2. Review of Literature
disintegrants, lubricants and glidants, colours, flavours and sweeteners (Fig 2.20). It might
also be necessary to add miscellaneous components such as buffers, depending on the
application. Common goals in pharmaceutical development and research work are to develop
formulations of required stability, with specific release profiles and to ensure that operating
conditions are robust during production (Peck et al., 1989).
Fig 2.20: Tablet and capsule manufacturing process
A need for new excipients
With the increasing number of new drug moieties with varying physicochemical and stability
properties, there is growing pressure on formulators to search for new excipients to achieve
the desired set of functionalities. The growing performance expectations of excipients to
address issues such as disintegration, dissolution, and bioavailability. The continued
popularity of solid dosage forms, a narrow pipeline of new chemical excipients, and an
increasing preference for the direct-compression process creates a significant opportunity for
the development of high-functionality excipients. . Fig 2.20 illustrates the different factors to
be taken in consideration when selecting a new excipient for the pharmaceutical tablet
production. The characterization of pharmaceutical excipients using a material science
approach has helped to design drug formulations to obtain a desired set of performance
properties. For tablets, a better understanding of the compression properties of the material
alone and in combination with other potential components helps in developing desirable
formulations as well as acceptable products (Ashok et al., 2006). When formulating tablets,
45
2. Review of Literature
the choice of excipients is extremely critical (Fig 2.21). It must fulfill certain requirements
such as compressibility, good binding functionality, powder crystallinity, flowability and
acceptable moisture content.
Fig 2.21: Factors considered when excipient is selected for oral solid dosage forms
2.7 Sources of new excipients
Pharmaceutical excipients are inorganic or organic compounds, which are necessary
formulation of medicinal preparations suitable for direct application to a patient, although,
they are not holders of any pharmacological activity. As with drug substances, excipients are
derived from natural sources or are synthesized either chemically or by other means. They
range from simple, usually highly characterized, organic, or inorganic molecules to highly
complex materials that are difficult to fully characterize. In earlier days, excipients were
considered inactive ingredients. Excipients are now known to have defined functional roles in
pharmaceutical dosage forms. These include (i) modulating solubility and bioavailability of
the active ingredient(s); (ii) enhancing stability of the active ingredient( s) in finished dosage
forms; (iii) helping active ingredients maintain a preferred polymorphic form or
conformation; (iv) maintaining pH and osmolarity of liquid formulations; (v) acting as
46
2. Review of Literature
antioxidants, emulsifying agents, aerosol propellants, tablet binders, and tablet disintegrants;
(vi) preventing aggregation or dissociation; and (vii) modulating the immunogenic response
of active ingredients (e.g., adjuvants) and many others. It is becoming increasingly apparent
that there is an important relationship between the properties of the excipients and the dosage
forms containing them ( Katdare and Mahesh, 2006)
The development of innovative pharmaceuticals is critical to improving the health care and
standard of living for billions of people around the globe. Commonly used excipients such as
corn starch, lactose, talc, and sucrose did not present significant questions of safety, and were
largely ignored by the regulatory community. Advancements in pharmaceutical technology
have rendered this view of excipients as simple inert pharmaceutical fillers obsolete.
Starch is usually called classical loosening agent. This effect is related not as much to the
swelling of starch grains (the degree of starch swelling in water at 37C does not exceed 5
10%) as to the ability of rendering tablets porous, which favors permeation of liquids
(Andreev, 2004). This behavior is consistent with the fact that starch as the loosening agent,
while neither significantly influencing water-soluble material nor improving the
disintegration characteristics of sugar-based tablets, is highly effective in lactose-based
tablets.
Both native starch and its modifications attract the attention of pharmacists developing drugs
with new compositions and pharmacological properties. The reasons for this interest are
predominantly as follows:
(i) The tendency to increased production of ready-to-use medicinal;
(ii) Variety of the structure and properties of starch produced from different raw materials;
(iii) Ability of native and modified starch to produce densification and stabilization of
various drug compositions;
(iv) The possibility of increasing the gel-forming and emulsifying properties of starch by
means of directed modification;
47
2. Review of Literature
(v) The possibility of increasing the stability of drugs under conditions of freezing thawing
cycles, high temperatures (sterilization), and strongly acid media ( Kul'fuis and ArendeScholte 2000, Bolhuis et al., 1981)
Starches from different botanical sources may not have identical properties with respect to
their intended use. Starch obtained from Ensete ventricosum, has been evaluated as tablet
binder using chloroquine phosphate, dipyrone and paracetamol as model drugs. Dioscorea
starch (composition on dry weight basis: 0.1% ash, 0.5% protein, 1% fat and 98.4% starch)
obtained from Dioscorea abysinica, was also evaluated as tablet binder (Odeku and PickerFreyer 2007). It showed better binding ability to that of maize starch and exhibiting
somewhat lower crushing strength and higher porosity. The binding performance of starches
obtained from taro (Colocassia esculenta) and sweet potato (Ipomoea batatas) was found to
be similar to that of commercial corn starch. Starches obtained from the seeds of Sorghum
bicolor, performed as well as maize starch and better than acacia in binding properties .The
uses of other alternative starches from rice, barley and wheat starches plantain starch from
Musa paradisiacia and tapioca, dried fibrous remnant material obtained from cassava,
Manihot esculenta have also been extensively reported in various tablet formulations
(Sanghvi et al., 1993).
Native starch upon such processing usually acquires a new superstructure. Precooked starch
described in the US Pharmacopoeia is good swelling and partly soluble. Swelling starch is
used in the production of tablets as a filling and binding component for wet granulation
process. The soluble part of a molecule acts as a binding agent, while the insoluble part
performs the role of filler possessing good disintegrating (loosening) properties. In
comparison with the tablets involving microcrystalline cellulose (MCC) and polyvinyl
pyrrolidone (PVP) as binding components, the compositions involving partly soluble
precooked starch possess comparable strength at a significantly shorter disintegration time.
The swelling starch in a powdered form can be introduced into the initial mixture prior to
granulation. During the granulation process, it is necessary to add water. This starch can be
additionally modified so as to improve its free-running ability (The USSR State
Pharmacopoeia 1989). The pregelatinization degree of starch paste influences the properties
of the resulting tablets (Itiola and Pilpel, 1986). A properly made paste is translucent rather
48
2. Review of Literature
than clear (which would indicate virtually complete conversion to glucose) and produces
cohesive tablets that are readily disintegrated when properly formulated (Marshall et al.,
1993)
Different chemically substituted starches are also used in the tablet production, starch acetate
and succinates are of special importance for pharmaceutical industry. Carboxymethyl starch
can be used in various tablet compositions, it is capable of preventing the detrimental
influence of hydrophobic lubricants (such as magnesium stearate) on the disintegration
characteristics (Eur. Patent No. 015963/1982 , Kul'fuis and Arende-Scholte 2000)
The European Pharmacopoeia (2002) defines tablets as solid preparations each
containing a single dose of one or more active substances and usually obtained by
compressing uniform volumes of particles. Tablets are intended for oral administration.
Some are swallowed whole, some after being chewed, some are dissolved or dispersed in
water before being administered and some are retained in the mouth where the active
substance is liberated. Despite the long and continuing history of the development of new
technologies for administration of drugs, the tablet form remains the most commonly used
dosage form
2.8 Tablet manufacturing process
The manufacture of conventional tablets is a cost-effective process and involves different
processing steps including operations where particles are engineered with the intention of
optimizing functional properties such as the technical performance during tabletting and drug
release properties. The manufacturing of tablets requires certain qualities of the powder, low
segregation tendency, good flowability and compactability being examples. It is also
important that the materials constituting a tablet, i.e. drug and excipients, are chemically and
physically stable during processing and storage (Jonas Berddren, 2003). The different step in
the tablet manufacturing process is shown in Fig.2.22. The manufacturing starts with the
granulation process and is summarized below.
49
2. Review of Literature
Fig 2.22: Different steps in tablet manufacture process.
Granulation
For the powder mixture to flow evenly and freely from the hopper into the dies, it is usually
necessary to convert the powder mixture to free flowing granules. The most important
reasons for a granulation step prior to tableting are to improve the flow properties of the mix
and hence the uniformity of the dose, to prevent segregation of the ingredients in the hopper
of tablet machines, to improve the compression characteristics of the tablet mixture and to
reduce dust during handling. A granule is an aggregation of component particles that is held
together by the presence of bonds of finite strength. Granulation usually refers to processes
whereby agglomerates with sizes ranging from 0.1 to 2mm are produced. There are various
techniques of producing granules such as dry and wet granulation, extrusion (Johansson et
al., 1998, 2001) or spray drying.
Dry granulation
Dry granulation is a valuable technique in situations where the effective dose of a drug is too
high for direct compaction and the drug is sensitive to heat, moisture or both, which
precludes wet granulation. The blend of powders is forced into dies of a large heavy-duty
tableting press and compacted to slugs. The slugs or roller compacts are then milled and
50
2. Review of Literature
screened in order to produce a granular form of tableting material which flows more
uniformly than the original powder mix (Davies and Newton 1996).
Wet granulation
The most frequent procedure for the preparation of aggregates or granules in this context is
wet granulation,. This is carried out by adding a liquid binder or an adhesive to the powder
mixture, passing the wetted mass through a screen of the desired mesh size, drying the
granulation and then passing through a second screen of smaller mesh to reduce further the
size of the granules. Granulation is mainly performed in planetary mixers with low speed and
low shear forces. In wet granulation, liquid bridges are developed between particles, and the
resulting tensile strength of these bonds increases as the amount of liquid increases. During
drying, inter-particulate bonds result from fusion or recrystallisation and curing of the
binding agent. The formation of crystal bridges has been shown to be a major influence on
the physical characteristics of tablets especially if the solid is more soluble in the granulating
fluid (Fell and Newton, 1971b, Sebhatu et al., 1997).
Variables that affect granulation properties
Effect of binders on granule properties
None of the pharmaceutical ingredients is more fundamental than the binding agents used in
the formulation of granules. Most binding agents used for wet granulations, such as starch
paste, acacia mucilage, gelatin solution, simple syrup, methylcellulose solution and corn
syrup are hydrophilic in nature. These binders increase the bulk density and reduce the
porosity of the powder, thereby diminishing the effective surface area for evaporation. The
most significant changes in the physical properties affected by binder dilution were found in
granule friability and bulk density. Specifically, the more dilute binder solution resulted in
less friable granules. Also considerable influence is observed on interparticulate porosity and
thus on flow rate while insignificant effects were observed on average particle size and
granule density (Sofia mattsson, 2000)
51
2. Review of Literature
Effect of processing variables on granule properties
Granule characteristics are reported to be influenced by equipment employed and processing
variables such as method of granulation, volume of granulating fluid, massing time and
method of compressing the granules to tablets. Investigations on influence of various
processing variables (impeller speed, granulating solution addition rate, total amount of
solution added in the granulation step, wet massing time, moisture content of the granulation
after drying, and screen size used for the dry milling) in granulation characteristics using high
shear mixer indicated that granulation growth (size) was enhanced by the increase in the
amount of added water, high impeller speeds and short wet massing time. It was also found
that moisture content had the largest impact on granulation compressibility. Increasing wet
massing time decreased granule porosity and fragmentation propensity hence increased
granule strength, which led to granulation compressibility
Binders as strength-enhancing materials in pharmaceutical tablets
A binder is a material that is added to a formulation in order to improve the mechanical
strength of a tablet. In direct compression, it is generally considered that a binder should have
a high compactibility to ensure the mechanical strength of the tablet mixture. Alternatively,
amorphous binders which undergo pronounced plastic deformation have been suggested to
provide an effective means of creating a large surface area available for bonding (Nystrm et
al., 1993).
Distribution of binders - comparison between direct compression and wet granulation
In direct compression, the binder is added in its dry state, whereas a liquid is employed in wet
granulation. Besides the common aim of enhancing the bonding properties between particles
or granules, the binder in wet granulation also aims at improving the binding between powder
particles during agglomeration. The binders used in wet granulation are generally polymeric
materials, which are amorphous or semi-crystalline, e.g. polyvinylpyrrolidone and gelatin.
These binders are considered plastically deformable, which is probably an important attribute
for their effective distribution. In direct compression, however, focus has mainly been on
using a binder with a high compactibility.
52
2. Review of Literature
Effect of binders on tablet properties
Binders are added to a material to increase bonding. Granulations with a more homogenous
distribution of binder in the granules generally produce tablets of a higher mechanical
strength than granulations with a peripheral localisation of binder. Studies on the effect of
various formulations and processing factors on the properties of some tablets by various
authors revealed that at a constant moisture level and packing fraction, an increase in binder
concentration generally results in increased tensile strength, disintegration and dissolution
times
(decreased
dissolution
rates),
reduced
capping
tendency,
ER/PC
(elastic
recovery/plastic compression) ratio and the brittle fracture index value (BFI- a measure of the
lamination tendency of tablets) of the tablets. Increasing molecular mass of binder (bloom
number for gelatin for example) increases tablet tensile strength when compressed to fixed
apparent density. Addition of a binder, which increases elasticity, can decrease tablet strength
because of the breakage of bonds as the compaction pressure is released (Nystrm et al.,
1982). The addition of a second component, such as a binder, to a compound has also been
reported to affect and modify the volume reduction behaviour of the compound (Wells and
Langridge, 1981, Yu et al., 1989; Larhrib and Wells, 1998.) Most binding agents used for
wet granulations, such as starch paste, acacia mucilage, gelatin solution, simple syrup,
methylcellulose solution and corn syrup are hydrophilic in nature. These binders increase the
bulk density and reduce the porosity of the powder, thereby diminishing the effective surface
area for evaporation. Hydrophilic binders also retard the rate of evaporation of moisture by
lowering the vapour pressure of liquid moisture. It was found that increasing the binder
concentration was followed by an increase in the mean particle size, harder granules,
decreased granule flowability and reduction in tapped density and hence reduction in granule
porosity (Sofia mattsson 2000) Specifically, the more dilute binder solution resulted in less
friable granules. Also considerable influence was observed on inter-particulate porosity and
thus on flow rate while insignificant effects were observed on average particle size and
granule density. A change in the intraparticulate pore spaces of the granules is needed to
effect change in granule density. The mechanical properties of the granules and the
53
2. Review of Literature
corresponding compacts are basically determined by the physicochemical interactions of the
substrate-binder interfacial layer (Newton et al., 1992)
2.9 Flow properties of powders and granules
Good flow characteristics are necessary because the mechanical action of the tablet press
requires a volume of fill. To achieve consistent tablet weights, the formula must be designed
to flow consistently and to fill volumetrically. Thus the powders in the formula must possess
a consistent particle-size distribution and density to attain proper flow and achieve volume of
fill (i.e., tablet weight). In other words, the powders must flow consistently to attain
consistent results
Granule flow.
The rheological behaviour of granules is closely related to tablet property (Li and Peck
1990).Glidants and lubricants such as talc, magnesium stearate are added to promote flow of
the tablet granulation. These glidants often possess a coefficient of friction less than that of
the bulk solid and hence improve the flowability thereby decreasing interparticulate friction.
Adequate mixing is needed for homogenous distribution of lubricants and satisfactory
granulation flow. The percent of fines, amount and type of granulating agent, particle size
distribution, and type of glidant all had a measurable effect on granule flow. Granules with
higher amount of fine (<100) and large particle size distribution will have poor flow from
hoppers and will cause weight variation of the dosage form. This is mainly caused from the
segregation of the fines. The smaller particles may fall through the voids between larger
particles and thus make their way towards the bottom of the mass (Cain, 2002). Many
methods are available to measure the extent of interparticle forces as index of flow Some of
the more common are measurements of bulk density (poured density), angle of repose, shear
strength and hopper flow rate measurements. The former three are indirect measures while
the latter is a direct measure of flow. Bulk density is the density calculated from the volume
of a poured granule of known weight. Tapped density is calculated from the volume obtained
by tapping the measuring cylinder mechanically at constant speed. A useful empirical guide
is given by the Carrs compressibility index (Carr, 1965), which is given by the equation:
54
2. Review of Literature
Carrs index (%) = [(tapped density bulk density)/tapped density] x 100
Carrs index of 5 15% indicate excellent flow, 12 16 good flow, and >23% indicate poor
flow. A similar index has been defined by Hausner (1967) by the equation:
Hausner ratio = Tapped density/ bulk density
Values less than 1.25 indicate good flow, while greater than 1.25 indicates poor flow.
Repose angle increases with increases in percentage of fines. Values for angles of repose
<300 usually indicate a free flowing material and angles <400 suggest a poorly flowing
material. Flow rates are also a function of particle diameter.
2.10 Compressibility and compactability of powders
Tablets are usually prepared by uniaxial compression using eccentric presses or rotary
tabletting machines. When a particle bed in a die is subjected to an external load its volume is
reduced and the interparticulate porosity is decreased. The term compressibility is often used
to describe the volume reduction ability of a material, whilst the compactability is the ability
of a material to form a tablet of a specified mechanical strength (Leuenberger, 1982). The
mechanism of volume reduction involves an initial rearrangement of the particles, followed
by fragmentation and deformation (Duberg and Nystrm, 1986). During compression, the
particles will be brought together into such close proximity that interparticulate bonds can be
created, resulting in a tablet of certain strength. It has been suggested that the mechanical
strength is governed by the interparticulate bonding mechanism and the area over which
these bonds interact (Nystrm et al., 1993). The strength of tablets is commonly determined
by diametral compression testing and subsequent calculation of the radial tensile strength
(Fell and Newton, 1970)
Tabletting of granulated materials
Granules are often prepared with the intention of improving tabletting properties such as the
compactability of a material. Granules are generally porous, therefore tablets of granulated
particles contain both inter and intragranular pores. It has been proposed that the volume
reduction process of granules (van Veen etal 200) includes four successive stages: (i) particle
55
2. Review of Literature
rearrangement and the filling of intergranular pores, (ii) fragmentation and plastic
deformation of the granules, (iii) filling of the intragranular pores with primary particles, i.e.,
the densification of the granule, and (iv) fragmentation and plastic deformation of the
primary particles. An increased intragranular porosity increased the degree of deformation,
resulting in formation of a closer intergranular pore structure during compression and,
therefore, stronger tablets (Johansson et al., 1995).
The compression shear strength of
granules during confined compression has also been found to be related to the intragranular
porosity (Nicklasson and Alderborn, 2000). The size and shape of the granules may also
influence the tabletting behaviour, e.g. decreasing the granule size has been claimed to
facilitate the formation of stronger tablets (Li and Peck, 1990, Riepma et al., 1993).
Fig 2.23: Granule compaction process during tablet production
In pharmaceutical tableting, an appropriate volume of granules in a die cavity is compressed
between an upper and lower punch to consolidate the material into a single solid matrix,
which is subsequently ejected from the die cavity as an intact tablet. The subsequent events
that occur in the process of compression are a) transitional repacking, b) deformation at
points of contact, c) fragmentation and/or deformation, d) bonding, e) deformation of the
solid body, f) decompression and finally ejection of the tablet. The process of compression is
described in terms of the relative volume (ratio of volume of the compressed mass to the
volume of th mass at zero voids) and applied pressure. This may be expressed by the Heckel
equation (Heckel, 1961) in terms of relative density rather than volume:
56
2. Review of Literature
Ln (1/(1-D)) = kP +A (1.3)
Where D is packing fraction of the tablet, P is applied pressure, constants k and A are
determined from the slopes and intercepts respectively of the extrapolated linear portion of
the plot of ln (1/(1-D)) vs P Materials have been characterised by comparing the behaviour of
a material in the compression and decompression phase Paracetamol though fragmenting
during compaction and consolidating by fragmentation to a large extent, its bonding capacity
is very poor, paracetamol rebounds or elastically recovers when the compressive load is
released. Paracetamol shows capping at higher compression forces and speeds.
2.11 Tablet properties
The qualitative evaluation and assessment of chemical, physical and bioavailability
properties of tablets are important in the design of tablets and to monitor product quality.
These properties are important in the design of tablets to monitor product quality, since
chemical breakdown or interactions between tablet components may alter the physical tablet
properties, and greatly affect the bioavailability of the tablet system. There are various
standards that have been set in the various pharmacopoeias regarding the quality of
pharmaceutical tablets. These include the diameter, size, shape, thickness, weight, hardness,
disintegration and dissolution characters. The diameters and the shape depends on the die and
punches selected for the compression of tablets. The remaining specifications assure that
tablets do not vary from one production lot to another. The following standards or quality
control tests should be carried out on compressed tablets.
Mechanical strength of tablets.
The mechanical strength of a tablet provides a measure of the bonding potential of the
material concerned and this information is useful in the selection of excipients. An
excessively strong bond may prevent rapid disintegration and subsequent dissolution of a
drug. Weak bonding characteristics may limit the selection and/or proportion of excipients,
such as lubricants, that would be added to the formulation. The mechanical properties of
pharmaceutical tablets are quantifiable by the friability, hardness or crushing strength,
crushing strength-friability values, tensile strength, and brittle factor index.
57
2. Review of Literature
Friability
The crushing strength test may not be the best measure of potential tablet behavior during
handling and packaging. The resistance to surface abrasion may be a more relevant
parameter, as exemplified by those tests that measure the weight loss on subjecting the tablets
to a standardized agitation procedure. The most popular (commercially available) version is
the Roche Friabilator.
Hardness or Crushing strength
Hardness crushing strength determinations are made during tablet production and are used to
determine the need for pressure adjustment on tablet machine. The most popular estimate of
tablet strength has been crushing strength, Se, which may be defined as that compressional
force (Fe) which, when applied diametrically to a tablet, just fractures it. If the tablet is too
hard, it may not disintegrate in the required period of time to meet the dissolution
specifications; if it is too soft, it may not be able to withstand the handling during subsequent
processing such as coating or packaging and shipping operations. The force required to break
the tablet is measured in kilograms and a crushing strength of 4kg is usually considered to be
the minimum for satisfactory tablets. Oral tablets normally have a hardness of 4 to 10kg.
Tablet hardness has been associated with other tablet properties such as density and porosity.
Tensile strength
A non-compendial method of measuring the mechanical strength of tablets that is now widely
used is the tensile strength. This is the force required to break a tablet in diametral
compression test.
Brittle fracture index (BFI)
The tabletting performance of pharmaceutical materials and stated that whether or non
fracture occurs during the shear deformation which accompanies decompression depends on
58
2. Review of Literature
the ability of the materials to relieve stresses by plastic deformation without undergoing
brittle fracture and this ability is a time-dependent phenomenon.
Tablet disintegration and porosity
Complete tablet disintegration is defined as that state in which any residue of the tablet,
except fragments of insoluble coating, remaining on the screen of the test apparatus is a soft
mass having no palpably firm core .The disintegration time of a tablet can be affected by the
pore structure and bonding structure within the tablet. A high porosity and the presence of
large pores facilitate rapid water penetration into the tablet with a subsequent rupture of
bonds, followed by disintegration of the tablet (Shangraw et al., 1980). Disintegration time
increases with increasing compression force and it is hardly affected by tablet formulation.
Tablet Dissolution
Dissolution is the process by which a solid solute enters a solution. In the pharmaceutical
industry, it may be defined as the amount of drug substance that goes into solution per unit
time under standardized conditions of liquids/solid interface, temperature and solvent
composition A drug given in an orally administered tablet must undergo dissolution before it
can be absorbed and transported into the systemic circulation. Disintegration of tablets plays
a vital role in the dissolution process since it determines the area of contact between the solid
and liquid. On coming into contact with water, a tablet disintegrates into granules and then
deaggregates into fine particles. Dissolution thus occurs from intact tablet, granules and fine
particles. Assuming that the dissolution rate is proportional to the surface area available, the
amount dissolved from the intact tablet will be negligible compared with that dissolved from
the granules and fine particles.
2.12 Pharmaceutical capsules
Capsule is the most versatile of all dosage forms. Capsules are solid dosage forms in which
one or more medicinal and inert ingredients are enclosed in a small shell or container usually
made of gelatin. There are two types of capsules, hard and soft. The hard capsule is also
called two piece as it consists of two pieces in the form of small cylinders closed at one
end, the shorter piece is called the cap which fits over the open end of the longer piece,
59
2. Review of Literature
called the body. The soft gelatin capsule is also called as one piece. Capsules are
available in many sizes to provide dosing flexibility. Unpleasant drug tastes and odors can be
masked by the tasteless gelatin shell. The administration of liquid and solid drugs enclosed in
hard gelatin capsules is one of the most frequently utilized dosage forms.
Advantages of Capsules
Capsules mask the taste and odor of unpleasant drugs and can be easily administered.
They are attractive in appearance
They are slippery when moist and, hence, easy to swallow with a draught of water.
As compared to tablets less adjuncts are required.
The shells are physiologically inert and easily and quickly digested in the gastrointestinal
tract.
They are economical
They are easy to handle and carry.
The shells can be opacified (with titanium dioxide) or colored, to give protection from light.
Disadvantages of Capsules
The drugs which are hygroscopic absorb water from the capsule shell making it brittle and
hence are not suitable for filling into capsules.
The concentrated solutions which require previous dilution are unsuitable for capsules
because if administered as such lead to irritation of stomach
Raw Materials for Capsules
The raw materials used in the manufacture of both hard and soft gelatin capsules are similar.
Both contain gelatin, water, colorants and optional materials such as process aids and
preservatives.
1. Gelatin gelatin is the major component of the capsules and has been the material from
which they have traditionally been made. Gelatin has been the raw material of choice because
of the ability of a solution to gel to form a solid at a temperature just above ambient
temperate conditions, which enables a homogeneous film to be formed rapidly on a mould
pin.
60
2. Review of Literature
Some of the disadvantages with using gelatin for hard capsules include: it has a high moisture
content, which is essential because this is the plasticizer for the film and, under International
Conference
on
Harmonization
of
Technical
Requirements
for
Registration
of
Pharmaceuticals for Human Use (ICH) conditions for accelerated storage testing, gelatin
undergoes a cross linking reaction that reduces its solubility. Gelatin is a translucent brittle
solid substance, colorless or slightly yellow, nearly tasteless and odorless, which is created
by prolonged boiling of animal skin connective tissue or bones.
2. Colourants The color of pharmaceutic product plays an important role in their use.
Color is used principally to identify a product in all stages of its manufacture and use. The
colorants that can be used in capsules are of two types: water soluble dyes or insoluble
pigments. To make a range of colors dyes and pigments are mixed together as solutions or
suspensions.
3. Process aids Preservatives and surfactants are added to the gelatin solution during
capsule manufacture to aid in processing. Gelatin solutions are an ideal medium for bacterial
growth at temperatures below 55 C. Preservatives are added to the gelatin and colourant
solutions to reduce the growth of microorganisms until the moisture content of the gelatin
film is below 16% w/v.
Some hard gelatin capsules may contain 0.15 % w/w of sodium lauryl sulphate which
functions as wetting agent, to ensure that the lubricated metal moulds are uniformly covered
when dipped into the gelatin solution. Capsules are available in many different sizes and
shapes and can be used for the administration of powders, semisolids and liquids.
One of the main disadvantages of gelatin is it is derived from animal tissue. There have been
attempts to find other substances to replace gelatin, although the successful manufacture of
starch capsules has only recently been achieved. The starch capsule, a unique solid oral
dosage form, is made of potato starch and represents a direct alternative to hard gelatin
capsules (Bhawna Bhatt, 2007).
The starch capsule can be considered to be equivalent to the hard gelatin capsule. However,
starch capsules feature several advantages: Dissolution is independent of pH, they are
61
2. Review of Literature
suitable for enteric coating moisture in the shell is tightly bound to starch, and the capsules
are tamper-evident and preservative-free and produced from non-animal-derived ingredients.
2.13 Capsule Manufacturing process
Recent advances in injection molding technology have permitted the manufacture of starch
capsules. Fig.2.23 illustrates the essential parts of a conventional injection-moulding machine
used. During the production process, starch in the form of powder, granules or pellets is
fed through the hopper onto a rotating reciprocating screw. The feed material moves along
the screw towards the tip. During this process the temperature is increased by means of
external heaters around the outside of the barrel and by the shearing action of the screw.
From the feed zone to the compression zone, the feed material is gradually melted down; it is
then conveyed through the metering zone, where homogenization of the melt occurs, to the
end of the tip. During this process, the temperature is increased by means of external heaters
around the outside of the barrel and by the shearing action of the screw. From the feed zone
to the compression zone, the feed material is gradually melted down; it is then conveyed
through the metering zone, where homogenization of the melt occurs, to the end of the
reciprocating screw. When sufficient melt is collected for injection, it is injected into the
mould. The rotation of the crew stops while the polymer in the mould cools sufficiently for
the mould to be opened and the moulded parts ejected. Pressures of between 7002000 bar
and temperatures of between 120180 0C are normally seen in the transport, injection and
moulding operations. Throughout the process the mould is maintained below the glass
transition temperature of starch, and the time for a complete cycle is usually a few seconds
(Bhawna Bhatt, 2007).
62
2. Review of Literature
Fig 2.24: Conventional injection moulding machine for the production of capsules (Bhawna
Bhatt, 2007).
2.14 Physical characteristics
Starch capsules can be manufactured in different sizes size numbers 0, 1, 2, 3 and 4 by
changing the moulds. An essential advantage of the capsule design is that the same sized cap
is used to fit different body lengths. The diameter of the junction of the cap and body is
always the same on all sizes of starch capsules. Unlike the lipped seal on a gelatin capsule,
the starch capsule cap fits evenly in place over the body, leading to a good surface finish.
This is a further advantage as the capsules are easily tamper-evident. The starch capsule is
odourless and rigid, and exhibits similar dissolution behavior to the gelatin capsules. In vitro
release studies of acetaminophen, as a model drug, using the United States Pharmacopeia
(USP) Apparatus 2, demonstrated that the release properties of starch capsules were
independent of pH (West Pharmaceutical Services, unpublished). The storage conditions,
especially the humidity, have significant influence on the integrity of all types of capsules.
Typically, the moisture content of the starch capsules ranges between 1214% w/w, with
more than 50% being tightly bound to the starch. The presence of bound moisture suggests
that starch capsules may provide better stability properties and reduced susceptibility to
changes on storage.
63
2. Review of Literature
2.15. Starch in edible film production
Starch and starch derivative films have been widely studied due to their great molding and
film forming properties, high oxygen barrier and good mechanical strength (Forssell et al.,
2002, Gilleland et al., 2001, Mali et al., 2002). Even though there have been numerous
studies conducted on the properties of starch based films, few studies have related starches
from different sources with the resulting film forming characteristics, mechanical and
physical properties. The overall performance of starch films and coatings is highly likely to
be customizable, because of the availability of a wide variety of starches and their capacity
for physical and/or chemical modifications (Ellis et al., 1998, Liu, 2002). Cereda et al.,
(2000) showed that the use of cassava starch films was promising, giving a good appearance,
without stickiness, exhibiting shininess and transparency. Vicentini et al., (2001) also
developed edible films based on a mixture of cassava starch and wheat gluten. Rosa et al.,
(2001) reported a methodology for preparing new polymer blends, containing different
quantities of starch, with poly (e-caprolactone), poly(-hydroxybutyrate) and poly (hydroxybutyrate-co-b-hydroxyvalerate). However, wide application of starch film is limited
by its efficient barrier against low polarity compound (Kester and Fennema, 1986). The
hydrophilic nature of starch is a major constraint that seriously limits the development of
starch-based materials; in fact, their properties depend on the ambient humidity (Shogren et
al., 1993). An alternative to reduce these drawbacks is the use of modified starches (Lafargue
et al., 2007).
Several studies have been carried out on starch-based films obtained by melt processing or
casting from a solution or gel with addition of a plasticizer (Wurzburg, 1986). The addition
of water (Hulleman et al., 1998,) or other plasticizers such as sorbitol (Gaudin et al., 2000)
and glycerol (Fishman et al., 2000), considerably improves mechanical properties. Many
studies have been reported on starch based films cast from solutions or gels since 1950 (Liu
2005, Lourdin et al., 1997). However, wide application of starch film is limited by its
efficient barrier against low polarity compound (Kester and Fennema, 1986). Many research
reported that film forming conditions have an effect on crystallinity of the starch films and,
therefore, their properties. Controlling film formulation allows tailoring the mechanical and
barrier properties of these materials improving the efficiency of packaged foods
64
2. Review of Literature
conservation. Generally, plasticizers are defined by two purposes which are to aid processing
and to modify the properties of the final product. In the case of starch-based films, plasticizer
addition overcomes film brittleness and improves flexibility and extensibility.
Plasticizers are added to polymers to increase the ductility of the material. Glycerol, sorbitol,
fructose, glucose, sucrose, xylose, lactic acid sodium, urea, diethylene glycol, polyethylene
glycol (PEG 200), and glycerol diacetate are materials used as plasticizers in starch films
(Kalichevsky et al., 1993, Arvanitoyannis et al., 1994, Lourdin et al., 1997b, Gaudin et al.,
1999).
The oxygen permeability of native starch films is low. Plasticizer content and the
surrounding air humidity have an effect: higher plasticizer content and/or a higher air
humidity lead to increasing oxygen permeability (Arvanitoyannis et al., 1997), whereas a
higher crystallinity leads to a reduction in gas (O2, N2, and CO2). Several studies have
reported changes in the mechanical properties of rubbery starch films during storage (Van
Soest and Knooren, 1997). The elongation of films decreases while the tensile strength
increases.
Even though there have been numerous studies conducted on the properties of starch based
films, few studies have related starches from different sources with the resulting film forming
characteristics, mechanical and physical properties. In a previous study, films were
developed using starches from different plant sources as the base raw materials (rice, sweet
rice, potato, sweet potato, mungbean, waterchestnut, amaranth, wheat, and buck wheat
starches). The physical and mechanical properties of the starch based films were evaluated
(Lawton, 1996, Lee and Rhim, 2000, Muetgeer et al., 1955). Among the starch films, potato,
sweet potato, mungbean and waterchestnut were selected due to their superior film-forming
properties when compared with synthetic films. The addition of water (Hulleman et al.,1998,
Lourdin et al., 1997) or other plasticizers such as sorbitol (Gaudin,et al., 2000) and glycerol
(Fishman et al., 2000), considerably improves mechanical properties. Plasticizers are added
to polymers to increase the ductility of the material.
65
2. Review of Literature
2.16 Regulatory status
Capsules prepared from starch are officially recognized in United States Pharmacopoeia 23
and National Formulary 18. The USP also permits the use of colouring agents, opacifying
agents such as titanium dioxide, dispersing and hardening agents, as well as preservatives.
European pharmacopoeias do not specifically include starch as an ingredient in capsules.
Instead, the wording is capsule shells are composed of gelatin or other materials. Such
alternatives to gelatin will be of interest to those who, for religious, cultural or other reasons
wish to avoid capsules made from animal derived components.
66
You might also like
- Rheology of FoodsFrom EverandRheology of FoodsR.P. BorwankarNo ratings yet
- Starch Cross LinkingDocument217 pagesStarch Cross LinkingMark VicsonNo ratings yet
- The Chemistry and Technology of Edible Oils and Fats and Their High Fat ProductsFrom EverandThe Chemistry and Technology of Edible Oils and Fats and Their High Fat ProductsRating: 4.5 out of 5 stars4.5/5 (3)
- Starch: Chemistry and TechnologyFrom EverandStarch: Chemistry and TechnologyRating: 4.5 out of 5 stars4.5/5 (5)
- The Chemistry and Technology of Edible Oils and Fats: Proceedings of a Conference Arranged by Unilever Limited at Research Department, Port Sunlight, March 10-12th 1959From EverandThe Chemistry and Technology of Edible Oils and Fats: Proceedings of a Conference Arranged by Unilever Limited at Research Department, Port Sunlight, March 10-12th 1959J. DevineRating: 2.5 out of 5 stars2.5/5 (2)
- Biscuit, Cookie and Cracker Manufacturing Manuals: Manual 4: Baking and Cooling of BiscuitsFrom EverandBiscuit, Cookie and Cracker Manufacturing Manuals: Manual 4: Baking and Cooling of BiscuitsNo ratings yet
- Polymer Blends and Composites: Chemistry and TechnologyFrom EverandPolymer Blends and Composites: Chemistry and TechnologyNo ratings yet
- Polymer BlendsFrom EverandPolymer BlendsD.R. PaulNo ratings yet
- 4 - Enzymes in Starch ModificationDocument28 pages4 - Enzymes in Starch ModificationLoredana Dana100% (1)
- Modified Starches For The Food IndustryDocument5 pagesModified Starches For The Food IndustryMiguelArceMonroyNo ratings yet
- GEA Westfalia-Starch From Corn-Separation Technology For CerealsDocument8 pagesGEA Westfalia-Starch From Corn-Separation Technology For CerealsMurali Krishna IndanaNo ratings yet
- Genencor PresentationDocument22 pagesGenencor PresentationI. Murali KrishnaNo ratings yet
- Chewing Gum - 3Document21 pagesChewing Gum - 3Siswand BIn Mohd Ali100% (1)
- Lecture Notes 1Document21 pagesLecture Notes 1Châu An NguyễnNo ratings yet
- Carboxy Methyl StarchDocument17 pagesCarboxy Methyl StarchJulie Spencer100% (1)
- Kuraray Technical Brochure - EnglishDocument28 pagesKuraray Technical Brochure - EnglishrbucholzNo ratings yet
- Palsgaard® PGPR 4125Document3 pagesPalsgaard® PGPR 4125Erik ChoqueNo ratings yet
- Complete Report On Formation of Poly Lac PDFDocument119 pagesComplete Report On Formation of Poly Lac PDFMadhukar Scribd0% (1)
- Cake EmulsifiersDocument9 pagesCake EmulsifiersakNo ratings yet
- A-Z of Practical Paper Chemistry v1.04 2Document437 pagesA-Z of Practical Paper Chemistry v1.04 2Arnoldo Sánchez D100% (1)
- Applications of StarchDocument5 pagesApplications of StarchvienzenyNo ratings yet
- Studies in Depolymerization of Natural Polysaccharides - PHD ThesisDocument268 pagesStudies in Depolymerization of Natural Polysaccharides - PHD ThesisSatish D ShewaleNo ratings yet
- Guar Gum PDFDocument9 pagesGuar Gum PDFRavindra V. Mokal0% (1)
- Native Wheat Starch Info SheetDocument4 pagesNative Wheat Starch Info SheetBuia DanutNo ratings yet
- Lactic Acid Industrial ProductionDocument20 pagesLactic Acid Industrial Productiondorei100% (1)
- Ion-Exchange and Adsorbent Resins For Food IndustryDocument28 pagesIon-Exchange and Adsorbent Resins For Food IndustryMario Darío Gatto0% (1)
- Info SorbitolDocument45 pagesInfo SorbitolSanti CarpNo ratings yet
- Amylase Development For Starch Liquefaction: Carsten Andersen Novozymes R&DDocument26 pagesAmylase Development For Starch Liquefaction: Carsten Andersen Novozymes R&DGXGGXGNo ratings yet
- StarchDocument449 pagesStarchAlejandra Navia100% (1)
- BindersDocument4 pagesBindersSariyyaHeydarovaNo ratings yet
- Starch. Advances in Structure and Function - T. L. Barsby PDFDocument233 pagesStarch. Advances in Structure and Function - T. L. Barsby PDFGrigore VladNo ratings yet
- Albanian Flour Mill Course Trial ResutlsDocument9 pagesAlbanian Flour Mill Course Trial ResutlsqeremiNo ratings yet
- Sorbitol Industry PDFDocument7 pagesSorbitol Industry PDFLucas Ferreira LozNo ratings yet
- Cellulose-Based MaterialsDocument74 pagesCellulose-Based Materialsjohannes karcherNo ratings yet
- The Application of Modified Starches at The Size PressDocument18 pagesThe Application of Modified Starches at The Size PressPeter de Clerck100% (1)
- Enzymatic Deinking - A Bright Solution With A Bright FutureDocument23 pagesEnzymatic Deinking - A Bright Solution With A Bright FuturehanifNo ratings yet
- Applications of Modified StarchDocument26 pagesApplications of Modified StarchHermes Alvarado100% (3)
- Surfactants - EmulsifiersDocument51 pagesSurfactants - EmulsifiersBishnu PrasadNo ratings yet
- General FERMENTOR PDFDocument66 pagesGeneral FERMENTOR PDFraghavan89100% (1)
- Specialty Starches For Snack Foods PDFDocument21 pagesSpecialty Starches For Snack Foods PDFKenya Bispo100% (3)
- CornWetMilling BrochureDocument8 pagesCornWetMilling BrochureAndrea HernandezNo ratings yet
- Emulsifiers in Bread Improvers PDFDocument20 pagesEmulsifiers in Bread Improvers PDFAnonymous X6GJjSFE100% (1)
- Shelf Life & GV - PET GuideDocument4 pagesShelf Life & GV - PET GuideSomasundaram Yamaraja100% (2)
- Corn Wet Milling Brochure 12209Document8 pagesCorn Wet Milling Brochure 12209amanawatNo ratings yet
- Starch For Corrugating - Tongaat HulettDocument20 pagesStarch For Corrugating - Tongaat HulettTomé SilvaNo ratings yet
- Ineos Polypropylene Processing GuideDocument18 pagesIneos Polypropylene Processing GuideLe Toan100% (1)
- GEA Complete Milk Powder Factory - tcm24-24064Document9 pagesGEA Complete Milk Powder Factory - tcm24-24064shella168No ratings yet
- Designing A Lactose Crystallization Process Based On Dynamic Metastable LimitDocument13 pagesDesigning A Lactose Crystallization Process Based On Dynamic Metastable LimitGian VillafverteNo ratings yet
- Dairy CatalogueDocument8 pagesDairy CatalogueponsaravanNo ratings yet
- Dieter Urban - Polymer Dispersion and Their Industrial Applications - 2002 PDFDocument417 pagesDieter Urban - Polymer Dispersion and Their Industrial Applications - 2002 PDFNop Pirom100% (2)
- CSNL An Environment Friendly Alternative PDFDocument15 pagesCSNL An Environment Friendly Alternative PDFDonald_12No ratings yet
- Aquarium Water Quality ManagementDocument8 pagesAquarium Water Quality ManagementPedro ViegasNo ratings yet
- Extreme Temperatures: Paul Fields Bhadriraju Subramanyam Raj HulasareDocument12 pagesExtreme Temperatures: Paul Fields Bhadriraju Subramanyam Raj Hulasarerajeevtyagi41No ratings yet
- Horne Alcoholandheatstabilityofmilkprotein JDS89Document15 pagesHorne Alcoholandheatstabilityofmilkprotein JDS89rajeevtyagi41No ratings yet
- Horne Alcoholandheatstabilityofmilkprotein JDS89Document15 pagesHorne Alcoholandheatstabilityofmilkprotein JDS89rajeevtyagi41No ratings yet
- ArticleText 70748 1 10 20140801Document5 pagesArticleText 70748 1 10 20140801rajeevtyagi41No ratings yet
- Coffee Stability MilkDocument128 pagesCoffee Stability Milkrajeevtyagi41No ratings yet
- Consent Form and Declaration-2Document1 pageConsent Form and Declaration-2rajeevtyagi41No ratings yet
- Chemistry Salt Analysis CheatsheetDocument4 pagesChemistry Salt Analysis CheatsheetSumit Dhall74% (50)
- Moisture Content AOAC 984.20 PDFDocument74 pagesMoisture Content AOAC 984.20 PDFWynona Basilio100% (1)
- Delhi Public School Gwalior: (Under The Aegis of DPS Society, New Delhi)Document1 pageDelhi Public School Gwalior: (Under The Aegis of DPS Society, New Delhi)rajeevtyagi41No ratings yet
- New Doc 2019-11-28 20.37.28Document14 pagesNew Doc 2019-11-28 20.37.28rajeevtyagi41No ratings yet
- 7462 c953b2Document26 pages7462 c953b2rajeevtyagi41No ratings yet
- Covid GuidelinesDocument20 pagesCovid Guidelinesrajeevtyagi41No ratings yet
- Covid GuidelinesDocument20 pagesCovid Guidelinesrajeevtyagi41No ratings yet
- ADocument1 pageArajeevtyagi41No ratings yet
- Almanac (IX-XII) (2021-22) With CovidDocument12 pagesAlmanac (IX-XII) (2021-22) With Covidrajeevtyagi41No ratings yet
- Articles: 1 Mark 4 MarksDocument14 pagesArticles: 1 Mark 4 Marksrajeevtyagi41No ratings yet
- Lesson 1, ComputerDocument12 pagesLesson 1, Computerrajeevtyagi41No ratings yet
- 26 - ChemistryDocument19 pages26 - Chemistryrajeevtyagi41No ratings yet
- 59 - Chemistry QPDocument9 pages59 - Chemistry QPrajeevtyagi41No ratings yet
- Hydrophilic Lipophilic BalanceDocument3 pagesHydrophilic Lipophilic Balancerajeevtyagi41No ratings yet
- Paneer FlowDocument1 pagePaneer Flowrajeevtyagi41No ratings yet
- Adsl Modem 220909Document51 pagesAdsl Modem 220909shreez_79No ratings yet
- List of Activities:-: Delhi Public School, GwaliorDocument2 pagesList of Activities:-: Delhi Public School, Gwaliorrajeevtyagi41No ratings yet
- HSN Product Code For GST PDFDocument855 pagesHSN Product Code For GST PDFkashyap_ajNo ratings yet
- Optimum Biotech: Product Code Degree of Substitution (D.S.) Viscosity (Mpa S, 2% H O Solution, 25 °C) Moisture PHDocument1 pageOptimum Biotech: Product Code Degree of Substitution (D.S.) Viscosity (Mpa S, 2% H O Solution, 25 °C) Moisture PHrajeevtyagi41No ratings yet
- 1.1 Background of The Study: UNIVERSITY OF SANTO TOMAS: Department of Medical Technology PAGE 1Document23 pages1.1 Background of The Study: UNIVERSITY OF SANTO TOMAS: Department of Medical Technology PAGE 1Aniza AbiaNo ratings yet
- Guar GumDocument13 pagesGuar GumDharmendra B Mistry100% (1)
- 9 - Upw Postpaid Plans-Trai FormatDocument1 page9 - Upw Postpaid Plans-Trai Formatrajeevtyagi41No ratings yet
- Shelf Life Testing: Procedures and Prediction Methods For Frozen FoodsDocument46 pagesShelf Life Testing: Procedures and Prediction Methods For Frozen FoodsJose O. Llaneta III50% (2)
- Dow Corning 200 FluidDocument6 pagesDow Corning 200 FluidProject Sales CorpNo ratings yet
- Sonovita AWS Presentation SKDocument34 pagesSonovita AWS Presentation SK1234abcdNo ratings yet
- 1974 - Die Swell and Melt Fracture - Effects of Molecular Weight DistributionDocument5 pages1974 - Die Swell and Melt Fracture - Effects of Molecular Weight Distributionmbarete2No ratings yet
- Hydrodynamics Equation SheetDocument1 pageHydrodynamics Equation SheetsubnautaNo ratings yet
- Nodia and Company: Gate Solved Paper Chemical Engineering 2011Document17 pagesNodia and Company: Gate Solved Paper Chemical Engineering 2011vijendra mauryaNo ratings yet
- Group 5Document11 pagesGroup 5vzimak2355No ratings yet
- SPH Main by Kelager.06Document88 pagesSPH Main by Kelager.06marthantyNo ratings yet
- Aquadyn - 1.scientific ReferencesDocument9 pagesAquadyn - 1.scientific Referencessavica pricopNo ratings yet
- C 4Document52 pagesC 4saur1No ratings yet
- 1 - Behaviour of Real FluidsDocument5 pages1 - Behaviour of Real FluidsfyqahNo ratings yet
- Physics Investigatory: ProjectDocument13 pagesPhysics Investigatory: ProjectswapnilNo ratings yet
- Appendix A TablesDocument3 pagesAppendix A TablesHtut Ko Ko YeeNo ratings yet
- Early Reservoir Appraisal Utilizing A Well Testing SystemDocument69 pagesEarly Reservoir Appraisal Utilizing A Well Testing Systemmadonnite3781100% (1)
- Waterborn Silicate PaintsDocument98 pagesWaterborn Silicate PaintsJane Ashworth100% (1)
- SPE-197616-MS A Novel Quantitative and Predictive Reservoir-Souring Approach To Assess Reservoir Souring During Planned-Waterflood Development PlanDocument23 pagesSPE-197616-MS A Novel Quantitative and Predictive Reservoir-Souring Approach To Assess Reservoir Souring During Planned-Waterflood Development PlanAmelia Van KallenNo ratings yet
- Relief, Bi-Directional CR10-28Document2 pagesRelief, Bi-Directional CR10-28tungNo ratings yet
- Piston / Valve Design General Characteristics: Flowmeter 1.3. Novatron TZ1-... GKDocument2 pagesPiston / Valve Design General Characteristics: Flowmeter 1.3. Novatron TZ1-... GKAstri NgentNo ratings yet
- Elastomeric Impression MaterialsDocument3 pagesElastomeric Impression MaterialsDuong LeNo ratings yet
- Kobe FSM PDFDocument57 pagesKobe FSM PDFSokha RunNo ratings yet
- STAD Balancing ValvesDocument10 pagesSTAD Balancing ValvesNestramiNo ratings yet
- Natural Gas Plant OperationDocument212 pagesNatural Gas Plant OperationMuhammad JahanzaibNo ratings yet
- Contributors of Fluid MechanicsDocument34 pagesContributors of Fluid MechanicsDaryl Ann BrionesNo ratings yet
- Yoon 1987Document10 pagesYoon 1987Dersein SaraozNo ratings yet
- Seleccion y Diseño GerotorDocument28 pagesSeleccion y Diseño GerotoremersonNo ratings yet
- Microscale Heat Transfer - Fundamentals and Applications PDFDocument516 pagesMicroscale Heat Transfer - Fundamentals and Applications PDFAndréRocha100% (1)
- Study On The Use of Banana Peels For Oil Spill RemovalDocument8 pagesStudy On The Use of Banana Peels For Oil Spill RemovalKilsys AlvaradoNo ratings yet
- Model Answer 15CE41T PDFDocument14 pagesModel Answer 15CE41T PDFPRAKASHA SNNo ratings yet
- Fluid Mechanics PDFDocument48 pagesFluid Mechanics PDFrakib hasanNo ratings yet
- How Fuel Properties Are Used in The Shell Marine Fuel Specification TablesDocument3 pagesHow Fuel Properties Are Used in The Shell Marine Fuel Specification TablesVilius BukysNo ratings yet
- A-Thurs-O2 Absorption-ReportDocument25 pagesA-Thurs-O2 Absorption-ReportQuang TranNo ratings yet