Professional Documents
Culture Documents
New Types of Lead-Free Solders and Their Properties: Jaromir - Drapala@vsb - CZ
Uploaded by
EidelsayedOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
New Types of Lead-Free Solders and Their Properties: Jaromir - Drapala@vsb - CZ
Uploaded by
EidelsayedCopyright:
Available Formats
23. - 25. 5.
2012, Brno, Czech Republic, EU
NEW TYPES OF LEAD-FREE SOLDERS AND THEIR PROPERTIES
Jaromr DRPALA1, Daniel PETLK1, Jitka MALCHARCZIKOV1, Vlastimil VODREK1, Kateina
KONEN1, Bedich SMETANA1, Simona ZL1, Gabriela KOSTIUKOV1, Jana SEIDLEROV1,
Stanislav LASEK1, Michal MADAJ1, Ale KROUPA2, Jan URBNEK3, Karel DUEK3, Josef
SEDLEK3, Valerij E. SIDOROV4
1
Vysok kola bsk Technical University of Ostrava, Faculty of Metallurgy and Materials Science, Av.
17. listopadu 15, 708 33 Ostrava Poruba, Czech Republic, Jaromir.Drapala@vsb.cz
2
Institute of Physics of Materials, Academy of Sciences of the Czech Republic, ikova 22, 61662 Brno,
Czech Republic
Czech Technical University in Prague, Faculty of Electrical Engineering, Technick 2, 16627 Prague 6,
Czech Republic
4
Ural State Pedagogical University, 26 Cosmonavtov Avenue, 620017 Ekaterinburg, Russia
Abstract
The aim of this work is an experimental study of lead-free solders. Ternary and binary alloys with different
ratios of individual elements Ag, Al, Bi, Cu, In, Mg, Sb, Sn and Zn were prepared experimentally. The study
of low-fusing solder alloys was performed with the aspect of observing their selected physical, chemical,
structural and technological properties. The following characteristics were studied: temperatures and
enthalpies of phase transformations (DTA, TG, DSC) of individual solders at the rates of re-heating and
cooling of specimens of about 4C/min, macro- and micro-structural analysis (optical metallography), microhardness, chemical analysis: ICP-AES, optical emission spectrometry (OES), X-ray micro-analysis of
individual phases in the structure of solders (WDX, EDX), measurement of density and electrical resistivity of
selected solders in dependence on the temperature, test of wettability with or without use of fluxes,
measurement of corrosion properties.
Key words:
Lead-free solders, tin alloys; DTA analysis; microstructure; properties; corrosion; density; electrical resistivity.
1.
INTRODUCTION
The international European project COST Action MP0602 Advanced Solder Materials for High Temperature
Application was focused, in the period 2008-2011, on the basic scientific research of materials suitable for
lead-free solders and on the problem of their practical application, reliability during long-term utilization in all
types of equipment and their recycling. The main objective of the COST Action MP0602 was to increase the
fundamental basic knowledge on possible alloy systems that can be used as lead-free solder materials and
to provide a scientific basis for deciding which of these materials should be used for different soldering
purposes in order to replace the currently used lead-containing solders in future.
Low-fusing lead-free solders have two main fields of applications: electronic assemblies and heat
exchangers. In both cases, replacement of lead causes problems, which are of entirely different kinds. The
electronic industry sees a change in melting temperature and processing ability to be major problems, while
manufacturers of heat exchangers are much more sensitive to cost and strength. There are a numerous
lead-free solders with varying properties available today. The problem is not only a question of developing
solders with the right properties, but also a lack of knowledge about the existing alternatives and their
properties. In this stage some of the most important properties, such as electrical and heat conductivity,
wettability, mechanical properties and melting temperature should be studied for the basic alloys considered
23. - 25. 5. 2012, Brno, Czech Republic, EU
in the literature survey. These properties are examined and compared to tin-lead solders [1-4].
In this work we present an experimental study of selected physical, chemical, structural and utility properties
of lead-free alloys on the base of tin with different ratios of individual elements.
2.
EXPERIMENTAL SAMPLES
Binary and ternary alloys with different ratios of individual elements Ag, Al, Bi, Cu, In, Mg, Sb, Sn and Zn
(see Table 1) were prepared experimentally. The purity of the starting metals was 3N5 minimally. The alloys
were prepared by smelting of input metals in a resistance furnace in evacuated quartz ampoules. After
smelting and homogenisation of the melt, the alloys were slowly cooled in the furnace to room temperature.
Table 1 Nominal chemical composition of alloys (charge), ICP-AES and OES chemical analyses.
No. Charge [at.%]
1 2.5 Al, 85 Sn, 12.5 Zn
ICP-AES [at.%]
OES [at.%]
1.6 Al, 82.3 Sn, 16.1 Zn
1 Al, 88.6 Sn, 10.4 Zn
4.9 Al, 70.4 Sn, 24.7 Zn
1.5 Al, 79.6 Sn, 18.9 Zn
1.4 Al, 75.3 Sn, 23.3 Zn
EDX (area) [at.%]
10 Al, 10 Sn, 80 Zn
12.8 Al, 16.5 Sn, 70.6 Zn
10.2 Al, 13.5 Sn, 76.3 Zn
10
10 Al, 70 Sn, 20 Zn
2.3 Al, 76.9 Sn, 20.8 Zn
2.5 Al, 79.4 Sn, 18.1 Zn
12
7 Al, 60 Sn, 33 Zn
1.7 Al, 64 Sn, 34.3 Zn
3.8 Al, 67 Sn, 29.2 Zn
15
9 Al, 39 Sn, 52 Zn
5.5 Al, 54.3 Sn, 40.2 Zn
7.3 Al, 44 Sn, 48.7 Zn
16
7 Al, 50 Sn, 43 Zn
7.1 Al, 51.2 Sn, 41.7 Zn
6.8 Al, 54.2 Sn, 39 Zn
21
15 Al, 20 Sn, 65 Zn
22.3 Al, 29.2 Sn, 48.5 Zn
14.5 Al, 36.2 Sn, 49.3 Zn
22
25 Al, 40 Sn, 35 Zn
20 Al, 45.2 Sn, 37.8 Zn
20 Al, 45.2 Sn, 34.8 Zn
23
18 Al, 40 Sn, 42 Zn
15.6 Al, 43.7 Sn, 40.7 Zn
8.9 Al, 51.3 Sn, 39.8 Zn
25
51.5 Al, 18.7 Sn, 29.8 Zn
48 Al, 20.5 Sn, 31.5 Zn
50.9 Al, 20.1 Sn, 29 Zn
26
40.5 Al, 20.5 Sn, 39 Zn
40 Al, 21.3 Sn, 38.7 Zn
37.8 Al, 8.2 Sn, 54 Zn
31
2.3 Bi, 3.4 Mg, 94.3 Sn
3.2 Bi, 0.4 Mg, 96.4 Sn
4.5 Bi, 0.1 Mg, 95.4 Sn
32
3.3 Mg, 7.7 Sn, 89 Zn
1.6 Mg, 7.2 Sn, 91.2 Zn
0.9 Mg, 11 Sn, 88 Zn
33
7.4 Mg, 81.1 Sn, 11.5 Zn
3.2 Mg, 84.9 Sn, 11.9 Zn
6.2 Mg, 84.7 Sn, 9.1 Zn
34
9.6 Mg, 90.4 Sn
4.5 Mg, 95.5 Sn
35
3.8 Ag, 96.2 Sn
3.3 Ag, 96.7 Sn
36
4 Ag, 7.1 Sb, 88.9 Sn
3.6 Ag, 7.4 Sb, 89 Sn
37
4 Ag, 94.4 Sn, 1.6 Zn
3.8 Ag, 95 Sn, 1.2 Zn
38
2.8 Ag, 94.7 Sn, 2.5 Zn
2.7 Ag, 94.7 Sn, 2.6 Zn
3.9 Ag, 94.7 Sn, 1.4 Zn
3.1 Ag, 95.3 Sn, 1.6 Zn
39
5.4 Ag, 92.4 Sn, 2.2 Zn
3.9 Ag, 94.1 Sn, 2 Zn
4 Ag, 94.8 Sn, 2.2 Zn
7 Ag, 90.2 Sn, 2.8 Zn
40
4 Ag, 92.4 Sn, 3.6 Zn
2.6 Ag, 95.2 Sn, 2.2 Zn
4 Ag, 96.7 Sn, 1.4 Zn
3.5 Ag, 94.5 Sn, 2 Zn
41
4.9 Ag, 1.7 Cu, 93.4 Sn
2.4 Ag, 0.6 Cu, 97 Sn
4 Ag, 1.9 Cu, 94.1 Sn
4 Ag, 1.2 Cu, 94.8 Sn
42
4.7 Ag, 94.8 Bi, 0.5 Cu
5.4 Ag, 94 Bi, 0.6 Cu
43
1.6 Ag, 2.2 Al, 96.2 Sn
1.3 Ag, 2.4 Al, 96.3 Sn
44
3.3 Ag, 0.9 Al, 95.8 Sn
3.9 Ag, 1.5 Al, 94.6 Sn
45
0.3 Cu, 93.5 Sn, 6.2 Zn
0.8 Cu, 96.5 Sn, 2.7 Zn
2.2 Cu, 94.2 Sn, 3.6 Zn
46
10 Cu, 85 Sn, 5 Zn
8 Cu, 89 Sn, 3 Zn
11.3 Cu, 84.2 Sn, 4.5 Zn
47
1.8 Cu, 7.4 Sb, 90.8 Sn
2 Cu, 6.9 Sb, 91.1 Sn
2 Cu, 7.4 Sb, 90.6 Sn
48
3.7 Cu, 20.2 Sb, 76.1 Sn
5.3 Cu, 16.4 Sb, 78.3 Sn
2.3 Cu, 20.2 Sb, 77.5 Sn
49
1.2 Cu, 5.2 In, 93.6 Sn
1.4 Cu, 4.9 In, 93.7 Sn
50
0.9 Al, 9 Mg, 90.1 Sn
0.9 Al, 4.9 Mg, 94.2 Sn
2.7 Mg, 97.3 Sn
4 Ag, 96 Sn
3.6 Ag, 96.4 Sn
3.8 Ag, 8 Sb, 88.2 Sn
4.7 Ag, 93.7 Sn, 1.6 Zn
7 Ag, 92.9 Bi, 0.1 Cu
3 Ag, 4.4 Al, 92.6 Sn
3.6 Ag, 0.1 Al, 95.3 Sn
1.2 Cu, 6.1 In, 92.7 Sn
3 Ag, 2.4 Al, 94.6 Sn
1.7 Cu, 3 In, 95.3 Sn
2.4 Al, 3.9 Mg, 93.7 Sn
23. - 25. 5. 2012, Brno, Czech Republic, EU
3.
EXPERIMENTAL METHODS
The following characteristics of prepared experimental solders were studied:
- Temperatures of phase transformations (DTA, TG, DSC) of individual solders at the rates of re-heating and
cooling of specimens of 4C/min Table 2.
- Microstructural analysis (optical metallography) Figs. 1 to 6 and micro-hardness of alloys - Table 2.
- Chemical analysis (ICP-AES and Optical Emission Spectrometry OES) - Table 1, micro-structural analysis
(SEI, BEI) and chemical microanalysis of individual phases in the microstructure of solders (X-ray
spectrometry WDX, EDX) see Figs. 1 to 6.
- Tests of wettability of solders with copper, nickel or brass wires using fluxes or without them - see Fig. 7
and Table 3, 4.
- Measurements of electrical resistivity and density of solders in dependence on the temperature Table 5,
Figs. 8a, 8b.
- Corrosion behaviour of individual alloys in various chemical corroding mediums Table 5, Figs. 9a, 9b.
The measurements of individual characteristics of solders were carried out at the Faculty of Metallurgy and
Materials Engineering (VB Technical University of Ostrava), Department of Non-ferrous Metals, Refining
and Recycling; Department of Physical Chemistry and the Theory of Technological Processes; Department
of Materials Engineering; Nanotechnology Centre; Institute of Physics of Materials AS CR in Brno; Czech
Technical University in Prague, Faculty of Electrical Engineering; Charles University, Prague; Ural State
Pedagogical University, Ekaterinburg.
Table 2 DTA analysis (heating and cooling rate 4 C/min) and micro-hardness HV0.01 of alloys.
Specimen
1
3
4
10
12
15
16
21
22
23
24
25
26
31
32
33
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
Charge - chemical
composition [at.%]
2.5 Al, 85 Sn, 12.5 Zn
4.9 Al, 70.4 Sn, 24.7 Zn
10 Al, 10 Sn, 80 Zn
10 Al, 70 Sn, 20 Zn
7 Al, 60 Sn, 33 Zn
9 Al, 39 Sn, 52 Zn
7 Al, 50 Sn, 43 Zn
15 Al, 20 Sn, 65 Zn
25 Al, 40 Sn, 35 Zn
18 Al, 40 Sn, 42 Zn
32.3 Al, 9.8 Sn, 57.9 Zn
51.5 Al, 18.7 Sn, 29.8 Zn
40.5 Al, 20.5 Sn, 39 Zn
2.3 Bi, 3.4 Mg, 94.3 Sn
3.3 Mg, 7.7 Sn, 89 Zn
7.4 Mg, 81.1 Sn, 11.5 Zn
3,8 Ag, 96,2 Sn
4 Ag, 7.1 Sb, 88.9 Sn
4 Ag, 94.4 Sn, 1.6 Zn
2.8 Ag, 94.7 Sn, 2.5 Zn
5.4 Ag, 92.4 Sn, 2.2 Zn
4 Ag, 92.4 Sn, 3.6 Zn
4.9 Ag, 1.7 Cu, 93.4 Sn
4.7 Ag, 94.8 Bi, 0.5 Cu
1.6 Ag, 2.2 Al, 96.2 Sn
3.3 Ag, 0.9 Al, 95.8 Sn
0.3 Cu, 93.5 Sn, 6.2 Zn
10 Cu, 85 Sn, 5 Zn
1.8 Cu, 7.4 Sb, 90.8 Sn
3.7 Cu, 20.2 Sb, 76.1 Sn
1.2 Cu, 5.2 In, 93.6 Sn
TE
[C]
198.8
197.6
196.7
197.6
197.9
197.3
197.9
197.5
197.5
197.6
196.9
197.4
197.8
215.7
180.0
182.1
220.3
TU
[C]
T2
[C]
277.3
327.1
277.5
277.5
277.6
277.8
277.8
277.4
277.4
277.4
325.3
297.0
311.6
344.5
333.8
302.9
T3
[C]
363.4
318.6
~412
~439
~332
TL
[C]
253.8
358.8
239.0
296.5
325.0
313.6
439.6
406.0
337.0
446.9
536.4
506.5
355
230.7
217.9
217.9
217.8
218.2
216.5
261.1
226.1
221.4
198.4
238.0
224.3
225.4
236.4
316.0
216.6
336.1
HV0.01 Sn
eutectic
15
16
19
16
16
17
16
17
18
16
21
26
13
13
24
14
15
10
14
15
14
18
14
13
9
21
17
14
HV0.01
phases
64 AlZn
94 AlZn
96 AlZn
83 AlZn
73 AlZn
87 AlZn
90 AlZn
77 AlZn
1170 Mg3Bi2
69 Zn; 334 Mg2Sn
87 Ag3Sn
318 AgZn
446 AgZn
56 AgZnSn
350 AgZn
156 AgAl
527 CuZn
468 CuZn
150 SnSb; 470 Cu6Sn5
360 Cu6Sn5
23. - 25. 5. 2012, Brno, Czech Republic, EU
4.
EXPERIMENTAL RESULTS
4.1
Differential thermal analysis
The differential thermal analysis (DTA) and the thermo-gravimetry & differential scanning calorimetry
(DG/DSC) were carried out in the device SETARAM SYSTEM 18TM. The results of the measurements of the
temperatures TE (eutectic reaction), TU (invariant reaction), and TL (liquid) were acquired at the heating rate
4C/min, see Table 2.
4.2
Metallographic study
Microstructures of specimens acquired by the microscope Neophot 32 using the camera Olympus DP 11
differed very slightly with respect to the history of their preparation. Metallographic analyses discovered
practically fine-grained structure, mostly of a eutectic type, often with present dendritic formations.
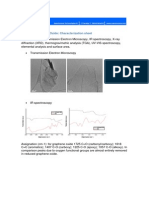
Microstructures of selected specimens of solders are shown in Figs. 1a to 6a. Results of microstructural a
chemical microanalysis of present phases in selected alloys are shown in Figs. 1b to 6b by using of X-ray
analysis EDX. Micro-hardness according to Vickers was measured in all the specimens by the microhardness tester LECO applying the load 0.01 N. The measurement results are presented in Table 2.
4.3
Measurement of wettability
Wettability is a property of metal surface that expresses the material diffluence. Formation of an intermetallic
compound is a necessary condition of good wetting and linking of the solder and wetted metal. A tested
component is suspended on the dynamometer above the vessel with the molten solder. The measurement
itself was carried out on the meniscograph solderability tester. The device consists of a measuring head with
a spring micro-balance, a holder for gripping the measured object, a soldering bath and electronic control
unit. The tested specimens of copper or nickel wires had the diameter 1 mm. Rinsing-less fluxes were used
for AlSnZn solders: types RX and RXZ. The wettability test was carried out at VUT in Prague. Cu and Ni
wires were tested at the temperature 50C above the liquid temperature applying various fluxes or without
any flux. The mean values of wetting forces [mN] on the plateau are summarized in the Table 3. An
example of the curves of wettability measurements is presented in Fig. 7.
Copper, nickel and brass wires ( 1 mm) were tested at the soldering temperature 250C applying various
fluxes too. All the combinations of the solders, fluxes and test specimens were measured. Several
measurements were performed for each combination solder-flux-test specimen. The wettability tests were
rated by marks from 1 (best wettable combination) to 10 (worst wettable combination). The classification was
carried out on the basis of the measured courses of the wetting force and on the basis of the visual control of
the measured specimens under the microscope. The results are summarized in Table 4.
4.4
Measurement of electrical resistivity
The electrical resistivity is an important property when determining suitable types of lead-free solders. To be
able to measure the resistivity of solders, it was necessary to roll down the specimens into the form of film
0.2 mm thick. Stampings of a required shape, 50 mm long, 5 0.1 mm wide, with notches 5 x 1 mm on both
sides, were prepared by means of a special cut jig with a flat stamping die. The measurements were carried
out at the Department of Physics of Materials, Charles University and at the Faculty of Electrical Engineering
of the Czech Technical University in Prague. The classical four-point method at room temperature 23C was
used for the resistivity measurement (Table 5). The electrical resistivity of metals is dependent on the purity
of individual elements and on the structural perfection of materials.
The electrical resistivity of AlSnZn alloys was measured at the Ural State Pedagogical University,
Ekaterinburg from the room temperature to 500C. It was determined by contactless method in rotating
magnetic field. The total uncertainty in resistivity values was 5%. The temperature dependencies of
resistivity were linear and fitted with linear function in solid and liquid states for all AlSnZn alloys Fig. 8a.
23. - 25. 5. 2012, Brno, Czech Republic, EU
Ternary
eutectic
SnZnAl
Alloy
90 Al10 Zn
[At.%]
Fig. 1a Microstructure of AlSnZn alloy No. 3.
Pure Zn
Fig. 1b EDX analysis of AlSnZn alloy No. 3.
Phase
88 Al12 Zn
[At.%]
Ternary
eutectic
SnZnAl
Fig. 2a Microstructure of AlSnZn alloy No. 16.
Pure Zn
Fig. 2b EDX analysis of AlSnZn alloy No. 16.
Pure Sn
Solid solution
97.6 Sn2.4 Sb
[At.%]
Phase
Ag3Sn
Fig. 3a Microstructure of AgSbSn alloy No. 36.
Fig. 3b EDX analysis of AgSbSn alloy No. 36.
23. - 25. 5. 2012, Brno, Czech Republic, EU
Pure Sn
Phase
(Ag,Zn)3Sn
Phase
44 Ag56 Zn
[At.%]
Fig. 4a Microstructure of AgSnZn alloy No. 40.
Fig. 4b EDX analysis of AgSnZn alloy No. 40.
Pure Ag
Phase
Cu6(Bi,Ag)5
Pure Bi
Fig. 5a Microstructure of AgBiCu alloy No. 42.
Fig. 5b EDX analysis of AgBiCu alloy No. 42.
Phase
Cu6(Sn,Sb)5
Solid solution
96.1 Sn3.9 Sb
[At.%]
Fig. 6a Microstructure of CuSbSn alloy No. 47.
Fig. 6b EDX analysis of CuSbSn alloy No. 47.
23. - 25. 5. 2012, Brno, Czech Republic, EU
Table 3 Mean values of wetting forces F [mN] of Sn-Zn-Al alloys on the plateau.
6
Alloy [wt.%]
91 Sn, 9Zn
91 Sn, 9Zn
85 Sn, 15 Zn
85 Sn, 15 Zn
70 Sn, 30 Zn
70 Sn, 30 Zn
90.4 Sn ,9 Zn, 0.6 Al
90.4 Sn, 9 Zn, 0.6 Al
75 Sn, 23 Zn, 2 Al
75 Sn, 23 Zn, 2 Al
70 Sn, 27 Zn, 3 Al
70 Sn, 27 Zn, 3 Al
56 Sn, 41 Zn, 3 Al
56 Sn, 41 Zn, 3 Al
18 Sn, 78 Zn, 4 Al
18 Sn, 78 Zn, 4 Al
Wire
1
mm
Cu
Ni
Cu
Ni
Cu
Ni
Cu
Ni
Cu
Ni
Cu
Ni
Cu
Ni
Cu
Ni
Without
flux
Flux
RX
Flux
RXZ
-2.22
-2.03
-2.347
-2.089
-2.68
-2.18
-2.295
-1.93
-2.183
-1.793
-3.64
-2.8
-2.96
-2.27
-3.82
-2.571
-2.56
-2
-2.652
-1.714
-2.61
-2.27
-3.05
-2.571
-3.031
-2.56
-3.6
-2.86
-2.83
-2.77
-3.922
-3.59
-2.64
-2.36
-2.451
-2.36
-2.54
-2.32
-2.851
-2.427
-2.941
-2.652
-3.38
-3.27
-2.9
-2.84
-4.134
-3.48
[mN]
Chart1 (mN)
Chart2 (mN)
Chart3 (mN)
Chart5 (mN)
Time [ms]
0
0
2000
4000
6000
8000
10000
12000
Chart7 (mN)
Chart8 (mN)
-2
Chart9 (mN)
-4
-6
Fig. 7 Wetting forces of the alloy 17.8 Sn, 78.2
Zn, 4 Al [wt.%]; Ni wire 1 mm, flux RXZ.
RX alcoholic flux, RXZ semi-aquatic flux
Table 4 Summary results of the wettability test.
Wire
1 mm
Copper
Nickel
Brass 66 wt.%
Cu
4.5
Flux
without flux
Epsilon M5
Epsilon 5
Epsilon 2
without flux
Epsilon M5
Epsilon 5
Epsilon 2
without flux
Epsilon M5
Epsilon 5
Epsilon 2
P1
10
8
8
8
10
7
5
3
10
8
6
4
P2
10
10
10
10
10
7
6
6
10
7
6
7
P3
10
9
8
4
10
2
2
2
10
7
6
3
Solder
P4 P5 P6
10 10 10
10 8 5
10 6 4
10 4 1
10 10 10
9 2 2
8 2 2
8 2 1
10 10 10
9 8 5
8 5 5
9 4 4
P7
10
9
9
9
10
8
5
3
10
9
6
4
Chart6 (mN)
P8
10
9
9
9
10
7
5
4
10
9
7
6
P9
10
9
8
7
10
5
3
3
10
8
6
4
Solders [wt.%]:
P1 Sn1 Cu
P2 Sn5 Sb
P3 Sn4 Ag
P4 Sn3.5 Sb1.5 Cu
P5 Sn3.8 Ag0.7Cu
P6 Sn37 Pb
P7 Sn3 Cu
P8 Sn 99.75
P9 Sn2 Bi1 Cu
Fluxes producer SLUVIS Prague.
Measurement of density of Al-Sn-Zn alloys in liquid state
Density of the Al-Sn-Zn melt was measured by absolute variant of gamma-absorption method at the Ural
State Pedagogical University. The uncertainty in absolute values of density was at the level of 0.3 % for the
sample containing 25 at.% Sn and 0.45 % for the sample with 91 at.% Sn. All the experiments were
performed during the heating and the following cooling with the rate of 1 K/min in helium atmosphere (the
-2
chamber was preliminary outgased up to 10 Pa) and crucibles from Al2O3. Fig. 8b represents the
temperature dependences of the melt density of five AlSnZn alloys. It is obvious that the melt density
decreases linearly with the temperature.
23. - 25. 5. 2012, Brno, Czech Republic, EU
80
70
No. 1
No. 2
No. 3
No. 4
No. 5
6700
6600
6500
6400
Resistivity [.cm]
Density [kg / m ]
6800
6300
6200
6100
250
60
50
40
30
heating No. 1
heating No. 3
heating No. 4
heating No. 5
heating No. 2
20
10
350
450
550
Temperature [C]
650
750
cooling No. 1
cooling No. 3
cooling No. 4
cooling No. 5
cooling No. 2
0
0
100
200
300
400
500
600
Temperature [C]
a)
b)
Fig. 8 Temperature dependences of the density a) and electrical resistivity b) of alloys: No. 1. Sn6.5 Zn2.5
Al, No. 2. Sn12 Zn8 Al, No. 3. Sn25 Zn5 Al, No. 4. Sn43 Zn7 Al, No. 5. Sn65 Zn10 Al [at.%]:
Table 5 The results of polarization measurements of lead-free solders using 0.1 mol/l NaCl solution,
salt spray test and electrical resistivity of solders (23C).
Alloy
No.
E3
E19
F1
F23
F25
F47
E5
E11
E14
F9
F17
F34
F36
F41
F42
F44
F45
F51
F52
F55
F56
Q1
P1
P2
P3
P4
P5
P6
Solder [wt.%]
Sn3 Ag2 Bi2 Sb
Sn2.5 Ag11.2 Bi5.5 In
Sn2 Ag7.5 Bi5.5 In
Sn0.5 Ag1.5 Bi3 Sb
Sn0.5 Ag56 Bi
Sn5 Bi1 In
Sn2.5 Ag2 Bi
Sn5 Ag8,6 In
Sn3,1 Ag6,1 Bi
Sn2 Ag7,5 Bi0,5 Cu
Sn3,4 Ag4,8 Bi
Sn3.33 Ag4.76 In
Sn2.91 Ag1.94 Bi2.91 In
Sn3.5 Ag7 Bi
Sn3.5 Ag10 Bi
Sn1.5 Ag3 Cu5 In
Sn0.5 Ag3 Cu5 In
Sn0.5 Ag1 Cu5 In
Sn1.5 Ag1 Cu5 In
Sn0.5 Ag5 In
Sn1.5Ag5In
Sn3.33 Ag4.83 Bi
Sn1Cu
Sn1.5 Cu3.5 Sb
Sn4 Ag
Sn5 Sb
Sn3.8 Ag0.7 Cu
Sn37 Pb
Sn2.7 Al13 Zn
Sn3 Al41 Zn
Sn0.6 Al7.4 Zn
Ecor
mc
Jp
2
[mV]
[mA/cm ]
[g/cm ]
[.cm]
-1064
-497
-585
-1054
-1043
-1001
-1059
-502
-1048
-1058
-1078
-570
-469
-1060
-1035
-533
-540
-495
-590
-640
-850
-1054
-1086
-1071
-1085
-1079
-1082
-490
-1074
2.18
0.011
N
2.79
2.21
0.799
2.86
N
2.704
3.014
2.67
0.135
0.27
3.49
2.32
0.061
0.085
0.099
0.119
0.116
0.128
2.22
3.61
3.84
3.75
2.78
3.4
2.4
4.03
3.98
4.65
2.67
3.5
9.86
11.8
4.24
3.58
4.8
4.65
7.96
7.61
3.42
9.67
8.48
9.5
10.2
8.67
20.2
3.18
0.547
0.795
0.503
1.71
2.32
2.32
15.4
20.9
15.3
17.2
13.8
17.3
15.6
15.8
15.1
16.1
16.0
15.6
15.9
17.3
16.4
16.8
16.4
16.7
16.8
15.1
12.2
15.8
13.8
15.6
12.0
15.3
10.8
9.3
9.8
Note:
Ecor corrosion potential
Jp critical passivation
current density
N polarization curves
without peak
mc corrosion loses on
samples after salt spray test
(exposition 240 h)
electrical resistivity
23. - 25. 5. 2012, Brno, Czech Republic, EU
4.6
Corrosion tests of lead-free solders
Electrochemical corrosion properties of selected lead-free solders were studied on the base of
potentiodynamic polarization method using 0.1 mol/l aqueous solution of NaCl at 25C. Relatively high
differences were found out among the values of corrosion potentials, critical current densities (Table 5) and
other measured parameters for lead-free solders. Lead-free solders containing Ag showed to be more
resistant to pitting corrosion in the neutral NaCl solution comparing to solders containing Mg or Zn see Fig.
9b. On the basis of accelerated corrosion test in salt spray, relatively small differences of corrosion losses
were found for selected lead-free solders on the base of tin. Considerable differences were discovered in the
corrosion products appearance. Corrosion in the form of dark points or spots in lead-free solders was
determined by the occurrence of phases with higher contents of Ag and Cu.
400
-200
Ecor
Edk [mV]
Erk [mV]
-200
Potential E [ mV] SCE
potential E [mV]
-400
Edk
Erk
200
Ecor [mV]
-600
-800
-1000
-400
-600
-800
-1000
-1200
-1400
-1200
0
20
40
60
content Zn [%]
80
100
31 32 33 34 36 37 38 39 40 42 43 44 45 46 47 48 49 50
-1600
Solder No.
a) AlSnZn solder alloys
b) solder alloys No. 31 to No. 50
Fig. 9 Comparison of potentials of pitting corrosion in 0.1 M NaCl solution.
Ecor corrosion (free) potential, Edk potential of depassivation, Erk potential of re-passivation.
5.
DISCUSSION
Metallographic analyses discovered practically in all the cases fine-grained two- or three-phase structures,
mostly of the eutectic type. Three phases pure Zn, pure Sn and AlZn alloy were found in AlSnZn alloys.
The alloys were relatively inhomogeneous in the volume of specimens due to different histories of the heat
treatment. Dendrites were observed in some cases at the surface of alloys. The eutectic structure containing
tin as a matrix, in which fine lamellae of zinc were segregated, was ascertained in all the alloys. Even finer
spots of AlZn phase were discovered on their surface, which was confirmed by the image analysis from the
area of ternary eutectic.
Furthermore, 20 different lead-free solders were prepared (specimens No. 31 to No. 50) see Table 1. The
chemical compositions of these alloys were selected so that the eutectic or invariant reaction could happen
at the crystallization. The matrix of alloys was mostly formed by tin. Classical methods of evaluation of
macro- and microstructural characteristics, chemical and thermodynamic properties of alloys were used for
the complex study of specimens, such as metallography, micro-hardness measurement, DTA, chemical
macro- and microanalysis (ICP AES, OES, EDX, WDX). Structures of all the alloys after crystallization in
evacuated SiO2 ampoules were mostly fine-grained except the alloy No. 48. Individual phases in structures
of alloys were identified by X-ray microanalysis (EDX). Demonstrations of microstructural analyses for four
types of solders are as an example shown in Figs. 3 to 6.
From the micro-hardness point of view, the solders of eutectic type exhibited a relatively low micro-hardness
(HVm = 11 to 18), while individual phases found in the alloys No. 31 to No. 50 had high values of microhardness see Table 2.
23. - 25. 5. 2012, Brno, Czech Republic, EU
It follows from the differential thermal analysis that our measured temperatures were almost in accordance
with the literature or differed only slightly. Some phase transformations were observed in alloys of AlSnZn
type, especially in specimens with high content of zinc. Two significant temperatures were ascertained in this
ternary system: eutectic TE = 197.4C and invariant temperature TU = 277.5C, which is in very good
agreement with the literature [5-7].
The wettability test was determined for the AlSnZn alloys. The tested Cu and Ni wires are not wetted for all
types of the solders if fluxes are not used. The better results of wettability were reached for nickel Table 3.
It should be noted that the AlSnZn alloys embody relatively bad wettability for used types of fluxes.
The wettability was determined for the other solders see Table 4. The wires of Cu, Ni and brass Ms66 were
used for the wettability and commercial rinsing-less fluxes Epsilon 2, Epsilon 5 and Epsilon M5 were applied
on their surface, in some cases no flux was used. All the materials of test specimens (Cu, Ni, Ms66) are not
wetted for all types of the solders if fluxes are not used. The best results were reached for nickel. The Sn37
Pb solder (sample P6) and from the lead-free solders the P5 solder had the best wetting properties. The
solder P4 is not wetted with all the fluxes mentioned above. The Epsilon 2 flux appears to be the most
universal flux. The fluxes Epsilon 5 and in particular Epsilon M5 have a considerably greater dispersion
variance among the measured courses of wetting forces compared to the flux Epsilon 2. These fluxes are
obviously much more volatile and can be recommended especially for manual soldering.
Electrical resistivity appeared constant vs. temperature for the samples 4 and 5 (AlSnZn alloys) in liquid
state. The sample 4 had the highest resistivity of all samples, in solid and especially in liquid state see Fig.
8a. The heating and cooling curves coincided with each other in liquid state. But they didnt quite coincide
with each other in solid state for samples 1 and 2, or one may say there is a hysteresis between them. It is
evident that irreversible transition took place in the melt after it had been heated up to 500C.
Corrosion tests. It was found out on the base of the potentiodynamic polarization method that the resistance
to pitting corrosion of lead-free solders SnZn and AlSnZn markedly decreased at the content 1015 at.%
Zn. At further increasing of the Zn content only slightly declining trend of reducing potentials of corrosion,
depassivation and re-passivation was recorded. The influence of aluminum on corrosion resistivity in terms
of the performed tests appears to be slightly negative. Attention has to be given to solders with zinc
considering their lowered corrosive resistance.
Potentiodynamic cyclic polarization methods and accelerated corrosion tests in salt spray were used for
evaluation of corrosive resistance of lead-free ternary solders: 9 alloys SnZn(Ag, Cu, Mg), 3 alloys Sn
Sb(Ag, Cu) and other 6 solders Sn(Ag, Al, Bi, Cu, In, Mg), including alloys on the base of zinc and
bismuth. Doping with Ag and Bi has a favourable influence on the corrosive resistance increase of examined
solders. On the contrary, a decrease of corrosive resistance was proved at doping with Zn and especially
with Mg. An abnormally high corrosion was discovered for SnZnMg solders. No essential differences were
ascertained for solders doped with Al, Cu, In and Sb. Solders with the addition of Sb and Cu showed a small
improvement of the corrosive resistance. Further investigation (influence of doping) is necessary for tin
solders doped with Mg and Zn in order to improve their corrosive resistance.
Overall evaluation of alternative solders
It seems from the performed analyses of AlSnZn alloys that an optimal candidate for alternative lead-free
solder is alloy No. 1, which has the relatively low eutectic temperature TE = 197C and its structure is
eutectic. This alloy has the aluminium content less than 1 wt.%. The alloy 16N, TL = 314C, can be
recommended for high-temperature applications of soldering, which is caused by the higher content of zinc.
The image analysis determined 9.6 vol.% of Zn in the eutectics. To enable a wider use of AlSnZn alloys, it
is necessary to prevent the aluminium oxidation during the melting and soldering itself, i.e. to find suitable
covering salts or soldering fluxes.
All the selected substitutions of lead solders No. 31 to No. 50 with different ratios of individual elements Ag,
23. - 25. 5. 2012, Brno, Czech Republic, EU
Al, Bi, Cu, In, Mg, Sb, Sn and Zn have melting temperatures in the range 198 to 236 C. The best candidates
are the following solders: No. 36: Sn3.6 Ag7.3 Sb, No. 41: Sn4.5 Ag0.9 Cu, No. 44: Sn3 Ag0.5 Al and
No. 49: Sn0.6 Cu4.9 In [at.%]. They have good structure characteristics, wettability, corrosion properties
and electrical conductivity too.
6.
CONCLUSION
The study of low-fusing solder alloys was performed with the aspect of observing their selected physical,
chemical, structural and technological properties. The experimental specimens of lead-free solders were
submitted to the following examinations: metallography, chemical and structural microanalysis, microhardness, differential thermal analysis, wettability, corrosion tests and resistometry. In the further stage, the
chosen types of alternative solders will be tested in working conditions.
ACKNOWLEDGEMENTS
This work was supported by Ministry of Education, Youth and Sport of the Czech Republic in the
project COST Action MP0602 Advanced solder materials for high temperature application - HISOLD
(OC 08032) and in the research project MSM 6198910013 Processes of preparation and properties of
high purity and structural defined materials.
LITERATURE
[1]
[2]
[3]
[3]
[5]
[6]
[7]
Database for Solder Properties with Emphasis on New Lead-Free solders. National Institute of Standards and
Technology and Colorado School of Mines, Golden, February 11, 2002.
SHANGGUAN D. Lead-Free Solder Interconnect Reliability. ASM International, Materials Park, Ohio, 2006, 292 p.
DINSDALE A., WATSON A., KROUPA A., VRESTAL J., ZEMANOVA A., VIZDAL J. Atlas of Phase Diagrams for
Lead-Free Soldering. COST 531, Lead-Free Solders, Vol. 1, 2008, 298 p.
SCHMETTERER C., IPSER H., PEARCE J. Handbook of Properties of SAC Solders and Joints. ELFNET, COST
531, Lead-Free Solders, Vol. 2, 2008, 210 p.
http://www.msiport.com/
http://www1.asminternational.org/asmenterprise/APD/
FRIES S.G., LUKAS H.L., KUANG S., EFFENBERG G. Calculation of the Al-Zn-Sn Ternary System. In "User
Aspects of Phase Diagrams", Ed. F. Hayes, The Institute of Metals, London, 1991, p. 280-286.
You might also like
- 2020 ISRI Scrap Specificiations CircularDocument62 pages2020 ISRI Scrap Specificiations CircularRod AguirreNo ratings yet
- PCI Board Review HandoutsDocument8 pagesPCI Board Review HandoutsChrissy LayugNo ratings yet
- Copper, Bronze, IronDocument15 pagesCopper, Bronze, IronDerick BrinNo ratings yet
- Inert Anode For Al ProductionDocument10 pagesInert Anode For Al ProductionErin Morales100% (1)
- Reflow ManualDocument47 pagesReflow Manuallcnblzr3877100% (1)
- Chem 210 Lab Report 1Document6 pagesChem 210 Lab Report 1Mxokzah Cmoh100% (1)
- Yamaha A-S701 PDFDocument77 pagesYamaha A-S701 PDFcharan100% (1)
- Exp7 Metal CorrosionDocument21 pagesExp7 Metal CorrosionArisha Ruzalani100% (1)
- Chemistry List of ExperimentDocument3 pagesChemistry List of ExperimentKaiswan GanNo ratings yet
- Thermodynamics and Agglomeration Behavior On SpineDocument16 pagesThermodynamics and Agglomeration Behavior On SpinePranjal SinghNo ratings yet
- Leaching of Gold, Silver and Accompanying Metals From Circuit Boards (PCBS) WasteDocument4 pagesLeaching of Gold, Silver and Accompanying Metals From Circuit Boards (PCBS) WasteDgek LondonNo ratings yet
- Synthesis and Characterization of CuFe2O4 CeO2 NanocompositesDocument8 pagesSynthesis and Characterization of CuFe2O4 CeO2 NanocompositesAlin DrucNo ratings yet
- Synthesis of Iron Nanoclusters by Pulsed Current Method: &) O. Rostami-OstadkalayehDocument14 pagesSynthesis of Iron Nanoclusters by Pulsed Current Method: &) O. Rostami-OstadkalayehsecateNo ratings yet
- 1 s2.0 S0925838806011017 Main PDFDocument5 pages1 s2.0 S0925838806011017 Main PDFeid elsayedNo ratings yet
- Optical Emission Spectrometer CalibrationDocument9 pagesOptical Emission Spectrometer CalibrationAli Mohsin100% (1)
- Segregation Effects Iii Welded Stairless Steels: Pinstech/Npd-121Document26 pagesSegregation Effects Iii Welded Stairless Steels: Pinstech/Npd-121Lenin CórdovaNo ratings yet
- Voltammetric Sensing of Mercury and Copper Cations at Poly (EDTA-like) Film Modified ElectrodeDocument10 pagesVoltammetric Sensing of Mercury and Copper Cations at Poly (EDTA-like) Film Modified ElectrodechimiutaNo ratings yet
- Polyhedron: Masoud Salavati-Niasari, Fatemeh Davar, Noshin MirDocument5 pagesPolyhedron: Masoud Salavati-Niasari, Fatemeh Davar, Noshin MirNILTHON FRANCO POMA HUARINGANo ratings yet
- Schiavi Electrochemical-Synthesis 2019Document8 pagesSchiavi Electrochemical-Synthesis 2019kkamolvisitNo ratings yet
- Ajp Jphyscol198748c666 PDFDocument7 pagesAjp Jphyscol198748c666 PDFtomy yunendroNo ratings yet
- Monika Hrubovčáková, Miriam Kupková, Andrea Fedorková, Renáta Oriňáková and Adam ZeleňákDocument5 pagesMonika Hrubovčáková, Miriam Kupková, Andrea Fedorková, Renáta Oriňáková and Adam ZeleňákManas Ranjan SahuNo ratings yet
- 12 PDFDocument5 pages12 PDFAngel JonNo ratings yet
- Begon, Garcìa - 2002 - Metal-Nanoparticles Based ElectroanalysisDocument11 pagesBegon, Garcìa - 2002 - Metal-Nanoparticles Based ElectroanalysisJimmy SimpsonNo ratings yet
- Corosion PDFDocument12 pagesCorosion PDFeid elsayedNo ratings yet
- Electrochemical Method For The Synthesis of SilverDocument9 pagesElectrochemical Method For The Synthesis of SilverilkerNo ratings yet
- Thermo-Chemistry of Non-Metallic Inclusions in Ductile Iron: ArticleDocument15 pagesThermo-Chemistry of Non-Metallic Inclusions in Ductile Iron: ArticleMonish KumarNo ratings yet
- Corrosion Behavior of Copper at Elevated Temperature: Int. J. Electrochem. Sci., 7 (2012) 7902 - 7914Document13 pagesCorrosion Behavior of Copper at Elevated Temperature: Int. J. Electrochem. Sci., 7 (2012) 7902 - 7914idanfriNo ratings yet
- Smith 2013Document7 pagesSmith 2013jhenyNo ratings yet
- Calculation of Sulfur Removal in Ladle Furnace Unit by Means of Ionic Theory of SlagsDocument7 pagesCalculation of Sulfur Removal in Ladle Furnace Unit by Means of Ionic Theory of Slagsheinz wieduwiltNo ratings yet
- Electrochemical Impedance Models For Molten Salt Corrosion: C.L. Zeng, W. Wang, W.T. WuDocument15 pagesElectrochemical Impedance Models For Molten Salt Corrosion: C.L. Zeng, W. Wang, W.T. WuVictor SabNo ratings yet
- Corrosion and Corrosion Inhibition of Pure Iron inDocument17 pagesCorrosion and Corrosion Inhibition of Pure Iron inKatNo ratings yet
- Air Cells Using Negative Metal Electrodes Fabricated by Sintering Pastes With Base Metal Nanoparticles For Efficient Utilization of Solar EnergyDocument7 pagesAir Cells Using Negative Metal Electrodes Fabricated by Sintering Pastes With Base Metal Nanoparticles For Efficient Utilization of Solar EnergySEP-PublisherNo ratings yet
- Electrochemical Method For The Synthesis of SilverDocument9 pagesElectrochemical Method For The Synthesis of SilverCaroNo ratings yet
- 258 PDFDocument47 pages258 PDFMichelle ArredondoNo ratings yet
- 1 s2.0 S0013468605008200 Main PDFDocument5 pages1 s2.0 S0013468605008200 Main PDFMarioNo ratings yet
- Nanosized Magnesium Doped Copper Chromites Spinel Particles Synthesis and CharacterizationDocument7 pagesNanosized Magnesium Doped Copper Chromites Spinel Particles Synthesis and CharacterizationSikander AzamNo ratings yet
- Corrosion of Aged and Annealed 18 Ni 250 Grade Maraging Steel in Phosphoric Acid MediumDocument16 pagesCorrosion of Aged and Annealed 18 Ni 250 Grade Maraging Steel in Phosphoric Acid MediumSharat ChandraNo ratings yet
- 615 FTPDocument3 pages615 FTPHendrawan LDNo ratings yet
- Effect of Niobium Addition On Microstructure and Mechanical Properties of Fe-7Al-0.35C Low Density SteelDocument13 pagesEffect of Niobium Addition On Microstructure and Mechanical Properties of Fe-7Al-0.35C Low Density SteelMarina PiermannNo ratings yet
- Gold Nanoparticles as Fuel Cell CatalystsDocument1 pageGold Nanoparticles as Fuel Cell CatalystsPavesh GangenNo ratings yet
- Pateli Et Al. 2020 Electrochemical Oxidation in DES PreprintDocument26 pagesPateli Et Al. 2020 Electrochemical Oxidation in DES PreprintEkRA GoRaYANo ratings yet
- The Study of Corrosion and Wear Resistance of CoppDocument9 pagesThe Study of Corrosion and Wear Resistance of CoppNoura Nour ElshamsNo ratings yet
- When Gold Is Not NobleDocument13 pagesWhen Gold Is Not NobleSumit KumarNo ratings yet
- AE-03 Analisis InstrumentalDocument7 pagesAE-03 Analisis InstrumentalJaime ReyesNo ratings yet
- 1 s2.0 S0022072899001540 MainDocument8 pages1 s2.0 S0022072899001540 MainwardaninurindahNo ratings yet
- Decopperization PDFDocument8 pagesDecopperization PDFrafly naufalNo ratings yet
- Vislavath Giri B201274MTDocument7 pagesVislavath Giri B201274MTSPARSH KUMAR AGARWALNo ratings yet
- Assessing IGC in 316(N) SS Using Electrochemical NoiseDocument7 pagesAssessing IGC in 316(N) SS Using Electrochemical Noiseandrew_yeap_2No ratings yet
- Abdur UmerSaeed 2Document9 pagesAbdur UmerSaeed 2Sari Ramadhani MeutuahNo ratings yet
- Magnetic Nanowires Via Template Electrodeposition: OriginalpaperDocument5 pagesMagnetic Nanowires Via Template Electrodeposition: OriginalpapermanikianNo ratings yet
- Analysis of Pure Copper - A Comparison of Analytical MethodsDocument12 pagesAnalysis of Pure Copper - A Comparison of Analytical Methodsban bekasNo ratings yet
- Inorganic Metal Oxides and Reduction of Environmental Pollution Using NanoparticlesDocument29 pagesInorganic Metal Oxides and Reduction of Environmental Pollution Using NanoparticlesMuhammad Shehzad HaiderNo ratings yet
- Corrosion Resistance Test of Electroplated MetalsDocument9 pagesCorrosion Resistance Test of Electroplated Metalsgolam kibriaNo ratings yet
- Trace Nickel Analysis Using Bismuth Film ElectrodesDocument6 pagesTrace Nickel Analysis Using Bismuth Film ElectrodesGabriel Ignacio SilvaNo ratings yet
- Influence of Mo, Nb, and Zr on microstructure of ternary uranium alloysDocument11 pagesInfluence of Mo, Nb, and Zr on microstructure of ternary uranium alloysNathanael Wagner MetalNo ratings yet
- Nanodispersed Fe Oxide Supported Catalysts With Tuned PropertiesDocument8 pagesNanodispersed Fe Oxide Supported Catalysts With Tuned PropertiesIsaac Andrés Díaz AburtoNo ratings yet
- Electrocatalytic Properties of Carbon Nanotube NanocompositesDocument10 pagesElectrocatalytic Properties of Carbon Nanotube NanocompositesKAMAL MANNANo ratings yet
- Selection of Stainless Steel For Cathode Plate in Hydrometallurgical ProcessDocument6 pagesSelection of Stainless Steel For Cathode Plate in Hydrometallurgical ProcessIbnu AndriNo ratings yet
- Effect of As SBDocument16 pagesEffect of As SBGabriel Ignacio SilvaNo ratings yet
- Materials Form Final Product ClassificationDocument30 pagesMaterials Form Final Product ClassificationBalakumarNo ratings yet
- Sing Hal 2005Document7 pagesSing Hal 2005Dita WulansariNo ratings yet
- Sintesis Serbuk Tembaga Dengan Metode Elektrolisis: Studi Perilaku Elektrokimia Dan Karakaterisasi SerbukDocument10 pagesSintesis Serbuk Tembaga Dengan Metode Elektrolisis: Studi Perilaku Elektrokimia Dan Karakaterisasi SerbukOnggy Aries SekaNo ratings yet
- ARC-Slag Remelting of Steel and AlloysDocument323 pagesARC-Slag Remelting of Steel and AlloysAlexiz Alvarado MendozaNo ratings yet
- Adsorption and Corrosion Inhibition of New Synthesized Pyridazinium-Based Ionic Liquid On Carbon Steel in 0.5 M H SODocument9 pagesAdsorption and Corrosion Inhibition of New Synthesized Pyridazinium-Based Ionic Liquid On Carbon Steel in 0.5 M H SOHyd BenNo ratings yet
- Analysis of The Electrodeposition Process of Rhenium and Rhenium Oxides in Alkaline Aqueous Electrolyte 2013 A. VargasDocument8 pagesAnalysis of The Electrodeposition Process of Rhenium and Rhenium Oxides in Alkaline Aqueous Electrolyte 2013 A. VargasCristianNo ratings yet
- 2590 PDFDocument2 pages2590 PDFeid elsayedNo ratings yet
- PDFDocument4 pagesPDFEidelsayedNo ratings yet
- Reduced Graphene Oxide: Characterization Sheet: Nanoinnova Technologies SL C/Faraday 7, 28049 MadridDocument3 pagesReduced Graphene Oxide: Characterization Sheet: Nanoinnova Technologies SL C/Faraday 7, 28049 Madrideid elsayedNo ratings yet
- GetPDFServlet PDFDocument3 pagesGetPDFServlet PDFEidelsayedNo ratings yet
- 2008 04e 05 PDFDocument1 page2008 04e 05 PDFeid elsayedNo ratings yet
- Fulltex2t PDFDocument4 pagesFulltex2t PDFEidelsayedNo ratings yet
- p4250 1 PDFDocument4 pagesp4250 1 PDFEidelsayedNo ratings yet
- 2010 02e 04 PDFDocument1 page2010 02e 04 PDFEidelsayedNo ratings yet
- Fulltex1t PDFDocument30 pagesFulltex1t PDFEidelsayedNo ratings yet
- 2008 04 17 Gruzberg I PDFDocument75 pages2008 04 17 Gruzberg I PDFeid elsayedNo ratings yet
- Brillouin Scattering Study of Zno: T. Azuhata M. Takesada and T. Yagi A. Shikanai Sf. ChichibuDocument5 pagesBrillouin Scattering Study of Zno: T. Azuhata M. Takesada and T. Yagi A. Shikanai Sf. ChichibuEidelsayedNo ratings yet
- Zno As A Material Mostly Adapted For The Realization of Room-Temperature Polariton LasersDocument4 pagesZno As A Material Mostly Adapted For The Realization of Room-Temperature Polariton LasersSoufiane BookiNo ratings yet
- Heterogeneous Photoreduction of Nitrogen To Ammonia On Tungsten OxideDocument5 pagesHeterogeneous Photoreduction of Nitrogen To Ammonia On Tungsten Oxideeid elsayedNo ratings yet
- Sdarticle PDFDocument4 pagesSdarticle PDFEidelsayedNo ratings yet
- Strong Biexcitonic Effects and Exciton-Exciton Correlations in ZnoDocument4 pagesStrong Biexcitonic Effects and Exciton-Exciton Correlations in ZnoEidelsayedNo ratings yet
- E153310 PDFDocument4 pagesE153310 PDFEidelsayedNo ratings yet
- JApplPhys 95 5498 PDFDocument4 pagesJApplPhys 95 5498 PDFEidelsayedNo ratings yet
- Jjap 41 L935 PDFDocument3 pagesJjap 41 L935 PDFEidelsayedNo ratings yet
- Heteroepitaxy of Hexagonal Zns Thin Films Directly On SiDocument4 pagesHeteroepitaxy of Hexagonal Zns Thin Films Directly On SiEidelsayedNo ratings yet
- Prof Chichibu PDFDocument4 pagesProf Chichibu PDFEidelsayedNo ratings yet
- JApplPhys 93 756 PDFDocument3 pagesJApplPhys 93 756 PDFEidelsayedNo ratings yet
- Phonon Scattering of Excitons and Biexcitons in Zno: K. Hazu and T. SotaDocument3 pagesPhonon Scattering of Excitons and Biexcitons in Zno: K. Hazu and T. SotaEidelsayedNo ratings yet
- Blue Light Emitting Diode Based on ZnO P-I-N HeterojunctionDocument3 pagesBlue Light Emitting Diode Based on ZnO P-I-N HeterojunctionHoracio SolacheNo ratings yet
- JApplPhys 93 2481 PDFDocument5 pagesJApplPhys 93 2481 PDFEidelsayedNo ratings yet
- Programm 28.8Document33 pagesProgramm 28.8EidelsayedNo ratings yet
- Jjap 44 3218 PDFDocument4 pagesJjap 44 3218 PDFEidelsayedNo ratings yet
- JApplPhys 95 7856 PDFDocument6 pagesJApplPhys 95 7856 PDFEidelsayedNo ratings yet
- Epitaxial Growth of Zno Films by Helicon-Wave-Plasma-Assisted SputteringDocument4 pagesEpitaxial Growth of Zno Films by Helicon-Wave-Plasma-Assisted SputteringEidelsayedNo ratings yet
- p157 - 1.pdf Fundmental Band PDFDocument3 pagesp157 - 1.pdf Fundmental Band PDFEidelsayedNo ratings yet
- Metals: Physical Properties of MetalDocument6 pagesMetals: Physical Properties of MetalAllen Jierqs SanchezNo ratings yet
- Sno2 ThesisDocument6 pagesSno2 ThesisLisa Muthukumar100% (1)
- Chemical Compositions of AlloysDocument40 pagesChemical Compositions of AlloysSampath Kumar50% (2)
- Asme BPV Scii Sgnfa: For Information 1/14Document14 pagesAsme BPV Scii Sgnfa: For Information 1/14최승원No ratings yet
- Electrodeposition Tin SN Cu PDFDocument9 pagesElectrodeposition Tin SN Cu PDFElenaNo ratings yet
- List of Cations and AnionsDocument2 pagesList of Cations and AnionsArvin MagtotoNo ratings yet
- Chapter 5 - Thermodynamics6Document8 pagesChapter 5 - Thermodynamics6pythagorus_banNo ratings yet
- #94 Talbot Bass 1690 PDFDocument40 pages#94 Talbot Bass 1690 PDFrodolfoNo ratings yet
- Duralumin Alloy Composition and PropertiesDocument49 pagesDuralumin Alloy Composition and PropertiesasjfgauojfgfNo ratings yet
- Die Attach Materials For High Temperature Applications - A ReviewDocument22 pagesDie Attach Materials For High Temperature Applications - A ReviewHanLe DuyNo ratings yet
- Know The Name of The Elements and Compounds KimiaDocument73 pagesKnow The Name of The Elements and Compounds KimiaNova SinagaNo ratings yet
- All Alloys PDFDocument72 pagesAll Alloys PDFFernando Pérez EsquivesNo ratings yet
- Yamaha RXV 461 DAB Service ManualDocument94 pagesYamaha RXV 461 DAB Service ManualBozsóki IstvánNo ratings yet
- PENGARUH VARIABEL SHAKING TABLEDocument6 pagesPENGARUH VARIABEL SHAKING TABLEFachrul RidwanNo ratings yet
- Year 8 Chapter 11 Worksheet 5Document3 pagesYear 8 Chapter 11 Worksheet 5Nicholas LeongNo ratings yet
- Exploded View & Part ListDocument8 pagesExploded View & Part ListThelma De Almeida PortilhoNo ratings yet
- KXMB 2230 JTDocument526 pagesKXMB 2230 JTkacperorNo ratings yet
- Tuto Chapter 2 Atoms. Molecules and StoichiometryDocument17 pagesTuto Chapter 2 Atoms. Molecules and StoichiometryNUR ALYSSA MYRA BINTI NULWHOFFAL ARSELANNo ratings yet
- JDocument4 pagesJJoylyn Hope BerinaNo ratings yet
- Hazmat S 23 16698Document40 pagesHazmat S 23 16698Ngô Ích SơnNo ratings yet
- Topic 09.3 Group IVDocument3 pagesTopic 09.3 Group IVzafarchem_iqbalNo ratings yet
- Yamaha RX-E810, RX-E410, NX-E800 Micro ComboDocument57 pagesYamaha RX-E810, RX-E410, NX-E800 Micro ComboPravin Mevada100% (1)
- Synthesis of Au-Sn Alloy Nanoparticles For Lead-Free Electronics With Unique PDFDocument5 pagesSynthesis of Au-Sn Alloy Nanoparticles For Lead-Free Electronics With Unique PDFeid elsayedNo ratings yet
- Yamaha RX v571 HTR 5064Document130 pagesYamaha RX v571 HTR 5064JorgeNo ratings yet