Professional Documents
Culture Documents
Catalysis ChE 481 581 Spring 2015 Complete
Uploaded by
joebug34Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Catalysis ChE 481 581 Spring 2015 Complete
Uploaded by
joebug34Copyright:
Available Formats
1
Gene and Linda Voiland School of Chemical Engineering and Bioengineering
Catalysis From
Fundamentals to
Applications
Professor Norbert Kruse
Prerequisites: Undergraduate courses of chemical reaction kinetics and
engineering
CHE 481/581: Catalysis (Spring 2015)
Prof. Norbert Kruse
Office: Wegner 155A
Email: Norbert.Kruse@wsu.edu
Office Phone: (509) 335 6601
Office Hours: Fridays 11:00 am 1 pm, with an appointment
Tanya Stewart, Secretary Senior
Office: Wegner 155C
Email: Tanya.Stewart@wsu.edu
Office Phone: (509) 335 1256
Course:
a) Aim: get acquainted with the fundamentals of (heterogeneous) catalysis. Think in terms of
kinetics and mechanisms and use the surface science approach for doing so. Get an overview
on the major large-scale applications of catalysis.
b) Textbooks: there is no unique textbook treating all the topics covered by the course. A
copy of the slides will be provided.
for Surface Chemistry: Gary Attard and Colin Barnes, Surfaces, Oxford Chemistry Primers
for Spectroscopy: J. W. Niemantsverdriet, Spectroscopy in Catalysis an Introduction ,
Wiley-VC
for Theory in Surface Chemistry: Roald Hoffman, Solids and Surfaces: A Chemists View of
Bonding in Extended Structures , VCH
c) Schedule: Terrell Lib. 24. Tu and Th 12pm to 1:15pm until May 1st. No classes on January
23rd, March 25th and 27th.
d) Methodology: Avoid a monologue, but rather try to engage a discussion when appropriate.
Slides provide a scaffold. They are to be completed by the students according to the
individual needs. Instructor jumps back to the basics when necessary. More detailed
considerations will be either developed at the blackboard or defined as homework. Try to
avoid lengthy mathematical derivation of formulas homework
Grading:
Oral (30 to 40 min each student): 70%. Project presentation: 30%
Projects will be assigned according to a list of subjects provided until end of March. A
subject may range from a hot topic in catalysis to the presentation of a large-scale catalytic
process not subject to the course. Every student has to present his/her project in PPT format
during 12 min., followed by questions during 6 min. Presentations take place on 22nd, 24th,
30th April and 1st May. Room TBD.
Homework will be defined occasionally and is intended to digest and deepen certain
aspects of a subject. No written homework is required, however, there is expectation of
background knowledge to support the new knowledge provided. The oral exam may include
questions on such homework issues.
Oral exams will take in my office at Wegner 155. Oral exam and PPT presentation will be
weighted as defined as above.
Grading Scale:
90-100%
77-79%
B-
87-89%
A-
73-76%
C+
83-86%
B+
70-72%
80-82%
0-69%
Course Website
The contents of this course will be available online on Angel: http://lms.wsu.edu or via email.
Students with Disabilities
Reasonable accommodations are available for students with a documented disability. If you
have a disability and need accommodations to fully participate in this class, please either visit
or call the Access Center (Washington Bldg 217; 509-335-3417) to schedule an appointment
with an Access Advisor. All accommodations MUST be approved through the Access
Center.
Academic Integrity
I encourage you to work with classmates on assignments. However, each student must turn in
original work. No copying will be accepted. Students who violate WSUs Standards of
Conduct for Students will receive an F as a final grade in this course, will not have the option
to withdraw from the course and will be reported to the Office of Student Standards and
Accountability. Cheating is defined in the Standards for Student Conduct WAC 50-26010(3). It is strongly suggested that you read and understand these definitions:
http://apps.leg.wa.gov/wac/default.aspx?cite=504-26-010
Safety
Washington State University is committed to maintaining a safe environment for its faculty,
staff, and students. Safety is the responsibility of every member of the campus community
and individuals should know the appropriate actions to take when an emergency arises. In
support of our commitment to the safety of the campus community the University has
developed a Campus Safety Plan, http://safetyplan.wsu.edu/. It is highly recommended that
you visit this web site as well as the University Emergency Management web site at
http://oem.wsu.edu/ to become familiar with information provided.
Caveat
The schedule and procedures outlined in this syllabus are subject to change in the event of
circumstances beyond the instructors control or in response to ongoing assessment of
learning.
Catalysis quo vadis? (Spring 2015)
Agatha Christie (1891 1976) wrote in 1930 (The Mysterious Mr. Quinn): Do
you happen to know anything about catalysis? The young man stared at him.
Never heard of it. What is it? Mr. Satterthwaite quoted gravely: A chemical
reaction depending for its success on the presence of a certain substance which
itself remains unchanged.
To appreciate Agatha Christies approach to catalysis we start this course by
reviewing the fundamentals of heterogeneous catalysis from the kinetic point of
view: adsorption, diffusion, reaction and desorption on model catalysts:
We continue by applying these concepts to real-world catalysis. Some major
industrial processes (homogeneous as well as heterogeneous) will be visited
before new lines of catalysis research to produce sustainable chemical feedstock
will be presented.
Catalysis Kinetic Phenomenon
Some early definitions:
A catalyst increases the rate of a reaction without being consumed by a reaction
Wilhelm Ostwald (1853-1932)
A chemical reaction has to be thermodynamically feasible in order to be
accelerated Alfred Mittasch (1869-1953) *
A catalyst doesnt appear in the stoichometric equation.
However: Catalysts undergo structural or chemical changes during the catalytic
reaction (mainly in heterogeneous reactions).
The catalyst is formed by the catalytic reaction.
Agatha Christie (1891-1976)
(The Mysterious Mr. Quinn, 1930): Do you happen to know anything about
catalysis? The young man stared at him. Never heard of it. What is it? Mr.
Satterthwaite quoted gravely, A chemical reaction depending for its success on
the presence of a certain substance which itself remains unchanged.
(Curtain, Poirots last case, 1940): So we get the curious result that we have here
a case of catalysis a reaction between two substances that takes place only in
the presence of a third substance, apparently taking no part in the reaction and
remaining unchanged. That is the position. It means that where X was present,
crimes took place but X did not actively take part in these crimes.
_____________________________________________________________________________________________
non-catalytic
catalytic
Some general comments on the importance of catalysis:
About 80% of the industrial production is based on the application of the catalytic
process at least in one intermediate stage.
High economic importance
About 90% of all catalytic processes are heterogeneous in nature.
Incidental remark:
homogeneous -heterogeneous catalysis
Reactants and catalyst in the same phase
not in the same phase
However: there is no general theory of catalysis Black Magic
Two examples for the importance of empirical research:
Ammonia synthesis:
N2 + 3H2 2NH3
About 20,000 different catalysts were tested
Fischer-Tropsch Reaction:
CO +H2
2O
About 15,000 different catalysts were tested
Eq. not equilibrated!
In both cases, metals are used to catalyze the reaction (mainly nano-sized
particles on an oxidic support.)
A historical example
Formation of water:
2H2 + O2 2H2O
Dbereiner (1822)
First construction of a lighter:
The lever e allows contacting a piece of zinc with
sulfuric acid in a glass vessel: Zn + 2H+ Zn2+ + H2
H2 is produced and mounts in the jack so as to mix up with O2. Sponge-like Pt
is placed inside the nozzle f and allows ignition of the reaction.
More basic kinetics:
AB
Reaction:
(ex: isomerization)
Thermodynamically feasible,
GR < O
Arrhenius:
Decrease of the reaction barrier (activation energy) by adding a catalyst
Potential Energy
a) Heterogeneous Catalysis:
AB*
EA
A
A ad
B
B ad
Reaction Advancement
The desorption process causes a separation from the catalyst.
Catalytic Cycle:
1) Adsorption
2) Reaction
3) Desorption
10
For a catalyst with a pore structure (texture) diffusion processes into the pores
and out of them have to be added.
b) Homogeneous Catalysis:
Potential Energy
Catalytic Cycle :
Reaction Advancement
A physical separation (unit operations like distillation, extraction, etc.) is
necessary in order to obtain the pure product.
Two examples involving multiple intermediate steps:
Ammonia Synthesis (heterogeneous catalysis):
All intermediate steps were clarified in a Nobel Prize winning work by G. Ertl
(Nobel Prize 2007)
11
Dissociation little activated
NH
+H ad
NH2,ad
2,ad + Had
Molecular adsorption
Dissociative adsorption
(Nad) + 3Had)
(N + 3H
ad
ad
Activation barriers for the ammonia synthesis were obtained from
experimental work with Fe model catalysts (monocrystals of (111)
orientation at low coverages: numerical values are in kJ/mol.
Hydroformylation or Oxo-process (homogeneous catalysis)
Note: The dissociation of molecularly bound nitrogen is only slightly
activated!
12
Hydroformylation (Homogeneous catalysis)
Catalysts are coordination compounds of Rh and Co
Note that the catalytic cycle above includes changes in the number of metal
valence electrons between 18 and 16, back and forth.
13
Kinetic compensation reality or fiction?
Isokinetic temperature
We can always find a temperature for which k0 has the same
numerical value, no matter which catalyst for a given
reaction is considered.
14
Example: methanation
CO + 3 H2
CH4 + H2O
ln k0
Ru
20
10
Fe
.
Pd.
. . .Co
. Ni
Rh
Pt
..Ir
60
80
100
120
EA kJ/mol
Compensation effect for the methanation of CO for a number of metals, both base
and noble.
15
Basic Kinetic Notions in Catalysis
Activity Selectivity
reaction rate in a homogeneous phase (gas or liquid):
for constant volume
for A B
since
reaction rate in heterogeneous catalysis:
intrinsic activity
S = surface area of the catalyst
we need to determine the surface area of the catalyst (method according to
Brunauer, Emmett, and Teller, BET).
Frequently also:
since m ~ S as long as the surface doesnt change
16
Best solution:
N number of active sites
since N ~ S
However: the nature of the active site may get redistributed during the reaction,
so they are subject to dynamic changes and should not be considered as fixed in
number. For example, the number of atoms defining an active site remains
unknown in most cases. Moreover, there may be similar active sites coexisting.
frequently in heterogeneous catalysis
Selectivity:
P
A
Reactions in parallel
APQ
Consecutive reactions
Define the conversion for the wanted product.
Example:
O
OH
OH
Butyraldehyde yield:
17
Conversion of crotonicaldehyde:
Butyraldehyde selectivity:
Note the distinction between:
TON is frequently used in homogeneous catalysis:
Processes start to become economic when TON > 20,000
18
Some statistical thermodynamics
Comparing reaction rates of heterogeneous catalysis with those of non-catalytic
reactions according to the Transition State Theory (TST)
Consider a bimolecular reaction (non-catalytic)
AB is the activated complex in homogeneous phase.
The same bimolecular reaction under catalytic conditions reads:
AB*2 is the activated complex bound to the surface of the catalyst.
The reaction rate according to TST then is:
19
in general:
k: Boltzmann constant
h: Planck constant
: Partition function of component i
Eo : Difference in energy of initial energy T=0 K
20
Hypothesis:
a)
b)
c)
d)
21
Heterogeneous Catalysis occurs at the surface of a solid
10nm
Transmission Electron Microscopy (TME) of metal
particles on a support (Ag/Al2O3).
Metal particles are well separated from each other
and the pore structure of the catalyst becomes visible
(texture).
Image blurring indicates carbon deposition
High Resolution Transmission
Electron Microscopy (HRTEM)
revealing the morphology of Rh
nanosized particles on a TiO2
support.
22
Ball model of a single metal grain to visualize the different sites exposed.
In white are: low coordination atoms have empty valence orbitals which are
supposed to be preferred binding sides for adsorbing atoms and molecules
(gasses or liquid molecules).
Field Ion Microscopy (FIM)
of a single Rh particle, in top
view, atom by atom. Not all atoms are
seen; only those in low coordination.
Miller indexes of individual surface
facets are also given.
23
Metal particles on a support should be nanosized so as to create as large a
surface area as possible.
r~N
Number of atoms located along the particle radius.
Ns = 2 N2
Number of atoms at the surface
Nt = 2/3 N3
Total number of atoms in the particle
Surface fraction is a function of
radius for a hemispherical particle
on a flat support.
24
Some comments on the nature of catalysts and their supports
Supports should provide high surface area so as to optimize the dispersion of the
catalytically active phase. For metal-based catalysts, frequently the following
supports are used.
Al2O3, SiO2, TiO2, ZnO, MgO, C
Some of these materials can be prepared with specific surface areas as large as
1,000 m2/g (equivalent to the size of a football field).
The catalytically active metal phase is frequently supplied by impregnation using
an aqueous solution of a suitable precursor compound such as Me-nitrate.
Impregnation can be performed wet using immersion techniques or by incipient
wetness. In the latter case, the volume of the aqueous precursor solution matches
the volume of the support pores so the solid doesnt appear wet. The preparation
in water enables solvated metal cations to bind to surface hydroxyl of the support.
The details of the binding mechanism depend on the pH conditions and the point
of zero charge (PZC) of the support.
25
pH > PZC cationic adsorption
pH < PZC anionic adsorption
A different type of catalyst are zeolites whose primary construction units are
tetrahedric [SiO4]4- and [AlO4]5- . These materials are highly crystalline with
specific surface areas sometimes exceeding 1,000 m2/g. They have acidic
properties and may or may not contain metallic cations.
[SiO4]4- and [AlO4]5- units share oxygen atoms so as to form Si-O-Al bridges.
Starting from a 3D SiO2 network the replacement of Si by Al creates localized
26
charges that have to be compensated in order to maintain electroneutrality (per
Al- either one H+ or one Me+ is needed).
General formula:
x [ (Me+, Me2+) AlO2] . y SiO2 . z H2O
The occurrence of protons causes Brnsted acidity which allows hydrocarbon
cracking in petrochemical industry.
Lewis Center
Heating
Heat treatment allows Brnsted acidity to turn into Lewis acidity.
Similar to the construction of secondary building blocks in silica highly
symmetric polyhedral units can be formed to build zeolites.
27
The number of basic zeolites can be constructed from sodalite units in which
for reasons of simplicity, the bent Si-O-Al bridges are considered as straight
lines. Corners contain either Si or Al.
Sodalite unit
Sodalite unit
Sodalite unit
Large cavity
a) Sodalite
b) Zeolite A
c) Faujasite (zeolites X and Y)
Windows vary between 2.6 in sodalite and 7.4 in faujasite.
28
More recent developments aim at synthesizing ordered silicas so as to create
mesopores molecular sieves.
Template-directed
Template
removal
Condensation
Inorganic Template
precursor
Template-oxide
Mesoporous
1992 - Scientists of Mobil Oil Corporation (USA) applied self-assembled
template (micelles of CTAB surfactant) and synthesized a family of ordered
mesoporous silicas named MCM (Mobil Composition of Matters) 41 and 48*
+
N(CH3)3 Br-
The CTAB molecule (cetyltrimethylammoniumbromide) consists of a long
(cetyl) hydrocarbon skeleton causing hydrophobic properties (tail) and a terminal
ionic group causing hydrophilic properties (head).
CTAB micelle
Silicate self-assembling around the micelle
*Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S., Nature, 1992, 359, 710
712
29
MCM-41 is characterized by a unique pore size distribution. Pores usually have
diameters between 2-5 nm with hexagonal structure. The total specific surface
area may range from 900-1,500 m2/g.
TEM image and model of MCM-41
V. Meynen et al. / Microporous and Mesoporous Materials 125 (2009) 170223
g = V/al
g packing parameter
V volume of the hydrophobic part of
surfactant
(including solubilized compounds)
a effective area of the hydrophilic head
(depends also on counter-ions)
Micelles can be shaped by use of proper surfactants.
30
Micelle shape and type versus g
More recent developments have lead to new silicates like SBA-15 or KIT-6
KIT-6 pore size vs HTT temperature
Model of KIT-6 double
gyroid Mesostructure
Y. Doi et al, Chem. Commun., 2010, 46, 63656367
31
Physisorption and Chemisorption
Forces
Physisorption
Chemisorption
dispersion
valence or electrostatic forces
create covalent and/or ionic
bonds
van der Waals
a) E Keesom
b) E Debye
c) E London
Hphys Hcond ~5-20 kJ/mol
Hchem 5 4
kJ/mol
32
Coverage
multilayers
monolayer limit
Reactants
all gasses below
the critical temperature
reactive gasses
Reversibility
yes
yes, in many cases,
but also irreversible.
Dependence on decreases with increasing
temperature
temperature
maybe complicated in
case of an activated
process
33
Potential diagrams
Chem
Edif
The above diagram applies to the case of physisorption on top of a chemisorbed
layer. The turnover from the physisorbed state to the chemisorbed state is nonactivated for the observer but can only occur as long as empty chemisorption sites
are available. A diffusion process in the physisorbed state is possible and occurs
with activation energy of Edif.
34
H chem
*Chemisorption site
H chem
The above potential diagram applies to the case of H2 adsorption on a Cu surface.
H chem
= 34 kJ/mol
EA
= 21 kJ/mol
E (*Cu - H) = 233 kJ/mol
Generally:
H chem = 2E (M - H) - E(H - H) = 34 kJ/mol
which is low for metals with a closed d-shell.
35
The turnover from the physisorbed state into the chemisorbed state is activated
for H2/Cu. In other cases, like CO on open d-shell metals the dissociation is nonactivated and leads to the deposition of carbon and oxygen. H chem can be
considerably larger in this case (between 100-160 kJ/mol). On the other hand, CO
chemisorption on Cu occurs without dissociation and is weak.
36
Dynamics of adsorption
Calculation of the surface residence time of molecules before thermoadsorption
For kinetic for order process:
surface residence time (lifetime)
6 . 1012 s-1 at 300K
Homework:
Calculate the surface lifetimes at 300K and 600K for the activation energies Ed =
4, 20, 40, 80, 160 kJ/mol.
Calculation of the concentration (surface coverage) of adsorbed species:
For physisorption:
phys = J . . wphys
wphys
probability of physisorption
For chemisorption:
chem = J . . s
impingement rate
[ L-2 . T-1]
s
sticking probability
37
calculation of the impingement rate:
<=
mean velocity (m s-1)
n number of molecules per m3
m molecular mass in kg per molecule
with:
and
J = 2.64 . 1024 p / (M . T) ( m-2 . s-1)
molecular mass in
atomic mass units
Homework:
Calculate the impingement rate per surface site for nitrogen molecules at 1 bar
and 273K. Assume the surface site to have a size of 10 2. Calculate the number
of multiple layers assuming Ed = 40 kJ/mol and a probability of physisorption
wphys = 1.
Calculation of the rate of chemisorption:
For a non-activated process:
Ra = J . s
This allows the sticking probability to be defined as:
S = Ra / J
> tM
38
tM = characteristic time of the measurement (molecules must be on the surface to
be measured)
S0 Sticking probability at zero coverage. It can be anticipated that values of
S0 are influenced by the surface structure and the temperature.
Example : N2/Fe
S0 7 . 10-8 for the (110) surface (densely packed)
S0 4 . 10-6 for the (111) surface (open structure)
It is interesting to see that the sticking probabilities for N2 chemisorption on Fe
vary in the same manner as the reaction rate of the ammonia synthesis
N2 + 3H2 2NH3
For comparison, the sticking probability S0 of the CO molecule on transition
metal surfaces varies between 1 and 0.1.
The Langmuir Isotherm:
(NA) . Ed = Hchem
with
(Hchem / RT)
39
at constant temperature:
n .p
Model assumptions made by Langmuir
1. all surface sites are treated in the same manner: no difference is
made between terrace sites, steps or kinks
2. adsorbed species do not interact laterally
3. incoming molecules hitting an occupied site are being reflected
without energy loss
The last argument leads to the following functional dependence of s vs.
coverage :
Assuming every incoming molecule hitting an empty site will get adsorbed,
s0 = 1, we will then receive:
with
For application purposes:
V / Vs
Vs
gas volume giving rise to
monolayer formation
40
linearization leads to:
The experiment consists of introducing a known volume of gas from a calibrated
reservoir into a reactor containing the catalyst sample and measuring the volume
consumed due to adsorption.
tg = 1/ Vs . kL
1/V
1/Vs
1/p
An equivalent derivation of the Langmuir equation would be to consider a
dynamic equilibrium between adsorption and thermal desorption.
) [ ] = kd .
[]
[ ] concentration of sites per
unit surfaca area
41
= ka /kd adsorption coefficient (equilibrium constant of adsorptiondesorption), will be denoted later as KA
1
T3
T2
T1
T3 < T2 < T1
42
Evaluation of the isosteric heat of adsorption:
T1
V / Vs
T2
T3
p1 p2
dln p / dT
=const
ln p = f (T)
p3
= - Hchem / RT2
analog:Clausius Clapeyron
Hchem
isosters
qst Hchem
isosteric heat of adsorption
(only equilibrium states are
considered)
43
Homework: How does the relative sticking probability s/s0 depend on the
coverage in case physisorption on top of a chemisorbed layer is taken into
account?
Langmuir Model for the co-adsorption of two species (competitive
adsorption for the same surface sites):
Adsorption and desorption rates for A species:
Ra = ka . pA (1 Rd = kd .
). [ ]
Dynamic equilibrium:
Ra = Rd = >
Analog for B:
Homework: Calculate the coverage ratio A/ B assuming both species have the
same pressure pA = pB for adsorption at room temperature. Species A is
considered to adsorb by 40kJ/mol stronger than B:
HA HB = 40 kJ/mol. Recall that the adsorption enthalpy is given by the
difference of the activation energies for adsorption and desorption.
44
Chemisorption of atoms and molecules on metal surfaces: simple
theoretical concepts
Formation of electron bands in solids:
45
Adsorption of an atom on the surface of a metal
Free atom
46
Moving the free atom to the surface would cause a broadening of the originally
sharp electron levels due to a resonance effect. Filled states above the Fermi level
may lose charge towards the metal.
Donation effect
Acceptor effect
Example: Alkalines adsorption
on transition metal
Halogens adsorption
47
back donation
48
Consideration of the d-band effect:
1s
jellium
back donation
d-bands are less broad than s-bands. Therefore, the interaction with d bands gives
rise to localized bonding. For the chemisorption of a hydrogen molecule, both the
occupied bonding molecular orbital of H2 and the unoccupied molecular orbital
have to be correlated with the metal d-band. The partial occupation of the
antibonding MO by charge transfer from the metal to the H2 gives rise to
weakening of the H-H bond.
49
Appropriate orbitals:
dz, dyz, dxz
To dissociate the CO molecule the relative position of the Fermi level is
important. Charge transfer into antibonding MO of the CO molecule causes bond
weakening which is the first step to dissociation. As a consequence the CO
molecule will tilt to allow its oxygen atom to contact the metal surface. This
process will finally lead to bond breaking with oxygen and carbon atoms being
deposited into next nearest neighbor sites of the catalyst surface.
50
CO molecular orbitals
Metallic Orbitals
dxz
dyz
dz2
5-dz2
dxzdyz-2 *
provides bonding
provides bonding
51
Symmetry of metallic surface planes: Miller indices
52
Basic planes of the cubic face-centered system:
Plane (323)
53
The direction in a crystal is defined by brackets: [uvw], expressed by the smallest
set of integers of a collinear vector of the indicated direction, such that hu+kv+lw
=0
Ordered overlayer structures
a) E.A. Wood
J. Appl. Phys. 35 (1964) 1306
Elementary vectors of the surface a1, a2
Elementary vectors of the adsorbate b1, b2
(|b1| / |a1| x |b2| / |a2|) + angle
b) R.L. Park, H.H. Madden
Surface Sci. 11 (1968) 188
b1 = m11 a1 + m12 a2
b2 = m21 a1 + m22 a2
M=(
fcc (100), (110), (111) M = (
)
)
54
Some examples:
55
Area of the elementary unit cell
General classification of overlayer structures:
1. mij are integers simple structure (M integer)
Adsorbed species are in well-defined local positions.
2. relation between (a1 a2) and (b1 b2) given by rational numbers (M fractional
number)
two periodic lattices with 3b1 = 4a1 or b1 = 4/3a1 (incommensurate)
|b1|
3. incoherent structure: irrational numbers between a and b (M irrational number)
56
Experimental evidence for ordered overlayer structures
Low Energy Electron Diffraction (LEED)
What means low?
Electron wave length :
acceleration of electrons by
application of a potential difference
e: elementary charge
( 5 4 )
for U = 100 V
electron microscope: U = 10 V
57
k = (2/) s
s = unit vector in the same
direction as k
58
Electron diffraction at a one-dimensional grating of atoms
electron gun
Spherical screen
Constructive interference is obtained for wavelets propagating along the surface
of the cones. Diffraction only occurs for certain angles which define the order
of diffraction. For a surface, a second series of cones has to be constructed. For a
rectangular lattice, constructive interference is obtained where the two sets of
cones intersect. The screen therefore contains a periodic arrangement of spots.
a1
a2 Bragg condition
59
r: radius of screen curvature in the center of which the sample is placed.
Since distances between spots on the screen are proportional to the reciprocal
of distances in real space, the reciprocal lattice can be designed as follows:
ai aj* = ij (i, j = 1,2)
60
Construction of the reciprocal lattice and the respective lattice vector:
Nodes of the reciprocal lattice
a1a2* = 0
a2a1* = 0
a1a1* = 1
a2a2* = 1
a1* = 1/ a1 sin
a2* = 1/ a2 sin
a 1 a2 *
a1 * a 2
angle between vectors
61
Periodic surface structures and ordered overlayers in real and reciprocal space:
*
aa
2 2
It can be shown using matrix calculates that:
(M* is the matrix of M inversely transposed,
62
An example:
reciprocal lattice
real space lattice
63
Kinetic parameters for elementary reaction steps: desorption energy
for thermal desorption
Evaluation of the data is based on the Polanyi-Wigner equation:
n - is order of the desorption
process
64
, Ed and n can all be evaluated from data
The experiment consists in heating the sample according to a temperature
program, which in most cases, is linear.
T = T0 + t
Determination of the temperature for which the pressure in the reaction chamber
reaches a maximum (the chamber is continuously evacuated)
)}
Hypothesis:
{n
for the maximum
)}
65
Substitution :
1st order kinetics:
2nd order kinetics:
Tm
Result:
f ()
for the 1st order process and
a constant activation energy
Tm if for a 2nd order process with
constant activation energy: or
for a 1st order process with
coverage dependence of the
activation energy
{Ed = g ()}
n(
66
for a 1st order process, the desorption trace is
asymmetric around the maximum
for a 2nd order process, we receive a symmetric curve
around Tm
lineshape analysis:
determination of Ed:
1st order:
a) hypothesis
b) variation of
Differentiation >
2nd order:
(symmetrical peak)
n(
verification of the kinetic order:
(
n
n n
problem: Ed and
extrapolation
n
frequently depend on
0 for increasing temperature T
Best data treatment: simulation of the thermal desorption spectrum by fitting with
the Polanyi-Wigner equation
67
Some examples for Temperature Programmed Desorption experiments:
area
H2/ Ni (100)
Coverage (atoms/cm2)
(a) 4.6 x 1013
(b) 8.8 x 1013
(c) 1.0 x 1014
(d) 1.7 x 1014
68
(II)
(I)
multiple states appear not
always resolved separately but
overlapping, so a deconvolution
has to be made. The appearance
of different states may depend
on the coverage.
CO/ Ni (100)
Desorption spectra of carbon monoxide from clean Ni(100) following
adsorption at 137 K. Coverages were 7.0 x 1013 molecules/cm2 (I) and 2.6
x 1014 molecules /cm 2 (II)
69
Back to real catalysis: determining the specific surface area of
materials
Adsorption in multiple layers
4
3
layer 33
couche
couche
layer 22
1 0
couche
layer 11
surface
i is the fraction of the surface covered with i layers. is then the fraction of the
surface remaining empty. Similar to the Langmuir model, the surface is
considered homogeneous.
i = i0
0 = =
i i
i 1
0 = 1 -
i
i1
Under dynamic equilibrium conditions i values remain constant
i= i0
is
i0 = Ji wi
i = Ji wi
70
i = i 1 heat of physisorption remains constant in all layers following the
first one (molecules in direct contact with the surface are assumed to have a
different heat of physisorption and therefore, a different mean lifetime).
With:
Wi = i-1
Ji 1i-1 = i 0
) i-2 =
i = (
i = (
) 0
)
i = i-1 = i-2 = 1
1 0
we can now express the overall coverage of the surface as:
with:
c i x
x = J1 / 0 and c = 0 / 1
1-
= 1 c
71
The above formula makes use of the following series expansion:
= (1 + x + x2
i) -1=
xi = x(1+2x +3x2
i-1)
using:
and
with:
=
we obtain:
V/Vs
if pq (condensation on the surface)
then we can identify q p0 as the saturation vapor pressure
literature: S. Brunauer, P.H. Emmett, E. Teller, J. Amer.Chem.Soc. 60 (1938) 309
72
one of the advantages of the BET equation is that it can be linearized:
p/V(p 0-p)
range of application:
0.05 p/p0 0.35
p/p0
determination of the constant c:
Hphys is usually larger than Hcond since the molecules interact more strongly
with the surface than with themselves in the multilayer. The larger the difference,
the steeper the slope in the above figure.
73
if c >> 1 we receive:
Vs = V (1 - p/ p0)
For pressures far below the saturation pressure, that is p/p0 << 1 we receive
which is formally identical to the Langmuir equation.
The BET equation as derived above allows determining the specific surface area
of the entire catalytic material since physisorption is non-specific and does not
distinguish between metallic particle surfaces and support.
A(
6.023 1023 10-20 As (2)
To determine the specific surface area, we need information about the mean
surface associated with the probing molecule which in many cases, is nitrogen.
As = f (M/ Na) 2/3
temperature C
mean surface 2
N2
Ar
CO
H2O
-196
16.2
-183
13.8
-183
16.8
0
10.6
In case of small specific surface areas (< 1m2/g) it is recommended to use a gas
whose vapor pressure is smaller than that of nitrogen at -196C
Ar, Kr, CH4
74
Some examples of BET measurements:
Similar to Langmuir:
indicates a microporous
material
p/p0
The catalyst surface is hydrophobic
and the formation of the first layer is
not visible.
2O/graphite
HH2O/graphite
Br2/SiO
2/SiO
2
Br
2
p/p0
75
Some criticism as to the BET equation:
Surfaces are energetically uniform.
Lateral interactions between physisorbed molecules do not exist.
Condensed molecules are treated identically to the liquid phase of these
molecules.
76
Catalytic reaction kinetics
For reasons of simplicity, we shall consider a sequence of steps involving
adsorption, reaction, and desorption. Each of these steps will follow first order
kinetics. The scheme therefore reads:
k1
k -1
Aad
k2
k -2
Bad
k3
k -3
It is assumed that the concentration of active sites is much smaller than the
concentration of reactants and products. The steady state kinetics of the reaction
will then be determined by the surface reaction step (the concentration of active
sites being the bottle-neck of the overall speed).
quasi-equilibrium
rate determining for the
overall reaction
quasi-equilibrium
R = Ri R-i = R2 R-2 = R3 R-3 = (k2 A k-2 . B) [ ]
using:
77
At the beginning of the reaction, with little product formation:
(1)
pA + pB = p
78
Five different case studies:
1) weak adsorption (A + B)
R = k2 KA PA [ ]
facilitates equation (1)
1st order kinetics with regard to A
Bad
Attention: HA is negative!
2) strong adsorption (A)
KB pB << 1
R = k2 . [ ]
KA pA >> 1
zero kinetic order with regard to A and B
79
1/T
3) strong adsorption (B)
KB pB >> KA pA
positive 1st order kinetics with regard to A, negative 1st order kinetics for B
the apparent activation energy is now larger than in case 1 (the desorption energy
is positive and equal to the adsorpton enthalpy).
4) adsorption determines the reaction rate
R = k1.pA. [ ]
surface coverage is low
5) desorption determines the reaction rate
R = k3 . B . [ ]
KB . pB >> 1
80
What changes for bimolecular reactions?
D
Assuming that the surface reaction is the slowest step (C and D are weakly
adsorbed) we receive:
both HA and HB are negative!
z is the number of next-nearest neighbor sites
for strong adsorption of A we receive:
;
reaction follows 1st order kinetics in A and negative 1st order kinetics in B ; the
overall reaction order is zero.
81
Mechanistic alternative for reactions on the surface of oxidic
catalysts:
example: SO2 + O2 SO3 catalyst:
V2O5
redox mechanism:
CatO + A
Cat- + AO
reduction
\
of catalyst
/
oxidation
Cat + O2 CatO
_____________________________
A + O2
Rred
AO
= R oxyd
kr . pA (1-) . [ ] = ko . pO2 . . [ ]
a) if kr pA >> k0pO2
sum reaction
for steady state conditions
fraction of the reduced surface
is close to 1 oxidation is
limiting
zero reaction order for A
82
is very small compared to
1 zero order reaction kinetics
for oxygen
b) if
R = kr . pA
The difference with respect to the Langmuir-Hinshelwood type mechanism on
metals is that according to the above Mars-Krevelen mechanism is a fully
reversible chemical alteration of the catalyst surface takes place while for L-H
this does not occur at all.
Examples with industrial application:
a. oxidation of hydrocarbons on mixed oxides of V, Mo
b. oxidation of ortho-xylene to phtalic anhydride on V2O5/SiC
You might also like
- Advanced and Modern MaterialsDocument10 pagesAdvanced and Modern MaterialsSachin RaneNo ratings yet
- 2 FJMJ: Denisty Computation-MetalsDocument2 pages2 FJMJ: Denisty Computation-MetalsBabette FreyNo ratings yet
- 1.1 Enzymology (Bravo)Document11 pages1.1 Enzymology (Bravo)Arman Carl DulayNo ratings yet
- Cleavable LinkersDocument12 pagesCleavable LinkersSrinivasa Reddy Telukutla100% (1)
- Structure and Function of Bio-Molecules: 9 2. Proteins 13Document62 pagesStructure and Function of Bio-Molecules: 9 2. Proteins 13Alex-Mihai CiubaraNo ratings yet
- Intro 2 MD SimulationDocument20 pagesIntro 2 MD SimulationachsanuddinNo ratings yet
- Catalysis For Co2 Conversion A Key Technology For Rapid Introduction of Renewable Energy in The Value Chain of Chemical IndustriesDocument20 pagesCatalysis For Co2 Conversion A Key Technology For Rapid Introduction of Renewable Energy in The Value Chain of Chemical IndustriesVictor SabNo ratings yet
- Air, Water and Land Pollution: UV-Visible and Infrared Spectroscopic Methods in Environmental AnalysisDocument72 pagesAir, Water and Land Pollution: UV-Visible and Infrared Spectroscopic Methods in Environmental AnalysisSaleem ShaikhNo ratings yet
- Thermo II Exam II Cheat SheetDocument1 pageThermo II Exam II Cheat SheetbengtglaveNo ratings yet
- CSIM2.24 - Signal TransductionDocument6 pagesCSIM2.24 - Signal TransductionAinahMahaniNo ratings yet
- BIO130Chapter2Notes PDFDocument4 pagesBIO130Chapter2Notes PDFjrenceNo ratings yet
- Chemical Energetics Notes EditedDocument12 pagesChemical Energetics Notes EditedDaniel Png100% (1)
- Carbon Nanostructures PDFDocument99 pagesCarbon Nanostructures PDFVishal WaghNo ratings yet
- CHM 414 Photochemistry & Pericycle Reactions PDFDocument119 pagesCHM 414 Photochemistry & Pericycle Reactions PDF24kemist_108741039No ratings yet
- Molecular DynamicsDocument54 pagesMolecular DynamicscesaggNo ratings yet
- Cheat Sheet 244Document2 pagesCheat Sheet 244torance44No ratings yet
- Gas Sensing Mechanism of Metal Oxides - The Role of Ambient Atmosphere, Type of Semiconductor and Gases - A ReviewDocument19 pagesGas Sensing Mechanism of Metal Oxides - The Role of Ambient Atmosphere, Type of Semiconductor and Gases - A ReviewNassar Al-EssawiNo ratings yet
- Catalysis PDFDocument104 pagesCatalysis PDFMandla Rebirth0% (1)
- Molecular Techniques For Detection, Species DifferentiationDocument43 pagesMolecular Techniques For Detection, Species DifferentiationUziel Castillo VelazquezNo ratings yet
- Amino Acids: Proteins and EnzymesDocument32 pagesAmino Acids: Proteins and EnzymesJay PandaNo ratings yet
- PolimerDocument22 pagesPolimerDhea Kana ZhafiraNo ratings yet
- Materials Chemistry and The Futurist Eco Friendlyapplications of Nanocellulose Status and ProspectDocument30 pagesMaterials Chemistry and The Futurist Eco Friendlyapplications of Nanocellulose Status and Prospectfrogi star100% (1)
- Role of Catalysis in Sustainable Development4thDocument59 pagesRole of Catalysis in Sustainable Development4thSwamiNo ratings yet
- Chapter 1 - Catalyst FundamentalsDocument48 pagesChapter 1 - Catalyst FundamentalsPedro LimaNo ratings yet
- Applications of Spectroscopic TechniquesDocument20 pagesApplications of Spectroscopic Techniquesamanbioq1No ratings yet
- Dennis TonnnDocument24 pagesDennis Tonnnhectorcflores10% (1)
- 01-Catalyst FundamentalsDocument12 pages01-Catalyst FundamentalsPrateek SoniNo ratings yet
- Beneficial Effects of The Amino Acid Glycine: Mini Reviews in Medicinal Chemistry June 2016Document19 pagesBeneficial Effects of The Amino Acid Glycine: Mini Reviews in Medicinal Chemistry June 2016riniNo ratings yet
- 22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndDocument70 pages22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndEnrique LiKeNo ratings yet
- And Auto Catalysis With Example), Catalytic PoisonDocument28 pagesAnd Auto Catalysis With Example), Catalytic Poisonvin2eethNo ratings yet
- Gujarat Technological University L.D. College of EngineeringDocument13 pagesGujarat Technological University L.D. College of EngineeringabdulqadirNo ratings yet
- Industrial Catalyst AssignmentDocument14 pagesIndustrial Catalyst AssignmentHarshitNo ratings yet
- Regular SolutionsDocument19 pagesRegular SolutionsRahul PandeyNo ratings yet
- Mitigation of CO2 by Chemical ConversionDocument21 pagesMitigation of CO2 by Chemical Conversiona_abbaspourNo ratings yet
- A Brief Review - Heavy Metal and Their AnalysisDocument7 pagesA Brief Review - Heavy Metal and Their AnalysisZakki LabiebNo ratings yet
- Statistical Methods and Thermodynamics - BatistaDocument97 pagesStatistical Methods and Thermodynamics - BatistapepeperezNo ratings yet
- Kinetics of Surface ReactionsDocument24 pagesKinetics of Surface ReactionsShehRoz KhanNo ratings yet
- Using Technology To Study Cellular and Molecular BiologyDocument138 pagesUsing Technology To Study Cellular and Molecular BiologybheeshmatNo ratings yet
- 11.2 Potentiometric Methods PDFDocument29 pages11.2 Potentiometric Methods PDFMohamad Abdul ChalimNo ratings yet
- Acids and BasesDocument26 pagesAcids and BasesGaayathiriNo ratings yet
- Syllabus For CHE 543 F2018Document6 pagesSyllabus For CHE 543 F2018Sena ErarslankılıçNo ratings yet
- Coordination Chemistry—XVI: XVIth International Conference on Coordination ChemistryFrom EverandCoordination Chemistry—XVI: XVIth International Conference on Coordination ChemistryNo ratings yet
- Chapter 36Document80 pagesChapter 36law05160% (1)
- 3-D Structure of ProteinsDocument22 pages3-D Structure of Proteinsraanja2No ratings yet
- Polymer Structures: Issues To Address..Document28 pagesPolymer Structures: Issues To Address..HaroonNo ratings yet
- Catalysis & Catalysts: Facts and Figures About CatalystsDocument88 pagesCatalysis & Catalysts: Facts and Figures About CatalystskeatyNo ratings yet
- Enzyme and Acid - Base CatalysisDocument64 pagesEnzyme and Acid - Base Catalysisbinseung skzNo ratings yet
- TK 411 - Lecture Note 6 - Reactor DesignDocument41 pagesTK 411 - Lecture Note 6 - Reactor DesignramaNo ratings yet
- Chemical EquilibriumDocument2 pagesChemical EquilibriumMichael Mohamed100% (6)
- Ap Chemistry: Kinetics Practice Problems: Rate of Reaction - (Clo (Clo (CL ) ) ) 3 2 T T TDocument13 pagesAp Chemistry: Kinetics Practice Problems: Rate of Reaction - (Clo (Clo (CL ) ) ) 3 2 T T TAbu Sufyan ButtNo ratings yet
- 1409302977chemical BondingDocument83 pages1409302977chemical Bondingparmodcobra360No ratings yet
- Lecture 5a CatalysisDocument22 pagesLecture 5a CatalysisSandeep ChawlaNo ratings yet
- Introduction of Surface Chemistry and CatalysisDocument7 pagesIntroduction of Surface Chemistry and CatalysisLetícia Lima100% (1)
- Plastics Piping Systems: Jeevan Bhar Ka Saath..Document28 pagesPlastics Piping Systems: Jeevan Bhar Ka Saath..Ritesh JhaNo ratings yet
- Bs and M.SC - Applied Chemistry PDFDocument4 pagesBs and M.SC - Applied Chemistry PDFZaid AhmadNo ratings yet
- An Introduction To Organic Chemistry:: The Saturated HydrocarbonsDocument73 pagesAn Introduction To Organic Chemistry:: The Saturated HydrocarbonsGabz Gabby0% (1)
- Collision TheoryDocument10 pagesCollision TheoryAnonymous pgjIAZoNo ratings yet
- Controlled Radical PolymerizationDocument13 pagesControlled Radical PolymerizationBeryl MawaridNo ratings yet
- Utilization of Solid Wastes Generated From Steel PlantDocument48 pagesUtilization of Solid Wastes Generated From Steel PlantAnonymous 2RduvkjgZNo ratings yet
- Joe Was A Toddler He Had A Formula For Everlasting Life Joe Sucked He Died Alone Ye (Document1 pageJoe Was A Toddler He Had A Formula For Everlasting Life Joe Sucked He Died Alone Ye (joebug34No ratings yet
- Islamophobia: The Roots of IntoleranceDocument7 pagesIslamophobia: The Roots of Intolerancejoebug34No ratings yet
- Bib 5390Document1 pageBib 5390joebug34No ratings yet
- Temperature Profile Along ReactorDocument3 pagesTemperature Profile Along Reactorjoebug34No ratings yet
- ChE 334 Appendix CodeDocument4 pagesChE 334 Appendix Codejoebug34No ratings yet
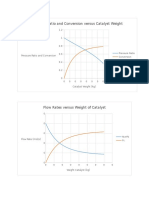
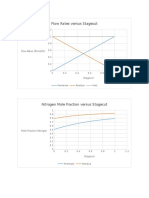
- Pressure Ratio and Conversion Versus Catalyst WeightDocument5 pagesPressure Ratio and Conversion Versus Catalyst Weightjoebug34No ratings yet
- Math423 HW#Document1 pageMath423 HW#joebug34No ratings yet
- Table of Van Der Waals ConstantsDocument2 pagesTable of Van Der Waals Constantsjoebug34No ratings yet
- Poop A Doop DoopsDocument1 pagePoop A Doop Doopsjoebug34No ratings yet
- H2o2 ApDocument12 pagesH2o2 Apjoebug34No ratings yet
- Enzyme ActivityDocument3 pagesEnzyme ActivityNabindra Ruwali100% (2)
- Mechanisms in Homogeneous Catalysis A Spectroscopi PDFDocument293 pagesMechanisms in Homogeneous Catalysis A Spectroscopi PDFCamiloHernándezNo ratings yet
- Enzyme Kinetics NotesDocument2 pagesEnzyme Kinetics NotesMarc Imhotep Cray, M.D.No ratings yet
- Solvation Effects On Quantum Tunneling ReactionsDocument11 pagesSolvation Effects On Quantum Tunneling ReactionsZdeněk ChvalNo ratings yet
- Interpretation of Batch Reactor DataDocument85 pagesInterpretation of Batch Reactor DataOath'zNo ratings yet
- KatalisDocument37 pagesKatalismeri hardina zdNo ratings yet
- Mekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringDocument77 pagesMekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringetayhailuNo ratings yet
- WWW Chemguide Co UkDocument4 pagesWWW Chemguide Co Ukgeoboom12No ratings yet
- Thermodynamic Investigation of Methanol and Dimethyl Ether Synthesis From CO HydrogenationDocument8 pagesThermodynamic Investigation of Methanol and Dimethyl Ether Synthesis From CO HydrogenationGonzalo TitoNo ratings yet
- Nickel by The Raney Process As A Catalyst of Hydrogenation 1932Document2 pagesNickel by The Raney Process As A Catalyst of Hydrogenation 1932masihNo ratings yet
- Esterification of Acetic Acid With Ethanol Catalysed by An AcidicDocument9 pagesEsterification of Acetic Acid With Ethanol Catalysed by An AcidicLord ZukoNo ratings yet
- Chemistry (IGCSE) 0620 - s07 - QP - 6Document16 pagesChemistry (IGCSE) 0620 - s07 - QP - 6Drizzle0% (1)
- Modelling of So2 Oxidation Kinetic Data of A CS/V Catalyst Rates Based On at High Pressures and ConversionsDocument6 pagesModelling of So2 Oxidation Kinetic Data of A CS/V Catalyst Rates Based On at High Pressures and ConversionsCarlos Alfredo Camacho PlazasNo ratings yet
- Design of A Separation ProcessDocument8 pagesDesign of A Separation Processdario delmoralNo ratings yet
- Cesium and Rubidium Salts of Keggin-TypeDocument146 pagesCesium and Rubidium Salts of Keggin-TypeChau MaiNo ratings yet
- Q-MAX Process For The Production of Cumene.Document15 pagesQ-MAX Process For The Production of Cumene.svo svoNo ratings yet
- Organometallics SukantaDocument34 pagesOrganometallics SukantaPavan KethavathNo ratings yet
- Questions Reaction Kinetics The EssentialsDocument2 pagesQuestions Reaction Kinetics The Essentialshernys NietoNo ratings yet
- Handout Powerpoint Chem 301 PharChm1Document101 pagesHandout Powerpoint Chem 301 PharChm1Mikee MeladNo ratings yet
- Catalysis in Organic Chemistry (1922) - Sabbatier PDFDocument442 pagesCatalysis in Organic Chemistry (1922) - Sabbatier PDFbabithyNo ratings yet
- Organometallic CatalysisDocument5 pagesOrganometallic CatalysisMuhammad Hassan ZiaNo ratings yet
- Chemistry IA - Bang D1 (Official)Document13 pagesChemistry IA - Bang D1 (Official)Kim-Bảng PhạmNo ratings yet
- Process For The Manufacture of Oxidized Starch, Oxidized Starch and Its UseDocument8 pagesProcess For The Manufacture of Oxidized Starch, Oxidized Starch and Its UseWahyu Dwi ChrismantoNo ratings yet
- International Journal of Biological Macromolecules: Abdallah R. Ismail, Kwang-Hyun BaekDocument16 pagesInternational Journal of Biological Macromolecules: Abdallah R. Ismail, Kwang-Hyun BaekAlex BaenaNo ratings yet
- Simulation of The Xylene Isomerization Catalytic ReactorDocument6 pagesSimulation of The Xylene Isomerization Catalytic ReactorAssia El IdrissiNo ratings yet
- Kinetics McqsDocument31 pagesKinetics McqsTayyaba SadaqNo ratings yet
- Patent Application Publication (10) Pub. No.: US 2017/0050899 A1Document15 pagesPatent Application Publication (10) Pub. No.: US 2017/0050899 A1Salsabil Nurazizah TANo ratings yet
- Enzymes - Life Sciences Questions and Answers - SanfoundryDocument8 pagesEnzymes - Life Sciences Questions and Answers - SanfoundryHUAWEI HUAWEINo ratings yet
- Thermally Double Coupled Reactor Coupling Aqueous Phase GlycerolDocument10 pagesThermally Double Coupled Reactor Coupling Aqueous Phase GlycerolMahdy HajienayatiNo ratings yet
- Format For Course CurriculumDocument4 pagesFormat For Course CurriculumJatinNo ratings yet