Professional Documents
Culture Documents
Brief Communications
Uploaded by
api-26343305Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Brief Communications
Uploaded by
api-26343305Copyright:
Available Formats
brief communications
Conservation biology
a c
Population size
200 12
‘Ghost’ alleles of the
(individuals)
100 N<50
Mauritius kestrel 0 11
1940
1974
1986
1994
1950
1960
1970
1990
1978
1982
Effective population size (ln∆ N e )
T
he population of Mauritius kestrels is Year 10 Ancestral
b b.p. Mauritius
thought to have recovered from a sin- 264
gle wild breeding pair in 19741, when 258 Ancestral
9
its prospects were considered to be hope- 246 Mauritius
less, to over 200 pairs today2. Here we evalu- 234 (museum skins) 8 Seychelles
228
ate the loss of genetic variation that resulted 222 Restored
from this bottleneck by typing 12 micro- 216
7 Mauritius
210
satellite DNA loci in museum skins up to b.p.
170 years old and from modern kestrels. We 228 Restored 6
222 6 8 10 12 14 16 18
find that ancestral variation was remarkably 216 Mauritius Species range (units)
high and comparable to continental kestrel 210
species. This shows that the unexpected
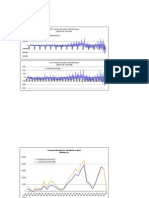
resilience of the population could not have Figure 1 The genetic diversity of the ancestral population of Mauritius kestrels is similar to those of continental populations. a, Known
been due either to benefits contributed by population size across the bottleneck; records from Mauritius naturalists (black bars) and the Mauritius Wildlife Foundation (red bars);
an undetected remnant population or to data calculated at 4-year intervals. b, Hexanucleotide microsatellite genotypes of Mauritius kestrel museum skins (top) include a mixture
reduction of the inbreeding genetic load by of alleles unique to the ancestral population (arrows) and those found in the restored population (bottom). c, The relationship between
a history of small population size3. estimates of effective population size and species range. The 95% confidence limits are shown for the linear regression through all
Following the widespread use of pesti- species, excluding the restored Mauritius (red) and Seychelles (white) population. Points show modal DNe values estimated using the like-
cides in 1940–602, the Mauritius kestrel lihood function for the k-alleles model; error bars show the 95% credible interval.
population was for a long time smaller than
the 50 individuals recommended as the variation9. Is this low genetic diversity a comparisons can only be made with the
minimum for viability4 (Fig. 1a). An inten- cause or a consequence of their vulnerabili- 5 years since intensive management has
sive conservation programme between 1983 ty? The relationship between our estimates ceased, but productivity was consistently
and 1993 led to a recovery to 400–500 of relative Ne (Table 1) and species range higher in each of these (P40.55*0.05),
birds5. Historical and post-bottleneck geno- (current population size) shows that the averaging 1.1 fledglings per nest (31%
types of Mauritius kestrels at one micro- ancestral Ne of the Mauritius kestrel lies increase). The recovery need not be a genet-
satellite locus show some of the ‘ghost’ within the range extrapolated from conti- ic response: growing populations can show
alleles lost through the bottleneck (Fig. 1b). nental populations (Fig. 1c). However, both surprising behavioural plasticity in coloniz-
Across all loci, allelic diversity fell by 55%, the restored Mauritius and Seychelles popu- ing habitat outside the native forest5.
and heterozygosity by 57% (Table 1). This lations are clearly outliers. The much higher The high ancestral level of genetic varia-
reduction is broadly in line with the value ancestral Ne on Mauritius suggests that the tion in the Mauritius kestrel shows that the
of 0.48 expected from estimates of popula- current low level of genetic diversity is a loss of variation associated with the arrival
tion size through the bottleneck6 (Fig. 1a). consequence of a recent population crisis. of humans on Mauritius and the Seychelles
Differences in genetic variation can be Unlike some other species10, the kestrel is without recent precedent. More encour-
measured more precisely from estimates of population has recovered without the addi- aging is that a population can recover from
effective population size, Ne . These take into tion of new genetic variation. The kestrel a bottleneck, even after a dramatic loss in
account variation in genetic diversity population’s resilience may be because its genetic variation. This may help endan-
among loci, small sample size and missing productivity was only weakly affected by the gered island endemics stake a claim for
data. The likelihood of the genotypes bottleneck. Laboratory studies of bottle- finite conservation resources.
observed at each locus was calculated as a necked species show that the effects on pro- Jim J. Groombridge*, Carl G. Jones†,
function of Nimj, where Ni is population size ductivity range from negligible to severe, Michael W. Bruford†‡, Richard A. Nichols§
and mj is the locus-specific mutation rate, and that populations may or may not *Institute of Zoology, Zoological Society of London,
which we parametrized as the differences recover from these in later generations11. Regents Park, London NW1 4RY, UK
from their means (Ni 4 á N &DNi , á mj = m & Comparison with other tropical falcons †Mauritius Wildlife Foundation,
Dmj). DNi and Dmi were estimated using the suggests that there has been some reduction Black River, Mauritius
Metropolis–Hastings algorithm7. in the fitness of the Mauritius kestrel5. §School of Biological Sciences, Queen Mary &
Endemic island species show high rates There is also evidence of an improvement Westfield College, London E1 4NS, UK
of historical extinction8 and low genetic since the height of the bottleneck. Direct ‡Present address: Cardiff School of Biosciences,
Cardiff University, Cathays Park,
Table 1 Genetic diversity of kestrel populations
Cardiff CF1 3TL, UK
Population Sample Mean number Heterozygosity* Estimate of logeDNi
1. Jones, C. G. in Studies of Mascarene Island Birds (ed. Diamond,
size (2N) of alleles
A. W.) 5–89 (Cambridge Univ. Press, Cambridge, 1987).
Mauritius (restored) 150* 1.41 0.10 7.38 2. Safford, R. J. & Jones, C. G. Biol. Conserv. 6, 1445–1451 (1997).
Mauritius (ancestral) 52 3.10 0.23 9.60 3. Bataillon, T. & Kirkpatric, M. Genet. Res. 75, 75–82 (2000).
Seychelles 8 1.25 0.12 7.84 4. Gilpin, M. E. & Soule, M. E. in Conservation Biology: The
Science of Scarcity and Diversity (ed. Soule, M. E.) 19–34
European 20 5.50 0.68† 11.2
(Sinauer, Sunderland, MA, 1986).
Canary Islands 16 4.41 0.64† 10.92 5. Jones, C. G. et al. Ibis 137, 173–180 (1995).
South African 20 5.00 0.63† 10.96 6. Nei, M., Maruyama, T. & Chakraborty, R. Evolution 29, 1–9 (1975).
7. Gelman, A., Carlin, J. B., Stern, H. S. & Rubin, D. B. Bayesian
Greater kestrel 20 4.50 0.59† 10.8
Data Analysis (Chapman & Hall, London, 1995).
Lesser kestrel 16 5.41 0.70† 11.36 8. Johnson, T. H. & Stattersfield, A. J. Ibis 132, 167–180 (1990).
*Methods for maximum likelihood estimates of heterozygosity and data on a further 225 individuals (including methods and genotypes for all populations) 9. Frankham, R. Heredity 78, 311–327 (1997).
are available at http://www.qmw.ac.uk/~ugbt112/programs/Mauritius_kestrel/ 10. Madsen, T. et al. Nature 402, 34–35 (1999).
†Significantly different from restored Mauritius population (Edwards likelihood ratio criterion, P*0.01). 11. Saccheri, I. et al. Evolution 50, 2000–2013 (1996).
616 © 2000 Macmillan Magazines Ltd NATURE | VOL 403 | 10 FEBRUARY 2000 | www.nature.com
You might also like
- Artemis - Rise of The Dragon - April 2021Document9 pagesArtemis - Rise of The Dragon - April 2021BerchadesNo ratings yet
- Growth of Objects in Earth Orbit 1957-2011Document1 pageGrowth of Objects in Earth Orbit 1957-2011VAWTmanNo ratings yet
- Lta Vasquez Orozco Miguel AlejandroDocument1 pageLta Vasquez Orozco Miguel AlejandroMiguel VásquezNo ratings yet
- Government Budget Deficits and SurplusesDocument33 pagesGovernment Budget Deficits and Surplusesblack pinkNo ratings yet
- Aborto e CancerDocument7 pagesAborto e CancerLara MarianaNo ratings yet
- Materi Geologi MigasDocument120 pagesMateri Geologi MigasAfdhal GansNo ratings yet
- Floods: The Awesome Power (Suzanne Van Cooten, PH.D)Document39 pagesFloods: The Awesome Power (Suzanne Van Cooten, PH.D)National Press FoundationNo ratings yet
- Cci2000 CTCDocument1 pageCci2000 CTCSmatrou RouchamouxNo ratings yet
- Evolucao Do Pensamento PedagogicoDocument1 pageEvolucao Do Pensamento PedagogicoEnayde Fernandes Silva DiasNo ratings yet
- Artigo - Especial-Grandes-Pensadores-Da-Educacao - ARVORE DO Pens Amen To PEDAGOGICODocument1 pageArtigo - Especial-Grandes-Pensadores-Da-Educacao - ARVORE DO Pens Amen To PEDAGOGICOMario Luis Tavares Ferreira0% (1)
- Árvore Do ConhecimentoDocument1 pageÁrvore Do ConhecimentoPedro Fauth Manhaes MirandaNo ratings yet
- Trends in U.S. CorrectionsDocument8 pagesTrends in U.S. CorrectionsYùscar Murasaki Lakasito'uNo ratings yet
- Cci2003 CTCDocument1 pageCci2003 CTCSmatrou RouchamouxNo ratings yet
- SBS DBIA Day 1Document154 pagesSBS DBIA Day 1Risho gitauNo ratings yet
- Midterm TimelineDocument1 pageMidterm TimelineDavid QuickNo ratings yet
- Cci2002 CTCDocument1 pageCci2002 CTCSmatrou RouchamouxNo ratings yet
- DIA 2010 Presentacion WDCDocument38 pagesDIA 2010 Presentacion WDCJesús Reinaldo Sanchez AmayaNo ratings yet
- Figure 1. Depicting Change in Vermont Loon Nesting Pairs, Chick Survival, and Territorial Pairs From 1978-2022.Document2 pagesFigure 1. Depicting Change in Vermont Loon Nesting Pairs, Chick Survival, and Territorial Pairs From 1978-2022.Kate SadoffNo ratings yet
- Single GraphDocument2 pagesSingle GraphKate SadoffNo ratings yet
- Dr. Kulıev - Istanbul 2016 - Practical PGDDocument14 pagesDr. Kulıev - Istanbul 2016 - Practical PGDcansu onerNo ratings yet
- NP TE L: Corporate FinanceDocument23 pagesNP TE L: Corporate Financevini2710No ratings yet
- Description: Tags: PresentnassgapDocument32 pagesDescription: Tags: Presentnassgapanon-856265No ratings yet
- Trends in US Corrections: Fact Sheet on Prison Population Rise and Mass IncarcerationDocument8 pagesTrends in US Corrections: Fact Sheet on Prison Population Rise and Mass IncarcerationJohnNo ratings yet
- 40 - KTS-CM-12Document8 pages40 - KTS-CM-12IrenataNo ratings yet
- North East Atlantic Ocean European Waters Mediterranean Sea - Small Scale ChartsDocument1 pageNorth East Atlantic Ocean European Waters Mediterranean Sea - Small Scale ChartsIlya BondarenkoNo ratings yet
- A Generation of Sociopaths - Reference MaterialDocument30 pagesA Generation of Sociopaths - Reference MaterialWei LeeNo ratings yet
- Ashokas Fund For Social Change in AfricaDocument16 pagesAshokas Fund For Social Change in AfricaAshoka: Innovators for the Public100% (1)
- Decision Support Model For Weekly Operation of Hydroelectric Reservoirs by Stochastic Nonlinear OptimizationDocument32 pagesDecision Support Model For Weekly Operation of Hydroelectric Reservoirs by Stochastic Nonlinear Optimizationpathy cocreNo ratings yet
- Booklet Final Draft SpreadDocument15 pagesBooklet Final Draft Spreadapi-277630063No ratings yet
- Article Newbuinessboomandbust UsDocument12 pagesArticle Newbuinessboomandbust UsjaynaNo ratings yet
- 059 Increasing Outputs at LKAB Iron Ore MinesDocument4 pages059 Increasing Outputs at LKAB Iron Ore MinesKenny CasillaNo ratings yet
- Long Run Correlations Money and Prices During HyperinflationsDocument25 pagesLong Run Correlations Money and Prices During HyperinflationsYuliya KulikovaNo ratings yet
- Sandweiss MoseleyFest 2009 OCRDocument16 pagesSandweiss MoseleyFest 2009 OCRDOMENICO FRANSCESC VILLAVICENCIO MERGONINo ratings yet
- PIA Reports GraphDocument1 pagePIA Reports GraphFoto ShackNo ratings yet
- The World Map of Political Conflicts: CONIAS Risk IntelligenceDocument19 pagesThe World Map of Political Conflicts: CONIAS Risk IntelligenceFerrari MihailescuNo ratings yet
- GW Modeling FactsDocument1 pageGW Modeling Factsayaz hasanNo ratings yet
- Green Economy & Trade: Industrial Policy: Challenges and OpportunitiesDocument2 pagesGreen Economy & Trade: Industrial Policy: Challenges and OpportunitiesLida PinzonNo ratings yet
- NP131 2011 Chart CatalogueDocument119 pagesNP131 2011 Chart CatalogueGintautas Balsys90% (10)
- Lecture4 CMDocument54 pagesLecture4 CMHarry SinghNo ratings yet
- Sea L Sto CK Ass Es Sme NTDocument16 pagesSea L Sto CK Ass Es Sme NTHafeniKaNo ratings yet
- CBMBA DividendPolicy 02march2019Document28 pagesCBMBA DividendPolicy 02march2019dialedin263No ratings yet
- India's Economic Growth StatewiseDocument46 pagesIndia's Economic Growth StatewiseKanishkKumarDhariwalNo ratings yet
- Treasury Tax RecieptsDocument3 pagesTreasury Tax Recieptskettle1No ratings yet
- Political Report April 2009Document7 pagesPolitical Report April 2009American Enterprise InstituteNo ratings yet
- ITA - Tin Market Outlook James Willoughby June - 22 PDFDocument12 pagesITA - Tin Market Outlook James Willoughby June - 22 PDFRodrigo RodrigoNo ratings yet
- AEI's Political Report May 2011: The Latest Polls On Obama, The Military, and The EconomyDocument6 pagesAEI's Political Report May 2011: The Latest Polls On Obama, The Military, and The EconomyAmerican Enterprise InstituteNo ratings yet
- Mid Ranja Concept Paper (05-07-2023)Document6 pagesMid Ranja Concept Paper (05-07-2023)Saiba AteeqNo ratings yet
- Map Colonial-AfricaDocument1 pageMap Colonial-AfricaMicheal KenaNo ratings yet
- Source: WDI, 2006: Indonesia: GDP Riil Per Kapita 2000 100, (RP)Document2 pagesSource: WDI, 2006: Indonesia: GDP Riil Per Kapita 2000 100, (RP)Ananda KhairiaNo ratings yet
- M&A Finance TrendsDocument51 pagesM&A Finance TrendsPaul GhanimehNo ratings yet
- Returning Cash To The Owners: Dividend Policy: Aswath DamodaranDocument69 pagesReturning Cash To The Owners: Dividend Policy: Aswath Damodarangioro_miNo ratings yet
- Presentacion Pallete enDocument24 pagesPresentacion Pallete enAbhishek ShahNo ratings yet
- Topic No.2Document28 pagesTopic No.2Viennalyn Gelicame VallentisNo ratings yet
- Sri Lanka Drought StatisticsDocument5 pagesSri Lanka Drought StatisticsArjuna SeneviratneNo ratings yet
- Organizational Environments and CulturesDocument34 pagesOrganizational Environments and CulturesTân NguyênNo ratings yet
- DeficitDocument2 pagesDeficitlkjfdoiNo ratings yet
- NiplesDocument59 pagesNiplesJose LiraNo ratings yet
- Midterm - TimelineDocument1 pageMidterm - TimelineChrisy LiNo ratings yet
- Statement of Business PrinciplesDocument6 pagesStatement of Business Principlesapi-25923204No ratings yet
- NullDocument9 pagesNullapi-26343305No ratings yet
- Jean-Raymond Boulle, Second US-SSA AGOA Forum - Boulle MediationDocument15 pagesJean-Raymond Boulle, Second US-SSA AGOA Forum - Boulle Mediationinvestorseurope offshore stockbrokersNo ratings yet
- NullDocument12 pagesNullapi-26343305No ratings yet
- Monday, December 8, 2003: Venue: J.W. Marriott Hotel, Grand Foyer Venue: J.W. Marriott Hotel, Salon III & IVDocument9 pagesMonday, December 8, 2003: Venue: J.W. Marriott Hotel, Grand Foyer Venue: J.W. Marriott Hotel, Salon III & IVapi-25923189No ratings yet
- Sierra Rutile Mine: Home - Sitemap - Email Update - ContactDocument2 pagesSierra Rutile Mine: Home - Sitemap - Email Update - Contactapi-26343305No ratings yet
- Statement of Business PrinciplesDocument6 pagesStatement of Business Principlesapi-25923204No ratings yet
- Operations at SRL: Sahr Wonday, Deputy General Manager Sierra Rutile LimitedDocument34 pagesOperations at SRL: Sahr Wonday, Deputy General Manager Sierra Rutile Limitedapi-26343305No ratings yet
- CNBC's Erin Burnett Introduces The 2009 Summit Presidential Plenary, ModeratedDocument12 pagesCNBC's Erin Burnett Introduces The 2009 Summit Presidential Plenary, Moderatedapi-26343305No ratings yet
- NullDocument5 pagesNullapi-26343305No ratings yet
- Adastra Minerals: Notable PersonnelDocument1 pageAdastra Minerals: Notable Personnelapi-26343305No ratings yet
- Opening Remarks by Stephen Hayes President, Corporate Council On Africa 2003 AGOA Forum Private Sector Session December 8, 2003 J.W. Marriott Hotel Washington, D.CDocument6 pagesOpening Remarks by Stephen Hayes President, Corporate Council On Africa 2003 AGOA Forum Private Sector Session December 8, 2003 J.W. Marriott Hotel Washington, D.Capi-25923189No ratings yet
- NullDocument6 pagesNullapi-25890976No ratings yet
- Adastra Minerals - Wikipedia, The Free EncyclopediaDocument1 pageAdastra Minerals - Wikipedia, The Free Encyclopediaapi-26343305No ratings yet
- NullDocument44 pagesNullapi-26343305No ratings yet
- Adastra Minerals - Wikipedia, The Free EncyclopediaDocument1 pageAdastra Minerals - Wikipedia, The Free Encyclopediaapi-26343305No ratings yet
- J. Field Ornithol., 70 (2) :230-235Document6 pagesJ. Field Ornithol., 70 (2) :230-235api-26343305No ratings yet
- Titanium Resources Group Preliminary ResultsDocument19 pagesTitanium Resources Group Preliminary Resultsapi-25890976No ratings yet
- Kestrelflyer Programme GuideDocument13 pagesKestrelflyer Programme Guideapi-26343305No ratings yet
- J. Field Ornithol., 70 (2) :230-235Document6 pagesJ. Field Ornithol., 70 (2) :230-235api-26343305No ratings yet
- HIV-Related Public-Private Partnerships and Health Systems StrengtheningDocument36 pagesHIV-Related Public-Private Partnerships and Health Systems Strengtheningapi-26343305No ratings yet
- Biodiversity and Protected Areas - MauritiusDocument7 pagesBiodiversity and Protected Areas - Mauritiusapi-26343305No ratings yet
- Biodiversity and Protected Areas - MauritiusDocument7 pagesBiodiversity and Protected Areas - Mauritiusapi-26343305No ratings yet
- Important Considerations For Intensive Management of EndangeredDocument36 pagesImportant Considerations For Intensive Management of Endangeredapi-26343305No ratings yet
- MWF - Newsletter NR 3. 2005Document5 pagesMWF - Newsletter NR 3. 2005api-26343305No ratings yet
- 2005 Summit Workshop Agenda: All Items Subject To ChangeDocument27 pages2005 Summit Workshop Agenda: All Items Subject To Changeapi-25923189No ratings yet
- Molecular Ecology (2001) 10, 593 - 602Document10 pagesMolecular Ecology (2001) 10, 593 - 602api-26343305No ratings yet
- November 16th-December 5th 2007Document1 pageNovember 16th-December 5th 2007api-26343305No ratings yet
- Malagasy Identifies 6.5Km Long Vanadium Zone: Ampanihy - Central / Fotadrevo (V-Ni-Cu-Mn)Document5 pagesMalagasy Identifies 6.5Km Long Vanadium Zone: Ampanihy - Central / Fotadrevo (V-Ni-Cu-Mn)api-26343305No ratings yet
- Government of Telangana Office of The Director of Public Health and Family WelfareDocument14 pagesGovernment of Telangana Office of The Director of Public Health and Family WelfareSidhu SidhNo ratings yet
- Computer Conferencing and Content AnalysisDocument22 pagesComputer Conferencing and Content AnalysisCarina Mariel GrisolíaNo ratings yet
- Jillian's Student Exploration of TranslationsDocument5 pagesJillian's Student Exploration of Translationsjmjm25% (4)
- Research Methods LessonDocument26 pagesResearch Methods LessonCarole Janne EndoyNo ratings yet
- It ThesisDocument59 pagesIt Thesisroneldayo62100% (2)
- Discover books online with Google Book SearchDocument278 pagesDiscover books online with Google Book Searchazizan4545No ratings yet
- Solar Presentation – University of Texas Chem. EngineeringDocument67 pagesSolar Presentation – University of Texas Chem. EngineeringMardi RahardjoNo ratings yet
- Prep - VN: Where Did The Polo Family Come From?Document1 pagePrep - VN: Where Did The Polo Family Come From?Phương LanNo ratings yet
- Interpersonal Communication LPDocument3 pagesInterpersonal Communication LPprincesslove.taduraNo ratings yet
- First Preliminary Examination in Tle 8 - Mechanical DraftingDocument6 pagesFirst Preliminary Examination in Tle 8 - Mechanical DraftingNefritiri BlanceNo ratings yet
- Intentional Replantation TechniquesDocument8 pagesIntentional Replantation Techniquessoho1303No ratings yet
- Extra Vocabulary: Extension Units 1 & 2Document1 pageExtra Vocabulary: Extension Units 1 & 2CeciBravoNo ratings yet
- MEE2041 Vehicle Body EngineeringDocument2 pagesMEE2041 Vehicle Body Engineeringdude_udit321771No ratings yet
- CV Finance GraduateDocument3 pagesCV Finance GraduateKhalid SalimNo ratings yet
- Dues & Scholarship Section: NotificationDocument6 pagesDues & Scholarship Section: NotificationMUNEEB WAHEEDNo ratings yet
- Group Assignment Topics - BEO6500 Economics For ManagementDocument3 pagesGroup Assignment Topics - BEO6500 Economics For ManagementnoylupNo ratings yet
- MID Term VivaDocument4 pagesMID Term VivaGirik BhandoriaNo ratings yet
- Puberty and The Tanner StagesDocument2 pagesPuberty and The Tanner StagesPramedicaPerdanaPutraNo ratings yet
- Business Planning Process: Chapter-Four Operations Planning and ControlDocument12 pagesBusiness Planning Process: Chapter-Four Operations Planning and ControlGemechis BussaNo ratings yet
- Derivatives 17 Session1to4Document209 pagesDerivatives 17 Session1to4anon_297958811No ratings yet
- UA-Series EN F2005E-3.0 0302Document25 pagesUA-Series EN F2005E-3.0 0302PrimanedyNo ratings yet
- Introduction To Alternative Building Construction SystemDocument52 pagesIntroduction To Alternative Building Construction SystemNicole FrancisNo ratings yet
- Dealer DirectoryDocument83 pagesDealer DirectorySportivoNo ratings yet
- The Free Little Book of Tea and CoffeeDocument83 pagesThe Free Little Book of Tea and CoffeeNgopi YukNo ratings yet
- Year 11 Physics HY 2011Document20 pagesYear 11 Physics HY 2011Larry MaiNo ratings yet
- Plo Slide Chapter 16 Organizational Change and DevelopmentDocument22 pagesPlo Slide Chapter 16 Organizational Change and DevelopmentkrystelNo ratings yet
- Cincinnati LaserNst PDFDocument204 pagesCincinnati LaserNst PDFedrf sswedNo ratings yet
- MCI FMGE Previous Year Solved Question Paper 2003Document0 pagesMCI FMGE Previous Year Solved Question Paper 2003Sharat Chandra100% (1)
- Platform Tests Forj Udging Quality of MilkDocument10 pagesPlatform Tests Forj Udging Quality of MilkAbubaker IbrahimNo ratings yet
- GravimetryDocument27 pagesGravimetrykawadechetan356No ratings yet