Professional Documents
Culture Documents
Microbiology: Laboratory Manual
Uploaded by
DevindraPrptOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Microbiology: Laboratory Manual
Uploaded by
DevindraPrptCopyright:
Available Formats
MICROBIOLOGY
Laboratory
Manual
RESPIRATORY SYSTEM
Cucunawangsih Cucunawangsih Cucunawangsih Cucunawangsih
Cucu/Mikro/FK-UPH/Nop.2008 2
General Laboratory Directions
To ensure your safety in the microbiology
laboratory some common practice should be
followed:
1. Bring only your laboratory manual,
writing instruments to the laboratory
bench. Leave all of your bags and other
belongings in a special area for these.
2. Always wearing your lab coat at all times
during laboratory classes. Dont remove
the lab coat during laboratory classes or
worn outside the laboratory.
3. Wash your hands thoroughly with
detergent prior entering and leaving the
laboratory. Clean and disinfect your
bench top before and after each
laboratory period.
4. Never eat, drink, or smoking in the
laboratory. Dont put anything in or near
your mouth (for examples fingers or
pencils), or rub your eyes without
washing your hands thoroughly.
5. If you have long hair, tie it back. Wear
shoes at all times.
6. The laboratory equipped with a Bunsen
burner, a wire transfer loop (sengkelit), a
matches for lighting the Bunsen burner
and/or other equipment. This equipment
is shared with other class, so do not
leave your personal belongings in the
laboratory.
7. Cultured to be incubated at 37C should
be placed on shelves with the
appropriate bench number. Make sure
that you cultures are clearly labeled to
avoid confusion. Incubate all plates in an
inverted position, unless otherwise
noted.
8. Be sure that you discard your slides and
cultures as soon as the exercise is
complete. All cultures, slides and other
materials to be discarded in the
containers provided. Never remove any
equipments and materials from the
laboratory.
9. Report all accidents to the instructor
immediately.
10. Turn off the Bunsen burner as soon as
you are done with them.
11. Never pipette by mouth. Always use the
mechanical devices provided.
12. If you break some glassware, call your
instructor immediately. Do not touch
broken glassware with bare hands. If
some of the broken glassware contained
microorganisms, cover them immediately
with a paper towel and saturate with
disinfectant. Call your instructor
immediately.
13. If a microbial culture is spilled, cover it
immediately with paper towel and
saturate with disinfectant. Call your
instructor immediately.
14. If you accidentally get microorganisms
on your hands immediately with
disinfectant. Scrub with soap for at least
30 seconds.
15. Before leaving the laboratory, CLEAN
your work space and wipe it down with
70% alcohol.
16. Handle your microscope with care. Be
sure to use lens paper to remove oil from
the oil immersion lens. The oil immersion
lens must be cleaned after each
laboratory period.
Cucu/Mikro/FK-UPH/Nop.2008 3
Description
Provide preparation for respiratory
system in microbiology laboratories; include
the important pathogens of tuberculosis, the
pathogenic cocci, and examination of
sputum.
Overall Course
Objective
Upon completion of this course,
students will be able to perform routine test
of acid fast bacilli in medical microbiology
and to identify the pathogenic of respiratory
infections. Also included in the course is
testing sputum for the presence of bacteria,
and understand the criteria of good sputum.
1. Students will understand and practice
basic principles of laboratory safety,
especially as related to acid fast bacilli
and sputum examinations. They will
practice safe handling of sputum
specimen and disposal of potentially
infectious materials.
2. Students will learn about many different
types of bacteriological media, including
proper use of each medium, organism
that grows well on each medium.
3. Students will be able to identify the
pathogenic organisms of respiratory
system. based on Gram stain result,
growth on specific media, and result of
biochemical tests.
4. Students will be able to list organisms
commonly isolated as pathogens and
normal flora from upper and lower
respiratory tract.
5. Students will be able to explain the Ziehl-
Neelsen staining of sputum from patient
with tuberculosis. They also be able to
discuss culture technique, media, and
growth requirements for mycobacterial
species.
Course Topics
1. An appropriate sputum specimen
2. The pathogenic cocci
Streptococcus sp.
S. pyogenes
S. pneumoniae
S. -hemolyticus non group A
Staphylococci
Staphylococcus aureus,
including MRSA
Staphylococcus epidermidis
3. Other pathogenic organisms of
respiratory infections
Haemophillus influenzae
Klebsiella pneumoniae
Pseudomonas aeruginosa
4. Acid fast-bacilli
Course Requirements
Students will be expected to submit
lab reports and study sheets.
There will be 1 unit examination on OSPE.
Course Information
Prior to beginning any lab exercises,
students will get an introduction about
laboratory safety and microbiology
examination on respiratory infection.
Cucu/Mikro/FK-UPH/Nop.2008 4
EXERCISE 1
The Gram-Positive Pathogenic Cocci
BACKGROUND
Most medically important cocci are
species of Neisseria, Staphylococcus, and
Streptococcus. The members of the genus
Neisseria, being Gram-negative diplococci,
are readily separated from the other two
genera which are Gram-positive. The genus
Staphylococcus is differentiated from the
genus Streptococcus on the basis of
microscopic and colonial morphology and by
its ability to produce the enzyme catalase.
Staphylococcus aureus, the
pathogen, is best distinguished from
Staphylococcus epidermidis and form the
Micrococcus and other Gram-positive cocci
by its ability to produce the enzyme
coaguase.
Hemolytic reaction, differences in
cellular antigents, the requirements for
growth, are used to separate the organisms
within the genus Streptococus. The two
major pathogens of the Streptococcus,
namely Streptococcus pyogenes and
Streptococcus pneumoniae, are
differentiated on the basis that the former is
hemolytic when grown on blood agar and
sensitive by the disk assay, to 0,04 unit of
the antibiotic bacitracin. The latter organism
is observed to be -hemolytic and sensitive
by the disk assay to 5 mcg of optochin (ethyl
hydrocupreine hydrochloride). They also
soluble in various surface tension
depressants such as sodium dodecyl (lauryl)
sulfate (SDS). This exercise is designed to
familiarize the student with some methods
commonly used to identify the pathogenic
Gram-positive cocci.
GRAM STAIN
Staphylococci are a major
component of human normal flora but also
include species that are important
pathogents. On staining, they are appearing
as Gram-positive cocci in cluster (fig.1).
Prepare and examine a Gram
stained smear of each slide provided. Record
your observations in the table provided in
your laboratory report.
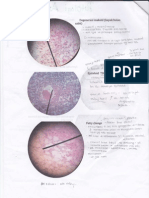
Fig.1 Staphylococcus sp. Gram stain from patients
specimen showing the typical Gram-positive cocci in
clusters. (Gram stain, x 1000)
Streptococci are Gram-positive cocci that
can be distinguished from staphylococci by
the catalase test. Streptococcus pyogenes,
a pathogen responsible for a range of
superficial and deep infections, is seen as
chain of cocci in Gram-stain preparation.
Cucu/Mikro/FK-UPH/Nop.2008
5
Streptococcus pneumoniae shows
characteristic diplococci on Gram staining.
Fig.2 Streptococcus pyogenes. Gram stain
showing typical Gram-positive cocci in chains.
Streptococci of different Lancefield groups have a
similar appearance. (Gram stain, x 1000).
Fig.3 Streptococcus pneumoniae. Gram stain of a
sputum specimen showing Gram-positive diplococci
with a lanceolate shape typical of S.pneumoniae
(pneumococcus). (Gram stain, x 1000)
CULTURE
Culture appearance of
Staphylococcus epidermidis and
Staphylococcus aureus on blood agar,
S.aureus is typically forms yellow or golden
colonies, and S.epidermidis or other
coagulase-negative staphylococci showing
white colonies. S.epidermidis is one of the
coagulase-negative staphylococci. They are
part of the normal skin flora, but may cause
infection in neonates, the
immunocompromised and patients with
indwelling devices.
Fig.4A Culture on blood agar (18 h at 37C).
Staphylococcus aureus showing typically form
yellow or golden colonies. Fig.4B Staphylococcus
epidermidis showing white colonies.
Fig.5 Staphylococcus aureus. Culture on mannitol
salt agar, showing yellow colonies. This is a selective
medium for recovering S.aureus when screening for
carriage in infection control investigations (Mannitol
salt agar, 18 h at 37C).
4A 4B
Cucu/Mikro/FK-UPH/Nop.2008
6
Fig.6 Staphylococcus epidermidis. Culture on
mannitol salt agar, showing growth but no
fermentation of manitol/pink colonies. (Mannitol salt
agar, 18 h at 37C).
Fig.7 Staphylococcus aureus. Culture on nutrient
slant agar, showing yellowgold pigment on the
colonies. Type of colonies are small, smooth, and
soliter.
TUBE COAGULASE TEST
The identification of Staphylococci is
based on microscope exam, colonial
morphology, and biochemical characteristics.
The ability for pathogenic Staphylococci, like
S.aureus to produce coagulase enzymes that
coagulate plasma continues to be the most
widely used and accepted criterion for
identification of these organisms. Coagulase
binds to plasma fibrinogen causing the
plasma to clot or positive reaction.
1. Homogenized one large loopful of
Staphylococcus aureus into a coagulase
plasma tube.
2. Incubate the tubes in the 37C incubator
for 24 hours. Tilt the tube gently when
examining. Do not shake! Any degree of
cloting is considered positive.
3. Record results on table provided in your
lab report.
Fig.8 Tube coagulase test
CATALASE TEST: AN EXAMPLE OF
A BIOCHEMICAL ASSAY
Test all of the slant culture for their
ability to produce the enzyme catalase. The
two genera differ in their ability to produce
this enzyme. Do not use culture grown on
blood agar for catalase test due to the
presence of catalase in red blood cells. Do
not reverse the order of procedure-this may
cause a false positive result.
Cucu/Mikro/FK-UPH/Nop.2008
7
1. With a sterile loop, place a heavy
inoculum from a slant culture on a clean
slide.
2. Add one drop 3% H2O over the
organisms on the slide.
3. Observe for immediate bubbling (oxygen
liberation) and record the result in the
laboratory report. Bubbling is a positive
reaction.
4. Repeat the procedure with the remaining
cultures.
5. Record results on table provided in your
lab report.
Fig.9 Catalase test. Member of the genus
Staphylococcus are catalase-positive, and members
of the genera Streptococcus and Enterococcus are
catalase-negative.
MRSA
The mecA gene is the structural
gene for a low-affinity Penicillin binding
protein that is found in methicillin/oxacillin-
resistance Staphylococcus aureus (MRSA)
strains.
Methods:
Solid Agar Media:
There are no universal standardized
methods for screening and isolation of
MRSA using solid agar media, 24-48 hrs
incubation is required for an acceptable
result.
o Mannitol Salt Agar containing 7%
NaCl with either 4mg/L methicillin or
2mg/L oxacillin;
o Oxacillin Resistant Screening Agar
with 5.5% NaCl and 2mg/L oxacillin;
o Baird Parker Medium with 8mg/L
ciprofloxacin;
o Mueller Hinton Agar with 4% NaCl
and 6mg/L oxacillin
Chromogenic media, agglutination
method, biochemical (commercial).
o CHROMagar MRSA(Bioconnections)
o MRSA Select (Bio-Rad)
o Oxacillin-resistant Staphylococcus
aureus agar (ORSA; Oxoid)
CHROMagar MRSA, MRSA Select are
MRSA broth all contained cefoxitin as the
selective agent. ORSA contained
oxacillin as the selective agent.
Broth based enrichment media
Molecular methods
Fig.10 Culture S.aureus on Mueller Hinton agar with
oxacillin disk, showing S.aureus resistant to oxacillin
disk. Methicillin resistance S.aureus positive.
Fig.11 MRSA growth on MRSA Select (Bio-Rad),
showing pink colonies.
Cucu/Mikro/FK-UPH/Nop.2008
8
BACITRACIN/OPTOCHIN TESTING
Streptococcus pyogenes is
susceptible to low levels of the antibiotic
bacitracin. Other -hemolytic Streptococci
are resistant to this antibiotic. A disk
containing bacitracin is placed in the heavily
inoculated area of a blood agar plate. After
overnight incubation, any zone of inhibition
around the disk is interpreted as susceptible
or a positive reaction.
Optochin susceptibility is used to
differentiate S.pneumoniae from other
Streptococci. A diskof optochin is placed on
the heavy area of the streak. After overnight
incubation, the zone of inhibition is
measured. A zone 14 mm indicative of
inhibition and indetifies the isolate as
S.pneumoniae.
1. On the most heavly inoculated areas of
your agar plate, using an alcohol flamed
forceps, place the P disk (Optochin)
and gently tap the disk for good surface
contact.
2. Incubate all blood plates for 24 hors at
37C.
Fig.12 Streptococcus pneumoniae. The plate
contains an optochin (P) disk, to wich
S.pneumoniae, in contrast to other hemolytic
Streptococci, is sensitive. (Blood agar, 18 h at 37C).
Fig.13 Streptococcus viridans. Culture on blood
agar, showing -hemolysis and optochin resistance.
Viridans streptococci are normal oral flora, but are an
important cause of bacterial endocarditis in patients
with congenital or acquired abnormalities og the heart
valves. (Blood agar, 18 h at 37C).
HEMOLYSIS TESTING
This testing will illustrate the
production of hemolysins by bacteria.
Hemolysin (enzymes) causes the lysis
(breakage) of red blood cell membranes. A
number of hemolysins are produced by
bacteria and differ from one another
according to the kind of red blood cell they
attact (human, rabbit, sheep, and horse), and
the type of lysis they cause. If red blood cells
(RBCs) are completely lysed, a clear area
surrounds the bacterial colony. This is called
-hemolysis. Partial destruction of red blood
cells yields a green, opaque zone around the
colony, is termed hemolysis.
To detect hemolysins, an agar
medium containing red blood cells (blood
agar plate) is used. A culture is streaked to
obtain isolated colonies. Hemolysins are
produced and secreted during microbial
growth. The hemolysins produced may
caused destruction of the RBC. Complete
hemolysis is called -hemolysis. Partial
hemolysis is called -hemolysis. The last
option is no hemolysis. Complete hemolysis
(-hemolysis) clears the area around the
Cucu/Mikro/FK-UPH/Nop.2008
9
colony because of complete RBC
destruction. When partial hemolysis (-
hemolysis) occurs, a cloudy zone (only by
S.aureus) or a greenish zone (all other
bacteria) around the colony is observed. The
color is due to the alteration of hemoglobin in
the RBC.
1. Streak a blood agar plate (to obtain
isolated colonies) with each of the
cultures provided.
2. Incubate the plates in the 37C incubator
for 24 hours.
Fig.14 Streptococcus pneumoniae (-hemolytic).
Culture on blood agar. Note the partial lysis of red
blood cells, producing a greenish discoloration. The
two most important groups are S.pneumoniae
(pneumococcus), a frequent cause of lobar
pneumonia, and the viridans group of streptococci.
(Blood agar, 18 h at 37C).
Fig.15 Streptococcus pyogenes (-hemolytic)
Culture on blood agar, showing complete lysis (beta)
of red blood cells and clearing of the medium around
the colony. Common pathogens which produce this
reaction are Groups A, B, C and some D streptococci,
as well as Staphylococcus aureus, the most common
pathogenic staphylococcus. (Blood agar, 18 h at
37C).
Cucu/Mikro/FK-UPH/Nop.2008 10
LABORATORY REPORT
EXERCISE 1
1. Look at the demonstration of Gram stain. Note the shape, size of bacteria, and their staining
properties.
2. Look at the demonstration carefully. Note the organisms which are catalase positive.
Cucu/Mikro/FK-UPH/Nop.2008
11
3. Look at the colony morphology carefully. Look closely for hemolysis by holding the plate up to
a light source (toward the window). Wchich ones are hemolytic? Which have -hemolysis? Are
they considered pathogenic? Which are hemolytic?
Cucu/Mikro/FK-UPH/Nop.2008 12
EXERCISE 2
The Other Pathogenics of Respiratory Infections
KLEBSIELLA PNEUMONIAE
Many aerobic gram negative bacilli
are classified under the family
Enterobacteriaceae, including Klebsiella
pneumoniae. The tribe Klebsiella includes
four major genera --- Klebsiella,
Enterobacter, Hafnia, and Serratia --- each of
which includes several species that are overt
and opportunistic pathogens in human.
Gram staining cannot differentiate
between the member of genera
Enterobacteriaceae.
Fig.16 Klebsiella pneumoniae. A Gram stain of
patients specimen showing characteristic short,
plump, Gram- negative, bacilli. typical member of the
Enterobacteriaceae (Gram stain, x 1000).
K.pneumoniae is most frequently
recovered from clinical specimens and can
cause a classic form of primary pneumonia.
It is infrequently found in the oropharynx of
normal persons (1-6% carrier rate), however
a prevalence as high as 20% may occur in
hospitalized patients. This colonization may
prove to be the source of lung infections that
generally occur in patients with debilitating
conditions such as alcoholism, diabetes
militus, and chronic obstructive pulmonary
disease.
Fig.17 Indian ink capsule stain of Klebsiella
pneumoniae showing white capsules (glycocalyx)
surrounding purple cells.
A wide range of sugar fermentation
and other biochemical tests may be used to
differentiate between the different
Enterobacteriaceae. Distinguishing
K.pneumoniae from other
Enterobacteriaceae is primarily done on the
basis of indole, methyl red voges-proskauer
and citrate (IMViC) test.
Cucu/Mikro/FK-UPH/Nop.2008
13
Fig.18 Klebsiella pneumoniae culture on blood agar,
showing the mocoid lactose-fermenting colonies. The
cells are protected by polysaccharide capsules (which
cause the slimy appearance of the colonies) against
defense reactions of the host. (18 h at 37C).
Fig.19 Biochemical set for K. pneumoniae:
Tube indole = Negative.
TSI slant = Acid / acid with some gas production, no
hydrogen disulfide production.
LIA slant = Lysine decarboxylase positive, deaminase
negative.
MIO agar = Motility negative, ornithine decarboxylase
negative.
Urea slant = Urease negative.
Citrate slant = Negative.
Sorbitol broth = Positive.
PSEUDOMONAS AERUGINOSA
Pseudomonas aeruginosa is a
Gram-negative, aerobic rod belonging to the
bacterial family Pseudomonadaceae.
Like other members of the genus,
Pseudomonas aeruginosa is a free-living
bacterium, commonly found in soil and water;
and has become increasingly recognized as
an emerging opportunistic pathogen of
human. It is occurrence as a nosocomial
pathogen and indicate that antibiotic
resistance is increasing in clinical solates.
It causes urinary tract infections, respiratory
system infections, dermatitis, soft tissue
infections, bacteremia, bone and joint
infections, gastrointestinal infections and a
variety of systemic infections, particularly in
patients with severe burns and in cancer and
AIDS patients who are immunosuppressed.
Minimum requirements for definitive
identification of Pseudomonas aeruginosa
are Gram-negative rods, oxidative positive,
typical smell (fruity grape-like odor or corn
tortilla), colony on blood or chocolate agar as
large colonies with metallic sheen-mucoid-
rough-or pigmented/pyocianin and often -
hemolytic.
Fig.20 Pseudomonas aeruginosa. A Gram stain of
patients specimen showing thin, gram-negative bacilli.
(Gram stain, x 1000)
Fig.21 Pseudomonas aeruginosa. Culture on
nutrient agar, produce a greenish pyocyanin pigmen,
non-lactose fermenter. It is oxidative positive. (18 h at
37C).
Cucu/Mikro/FK-UPH/Nop.2008
14
HAEMOPHILLUS INFLUENZAE
Haemophillus spp. include a number
of species pathogenic to humans. Members
of genus Haemophillus are small, nonmotile,
gram-negative bacilli that require factors
present in blood for growth. Some
Haemophillus species require X factors, that
are provided by several iron-containing
pigments (e.g., hemin, hematin,
protoporphyrin IX). These compounds are
used in the synthesis of catalase,
peroxidase, and cytochromes of the electron
transport system). Most Haemophillus spp.
also require V factors, which is
NAD/coenzyme I or NADP/coenzyme II. Both
X and V factors are found within red blood
cells, including the sheep erythrocytes found
in blood agar formulation routinely used in
clinical laboratory.
Haemophillus spp. include a number
of species pathogenic to humans.
Haemophillus influenzae has both
capsulated and non-capsulated strains and
requires both factors (X and V), which are
provided by heated blood agar (chocolate
agar).
Fig.22 Haemophillus influenzae,Gram stain of sputum
showing gram-negative cocco bacilli. (Gram stain, x
1000)
Fig.23 Haemophillus influenzae, chocolate agar. This
organism require the growth factors hemin (X factor)
and nicotinamide adenine dinucleotide (NAD, V
factor). V factor is released when blood is heated. The
colonies are grey with mucoid appreance. (Heated
blood agar, 18 h at 37C).
Fig.24A Growth factor test for Haemophillus
influenzae. Growth on nutrient agar with X, V, and XV
disks. H.influenzae requires both X and V factors, and
only grows around the disk containing X and V.
(nutrient agar, 18 h at 37C)
Cucu/Mikro/FK-UPH/Nop.2008
15
Fig.24B Growth factor test for Haemophillus
influenzae. Growth on nutrient agar with X, V, and XV
disks. H.influenzae requires both X and V factors, and
only grows around the disk containing X and V.
(nutrient agar, 18 h at 37C)
Cucu/Mikro/FK-UPH/Nop.2008 16
LABORATORY REPORT
EXERCISE 2
1. Look at the demonstration of Gram stain. Note the shape, size of bacteria, and their staining
properties.
2. Look at the demonstration carefully. Note and look closely the colony morphology carefully.
Cucu/Mikro/FK-UPH/Nop.2008
17
3. Look at the demonstration of biochemical test carefully. Note the results of the biochemical test
according to the organism.
Cucu/Mikro/FK-UPH/Nop.2008 18
EXERCISE 3
Acid Fast Bacilli
Mycobacteria, include the important
pathogens of tuberculosis and leprosy, as
well as a large number of environmental
strains that opportunistic pathogens.
Mycobacteria can be recovered from a
variety of clinical specimens, including upper
respiratory specimens (sputum, bronchial
washes, bronchoalveolar lavage, and
bronchial biopsies); urine, feces,
cerebrospinal fluid (CSF); tissue biopsies,
and deep needle aspirations of virtually any
tissue or organ. Specimen that may contain
normal bacterial flora should be processed
as soon as possible to minimize the degree
of overgrowth with specimen contaminants.
SPUTUM SPECIMEN
Sputum samples collected by
expectoration or by ultrasonic nebulization
are best obtained shortly after the patient
awakens in the morning, when mycobacteria
are at their highest concentration.
Culture obtained from patients with
pulmonary or renal tuberculosis, in particular,
may be positive one day but particular, may
be positive one day but negative the next;
thus, a minimum of three early-morning
sputum or five early-morning urine
specimens should be collected on
successive 24-hours periods to maximize the
chance of recovery of mycobacteria. All
specimens should be transported promptly to
the laboratory and refrigerated if processing
is delayed.
Twenty-four-hour collections are now
discouraged because the sample containing
the highest concentration of mycobacteria
will be proportionately diluted by subsequent
low-yield-samples, and the chances for
bacterial and fungal overgrowth during the
prolonged collection process are significantly
increased.
Fig.25 Purulent sputum from the lower respiratory
tract.
Fig.26 This Gram-staining of a sputum from the "Atlas of
Bacteriology" (E. & S. Liningstone, Ltd., Edinburgh, 1947)
shows two chains of four and five cells, respectively, of
Gram-positive Enterococcus hirae (formerly called
Streptococcus faecalis or faecium). Also present are Gram-
negative Haemophilus influenza (the plentiful small rods)
and Diplococcus catarrhalis (the pairs in the upper right) as
well as some yeast cells.
Cucu/Mikro/FK-UPH/Nop.2008
19
ACID FAST STAINING
The cell walls of mycobacteria,
because of their high lipid content, have the
unique capability of binding the fuschin dye
so that it is not removed (destained) by acid
alcohol. This acid fast staining reaction of
mycobacteria, along with their characteristic
size and shape, is a valuable aid in the early
detection of infection and in the morning
therapy for mycobacterial disease.
Acid fast smears are also useful in
following response to treatment. After
antimycobacterial drugs are started, cultures
become negative before the smear do,
suggesting that the organisms are not
capable of replicating but are capable of
binding the stain.
Two types of acid fast stains are
commonly used:
1. Carbolfuchsin stain: a mixture of fuchsin
with phenol (carbolic acid)
Ziehl-Neelsen (hot stain)
Kinyoun (cold stain)
2. Fluorochrome stain: auramine O, with or
without a second fluorochrome,
rhodamine
Mycobacteria are stained by Ziehl-Neelsen
method, showing pink, acid fast bacilli.
Fig.27 Mycobcaterium tuberculosis. ZN staining of
sputum from a patient with tuberculosis showing pink,
acid fast bacilli against a blue background of pus cells.
(ZN stain, x1000).
Fig.28 Mycobacterium tuberculosis, auramine phenol
fluorochrome. Auramine is a fluorochrome, a dye that
fluorescen when illuminated by UV light. Tubercle
bacilli fluoresce a white-yellow color. (fluorochrome
stain, x1000).
Ziehl-Neelsen Procedures
Carbolfuchsin: dissolve 3 g of basic fuchsin
in 10 ml of 90-95% ethanol. Add 90 ml of 5%
aqueous solution of phenol.
Acid-alcohol: add 3 ml of concentrated HCL
slowly to 97 ml of 90-95% ethanol, in the
order. Solution may get hot!!
Methylene blue counterstain: dissolve 0,3
g of methylene blue chloride in 100 ml of
distilled
Procedure:
1. Apply of carbolfuchsin stain to thoroughly
moistened filter paper.
2. Heat the stain-covered slide to steaming,
but do not allow drying.
3. Heating may be done by gas burner or
over an electric staining rack.
4. Stay the carbolfuchsin stain for about 5
minute.
5. Rinse slide with water, and drain.
6. Decolorized with acid-alcohol until no
more stain appears in the washing.
7. Counterstain with methylene blue for 10-
20 second.
8. Rinse, drain, and air dry.
9. Examine with 100 x oil-immersion
objective.
Cucu/Mikro/FK-UPH/Nop.2008
20
Quantitative scale
ACID FAST STAINING
Culture media containing inhibitory
agent are used to suppress bacterial and
fungal contamination.
Mycobacteria have exacting
nutritional requirements, and will not grow on
ordinary media. Lowenstein-Jensen (LJ)
medium has ingredients including eggs,
glycerol and malachite green (an inhibitory
agent). Most mycobacteria are slow growing,
taking 4-12 weeks to produce visible
colonies.
Fig. 29 Mycobacterium tuberculosis, Lowenstein-
Jensen (LJ) medium. Culture on LJ medium after 6
weeks. The mycobacteria produce creamy,
breadcrumb-like colonies (LJ medium, 6 weeks at
37C)
NIACIN ACCUMULATION TEST
All Mycobacterium species produce
niacin ribonucleotide; however, virtually all
strains of M.tuberculosis lake the enzyme to
further convert niacin to nicotinamide
adenine dinucleotide (NAD). Niacin
accumulates in the culture medium, from
which it can be extracted with sterile water or
physiologic saline. The extract is placed in a
small test tube to which is added a reagent-
impregnated niacin filter strip.
Fig.30 A niacin-accumulation test. Mycobacterium
tuberculosis, the fluid in the tube on the right had been
extracted from the surface of LJ culture medium. The
development of a yellow color on the strip and in the
underlying fluid indicates the presence of niacin.
Cucu/Mikro/FK-UPH/Nop.2008 21
LABORATORY REPORT
EXERCISE 3
1. Look at the demonstration of Ziehl-Neelsen stain. Compare with yours ZN-stain, sketch
representative field of each slide, and indicate which organisma are acid fast bacilli.
2. Look at the demonstrations carefully. Note and sketch all the demonstration.
Cucu/Mikro/FK-UPH/Nop.2008
22
Cucu/Mikro/FK-UPH/Nop.2008 23
REFERENCE
1. Bey FR. Microbiology Laboratory Manual. Wadsworth Group-Brooks/Cole. 2001
2. Winn W., Allen S., Janda W., Koneman E., Procop G., et.al. Color atlas and textbook of
diagnostic microbiology. 6
th
ed. Lippincott William and Wilkins. USA. 2006.
3. Hart T., Shears P. Color atlas of medical microbiology. 2
th
ed. Elsevier. USA. 2004.
4. Partakusuma LG., Sudiro TM., Kadarsih R., Trisnawati E., Murwheni WD., Harun S., et.al.
Pemeriksaan mikroskopis tuberculosis. Departemen Kesehatan RI. Jakarta. 2006.
5. http://www.mgm.ufl.edu/
6. http://teaching.path.cam.ac.uk/partlB_pract/P20
You might also like
- Microbiology PDFDocument71 pagesMicrobiology PDFDanny Alexander TullumeNo ratings yet
- Standard Operating Procedure for Eye CultureDocument2 pagesStandard Operating Procedure for Eye CultureSemeeeJuniorNo ratings yet
- Microbiology Lab Manual - Revised Spring 2013Document117 pagesMicrobiology Lab Manual - Revised Spring 2013Mbiko SabeyoNo ratings yet
- Ear Cultures Principle: 3.6.12 Sop: Ear Culture Page 1 of 2Document2 pagesEar Cultures Principle: 3.6.12 Sop: Ear Culture Page 1 of 2SemeeeJuniorNo ratings yet
- Campylobacter: Features, Detection, and Prevention of Foodborne DiseaseFrom EverandCampylobacter: Features, Detection, and Prevention of Foodborne DiseaseGünter KleinNo ratings yet
- Identification of BacteriaDocument4 pagesIdentification of BacteriaExamville.comNo ratings yet
- Food Microbiology PDFDocument20 pagesFood Microbiology PDFbyagniNo ratings yet
- Experiment 3 Cultivation and Sub-Culturing of Microbes: StructureDocument8 pagesExperiment 3 Cultivation and Sub-Culturing of Microbes: StructureGurpreet Singh100% (2)
- Pseudomonas aeruginosa Characteristics and Laboratory DiagnosisDocument6 pagesPseudomonas aeruginosa Characteristics and Laboratory DiagnosisankitamicroNo ratings yet
- Culture Media and MethodsDocument49 pagesCulture Media and Methodscj bariasNo ratings yet
- Exercise 1: Tools in The Laboratory: 1. AutoclaveDocument48 pagesExercise 1: Tools in The Laboratory: 1. AutoclaverayaimNo ratings yet
- rr212302 MicrobiologyDocument4 pagesrr212302 MicrobiologySrinivasa Rao GNo ratings yet
- Equipments and Materials Used in MCB LabDocument14 pagesEquipments and Materials Used in MCB LabBarry Allen100% (1)
- Secondary MetabolliteDocument20 pagesSecondary MetabolliteChindieyciiEy Lebaiey CwekZrdoghNo ratings yet
- Preparation, Dispensing and Sterilizing of Media For Cultivation of MicroorganismDocument11 pagesPreparation, Dispensing and Sterilizing of Media For Cultivation of Microorganismkolita kamal100% (1)
- B.sc. Medical Laboratory Sciences Medical Bacteriology (MLS 2413) (PDFDrive)Document101 pagesB.sc. Medical Laboratory Sciences Medical Bacteriology (MLS 2413) (PDFDrive)Isah MohammedNo ratings yet
- Measurement of Microbial GrowthDocument27 pagesMeasurement of Microbial GrowthDr. Kalavati PrajapatiNo ratings yet
- Chapter 1 Microbiology PDFDocument79 pagesChapter 1 Microbiology PDFpizzaNo ratings yet
- Bacteriology of Water and Analysis: BasicsDocument37 pagesBacteriology of Water and Analysis: Basicstummalapalli venkateswara raoNo ratings yet
- Other Body FluidsDocument34 pagesOther Body FluidsNajibah A. CasimNo ratings yet
- Medical Parasitology Lab GuideDocument10 pagesMedical Parasitology Lab Guideskeltenboi100% (1)
- 2.medical HelminthologyDocument148 pages2.medical HelminthologyHanifatur Rohmah100% (2)
- SerialDilutions PDFDocument5 pagesSerialDilutions PDFAmelia_KharismayantiNo ratings yet
- Techniques For Collection, Isolation and Preservation of MicroorganismsDocument42 pagesTechniques For Collection, Isolation and Preservation of Microorganismsaziskf100% (2)
- Laboratory Exercises in Microbiology - Discovering The Unseen WorlDocument208 pagesLaboratory Exercises in Microbiology - Discovering The Unseen WorlJennybabe PartozaNo ratings yet
- Guidelines for Lab Safety and EquipmentDocument61 pagesGuidelines for Lab Safety and EquipmentMadhu ShaliniNo ratings yet
- MICR3213 - Microbial Ecology - 2018 PDFDocument44 pagesMICR3213 - Microbial Ecology - 2018 PDFCalesia FearonNo ratings yet
- Sterilization and Aseptic TechniqueDocument3 pagesSterilization and Aseptic TechniqueE.coilman100% (3)
- Isolation of BacteriaDocument7 pagesIsolation of BacteriaMuhammad Faraz SaleemNo ratings yet
- Specimen Collection and ProcessingDocument18 pagesSpecimen Collection and ProcessingShopee PremiumismeNo ratings yet
- Anaerobic Culture .: Ahmed GomaaDocument42 pagesAnaerobic Culture .: Ahmed Gomaaah7510No ratings yet
- Collection, Transport and Storage of Specimens For Laboratory DiagnosisDocument25 pagesCollection, Transport and Storage of Specimens For Laboratory DiagnosisBrihadishwaran ThiyagarajanNo ratings yet
- Fungal Infections in IcuDocument195 pagesFungal Infections in IcubhumikaNo ratings yet
- Viral ReplicationDocument1 pageViral ReplicationMoh'd Ghanayem100% (1)
- BT-303 Lab ManualDocument21 pagesBT-303 Lab ManualZakaullah Akhtar50% (2)
- Microbiology PosterDocument1 pageMicrobiology PosterAgnieszkaNo ratings yet
- Growth-Requirement-of-Bacteria-Growth-Curve2 - Culture Media Updated-1Document72 pagesGrowth-Requirement-of-Bacteria-Growth-Curve2 - Culture Media Updated-1Tania.dmp20No ratings yet
- Antibiotic Sensitivity TestDocument2 pagesAntibiotic Sensitivity Testganesh2gigNo ratings yet
- 3 Concentration Techniques SedimentationDocument18 pages3 Concentration Techniques SedimentationFatihah JahsmiNo ratings yet
- Microorganism Classification OverviewDocument4 pagesMicroorganism Classification OverviewMaeve Ylain SeanNo ratings yet
- Lec 9 MalariaDocument42 pagesLec 9 MalariaMye AkmaNo ratings yet
- Appropriate Clinical Specimens Collection and Transport For Diagnostic VirologyDocument48 pagesAppropriate Clinical Specimens Collection and Transport For Diagnostic Virologylong thomNo ratings yet
- Biotechnology Lab ManualDocument34 pagesBiotechnology Lab Manualanon_348923763No ratings yet
- Pathogens - TRAINING IN MICROBIOLOGICAL ANALYSIS OF FOODDocument52 pagesPathogens - TRAINING IN MICROBIOLOGICAL ANALYSIS OF FOODclairealbertiniNo ratings yet
- Major and Minor Elements.Document5 pagesMajor and Minor Elements.Md Ahsanul HaqueNo ratings yet
- 1 Staphylococcus Lecture 1 Last YearDocument39 pages1 Staphylococcus Lecture 1 Last YearKeshant Samaroo100% (1)
- StaingDocument29 pagesStaingVijay Bhasker TekulapallyNo ratings yet
- NMEICT-MHRD Fermentation Media DesignDocument4 pagesNMEICT-MHRD Fermentation Media DesignriyaNo ratings yet
- Microbiology Poster KeeleDocument1 pageMicrobiology Poster KeeleAgnieszkaNo ratings yet
- Malaysia: JIB MicrobiologyDocument4 pagesMalaysia: JIB MicrobiologyArunaNo ratings yet
- Xylose Lysine Desoxycholate (XLD) Agar: National Standard MethodDocument7 pagesXylose Lysine Desoxycholate (XLD) Agar: National Standard MethodMohamedNo ratings yet
- Sample CollectionDocument20 pagesSample Collectionmaan khanNo ratings yet
- Factors Affecting The Growth of BACTERIADocument4 pagesFactors Affecting The Growth of BACTERIAfarijan100% (1)
- Classification and Indentification of BacteriaDocument77 pagesClassification and Indentification of BacteriaAnand MadhavanNo ratings yet
- The Microbiology Manual PDFDocument136 pagesThe Microbiology Manual PDFMohammad Yasir100% (1)
- Microbiology Methods of Monitoring PopulationsDocument17 pagesMicrobiology Methods of Monitoring PopulationsStephen MooreNo ratings yet
- Steri ZL IzationDocument19 pagesSteri ZL Izationأبو محمدNo ratings yet
- A. Neisseria Gonorrhoeae B. Moraxella Catarrhalis C. Neisseria Meningitidis D. Neisseria LactamicaDocument7 pagesA. Neisseria Gonorrhoeae B. Moraxella Catarrhalis C. Neisseria Meningitidis D. Neisseria LactamicaWynlor AbarcaNo ratings yet
- Module 4 Annex Part 1 MKDocument95 pagesModule 4 Annex Part 1 MKtata_77No ratings yet
- Cyto Presentation 1Document12 pagesCyto Presentation 1DevindraPrptNo ratings yet
- Img 0004Document1 pageImg 0004DevindraPrptNo ratings yet
- Degenerasi jaringan lemak dan epiteloid sel TBCDocument1 pageDegenerasi jaringan lemak dan epiteloid sel TBCDevindraPrptNo ratings yet
- Brainstem Lesions GuideDocument107 pagesBrainstem Lesions GuideAndrei ChyzhNo ratings yet
- Grey'sDocument2 pagesGrey'sDevindraPrptNo ratings yet
- Parents Unaware of Backpack Weight and ContentsDocument15 pagesParents Unaware of Backpack Weight and ContentsDevindraPrptNo ratings yet
- Heavy Backpacks Harmful To Students, Simmons Study SaysDocument9 pagesHeavy Backpacks Harmful To Students, Simmons Study SaysDevindraPrptNo ratings yet
- Pediatric NotesDocument20 pagesPediatric Notestweet196% (23)
- UKDIDocument1 pageUKDIDevindraPrptNo ratings yet
- Sepsis Flow Chart FinalDocument2 pagesSepsis Flow Chart FinalDevindraPrptNo ratings yet
- Lesson 1Document20 pagesLesson 1Irish Jean AgsawayNo ratings yet
- MCQ on Types of GlomerulonephritisDocument3 pagesMCQ on Types of GlomerulonephritisIM CT50% (2)
- Femur and Pelvis Fracture (Trauma)Document20 pagesFemur and Pelvis Fracture (Trauma)Ree YahNo ratings yet
- C Anticholinergic Drugs PDFDocument53 pagesC Anticholinergic Drugs PDFHitesh karnNo ratings yet
- Everything You Need to Know About Artificial EyesDocument24 pagesEverything You Need to Know About Artificial Eyesapoorva1509No ratings yet
- IV Therapy SsDocument56 pagesIV Therapy Sssaeed_chohan100% (8)
- Kurt Cobain Case StudyDocument11 pagesKurt Cobain Case StudyKC Respicio67% (3)
- GetPdf Cgi PDFDocument9 pagesGetPdf Cgi PDFAKNTAI002No ratings yet
- Body's Battles (gnv64) PDFDocument96 pagesBody's Battles (gnv64) PDFameyparanjape100% (1)
- Diabetes Care GuideDocument14 pagesDiabetes Care GuideadaezeNo ratings yet
- The China Study PDFDocument7 pagesThe China Study PDFPatrice108365No ratings yet
- Dementia Incidence, Burden and Cost of Care: A Filipino Community-Based StudyDocument9 pagesDementia Incidence, Burden and Cost of Care: A Filipino Community-Based StudyVanessa PalomaNo ratings yet
- Elevated Serum Immunoglobulin E Level As A Marker For Progression Ofimmunoglobulin A NephropathyDocument5 pagesElevated Serum Immunoglobulin E Level As A Marker For Progression Ofimmunoglobulin A Nephropathyagustinaw1981No ratings yet
- Feline Asthma: Laura A. Nafe, DVM, MS, Dacvim (Saim)Document5 pagesFeline Asthma: Laura A. Nafe, DVM, MS, Dacvim (Saim)Miruna ChiriacNo ratings yet
- Nephrons (Functional Unit)Document44 pagesNephrons (Functional Unit)Nur SanaaniNo ratings yet
- 6 5 Nerves Hormones and HomeostasisDocument19 pages6 5 Nerves Hormones and Homeostasisapi-235355872No ratings yet
- How To Maintain Throat HygieneDocument9 pagesHow To Maintain Throat Hygieneankita singhNo ratings yet
- Cataract BookDocument261 pagesCataract Bookstarsk777No ratings yet
- Stanford Letter Criticizes Trump COVID-19 AppointeeDocument11 pagesStanford Letter Criticizes Trump COVID-19 AppointeeBayAreaNewsGroup100% (2)
- The Natural Way To A Healthy HealingDocument111 pagesThe Natural Way To A Healthy HealingAnjanaNo ratings yet
- KenyaEMR v17.3.1 Release Notes SummaryDocument3 pagesKenyaEMR v17.3.1 Release Notes SummaryMigori Art DataNo ratings yet
- Definition of NephrolithiasisDocument30 pagesDefinition of Nephrolithiasiszz_13No ratings yet
- Hangnails and HomoeopathyDocument7 pagesHangnails and HomoeopathyDr. Rajneesh Kumar Sharma MD HomNo ratings yet
- A Review of Subdural Empyema and Its Management.6Document5 pagesA Review of Subdural Empyema and Its Management.6PatriciaChRistianiNo ratings yet
- Physical Examination AbdomenDocument249 pagesPhysical Examination AbdomenDrbee10100% (1)
- Abu Dhabi DRGDocument62 pagesAbu Dhabi DRGDolly creationsNo ratings yet
- US Elsevier Health Bookshop - Mosby, Saunders, Netter & MoreDocument4 pagesUS Elsevier Health Bookshop - Mosby, Saunders, Netter & MoreWilmer Zambrano GuerreroNo ratings yet
- CH 14 Part 1 Principles of Disease & Epidemiology (FA20)Document15 pagesCH 14 Part 1 Principles of Disease & Epidemiology (FA20)sammy alanNo ratings yet
- List of Empanelled Hospitals in CGHS NagpurDocument58 pagesList of Empanelled Hospitals in CGHS NagpurRajatNo ratings yet
- Windkessel EffectDocument11 pagesWindkessel EffectAkhmad HidayatNo ratings yet