Professional Documents
Culture Documents
Diagnosis and Management For Urosepsis
Uploaded by
FitrianiMappasere0 ratings0% found this document useful (0 votes)
28 views8 pagesUrosepsis is defined as sepsis caused by a urogenital tract infection. The urinary tract is the infection site of severe sepis or septic shock in approximately 10-30% of cases.
Original Description:
Original Title
iju12200.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentUrosepsis is defined as sepsis caused by a urogenital tract infection. The urinary tract is the infection site of severe sepis or septic shock in approximately 10-30% of cases.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
28 views8 pagesDiagnosis and Management For Urosepsis
Uploaded by
FitrianiMappasereUrosepsis is defined as sepsis caused by a urogenital tract infection. The urinary tract is the infection site of severe sepis or septic shock in approximately 10-30% of cases.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 8
Review Article
Diagnosis and management for urosepsis
Florian ME Wagenlehner,
1
Christoph Lichtenstern,
2
Caroline Rolfes,
4
Konstantin Mayer,
3
Florian Uhle,
2
Wolfgang Weidner
1
and Markus A Weigand
2
1
Clinic of Urology, Pediatric Urology and Andrology,
2
Clinic of Anesthesiology and Surgical Intensive Care Medicine,
3
Clinic of
Medicine II, Justus-Liebig-University Gieen, Giessen, and
4
Clinic of Anesthesiology and Surgical Intensive Care Medicine,
Phillips-University, Marburg, Germany
Abbreviations & Acronyms
aPTT = activated partial
thromboplastin time
ARDS = acute respiratory
distress syndrome
BLI = b-lactamase inhibitor
HMGB1 = high-mobility
group box 1 protein
IL = interleukin
INR = international
normalized ratio
MAP = mean arterial
pressure
PAF = platelet-activating
factor
PAMP = pathogen-associated
molecular patterns
PRR = pattern-recognition
receptors
SBP = systolic blood
pressure
SD = standard deviation
SIRS = systemic
inammatory response
syndrome
TLR = Toll-like receptors
TNF = tumor necrosis
factor
UTI = urinary tract
infections
WBC = white blood cells
Correspondence: Florian ME
Wagenlehner M.D., Ph.D., Clinic
of Urology, Pediatric Urology
and Andrology,
Justus-Liebig-University
Gieen, Rudolf-Buchheim Street
7, Giessen 35392, Germany.
Email: wagenlehner@aol.com
Received 2 March 2013;
accepted 29 April 2013.
Online publication 29 May 2013
Abstract: Urosepsis is dened as sepsis caused by a urogenital tract infection. Uro-
sepsis in adults comprises approximately 25% of all sepsis cases, and is in most cases
due to complicated urinary tract infections. The urinary tract is the infection site of
severe sepsis or septic shock in approximately 1030% of cases. Severe sepsis and septic
shock is a critical situation, with a reported mortality rate nowadays still ranging from
30% to 40%. Urosepsis is mainly a result of obstructed uropathy of the upper urinary
tract, with ureterolithiasis being the most common cause. The complex pathogenesis of
sepsis is initiated when pathogen or damage-associated molecular patterns recognized
by pattern recognition receptors of the host innate immune system generate pro-
inammatory cytokines. A transition from the innate to the adaptive immune system
follows until a T
H2
anti-inammatory response takes over, leading to immunosuppres-
sion. Treatment of urosepsis comprises four major aspects: (i) early diagnosis; (ii) early
goal-directed therapy including optimal pharmacodynamic exposure to antimicrobials
both in the plasma and in the urinary tract; (iii) identication and control of the compli-
cating factor in the urinary tract; and (iv) specic sepsis therapy. Early adequate tissue
oxygenation, adequate initial antibiotic therapy, and rapid identication and control of
the septic focus in the urinary tract are critical steps in the successful management of a
patient with urosepsis, which includes early imaging, and an optimal interdisciplinary
approach encompassing emergency unit, urological and intensive-care medicine
specialists.
Key words: sepsis therapy, systemic inammatory response syndrome, urinary tract
infections, urosepsis.
Introduction
Severe sepsis and septic shock is a critical situation, with recently reported mortality rates
ranging from 28.3% to 41.1%.
1
The infection sites leading to severe sepsis or septic shock
are pneumonia in approximately 45% of patients, and urinary tract infections ranging from
9% to 31% of patients, depending on the geographic region, followed by abdominal sites
ranging from 19% to 32%.
1
Frequent causes for urosepsis are obstructive diseases of the urinary tract, such as ureteral
stones, anomalies, stenosis or tumor. Urosepsis can also occur after interventions in the
urogenital tract, such as intrarenal surgery or transrectal prostate biopsy.
2,3
In patients with
nosocomial UTI treated in urology, the prevalence of urosepsis was on average approxi-
mately 12%,
4
whereas in patients with nosocomial UTI treated in other specialities, the
prevalence for severe sepsis was 2% and for septic shock 0.3%.
5
Denition of urosepsis
Urosepsis is dened as sepsis caused by infection of the urogenital tract, and is a systemic
response to infection. The signs and symptoms of SIRS that were initially considered to be
mandatory for the diagnosis of sepsis
6,7
are now considered to be alerting symptoms
(Table 1).
8
Many other clinical or biological symptoms must be considered.
bs_bs_banner
International Journal of Urology (2013) 20, 963970 doi: 10.1111/iju.12200
2013 The Japanese Urological Association 963
The diagnostic criteria of sepsis should enable physicians
and emergency doctors to identify patients at an early stage
of the syndrome, to allow for early therapy and treatment
within the rst hours.
Pathophysiology of urosepsis
Despite microbial pathogens being the underlying factor,
eventually the host is driving the disease.
PAMP of the bacterial cells, such as lipopolysaccharides,
interact with PRR on cell membranes of the cells of the
innate and adaptive immune system of the host.
Complete bacteria or bacterial ingredients of the cell wall,
such as lipopolysaccharides, of Gram-negative bacteria or
peptidoglycane, teichon- or lipoteichon acids of Gram-
positive bacteria (PAMP)
9
bind to cellular receptors and
coreceptors, such as CD 14, Toll-like receptors TLR2 and
TLR4, CD 18 and selectin (PRR) on the surface of macro-
phages, neutrophils, endothelial cells or even urothelial
cells. Intracellular messengers, such as nuclear factor-kB
and protein-kinase C, are activated, which induce transcrip-
tion of mediator cytokines, such as IL-1, IL-6, IL-8, TNF
and PAF. These factors can be cooperative or antagonistic,
incorporating other mediators, such as chemokines, prostag-
landines, thromboxane, leukotriene or nitric oxide.
1013
IL-17 cytokines provide the crosstalk between lymphocytes
and phagocytes, and thus link the innate and the adaptive
immune system.
14
In the course of sepsis and the treatment of it, cells might
undergo necrosis, releasing so-called alarmins, such as
HMGB1, that are also able to stimulate PRR. Alarmins and
PAMP are nowadays seen together, and termed as damage-
associated molecular patterns.
14
Initial activation of the complement system and cellular
innate immune responses by bacteria in the urogenital
tract produces an overwhelming pro-inammatory reaction
involving neutrophils and macrophages. Hematopoetic
growth factors are stimulated leading to the formation
of new neutrophils and the release of stored neutrophils.
The neutrophils are additionally activated and produce
bactericidal substances, such as proteases and oxygen
radicals. B- and T-lymphocytes are stimulated for synthesis
of antibodies and cellular immune-reaction. Secondary
mediators amplify this process leading to a severe
pro-inammatory burst. In this process, the muscular
protein is degraded, and the released amino acids are used
for antibody synthesis. In endothelial cells, the production
of PAF and nitric oxide is triggered, which leads to a
decreased vessel tone resulting in hypotension. The
endothelial cells are damaged and an increased permeabil-
ity results, leading to edema formation in the various
tissues.
Most patients, however, survive this initial pro-
inammatory phase. This initial phase is followed by a
Table 1 Clinical diagnostic criteria for sepsis and severe
sepsis
6,8,28
Infection, documented or suspected, and some of the
following:
General variables
Fever (>38.3C)
Hypothermia (core temperature <36C)
Heart rate >90/min or more than two SD above the normal
value for age
Tachypnea
Altered mental status
Signicant edema or positive uid balance (>20 mL/kg over
24 h)
Hyperglycemia (plasma glucose >140 mg/dL or 7.7 mmol/L)
in the absence of diabetes
Inammatory variables
Leukocytosis (WBC count >12 000/mL)
Leukopenia (WBC count <4 000/mL)
Normal WBC count with greater than 10% immature forms
Plasma C-reactive protein more than two SD above the
normal value
Plasma procalcitonin more than two SD above the normal
value
Hemodynamic variables
Arterial hypotension (SBP <90 mmHg, MAP <70 mmHg, or
an SBP decrease >40 mmHg in adults or less than two SD
below normal for age)
Organ dysfunction variables
Arterial hypoxemia (Pao2/Fio2 <300)
Acute oliguria (urine output <0.5 mL/kg/h for at least 2 h
despite adequate uid resuscitation)
Creatinine increase >0.5 mg/dL or 44.2 mmol/L
Coagulation abnormalities (INR >1.5 or aPTT >60 s)
Ileus (absent bowel sounds)
Thrombocytopenia (platelet count <100 000/mL)
Hyperbilirubinemia (plasma total bilirubin >4 mg/dL or
70 mmol/L)
Tissue perfusion variables
Hyperlactatemia (>1 mmol/L)
Decreased capillary rell or mottling
Severe sepsis denition = sepsis-induced tissue hypoperfusion
or organ dysfunction (any of the following thought to be
due to the infection)
Sepsis-induced hypotension
Lactate above upper limits laboratory normal
Urine output <0.5 mL/kg/h for more than 2 h despite
adequate uid resuscitation
Acute lung injury with Pao2/Fio2 <250 in the absence of
pneumonia as infection source
Acute lung injury with Pao2/Fio2 <200 in the presence of
pneumonia as infection source
Creatinine >2.0 mg/dL (176.8 mmol/L)
Bilirubin >2 mg/dL (34.2 mmol/L)
Platelet count <100 000 mL
Coagulopathy (international normalized ratio >1.5)
FME WAGENLEHNER ET AL.
964 2013 The Japanese Urological Association
counterregulatory anti-inammatory response syndrome,
leading to an immunosuppressive state, which accounts for
the mortality in the longer course of sepsis.
15
In this phase,
for example, macrophages and neutrophils can undergo
complete immune paralysis, but do not go into apoptosis,
whereas lymphocytes and dendritic cells undergo increased
apoptosis in a large quantity. The increased number of dys-
functional macrophages and neuthrophils can also increase
the bystander damage of those cells. Interestingly, for
example, HMGB1 is released on cell and tissue necrosis, but
is also actively produced by macrophages during the later
stages of sepsis.
The inammatory system, however, is not the only system
affected in sepsis. Other biological systems, such as the
coagulation system, the autonomic nervous system or the
endocrine system, are equally affected.
15
The complement system, which is hyperactivated in
sepsis, is intimately connected with the coagulation and
brinolysis systems by multiple interactions of serine pro-
teases. Surface receptors of endothelial cells and neutrophils
are upregulated, which increases the mutual adhesiveness.
Additionally, the endothelial procoagulatory activity and
the synthesis of a plasminogenactivator inhibitor substance
is increased, which activates the blood coagulation
system.
913,16
This activated coagulation system predisposes
to thrombosis and disseminated intravascular coagulation.
In parallel, anticoagulant mechanisms, such as the protein-C
pathway, are inhibited, augmenting the systemic coagula-
tion. This leads to tissue and cell hypoxemia.
The autonomic nervous system and the hypothalamo
pituitaryadrenocortical axis show a cross-talk to the
immune system and regulate each other.
17
The autonomic
nervous system has three components, which are the para-
sympathetic system, the sympathetic system and the enteric
nervous system.
The sympathetic system innervates and modulates lym-
phoid organs. Under pathological circumstances, noradrena-
lin can stimulate a
2
-receptors on the surface of macrophages
and stimulate release of TNF-a,
18
whereas stimulation of
b-receptors inhibits release of pro-inammatory cytokines.
Pro-inammatory cytokines stimulate hypothalamic centers
activating the sympathetic system and the hypothalamo
pituitaryadrenocortical axis inducing the expression of
corticotropin-releasing hormone and an adrenocorticotropic
hormone in the pituitary gland. Consecutively, cortisol is
released from the adrenal glands leading to an anti-
inammatory response by suppressing nuclear factor-kB,
and increasing anti-inammatory IL-4 and 10.
19
The parasympathetic system acts through the vagal nerve
or by acetylcholine receptors sensing inammation and
informing specic centers in the brain. Release of acetyl-
choline reduces pro-inammatory cytokines, such as
TNF-a, as well as release of HMGB1, which is termed the
cholinergic anti-inammatory pathway.
14,20,21
Importantly,
vagal branches to the spleen might be able to suppress
cytokine synthesis in the spleen.
14
Cholinesterase inhi-
bitors therefore have been demonstrated to show anti-
inammatory effects in experimental sepsis.
17
Certain hormone systems are affected in the course of
sepsis, especially steroid hormones, such as corticosteroids
or sex hormones, especially testosterone. The macrophage-
inhibitory factor is secreted by the hypothalamus, pituitary
and adrenal glands, and antagonizes corticosteroid anti-
inammatory activity.
14
There is apparently also a sex difference in the host
response to sepsis, which is mainly attributed to the sex
hormones. Acute infection has been shown, for example, to
inhibit gonadal steroidogenesis in the Leydig cells through
reactive oxygen species-mediated disruption of Leydig cell
mitochondria.
23
Central hubs in the pathophysiology of sepsis link key
molecules with these different systems. Complement C5a
links inammation with coagulation and through
macrophage-inhibitory factor inammation with the endo-
crine system. HMGB1 links inammation with the auto-
nomic nervous system.
15
Surviving Sepsis Campaign
Guidelines
Mortality from severe sepsis and septic shock has been
shown to be signicantly different from region to region,
ranging from 25% to 80%, over the past few decades.
23
Many factors were dened to explain this difference one
was the difference in management of septic patients. In
2004, the Surviving Sepsis Campaign rst introduced guide-
lines for the management of severe sepsis and septic shock,
as well as strategies for bedside implementation,
16,24,25
which
were updated in 2008,
26
and again in 2012.
27,28
The treatment
recommendations were organized in so-called sepsis
bundles, such as a resuscitation bundle (tasks to begin
immediately and to be accomplished within 6 h) and a man-
agement bundle (tasks to be completed within 24 h). In the
follow up of the Surviving Sepsis Campaign, important
developments have been made considering the role of
steroid administration, activated protein C or vasopressor
choice; intravenous hydrocortisone is not longer indicated as
a primary treatment, activated protein C is no longer on the
market after negative results in the last studies and the vaso-
pressor of choice is norepinephrine.
27,28
Other strategies,
such as modern endotoxin inhibitors, have been investigated
in clinical trials, but are not yet included in the current
recommendations.
29
Key recommendations for adults with severe sepsis and
septic shock are listed by category and comprise the follow-
ing recommendations:
27,28
Early quantitative resuscitation of the septic patient
during the rst 6 h after recognition.
Items in urosepsis
2013 The Japanese Urological Association 965
Blood cultures before antibiotic therapy.
Imaging studies carried out promptly to conrm a poten-
tial source of infection.
Administration of broad-spectrum antimicrobials
therapy within 1 h of recognition of septic shock and
severe sepsis without septic shock as the goal of therapy.
Reassessment of antimicrobial therapy daily for
de-escalation, when appropriate.
Infection source control with attention to the balance of
risks and benets of the chosen method within 12 h of
diagnosis.
Initial uid resuscitation with crystalloid and considera-
tion of the addition of albumin in patients who continue
to require substantial amounts of crystalloid to maintain
adequate mean arterial pressure and the avoidance of
hetastarch formulations.
Initial uid challenge in patients with sepsis-induced
tissue hypoperfusion and suspicion of hypovolemia to
achieve a minimum of 30 mL/kg of crystalloids (more
rapid administration and greater amounts of uid might
be needed in some patients).
Fluid challenge technique continued as long as there is
hemodynamic improvement, as based on either dynamic
or static variables.
Norepinephrine as the rst-choice vasopressor to main-
tain mean arterial pressure 65 mmHg. Epinephrine
when an additional agent is required to maintain
adequate blood pressure. Vasopressin (0.03 U/min) can
be added to norepinephrine to either raise mean arterial
pressure to target or to decrease norepinephrine dose, but
should not be used as the initial vasopressor. Dopamine
is not recommended, except in highly selected circum-
stances. Dobutamine infusion administered or added to
vasopressor in the presence of: (i) myocardial dysfunc-
tion, as suggested by elevated cardiac lling pressures
and low cardiac output; or (ii) ongoing signs of hypop-
erfusion despite achieving adequate intravascular
volume and adequate mean arterial pressure.
Avoiding use of intravenous hydrocortisone in adult
septic shock patients if adequate uid resuscitation and
vasopressor therapy are able to restore hemodynamic
stability.
Hemoglobin target of 79 g/dL in the absence of tissue
hypoperfusion, ischemic coronary artery disease or acute
hemorrhage.
Low tidal volume and limitation of inspiratory plateau
pressure for ARDS. Application of at least a minimal
amount of positive end expiratory pressure in ARDS. A
higher rather than a lower level of positive end expiratory
pressure for patients with sepsis-induced moderate or
severe ARDS.
Recruitment maneuvers in sepsis patients with severe
refractory hypoxemia as a result of ARDS. Prone posi-
tioning in sepsis-induced ARDS patients with a Pao2/
Fio2 ratio of 100 mmHg in facilities that have
experience with such practices. Head-of-bed elevation in
mechanically ventilated patients unless contraindicated.
A conservative uid strategy for patients with
established ARDS who do not have evidence of tissue
hypoperfusion.
Protocols for weaning and sedation.
Minimizing use of either intermittent bolus sedation or
continuous infusion sedation targeting specic titration
end-points.
Avoidance of neuromuscular blockers if possible in the
septic patient without ARDS. A short course of neu-
romuscular blocker (no longer than 48 h) for patients
with early ARDS and a Pao2/Fio2 <150 mmHg.
A protocolized approach to blood glucose management
commencing insulin dosing when two consecutive blood
glucose levels are >180 mg/dL, targeting an upper blood
glucose 180 mg/dL.
Equivalency of continuous veno-venous hemoltration
or intermittent hemodialysis.
Prophylaxis for deep vein thrombosis.
Use of stress ulcer prophylaxis to prevent upper gastroin-
testinal bleeding in patients with bleeding risk factors.
Oral or enteral (if necessary) feedings, as tolerated,
rather than either complete fasting or provision of only
intravenous glucose within the rst 48 h after a diagnosis
of severe sepsis/septic shock.
Addressing goals of care, including treatment plans and
end-of-life planning (as appropriate), as early as feasible,
but within 72 h of intensive care unit admission.
Implications of the Surviving Sepsis
Campaign Guidelines for the
management of urosepsis
A rapid diagnosis is critical to meet the requirements of the
Surviving Sepsis Campaign Guidelines. As the urogenital
tract is one of the most frequent sites of infection in sepsis in
general and, depending on the data of geographic regions,
also in severe sepsis and septic shock, ranging from 8.6% to
30.6%,
1
assessment of the urinary tract should be regularly
carried out in a septic patient. A diagnosis and management
algorithm is therefore helpful (Fig. 1).
Initial clinical picture
The clinical picture of a septic patient in a hyperdynamic
state might show warm skin, bounding pulse and hyperdy-
namic circulation. In the later stages of the septic process,
the patient might show signs of vasoconstriction and periph-
eral cyanosis. The internationally accepted criteria for diag-
nosis of sepsis (Table 1) should be checked immediately. If
the SIRS criteria are positive, rst uid and oxygen resus-
citation should be started immediately during the rst 6 h
after recognition. Early goal-directed therapy
30
is simply a
FME WAGENLEHNER ET AL.
966 2013 The Japanese Urological Association
protocol derived from components that have long been rec-
ommended as standard care for the septic patient to optimize
hemodynamics and oxygen supply (Table 2).
Clinical diagnosis
Signs and symptoms indicating that the urogenital tract is
the septic source should be assessed; ank pain, costoverte-
bral tenderness, pain at micturition, urinary retention, pros-
tatic or scrotal pain should therefore be examined. Acute
prostatitis should be ruled out by digital rectal examination
of the prostate, and epididymitis can be assessed by palpa-
tion of the testicles. Microbiological analysis should include
urine and blood cultures.
Initial imaging
The rst rapid imaging studies by sonographic examination
of the urogenital organs can be carried out in the emergency
unit. As obstruction of the upper urinary tract is the predomi-
nant cause of urosepsis, sonographic examination of the
kidneys, ruling out dilation of the renal pelvis, is a good rst
imaging study. Additional ultrasound of the bladder, ruling
out urinary retention, and the prostate, to rule out a large
prostatic abscess, is recommended. If the ultrasound investi-
gations showed suspicious ndings pointing to the urogenital
tract, further radiographic investigations (e.g. computed tom-
ography scan, urography) of the urogenital tract are now
generally applied to specify the complicating factor.
Antimicrobial therapy
After microbiological sampling of urine, blood and suspi-
cious secretions, empirical effective broad spectrum antibi-
otic therapy should be instigated parenterally.
Parameters of antimicrobial therapy
Antimicrobials are among the most important drugs in the
management of patients with severe infections.
31
Inappro-
priate use of antimicrobials can cause therapeutic failure in
the individual patient, and additionally can contribute
towards promoting the emergence of resistant pathogens,
which might also readily spread in the hospital setting.
32
An
adequate initial (e.g. in the rst hour) antibiotic therapy
ensures improved outcome in septic shock,
33,34
and is also
critical in severe UTI,
35
as it has been shown with other
infections as well.
36,37
Empirical antibiotic therapy considers
the expected bacterial spectrum, the institutional specic
resistance rates and the individual patients requirements.
38
Empirical initial treatment should provide effective broad
antimicrobial coverage, and should later be adapted to the
culture results.
The treatment of sepsis using hemodynamically active
drugs, such as catecholamines and furosemide, usually
increases the renal clearance by means of enhanced cardiac
output and/or renal blood ow,
39
which also results in higher
clearances of antibacterial drugs.
40
The volume of distribu-
tion is increased in sepsis and will lead to underexposure of
certain antimicrobials, such as b-lactams and aminoglyco-
sides.
41
Only if organ dysfunctions, such as hepatic or renal
dysfunctions, are present clearance of antibacterial drugs
may be decreased. Individualized dosing of antibacterial
Clinical status indicative for sepsis
SIRS/ sepsis criteria positive
Initial oxygen + fluid resuscitation
Microbiology + initial imaging
Signs/ symptoms indicative for urosepsis
Early goal directed therapy + antibiotic therapy
Further imaging studies
Complicating factor in urogenital tract
Source control
Supportive/ adjunctive sepsis therapy
1h 6h
Fig. 1 Algorithmfor management of urosepsis adapted from
Grabe et al.
3
Table 2 Target parameters of early goal directed
therapy
27,28,30
1. Protocolized, quantitative resuscitation of patients with
sepsis- induced tissue hypoperfusion (hypotension
persisting after initial uid challenge or blood lactate
concentration 4 mmol/L). Goals during the rst 6 h of
resuscitation:
Parameter Target
Central venous pressure 812 mmHg
Mean arterial pressure 6590 mmHg
Central venous (superior vena cava)
or mixed venous oxygen saturation
70% or 65%,
respectively
Urine output >0.5 mL/kg/h
2. In patients with elevated lactate levels targeting
resuscitation to normalize lactate
Items in urosepsis
2013 The Japanese Urological Association 967
agents is therefore necessary, in order to prevent therapeutic
failure of antimicrobial therapy or severe side-effects.
Post-renal obstruction is one of the most frequent causes
of urosepsis. Obstruction hereby is the cause of the sepsis on
one side, and on the other side severely inuences the
urinary and sometimes also the systemic pharmacokinetics
of the antimicrobial drugs. The urinary concentrations of an
antimicrobial at the affected site depend on whether the
obstruction is acute or chronic, uni-or bilateral, and are also
signicantly inuenced by the function of the contralateral
kidney. The renal tissue concentration is complex, and is a
function of renal blood ow, glomerular ltration, tubular
secretion and reabsorption, pyelovenous and lymphatic
backow, and the number of intact nephrons. Renal lymph
concentrations resemble renal tissue concentration to some
extent.
42
Renal lymph concentrations in unobstructed,
normal and unobstructed, but infected kidneys, showed that
b-lactam antibiotics and aminoglycoside concentrations
were generally lower than the corresponding arterial plasma
concentrations, which suggests that there is no concentra-
tion effect in the renal interstitial space.
In acute obstruction, urinary concentrations of ltered
and secreted substances will at rst reach a high plateau. If
the ureteral pressure rises and exceeds one-third of the mean
blood pressure, an increasing number of nephrons will cease
ltering, resulting in a decrease of glomerular ltration and
also in a decrease of urinary concentrations.
42
This process
is very much enhanced by infection of an obstructed kidney,
resulting in urosepsis due to obstruction, and high doses of
antimicrobials mainly excreted by the kidneys are necessary.
Bacterial spectrum in urosepsis
There are not many publications on the specic bacterial
spectrum in urosepsis. Mainly the bacterial spectrum of
complicated and nosocomially acquired UTI are taken as
representative for urosepsis as well, which in general might
be correct.
43
The bacterial spectrum in urosepsis can also
vary depending on whether the origin is primary (mostly
community acquired) or secondary (after urological inter-
vention and/or indwelling urinary catheters or devices).
The German septicemia study in 2002 discriminated the
bacterial spectrum of blood culture isolates according to
their origin. If the urinary tract was the source for the sep-
ticemia, the bacterial spectrum consisted of approximately
61% Escherichia coli, 16% other enterobacteria, 8% Sta-
phylococcus aureus and 6% enterococi, underlining the pre-
dominant role of E. coli.
44
If host defence is impaired, less
virulent organisms, such as enterococci, coagulase negative
staphylococci or Pseudomonas aeruginosa, are seen more
frequently and can also cause urosepsis.
Selection of antimicrobials for empiric therapy
As effective antimicrobial therapy has to be initiated during
the rst hour when sepsis is diagnosed, empiric intravenous
therapy should be initiated immediately after microbiologi-
cal sampling. For the selection of appropriate antimicrobi-
als, it is important to know the site of origin and underlying
diseases; whether the sepsis is primary or secondary, or
community or nosocomially acquired. In addition, the pre-
ceding antimicrobial therapies have to be recorded as pre-
cisely as possible. If the pretreatment history is known, the
same group of antimicrobials should be avoided. All antibi-
otics have to be selected in consideration of the local sus-
ceptibility patterns. For empiric treatment of severe
infections, a resistance threshold of 10% is discussed.
In consideration of the local susceptibility data, a third
generation cephalosporin, piperacillin, plus a BLI, or a uo-
roquinolone, for example, ciprooxacin or levooxacin,
might be appropriate if there is no history of uoroqui-
nolone therapy in the past 6 months (Table 3). In secondary
urosepsis after urological interventions, P. aeruginosa
should be considered, and antipseudomonal active antibiot-
ics, such as pseudomonas active third generation cepha-
losporins, piperacillin/BLI or a carbapenem, might be
necessary. In areas with a high rate of Enterobacteriaceae
with extended spectrum beta-lactamases, a therapy with a
Table 3 Antibiotics recommended for the treatment of urosepsis
Most frequent pathogens/species Initial, empirical antimicrobial therapy Therapy duration
E. coli
Other enterobacteria
Cephalosporin (group 3a/b)
Fluoroquinolone
35 days after defervescence
or control/elimination of
complicating factor After urological interventions or if multi-resistant pathogens are suspected
Pseudomonas spp.
Proteus spp.
Serratia spp.
Enterobacter spp.
Anti-pseudomonas active acylaminopenicillin/BLI
Cephalosporin (group 3b)
Carbapenem
Aminoglycoside
Fluoroquinolone
Only in regions where uoroquinolone resistance is below 10% and if not used in the past 6 months. Adapted from Grabe et al.
3
FME WAGENLEHNER ET AL.
968 2013 The Japanese Urological Association
carbapenem might be necessary for initial empiric therapy.
To cover a broader bacterial spectrum, including multiresist-
ant pathogens, combination antibiotic therapy including
uoroquinolones, aminoglycosides and carbapenems might
be indicated, although the advantage of combination antibi-
otic therapy in sepsis has not yet been shown.
Urological source control
If a complicating factor in the urinary tract warranting treat-
ment is identied, control and/or removal of the complicat-
ing factor should follow in the rst 6 h. This procedure is
frequently carried out in two stages:
1 Immediate low-level invasive treatment (e.g. insertion of
an indwelling bladder-catheter, JJ-stent or percutaneous
nephrostomy) for control of the complicating factor.
2 After easing off the septic symptoms, denitive elimi-
nation of the complicating factor by adequate urological
techniques.
There is no evidence of superiority of either treatment, if
comparing ureteral stents versus percutaneous nephrostomy
in septic patients with obstructed kidneys.
45
The data are,
however, scarce; although the procedures are extremely
frequent.
In parallel with the urological control of the septic focus,
the sepsis bundles mentioned in the Surviving Sepsis
Campaign Guidelines should be followed by the attending
physicians.
Prevention
The most effective methods to prevent nosocomial urosepsis
are the same as those used to prevent other nosocomial
infections. As most urosepsis cases are as a result of obstruc-
tion of the urinary tract at some level, the development of the
full picture of septic shock can frequently be prevented by
carrying out an early deobstruction procedure. A patient
with a so-called infected hydronephrosis is an absolute
emergency. Before starting the deobstruction procedure, an
empirical antibiotic treatment needs to be administered.
Despite antimicrobial therapy, appropriate urological inter-
ventions are very important. Of 49 patients with urosepsis as
a result of pyonehrosis, 22% died despite intensive care, but
no patient died if pyonephrosis was treated by nephrectomy,
or in a few cases by nephrostomy drainage at that time
before urosepsis developed.
46
Conclusion
Sepsis syndrome in urology remains a severe situation, with
a mortality rate as high as 2040% in severe sepsis. A
campaign, Surviving Sepsis Guidelines, aimed at reduc-
ing mortality by 25% in the next few years has been pub-
lished.
16
Early recognition of the symptoms can decrease the
mortality by timely treatment of urinary tract disorders; for
example, obstruction and urolithiasis. Adequate life-support
measures and appropriate antibiotic treatment including
optimized dosing provide the best conditions for improving
patients survival. The prevention of sepsis syndrome is
dependent on good practice to avoid nosocomial infections,
and using antibiotic prophylaxis and therapy in a prudent
and well-accepted manner.
Conict of interest
None declared.
References
1 Levy MM, Artigas A, Phillips GS et al. Outcomes of the
Surviving Sepsis Campaign in intensive care units in the
USA and Europe: a prospective cohort study. Lancet Infect.
Dis. 2012; 12: 91924.
2 Raz R, Naber KG, Raizenberg C et al. Ciprooxacin
250 mg twice daily versus ooxacin 200 mg twice daily in
the treatment of complicated urinary tract infections in
women. Eur. J. Clin. Microbiol. Infect. Dis. 2000; 19:
32731.
3 Grabe M, Bjerklund-Johansen TE, Botto H et al.
Guidelines on urological infections. In: Urology EA (ed.).
European Association of Urology Guidelines. European
Association of Urology, Arnhem, The Netherlands, 2012;
1112.
4 Bjerklund Johansen TE, Cek M, Naber K, Stratchounski L,
Svendsen MV, Tenke P. Prevalence of hospital-acquired
urinary tract infections in urology departments. Eur. Urol.
2007; 51: 110012.
5 Bouza E, San Juan R, Munoz P, Voss A, Kluytmans J. A
European perspective on nosocomial urinary tract infections
II. Report on incidence, clinical characteristics and outcome
(ESGNI-004 study). European Study Group on Nosocomial
Infection. Clin. Microbiol. Infect. 2001; 7: 53242.
6 Bone RC, Balk RA, Cerra FB et al. Denitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee. American College of Chest
Physicians/Society of Critical Care Medicine. Chest 1992;
101: 164455.
7 Bone RC, Sprung CL, Sibbald WJ. Denitions for sepsis
and organ failure. Crit. Care Med. 1992; 20:
7246.
8 Levy MM, Fink MP, Marshall JC et al. 2001
SCCM/ESICM/ACCP/ATS/SIS International Sepsis
Denitions Conference. Crit. Care Med. 2003; 31:
12506.
9 Dinarello CA. The endogenous pyrogens in host-defense
interactions. Hosp. Pract. (Off. Ed.) 1989; 24: 11115, 118,
121 passim.
10 Hotchkiss RS, Karl IE. The pathophysiology and treatment
of sepsis. N. Engl. J. Med. 2003; 348: 13850.
11 Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors,
mediators, and mechanisms involved in bacterial sepsis and
septic shock. Clin. Microbiol. Rev. 2003; 16: 379414.
12 Russell JA. Management of sepsis. N. Engl. J. Med. 2006;
355: 1699713.
Items in urosepsis
2013 The Japanese Urological Association 969
13 Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro-
versus anti-inammatory cytokine prole in patients with
severe sepsis: a marker for prognosis and future therapeutic
options. J. Infect. Dis. 2000; 181: 17680.
14 Rittirsch D, Flierl MA, Ward PA. Harmful molecular
mechanisms in sepsis. Nat. Rev. Immunol. 2008; 8:
77687.
15 Astiz ME, Rackow EC. Septic shock. Lancet 1998; 351:
15015.
16 Dellinger RP, Carlet JM, Masur H et al. Surviving Sepsis
Campaign guidelines for management of severe sepsis and
septic shock. Crit. Care Med. 2004; 32: 85873.
17 Weismuller K, Bauer M, Hofer S, Weigand MA. The
neuroendocrine axis and the pathophysiology of sepsis.
Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2010;
45: 5748, quiz 9.
18 Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel
SL. Stimulation of alpha-adrenergic receptor augments the
production of macrophage-derived tumor necrosis factor.
J. Immunol. 1990; 145: 14304.
19 John CD, Buckingham JC. Cytokines: regulation of the
hypothalamo-pituitary-adrenocortical axis. Curr. Opin.
Pharmacol. 2003; 3: 7884.
20 Borovikova LV, Ivanova S, Zhang M et al. Vagus nerve
stimulation attenuates the systemic inammatory response
to endotoxin. Nature 2000; 405: 45862.
21 Huston JM, Gallowitsch-Puerta M, Ochani M et al.
Transcutaneous vagus nerve stimulation reduces serum high
mobility group box 1 levels and improves survival in
murine sepsis. Crit. Care Med. 2007; 35: 27628.
22 Allen JA, Diemer T, Janus P, Hales KH, Hales DB.
Bacterial endotoxin lipopolysaccharide and reactive oxygen
species inhibit Leydig cell steroidogenesis via perturbation
of mitochondria. Endocrine 2004; 25: 26575.
23 Angus DC, Wax RS. Epidemiology of sepsis: an update.
Crit. Care Med. 2001; 29 (7 Suppl): S10916.
24 Dellinger RP, Carlet JM, Gerlach H, Ramsey G, Levy M.
The surviving sepsis guidelines: not another groundhog
day. Crit. Care Med. 2004; 32: 16012.
25 Dellinger RP, Carlet JM, Masur H et al. Surviving Sepsis
Campaign guidelines for management of severe sepsis and
septic shock. Intensive Care Med. 2004; 30: 53655.
26 Dellinger RP, Levy MM, Carlet JM et al. Surviving Sepsis
Campaign: international guidelines for management of
severe sepsis and septic shock. Crit. Care Med. 2008; 36:
296327.
27 Dellinger RP, Levy MM, Rhodes A et al. Surviving Sepsis
Campaign: international guidelines for management of
severe sepsis and septic shock, 2012. Intensive Care Med.
2013; 39: 165228.
28 Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis
campaign: international guidelines for management of
severe sepsis and septic shock: 2012. Crit. Care Med. 2013;
41: 580637.
29 Barochia A, Solomon S, Cui X, Natanson C, Eichacker PQ.
Eritoran tetrasodium (E5564) treatment for sepsis: review
of preclinical and clinical studies. Expert Opin. Drug
Metab. Toxicol. 2011; 7: 47994.
30 Rivers E, Nguyen B, Havstad S et al. Early goal-directed
therapy in the treatment of severe sepsis and septic shock.
N. Engl. J. Med. 2001; 345: 136877.
31 Paterson DL. Restrictive antibiotic policies are appropriate
in intensive care units. Crit. Care Med. 2003; 31 (1 Suppl):
S258.
32 Eggimann P, Pittet D. Infection control in the ICU. Chest
2001; 120: 205993.
33 Kreger BE, Craven DE, McCabe WR. Gram-negative
bacteremia. IV. Re-evaluation of clinical features and
treatment in 612 patients. Am. J. Med. 1980; 68: 34455.
34 Kreger BE, Craven DE, Carling PC, McCabe WR.
Gram-negative bacteremia. III. Reassessment of etiology,
epidemiology and ecology in 612 patients. Am. J. Med.
1980; 68: 33243.
35 Elhanan G, Sarhat M, Raz R. Empiric antibiotic treatment
and the misuse of culture results and antibiotic sensitivities
in patients with community-acquired bacteraemia due to
urinary tract infection. J. Infect. 1997; 35: 2838.
36 Kollef MH, Ward S. The inuence of mini-BAL cultures on
patient outcomes: implications for the antibiotic
management of ventilator-associated pneumonia. Chest
1998; 113: 41220.
37 Luna CM, Vujacich P, Niederman MS et al. Impact of BAL
data on the therapy and outcome of ventilator-associated
pneumonia. Chest 1997; 111: 67685.
38 Singh N, Yu VL. Rational empiric antibiotic prescription in
the ICU. Chest 2000; 117: 14969.
39 Pea F, Furlanut M. Pharmacokinetic aspects of treating
infections in the intensive care unit: focus on drug
interactions. Clin. Pharmacokinet. 2001; 40: 83368.
40 Roberts JA, Lipman J. Antibacterial dosing in intensive
care: pharmacokinetics, degree of disease and
pharmacodynamics of sepsis. Clin. Pharmacokinet. 2006;
45: 75573.
41 Pea F, Viale P, Furlanut M. Antimicrobial therapy in
critically ill patients: a review of pathophysiological
conditions responsible for altered disposition and
pharmacokinetic variability. Clin. Pharmacokinet. 2005;
44: 100934.
42 Naber KG, Madsen PO. Renal function during acute total
ureteral occlusion and the role of the lymphatics: an
experimental study in dogs. J. Urol. 1973; 109: 3308.
43 Foz A. Sepsis of urological origin: microbiological aspects.
Antibiot. Chemother. 1976; 21: 6972.
44 Rosenthal EJ. Epidemiology of septicaemia pathogens.
Dtsch. Med. Wochenschr. 2002; 127: 243540.
45 Ramsey S, Robertson A, Ablett MJ, Meddings RN, Hollins
GW, Little B. Evidence-based drainage of infected
hydronephrosis secondary to ureteric calculi. J. Endourol.
2010; 24: 1859.
46 Schilling A, Marx FJ, Hofstetter A, Jesch F. Septic shock
in the urologic patient. IV. monitoring and therapy (authors
transl). Urologe A 1977; 16: 3515.
FME WAGENLEHNER ET AL.
970 2013 The Japanese Urological Association
You might also like
- Koran Kamis, 18 September 2014 Rsuh Lantai 3 Kamar 318Document2 pagesKoran Kamis, 18 September 2014 Rsuh Lantai 3 Kamar 318FitrianiMappasereNo ratings yet
- Total Urgency and Frequency Score Measures OAB SymptomsDocument9 pagesTotal Urgency and Frequency Score Measures OAB SymptomsFitrianiMappasereNo ratings yet
- Iju12238 PDFDocument7 pagesIju12238 PDFFitrianiMappasereNo ratings yet
- JurnalDocument9 pagesJurnalFitrianiMappasereNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Acute Hemoabdomen: Topic OverviewDocument23 pagesThe Acute Hemoabdomen: Topic OverviewWeiwei CierraNo ratings yet
- Pararescue Medication and Procedure Handbook-1Document137 pagesPararescue Medication and Procedure Handbook-1Charles Harris100% (1)
- 081-831-1005 (First Aid To Prevent or Control Shock)Document13 pages081-831-1005 (First Aid To Prevent or Control Shock)sny007No ratings yet
- Haemodynamic Optimisation: Understanding the Key Physiological VariablesDocument86 pagesHaemodynamic Optimisation: Understanding the Key Physiological VariablesRhyno FebriyantoNo ratings yet
- Important Developments in Burn Care: Learning Objectives: After Studying This Article, The Participant Should Be AbleDocument19 pagesImportant Developments in Burn Care: Learning Objectives: After Studying This Article, The Participant Should Be AbleRatu IntaniaNo ratings yet
- แนวทางการรักษา variceal bleeding 2011Document18 pagesแนวทางการรักษา variceal bleeding 2011เด็กชายสมันตภัทร แฟนคลับอาจารย์กวงNo ratings yet
- Weaning From IABPDocument22 pagesWeaning From IABPdrjigneshnhl100% (1)
- Obstructed Labor-Bandl's RingDocument43 pagesObstructed Labor-Bandl's RingJune DumdumayaNo ratings yet
- AkiDocument20 pagesAkiEnvhy AmaliaNo ratings yet
- Stages of ShockDocument13 pagesStages of ShockA. P.No ratings yet
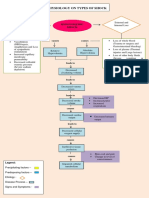
- Pathophysiology On Types of ShockDocument4 pagesPathophysiology On Types of ShockJessa Mae Alforque AsentistaNo ratings yet
- Challenges in Conducting Literature ReviewDocument11 pagesChallenges in Conducting Literature Reviewafmzfsqopfanlw100% (1)
- Atherosclerosis-Thrombosis QuestionsDocument42 pagesAtherosclerosis-Thrombosis QuestionsJim Jose Antony100% (17)
- PATHOPHYSIOLOGY OF SHOCKDocument56 pagesPATHOPHYSIOLOGY OF SHOCKDr. Haricharan ANo ratings yet
- Assessing Vital Signs and Poisoning SymptomsDocument76 pagesAssessing Vital Signs and Poisoning SymptomsmasorNo ratings yet
- Traumatic ShockDocument22 pagesTraumatic ShockOlga GoryachevaNo ratings yet
- Pathophys BURNDocument2 pagesPathophys BURNpaupaulala83% (6)
- Septic ShockDocument11 pagesSeptic ShockJonna Mae Agcaoili SalameroNo ratings yet
- PALS Study Guide Short FormDocument11 pagesPALS Study Guide Short FormLenTheRN88% (8)
- Neurogenic Shock Causes and TreatmentDocument10 pagesNeurogenic Shock Causes and TreatmentroyNo ratings yet
- Survival Guide For Medical InternshipDocument16 pagesSurvival Guide For Medical Internshipsuggaplum0% (1)
- Crim Pe 3 (First Aid and Water Safety)Document32 pagesCrim Pe 3 (First Aid and Water Safety)Marc Daryl FelipeNo ratings yet
- Hypovolemic Shock Sample NCPDocument14 pagesHypovolemic Shock Sample NCPRENEROSE TORRES100% (1)
- Trauma Respuesta Ishikawa2013Document7 pagesTrauma Respuesta Ishikawa2013María José BencomoNo ratings yet
- 04-Infectious and Parasitic DiseasesDocument16 pages04-Infectious and Parasitic DiseasesAdityaNo ratings yet
- Critical Care Nephrology Core Curriculum 2019Document34 pagesCritical Care Nephrology Core Curriculum 2019Edmilson R. LimaNo ratings yet
- Complication of IV TherapyDocument3 pagesComplication of IV TherapyRayl RsNo ratings yet
- Paediatric Guidelines 2013-2014Document251 pagesPaediatric Guidelines 2013-2014Emad Adel100% (2)
- Chapter 23: Alterations of Cardiovascular FunctionDocument5 pagesChapter 23: Alterations of Cardiovascular Functionw1111am0% (1)
- Prehospital CTAS 5x7 Field Reference Guide - Version 1.0 PDFDocument36 pagesPrehospital CTAS 5x7 Field Reference Guide - Version 1.0 PDFSaifullah RachmanNo ratings yet