Professional Documents
Culture Documents
Eutectics: Lecture 3 Manufacturing Technology
Uploaded by
Ayush BhadauriaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Eutectics: Lecture 3 Manufacturing Technology
Uploaded by
Ayush BhadauriaCopyright:
Available Formats
Eutectics
Lecture 3 Manufacturing Technology
1
How to construct phase diagrams
Cooling curves:
usedtodeterminephasetransitiontemperature
record T (Temperature) of material vs time, as it cools
fromits moltenstatethroughsolidificationandfinallyto
RT(at aconstant pressure!!!)
The cooling curve of a pure metal
BC: plateaueor region of
thermal arrest; in this region
material is in the form of
solid and liquid phases
CD: solidification is
completed, T drops
2
Cooling curve of pure iron (1 atm)
3
Coolingcurvestoisomorphousbinaryphasediagram
4
Invariant Reactions
5
The possible types of invariant reaction areshown below. Thevertical greenbar identifies
thecompositionrequiredfor theinvariant reaction, thehorizontal lineisthetemperatureof
thereaction, andthephasepointsontheisothermrepresent their compositionsduringthe
reaction.
6
Eutectoid one solidphase transforms to two other solidphases
Solid
1
Solid
2
+Solid
3
+Fe
3
C (For Fe-C, 727C, 0.76 wt% C)
Eutectic, Eutectoid, & Peritectic
Eutectic- liquidtransforms to two solidphases
L + (For Pb-Sn, 183C, 61.9 wt% Sn)
cool
heat
Peritectic- liquidand one solidphase transform to a 2nd solidphase
Solid
1
+Liquid Solid
2
+L (For Cu-Zn, 598C, 78.6 wt% Zn)
cool
heat
cool
heat
7
Eutectic
A eutecticor eutecticmixtureis a mixture of
two or morephasesat acompositionthat has
thelowest meltingpoint.
It is where the phases simultaneously
crystallizefrommoltensolution.
The proper ratios of phases to obtain a
eutecticisidentifiedbytheeutecticpoint ona
binaryphasediagram.
The term comes from the Greek 'eutektos',
meaning'easilymelted.
8
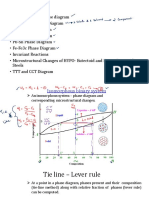
The phase diagramdisplays asimple binary systemcomposed of two components, A and
B, whichhasaeutecticpoint.
The phase diagram plots relative concentrations of A and B along the X-axis, and
temperature along the Y-axis. The eutectic point is the point where the liquid phase
borders directly on the solid + phase; it represents the minimum melting
temperatureof anypossibleABalloy.
Thetemperaturethat corresponds tothispoint isknownastheeutectic temperature.
Not all binary systemalloys have a eutectic point: those that forma solid solution at all
concentrations, such as the gold-silver system, have no eutectic. Analloy systemthat has
aeutecticisoftenreferredtoasaeutecticsystem, or eutecticalloy.
Solid products of a eutectic transformation can often be identified by their lamellar
structure, as opposed to the dendritic structures commonly seen in non-eutectic
solidification. Thesameconditionsthat forcethematerial toformlamellaecaninsteadform
anamorphoussolidif pushedtoanextreme.
9
2 components
has a special composition
with a min. melting T.
Binary-Eutectic Systems
3 single phase regions
(L, , )
Limited solubility:
: mostly Cu
: mostly Ag
T
E
: No liquid below T
E
: Composition at
temperature T
E
C
E
Cu-Ag system
L (liquid)
L +
L+
+
C , wt% Ag
20 40 60 80 100 0
200
1200
T(C)
400
600
800
1000
C
E
T
E
8.0 71.9 91.2
779C
Ag) wt% 1.2 9 ( Ag) wt% .0 8 ( Ag) wt% 9 . 71 ( + L
cooling
heating
Eutectic reaction
L(C
E
) (C
E
) +(C
E
)
Copper-Silver Phase Diagram
10
11
Eutectic Reaction
An isothermal, reversible reaction between two (or more) solid
phases during the heating of a system where a single liquid
phase is produced.
Eutectic reaction
L(C
E
) (C
E
) +(C
E
)
Ag) wt% 1.2 9 ( Ag) wt% .0 8 ( Ag) wt% 9 . 71 ( + L
cooling
heating
Solvus (solid solubility line) BC, GH
Solidus AB, FG, BEG (eutectic isotherm)
Liquidus AEF
Maximum solubility: =8.0 wt% Ag, =8.8 wt %Cu
Invariant point (where 3 phases are in equilibrium) is at E; C
E
=
71.9 wt% Ag,T
E
=779C (1434F).
Pb-SnPhase Diagram
Liquidus
Solidus
Solidus
Solidus
Solvus
Solvus
12
Cooling 60%Pb 40%Sn system
at eutectic point
13
14
Solidification of Eutectic Mixtures
Mixturesof somemetals, suchascopper &nickel, arecompletelysoluble
inbothliquidandsolidstatesfor all concentrationsof bothmetals. Copper
&nickel have the same crystal structure (FCC) and have nearly the same
atomic radii. The solid formed by cooling can have any proportion of
copper & nickel. Such completely miscible mixtures of metals are called
isomorphous.
Bycontrast, amixtureof lead&tinthat iseutecticisonlypartiallysoluble
when in the solid state. Lead & tin have different crystal structures (FCC
versusBCT) andleadatomsaremuchlarger. No morethan18.3weight %
solidtincandissolveinsolidleadandnomorethan2.2%of solidleadcan
dissolveinsolidtin(accordingtopreviousphasediagram).
The solid lead-tin alloy consists of a mixture of two solid phases, one
consisting of a maximum of 18.3 wt%tin (the alpha phase) and one
consistingof amaximumof 2.2wt%lead(thebetaphase).
15
Pb-Sn
For lead & tin the eutectic composition is
61.9 wt%tin and the eutectic temperature is
183C -- which makes this mixture useful as
solder.
At 183C, compositions of greater than
61.9wt%tinresult inprecipitationof atin-rich
solid in the liquid mixture, whereas
compositions of less than 61.9 wt%tin result
inprecipitationof lead-richsolid.
16
For alloys where
C
0
<2 wt% Sn
Result at room temperature is a
polycrystalline with grains of
phase having composition C
0
Microstructural Developments
in Eutectic Systems - I
0
L
+
200
T(C)
C , wt% Sn
10
2
20
C
0
300
100
L
30
+
400
(room T solubility limit)
T
E
L
L: C
0
wt%Sn
: C
0
wt%Sn
Pb-Sn
system
17
2 wt% Sn <C
0
<18.3 wt% Sn
Results in polycrystalline
microstructure with grains and
small -phaseparticles at lower
temperatures.
Microstructural Developments
in Eutectic Systems - II
L
+
200
T(C)
C, wt% Sn
10
18.3
20 0
C
0
300
100
L
30
+
400
(sol. limit at T
E
)
T
E
2
(sol. limit at T
room
)
L
L: C
0
wt%Sn
: C
0
wt%Sn
Pb-Sn
system
Microstructures in Eutectic Systems - III
Pb-Sn
system
C
o
=C
E
Results in a
eutectic
microstructure
with alternating
layers of and
crystals.
Sn) wt% 7.8 9 ( Sn) wt% .3 8 (1 Sn) wt% 9 . 61 ( + L
cooling
heating
18
19
Lamellar Eutectic Structure
A 2-phase microstructure
resulting from the
solidification of a liquid
having the eutectic
composition where the
phases exist as a lamellae
that alternate with one
another.
Formationof eutecticlayered
microstructure in the Pb-Sn
systemduringsolidificationat
the eutectic composition.
Compositions of and
phases are very different.
Solidification involves
redistribution of Pb and Sn
atomsbyatomicdiffusion.
Pb-rich
Sn-rich
20
Pb-SnMicrostructures
The dark layers are Pb-rich
phase, the light layers are the Sn-
rich phase.
21
For alloys with18.3 wt% Sn <C
0
<61.9 wt% Sn
Result: phase particles and a eutectic microconstituent
Microstructures in Eutectic Systems -
IV
18.3 61.9 97.8
eutectic
eutectic
W
L
=(1- W
) =0.50
C
=18.3 wt% Sn
C
L
=61.9 wt% Sn
W
= =0.50
Just above T
E
:
Just below T
E
:
C
=18.3 wt% Sn
C
=97.8 wt% Sn
W
= =0.727
W
=0.273 wt% Sn
Pb-Sn
system
L+
200
T(C)
C, wt% Sn
20 60 80 100 0
300
100
L
L+
40
+
T
E
L: C
0
wt% Sn
L
C
L
- C
0
C
L
- C
- C
0
C
- C
Primary
Binary PeritecticAlloy System
Themeltingpointsof thetwocomponentsarequitedifferent
Aliquidphasereactswiththesolidphasetoformanewanddifferent
solidphase
Liquid+
22
Binary PeritecticAlloy System
23
Binary MonotecticSystems
24
You might also like
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Phase DiagramsDocument79 pagesPhase DiagramsArun V NairNo ratings yet
- Ch10 Phase DiagramsDocument79 pagesCh10 Phase DiagramsDhileepan KumarasamyNo ratings yet
- Lecture 08 Phase Diagram Type III GroupDocument20 pagesLecture 08 Phase Diagram Type III GroupAhmad NawazNo ratings yet
- Chapter 4-Phase DiagramDocument16 pagesChapter 4-Phase Diagramtky96No ratings yet
- Chapter 9: Phase Diagrams: Issues To Address..Document41 pagesChapter 9: Phase Diagrams: Issues To Address..Faiz AkhtarNo ratings yet
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongNo ratings yet
- Lecture06and07S Oct12Document22 pagesLecture06and07S Oct12ali_b1367No ratings yet
- Phase Diagrams and Phase TransformationsDocument38 pagesPhase Diagrams and Phase TransformationsNameIs RajNo ratings yet
- Phase Diagrams & Heat Treatment of Carbon SteelDocument84 pagesPhase Diagrams & Heat Treatment of Carbon SteelTanmay DuttaNo ratings yet
- Phase DiagramsDocument50 pagesPhase DiagramsIbrahim MalikNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- Phase Diagrams: Lecture 2 (Manufacturing Technology)Document21 pagesPhase Diagrams: Lecture 2 (Manufacturing Technology)Ayush BhadauriaNo ratings yet
- Chapter 8 - Phase Diagram PART2Document26 pagesChapter 8 - Phase Diagram PART2Mohd IqbalNo ratings yet
- Review MetalDocument38 pagesReview MetalnisannisaNo ratings yet
- Phase Diagrams Material ScienceDocument46 pagesPhase Diagrams Material ScienceSabir Ali100% (1)
- Lab 7 - Phase DiagramsDocument7 pagesLab 7 - Phase Diagramsabd333No ratings yet
- 02 Phase DiagramsDocument24 pages02 Phase DiagramsPalash SwarnakarNo ratings yet
- Lect 10Document37 pagesLect 10MikeNo ratings yet
- Anup Sir PPT All PDFDocument222 pagesAnup Sir PPT All PDFFAIQNo ratings yet
- Engineering Materials 24-26Document31 pagesEngineering Materials 24-26Sanu SouravNo ratings yet
- Phase Diagram ExperimentDocument8 pagesPhase Diagram ExperimentAnand PatelNo ratings yet
- Che 414Document21 pagesChe 414Looking forwardNo ratings yet
- MM235 - Phase Diagram - SMDocument18 pagesMM235 - Phase Diagram - SMUtkarsh MishraNo ratings yet
- 4Document63 pages4ARJUN PESARU 19BME0161No ratings yet
- Unit-3 1Document70 pagesUnit-3 1Mruganesh SonarNo ratings yet
- Phase Diagram - : Dr. Aneela WakeelDocument21 pagesPhase Diagram - : Dr. Aneela WakeelHassan KhanNo ratings yet
- Chapter 9: Phase Diagrams: Issues To Address..Document38 pagesChapter 9: Phase Diagrams: Issues To Address..yunlu0705No ratings yet
- MSE 09 Phase Diagrams (39) 091116Document39 pagesMSE 09 Phase Diagrams (39) 091116eeng.ali6515No ratings yet
- Issues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..Document34 pagesIssues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..arif_ashraf94No ratings yet
- Intro PhaseDiagrams Dec09AS PDFDocument19 pagesIntro PhaseDiagrams Dec09AS PDFSH1961No ratings yet
- Question & Answer Set-7Document12 pagesQuestion & Answer Set-7eeng.ali651550% (2)
- Phase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingDocument35 pagesPhase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingSiratullah ShahNo ratings yet
- Phase DiagramDocument33 pagesPhase DiagramAlan TehNo ratings yet
- Phases PDFDocument13 pagesPhases PDFc1a5c7No ratings yet
- Unit - III: Me432P: Material EnggDocument37 pagesUnit - III: Me432P: Material Engghans groupNo ratings yet
- (Chapter 8) LC 8Document44 pages(Chapter 8) LC 8venosyah devanNo ratings yet
- Solidification of Pure Metal and Alloys (CHAPTER 2)Document52 pagesSolidification of Pure Metal and Alloys (CHAPTER 2)imfendi0% (1)
- 10.phase Diagrams PDFDocument24 pages10.phase Diagrams PDFMumpuniLuthfiNo ratings yet
- Phase DiagramDocument47 pagesPhase DiagramMuhammed MansNo ratings yet
- Chapter 6 (I-II) Phase DiagramDocument34 pagesChapter 6 (I-II) Phase Diagrammdipanwita48No ratings yet
- Engineering Materials - Manufacturing Engineering and TechnologyDocument12 pagesEngineering Materials - Manufacturing Engineering and Technologyhans vNo ratings yet
- Unit-2: Phase DiagramDocument37 pagesUnit-2: Phase DiagramPrasad Govind KumbharNo ratings yet
- Lecture 3-4 PPT Slides - Phase DiagramsDocument48 pagesLecture 3-4 PPT Slides - Phase DiagramshenryNo ratings yet
- Eutectic System - WikipediaDocument10 pagesEutectic System - Wikipediasterling goinNo ratings yet
- Chapter 8 (Heat Treatment)Document12 pagesChapter 8 (Heat Treatment)Shekinah KanindaNo ratings yet
- ENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachDocument2 pagesENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachterratempestNo ratings yet
- Definitions: Components and PhasesDocument42 pagesDefinitions: Components and Phasesabhilash reddyNo ratings yet
- Phase DiagramDocument36 pagesPhase Diagramzainal arifinNo ratings yet
- Phase DiagramsDocument30 pagesPhase DiagramsMervielle D ValiNo ratings yet
- Microstructure of A Lead-Tin AlloyDocument55 pagesMicrostructure of A Lead-Tin AlloyThaya GanapathyNo ratings yet
- 06 Diagram Phase PDFDocument24 pages06 Diagram Phase PDFTeknik PemesinanNo ratings yet
- Alloys & Their Phase Diagrams Alloys & Their Phase DiagramsDocument52 pagesAlloys & Their Phase Diagrams Alloys & Their Phase DiagramselgawadhaNo ratings yet
- Binary Phase DiagramsDocument60 pagesBinary Phase DiagramsmaryzeenNo ratings yet
- Module 4Document147 pagesModule 4Xavier HunterNo ratings yet
- Structural Analysis of Nanomaterials: Lecture 04: Phase Diagram: Determination of PhasesDocument31 pagesStructural Analysis of Nanomaterials: Lecture 04: Phase Diagram: Determination of PhaseswinnieNo ratings yet
- Assignment 8 SolutionDocument6 pagesAssignment 8 SolutionBrishen Hawkins100% (1)
- Phase DiagramsDocument80 pagesPhase DiagramsWilliams AkandiNo ratings yet
- Toilet 1.75 LacDocument1 pageToilet 1.75 LacAyush BhadauriaNo ratings yet
- Delhi ReportDocument2 pagesDelhi ReportAyush BhadauriaNo ratings yet
- Bill Position - 2ndDocument3 pagesBill Position - 2ndAyush BhadauriaNo ratings yet
- Guidelines For Short Film Making CompetitionDocument1 pageGuidelines For Short Film Making CompetitionAyush BhadauriaNo ratings yet
- LiFE MovementDocument5 pagesLiFE MovementAyush BhadauriaNo ratings yet
- Mechanical 5Document7 pagesMechanical 5Ayush BhadauriaNo ratings yet
- IIT5Document31 pagesIIT5Ayush BhadauriaNo ratings yet
- 733 Assignment 3.3: 1. 2.942821 3. 1.875 7. (I) 1.860, .2541 13. - 1.25115 and 0.55000Document1 page733 Assignment 3.3: 1. 2.942821 3. 1.875 7. (I) 1.860, .2541 13. - 1.25115 and 0.55000Ayush BhadauriaNo ratings yet
- UNSC ReformsDocument5 pagesUNSC ReformsAyush BhadauriaNo ratings yet
- 738 Assignment 6.3: C - B N S TDocument1 page738 Assignment 6.3: C - B N S TAyush BhadauriaNo ratings yet
- Eu 2Document15 pagesEu 2Ayush BhadauriaNo ratings yet
- Assignment 6.3: Z 1, Z 3, Z 3 y (X) X + XDocument13 pagesAssignment 6.3: Z 1, Z 3, Z 3 y (X) X + XAyush BhadauriaNo ratings yet
- Runge Kutta MethodDocument53 pagesRunge Kutta MethodAyush Bhadauria0% (1)
- Regula Falsi PDFDocument16 pagesRegula Falsi PDFAyush Bhadauria50% (2)
- Capital Market PDFDocument12 pagesCapital Market PDFAyush BhadauriaNo ratings yet
- N S O D E: Umerical Olution OF Rdinary Ifferential QuationsDocument3 pagesN S O D E: Umerical Olution OF Rdinary Ifferential QuationsAyush BhadauriaNo ratings yet
- Ethics and Human Interface PDFDocument9 pagesEthics and Human Interface PDFAyush BhadauriaNo ratings yet
- 732 Assignment 2.2: C - B N S TDocument1 page732 Assignment 2.2: C - B N S TAyush BhadauriaNo ratings yet
- Partition of BengalDocument2 pagesPartition of BengalAyush BhadauriaNo ratings yet
- Lgebraic AND Ranscendental Quations: Chapt ErDocument18 pagesLgebraic AND Ranscendental Quations: Chapt ErAyush BhadauriaNo ratings yet
- Foreign Investment Promotion Board (FIPB)Document4 pagesForeign Investment Promotion Board (FIPB)Ayush BhadauriaNo ratings yet
- 17A. Shale GasDocument8 pages17A. Shale GasAyush BhadauriaNo ratings yet
- Environment Impact AssessmentDocument15 pagesEnvironment Impact AssessmentAyush BhadauriaNo ratings yet
- International Relations Current Affairs 2017 Rohingya CrisisDocument4 pagesInternational Relations Current Affairs 2017 Rohingya CrisisAyush BhadauriaNo ratings yet
- Modern Indian History Congress-Khilafat Swarajya Party (Swarajya Party)Document4 pagesModern Indian History Congress-Khilafat Swarajya Party (Swarajya Party)Ayush Bhadauria0% (1)
- Insolvency and Bankruptcy Code, 2016Document4 pagesInsolvency and Bankruptcy Code, 2016Ayush BhadauriaNo ratings yet
- Model Answers (PURA)Document3 pagesModel Answers (PURA)Ayush BhadauriaNo ratings yet
- Social Justice PYQ AnalysisDocument8 pagesSocial Justice PYQ AnalysisAyush BhadauriaNo ratings yet
- Field Extensions Splitting Field and Perfect FieldsDocument18 pagesField Extensions Splitting Field and Perfect FieldsAyush BhadauriaNo ratings yet
- DOERING Produktinfo Cylpebs EnglischDocument4 pagesDOERING Produktinfo Cylpebs EnglischrecaiNo ratings yet
- Differences Between Metals and Non-MetalsDocument20 pagesDifferences Between Metals and Non-MetalsFera Cherilyn JulianNo ratings yet
- AIRCRAFT STRUCTURE MATERIAL METAL Pictures OnlyDocument27 pagesAIRCRAFT STRUCTURE MATERIAL METAL Pictures OnlyYasser ZubaidiNo ratings yet
- Valvula de Alivio de PresionDocument4 pagesValvula de Alivio de PresioneselcosacNo ratings yet
- Cga g-4 1 1985 PDFDocument22 pagesCga g-4 1 1985 PDFbkanoNo ratings yet
- Fixed Orthodontic ApplianceDocument43 pagesFixed Orthodontic ApplianceKhalil Raziq100% (3)
- Limit TestDocument34 pagesLimit TestAbhinav kumarNo ratings yet
- IUPAC Periodic Table-1May13Document1 pageIUPAC Periodic Table-1May13Anabel Bianco ArtajonaNo ratings yet
- Mro 082016Document172 pagesMro 082016American Hotel Register CompanyNo ratings yet
- Practice Test (Dolgos) - Periodic Table - W KeyDocument7 pagesPractice Test (Dolgos) - Periodic Table - W Keychandro57100% (1)
- ASME Section II PartA SA193 2007 PDFDocument18 pagesASME Section II PartA SA193 2007 PDFhoustonhimselfNo ratings yet
- MetalisEnergy TechnicalBrochure PDFDocument60 pagesMetalisEnergy TechnicalBrochure PDFAshok SureshNo ratings yet
- API 936 - Model ExamDocument13 pagesAPI 936 - Model ExamMohammed Ahtesham100% (1)
- Plumbing Materials and Various ToolsDocument8 pagesPlumbing Materials and Various ToolsJayson G. GunioNo ratings yet
- 816 (Welding)Document29 pages816 (Welding)Subhash SharmaNo ratings yet
- 2006 Fire Resitant Gypsum ManualDocument28 pages2006 Fire Resitant Gypsum Manuallangley.zhu.cnNo ratings yet
- Duplex Welding GuidelinesDocument13 pagesDuplex Welding GuidelinesadelNo ratings yet
- Astm b850 PDFDocument3 pagesAstm b850 PDFzhiqianxuNo ratings yet
- Hardness Test What Is Hardness?Document54 pagesHardness Test What Is Hardness?Parvatham RamalingamNo ratings yet
- Online Solution of Assignment of Chemistry On Electrochemistry PDFDocument6 pagesOnline Solution of Assignment of Chemistry On Electrochemistry PDFrvignesh2809No ratings yet
- Welding Super Duplex Stainless Steel Lincoln NorweldSmitweld NITO ConferenceDocument14 pagesWelding Super Duplex Stainless Steel Lincoln NorweldSmitweld NITO ConferencekbldamNo ratings yet
- PhosphatingDocument9 pagesPhosphatingnirai101259No ratings yet
- Radiographic Testing ProcedureDocument8 pagesRadiographic Testing ProcedureRai Singh MalhiNo ratings yet
- Etals Nternational Imited: Significant Welding Variables Brazing ReferencesDocument2 pagesEtals Nternational Imited: Significant Welding Variables Brazing ReferencesSSMNo ratings yet
- H-E Parts Data Sheet PT-60 Chromium CarbideDocument1 pageH-E Parts Data Sheet PT-60 Chromium CarbideJorge VillalobosNo ratings yet
- A 917Document3 pagesA 917bennNo ratings yet
- Creusabro 4800: Advanced Technology in WearDocument8 pagesCreusabro 4800: Advanced Technology in Wearsobhan61No ratings yet
- Recreation of Ancient Iron Smelting ProcessDocument7 pagesRecreation of Ancient Iron Smelting ProcessDILRUKSHINo ratings yet
- Engineering Materials: Mechanism in MetalsDocument38 pagesEngineering Materials: Mechanism in Metalssamurai7_77No ratings yet