Professional Documents
Culture Documents
Chemistry Teacher Book Chapter 5.1
Uploaded by
BryceWallsCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry Teacher Book Chapter 5.1
Uploaded by
BryceWallsCopyright:
Available Formats
CHAPTER
5
148 Chapter 5
148 Chapter 5
CHAPTER
5 Study Guide
Key Concepts
withChemASAP
5.1 Models of the Atom
Rutherfords planetary model could not
explain the chemical properties of elements.
Bohr proposed that electrons move only in
specific circular paths, or orbits, around the
nucleus.
The quantum mechanical model determines
the allowed energies an electron can have
and how likely it is to be found in various
locations around the nucleus.
Each sublevel of a principal energy level cor-
responds to an orbital shape describing
where the electron is likely to be found.
5.2 Electron Arrangement in Atoms
Three rulesthe aufbau principle, the Pauli
exclusion principle, and Hunds ruletell
you how to find the electron configurations
of atoms.
Some actual electron configurations differ
from those assigned using the aufbau princi-
ple because half-filled levels are not as stable
as filled levels, but they are more stable than
other configurations.
5.3 Physics and the Quantum Mechanical Model
The wavelength and frequency of light are
inversely proportional to each other.
When atoms absorb energy, electrons move
into higher energy levels, and then lose
energy by emitting light when the electrons
drop back to lower energy levels.
The light emitted by an electron moving
from a higher to a lower energy level has a
frequency directly proportional to the energy
change of the electron.
Classical mechanics adequately describes
the motions of bodies much larger than
atoms, while quantum mechanics describes
the motions of subatomic particles and
atoms as waves.
amplitude (p. 138)
atomic emission spectrum
(p. 141)
atomic orbital (p. 131)
aufbau principle (p. 133)
electromagnetic radiation
(p. 139)
electron configurations (p. 133)
energy levels (p. 128)
frequency (p. 138)
ground state (p. 142)
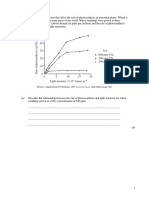
Heisenberg uncertainty
principle (p. 145)
hertz (p. 138)
Hunds rule (p. 134)
Pauli exclusion principle
(p. 134)
photons (p. 144)
quantum (p. 128)
quantum mechanical model
(p. 130)
spectrum (p. 139)
wavelength (p. 138)
c
Use these terms to construct a
concept map that organizes the
major ideas of this chapter.
electron
configuration
Concept Map 5 Solve the
concept map with the help of
an interactive guided tutorial.
Key Equation
Vocabulary
Organizing Information
aufbau
principle
energy
level
quantum
mechanical
model
Pauli exclusion
principle
Hunds rule
quantum
Study Guide
Study Tip
Summarizing Summarizing requires
students to identify key ideas and state
them briey in their own words. Tell
students that they will remember the
content of an entire section better
even if they summarize only a portion
of the section.
with ChemASAP
If your class subscribes to the Inter-
active Textbook with ChemASAP,
your students can go online to
access an interactive version of the
Student Edition and a self-test.
Chapter Resources
Print
Core Teaching Resources, Chapter 5,
Practice Problems, Vocabulary Review, Quiz,
Chapter Test A, Chapter Test B
Technology
Computer Test Bank, Chapter 5 Test
Interactive Textbook with ChemASAP,
Chapter 5
Virtual Chem Labs, Labs 47
Electrons in Atoms 149
CHAPTER
5 ASSESSMENT
22. could not explain why metals and
metal compounds give off charac-
teristic colors when heated, nor
could it explain the chemical prop-
erties of the elements; electrons
23. that electrons traveled in circular
paths around the nucleus
24. In Rutherfords model, negatively
charged electrons surround a
dense, positively charged nucleus.
In Bohrs model, the electrons are
assigned to concentric circular
orbits of xed energy.
25. An electron is found 90% of the
time inside this boundary.
26. a region in space around the
nucleus in which there is a high
probability of nding an electron
27. 3
28. The 1s orbital is spherical. The 2s
orbital is spherical with a diameter
larger than that of the 1s orbital.
The three 2p orbitals are dumbbell
shaped and oriented at right
angles to each other.
29. a. 1 b. 2 c. 3 d. 4
30. a. 2 b. 1 c. 3 d. 6
31. Electrons occupy the lowest possible
energy levels. An atomic orbital can
hold at most two electrons. One
electron occupies each of a set of
orbitals with equal energies before
any pairing of electrons occurs.
32. a. 1s
2
2s
2
2p
6
3s
2
3p
3
b. 1s
2
2s
2
2p
6
3s
2
c. 1s
2
2s
2
2p
5
d. 1s
2
2s
2
2p
6
3s
2
3p
6
33. The p orbitals in the third quantum
level have three electrons.
34. a.1s
2
2s
2
2p
6
3s
1
b. 1s
2
2s
2
2p
6
3s
2
3p
4
c. 1s
2
2s
2
2p
6
3s
2
d. 1s
2
2s
2
2p
6
e. 1s
2
2s
2
2p
6
3s
2
3p
6
4s
1
35. b and c
36. a. 2 b. 6 c. 2 d. 10 e. 6
f. 2 g. 14 h. 6
37. 2s, 3p, 4s, 3d
38. a. 8 b. 8 c. 8
39. a. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
4
b. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
3
4s
2
c.1s
2
2s
2
2p
6
3s
2
3p
6
3d
8
4s
2
d.1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
40. Violet, indigo, blue, green, yellow,
orange, red
41. Frequency is the number of wave
cycles that pass a given point per
unit time. Frequency units are cycles/s or
s
-1
or Hertz. Wavelength and frequency are
inversely related.
42. diagrams similar to those in Figure 5.9
43. Classical physics views energy changes as
continuous. In the quantum concept,
energy changes occur in tiny discrete units
called quanta.
44. Both travel at the same speed. Ultraviolet is
short wavelength and high frequency; micro-
wave is long wavelength and low frequency.
45. a. v, vi, iv, iii, i, ii b. It is the reverse.
46. Students may say that ultraviolet is used for
tanning the skin and growing plants, X-rays
for taking pictures of the interior of the
body, visible for seeing, infrared for warmth,
radio waves for communication, and micro-
waves for cooking.
47. The electron of the hydrogen atom is
raised (excited) to a higher energy level.
48. The outermost electron of sodium absorbs
photons of wavelength 589 nm as it jumps
to a higher energy level.
49. visible spectrum, Balmer series
CHAPTER
5 Assessment
Reviewing Content
Assessment 149
5.1 Models of the Atom
22. What was inadequate about Rutherfords model
of the atom? Which subatomic particles did
Thomson include in the plum-pudding model of
the atom?
23. What did Bohr assume about the motion of
electrons?
24. Describe Rutherfords model of the atom and
compare it with the model proposed by his stu-
dent Niels Bohr.
25. What is the signicance of the boundary of an
electron cloud?
26. What is an atomic orbital?
27. How many orbitals are in the 2p sublevel?
28. Sketch 1s, 2s, and 2p orbitals using the same
scale for each.
29. How many sublevels are contained in each of
these principal energy levels?
a. n 1 b. n 2 c. n 3 d. n 4
5.2 Electron Arrangement in Atoms
30. How many electrons are in the highest occupied
energy level of these atoms?
a. barium b. sodium
c. aluminum d. oxygen
31. What are the three rules that govern the lling of
atomic orbitals by electrons?
32. Write electron congurations for the elements
that are identied only by these atomic numbers.
a. 15 b. 12 c. 9 d. 18
33. What is meant by 3p
3
?
34. Give electron congurations for atoms of these
elements:
a. Na b. S c. Mg d. Ne e. K
35. Which of these orbital designations are invalid?
a. 4s b. 3f c. 2d d. 3d
36. What is the maximum number of electrons that
can go into each of the following sublevels?
a. 2s b. 3p c. 4s d. 3d
e. 4p f. 5s g. 4f h. 5p
37. Arrange the following sublevels in order of
increasing energy:
3d, 2s, 4s, 3p.
38. How many electrons are in the second energy
level of an atom of each element?
a. chlorine b. phosphorus c. potassium
39. Write electron congurations for atoms of these
elements.
a. selenium b. vanadium
c. nickel d. calcium
5.3 Physics and the Quantum Mechanical Model
40. List the colors of the visible spectrum in order of
increasing wavelength.
41. What is meant by the frequency of a wave? What
are the units of frequency? Describe the rela-
tionship between frequency and wavelength.
42. Use a diagram to illustrate each term for a wave.
a. wavelength
b. amplitude
c. cycle
43. Explain the difference between the energy lost
or gained by an atom according to the laws of
classical physics and according to the quantum
model of an atom.
44. How are ultraviolet radiation and microwave
radiation the same? How are they different?
45. Consider the following regions of the electromag-
netic spectrum: (i) ultraviolet, (ii) X-ray, (iii) visible,
(iv) infrared, (v) radio wave, (vi) microwave.
a. Use Figure 5.10 to arrange them in order of
decreasing wavelength.
b. How does this order differ from that of
decreasing frequency?
46. List one way in which each of the radiations
listed in Question 45 is used.
47. What happens when a hydrogen atom absorbs a
quantum of energy?
48. When white light is viewed through sodium
vapor in a spectroscope, the spectrum is contin-
uous except for a dark line at 589 nm. How can
you explain this observation?
49. The transition of electrons from higher energy
levels to the n 2 energy level results in the
emission of light from hydrogen atoms. In what
part of the spectrum is the emitted light, and
what is the name given to this transition series?
150 Chapter 5
ASSESSMENT
continued
CHAPTER
5
50. a. Ar b. Ru c. Gd
51. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
3
; level 1,
2; level 2, 8; level 3, 18; level 4, 5; The
fourth energy level is not lled.
52. a. 2 b. 4 c. 10 d. 6
53. 1s
2
2s
2
2p
3
nitrogen; 3
54. 2.61 10
4
cm
55. a. 4.36 10
-5
cm b. visible
c. 6.88 10
14
s
-1
56. a. 5.890 10
-5
cm and
5.896 10
-5
cm
b. 5.090 10
14
s
-1
(Hz) and
5.085 10
14
s
-1
(Hz)
c. yellow
57. a. Na, sodium
b. N, nitrogen
c. Si, silicon
d. O, oxygen
e. K, potassium
f. Ti, titanium
58. The frequency is inversely propor-
tional to the wavelength, so if the
frequency increases by a factor of
1.5, the wavelength will decrease
by a factor of 1.5.
59. It is not possible to know both the
position and the velocity of a parti-
cle at the same time.
60. 2
61. c
62. c
63. a
64. a
150 Chapter 5
CHAPTER
5 Assessment continued
50. Give the symbol for the atom that corresponds
to each electron conguration.
a. 1s
2
2s
2
2p
6
3s
2
3p
6
b. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
4d
7
5s
1
c. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
4d
10
4f
7
5s
2
5p
6
5d
1
6s
2
51. Write the electron conguration for an arsenic
atom. Calculate the total number of electrons in
each energy level and state which energy levels
are not full.
52. Howmany paired electrons are there in an atom
of each element?
a. helium b. boron
c. sodium d. oxygen
53. An atom of an element has two electrons in the
rst energy level and ve electrons in the second
energy level. Write the electron conguration for
this atom and name the element. How many
unpaired electrons does an atom of this element
have?
54. Suppose your favorite AM radio station broad-
casts at a frequency of 1150 kHz. What is the
wavelength, in centimeters, of the radiation
from the station?
55. A mercury lamp, such as the one below, emits
radiation with a wavelength of 4.36 10
7
m.
a. What is the wavelength of this radiation in
centimeters?
b. In what region of the electromagnetic spec-
trum is this radiation?
c. Calculate the frequency of this radiation.
56. Sodium vapor lamps are used to illuminate
streets and highways. The very bright light emit-
ted by these lamps is actually due to two closely
spaced emission lines in the visible region of the
electromagnetic spectrum. One of these lines
has a wavelength of 5.890 10
7
m, and the
other line has a wavelength of 5.896 10
7
m.
a. What are the wavelengths of these radiations
in centimeters?
b. Calculate the frequencies of these radiations.
c. Inwhat region of the visible spectrum do these
lines appear?
57. Give the symbol and name of the elements that
correspond to these congurations of an atom.
a. 1s
2
2s
2
2p
6
3s
1
b. 1s
2
2s
2
2p
3
c. 1s
2
2s
2
2p
6
3s
2
3p
2
d. 1s
2
2s
2
2p
4
e. 1s
2
2s
2
2p
6
3s
2
3p
6
4s
1
f. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
2
4s
2
58. Describe how the wavelength changes if the fre-
quency of a wave is multiplied by 1.5.
59. State the Heisenberg uncertainty principle.
60. What is the maximum number of electrons that
can be found in any orbital of an atom?
61. Pieces of energy are known as
a. isotopes b. particles
c. quanta d. line spectra
62. The lowest sublevel in each principal energy
level is represented by the symbol
a. f b. p c. s d. d
63. Which electron transition results in the emission
of energy?
a. 3p to 3s
b. 3p to 4p
c. 2s to 2p
d. 1s to 2s
64. Which is the ground state conguration of a
magnesium atom?
a. 1s
2
2s
2
2p
6
3s
2
b. 1s
2
2s
2
2p
6
3s
1
c. 1s
2
2s
2
3s
2
2p
6
d. 1s
2
2s
2
2p
4
3s
2
Understanding Concepts
Electrons in Atoms 151
65. An orbit connes the electron to a
xed circular path around the
nucleus; an orbital is a region
around the nucleus in which elec-
trons are likely to be found.
66. Answers will vary. Some students
may note that radio waves have
the lowest energy in the electro-
magnetic spectrum, and thus
would not be energetic enough to
cook food. Others may reason that
if microwaves cook food faster
than infrared radiation, then radio
waves would cook food even
faster.
67. Answers will vary. The model of the
atom is based on the abstract idea
of probability. Light is considered a
particle and a wave at the same
time. Atoms and light cannot be
compared to familiar objects or
observations because humans
cannot experience atoms or pho-
tons directly and because matter
and energy behave differently at
the atomic level than at the level
humans can observe directly.
68. a. Electrons in 2p boxes should not
be pairedthere should be one
electron in each.
b. Magnesium has 12 electrons.
Two more electrons need to be
added to 3s.
69. a. n = 1 level
b. n = 4 level
c. n = 4 level
d. n = 1 level
70. a. uorine b. germanium
c. vanadium
71. a. potassium, excited state, valence
electron has been promoted from
4s to 5p b. potassium, ground
state, correct electron conguration
c. impossible conguration, 3p
orbitals can hold a maximum of 6
electrons, not 7
72. The electrons obey Hunds rule.
73. a. Frequency(s
-1
): 5.20 10
12
; 4.40 10
13
;
9.50 10
13
; 1.70 10
14
; 2.20 10
14
;
4.70 10
14
b.
c. 6.63 10
-34
joulesecond.
d. The slope is Plancks constant.
74. 6.93 10
2
s
75. H: (n = 1), 1312kJ/mol); (n = 2), 328 kJ
Li
+
: (n = 1), 1.18 10
4
kJ
76. 2.12 10
-22
J
0
0.5
1.0
1.5
2.0
200 100 0
E
n
e
r
g
y
(
x
1
0
1
9
J
)
Frequency (x10
12
s
1
)
2.5
3.0
300 400 500
Concept Challenge Critical Thinking
Assessment 151
65. Explain the difference between an orbit in the
Bohr model and an orbital in the quantum
mechanical model of the atom.
66. Traditional cooking methods make use of infra-
red radiation (heat). Microwave radiation cooks
food faster. Could radio waves be used for cook-
ing? Explain.
67. Think about the currently accepted models of
the atom and of light. In what ways do these
models seem strange to you? Why are these mod-
els not exact or denite?
68. Orbital diagrams for the ground states of two
elements are shown below. Each diagram shows
something that is incorrect. Identify the error in
each diagram and then draw the correct diagram.
a. Nitrogen
b. Magnesium
69. Picture two hydrogen atoms. The electron in the
rst hydrogen atom is in the n 1 level. The
electron in the second atom is in the n 4 level.
a. Which atom has the ground state electron
conguration?
b. Which atom can emit electromagnetic
radiation?
c. In which atom is the electron in a larger
orbital?
d. Which atom has the lower energy?
70. Identify the elements whose electrically neutral
atoms have the following electron congurations.
a. 1s
2
2s
2
2p
5
b. 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
2
c. 1s
2
2s
2
2p
6
3s
2
3p
6
3d
3
4s
2
71. Which of the following is the ground state of an
atom? Which is its excited state? Which is an
impossible electron conguration? Identify the
element and briey explain your choices.
a. 1s
2
2s
2
2p
6
3s
2
3p
6
5p
1
b. 1s
2
2s
2
2p
6
3s
2
3p
6
4s
1
c. 1s
2
2s
2
2p
6
3s
2
3p
7
72. Why do electrons occupy equal energy orbitals
singly before beginning to pair up?
1s 2s 2p
1s 2s 2p 3s
73. The energy of a photon is related to its wave-
length and its frequency.
a. Complete the table above.
b. Plot the energy of the photon (y-axis) versus
the frequency (x-axis).
c. Determine the slope of the line.
d. What is the signicance of this slope?
74. The average distance between Earth and Mars is
about 2.08 10
8
km. How long does it take to
transmit television pictures from the Mariner
spacecraft to Earth from Mars?
75. Bohrs atomic theory can be used to calculate
the energy required to remove an electron from
an orbit of a hydrogen atom or an ion containing
only one electron. This is the ionization energy
for that atom or ion. The formula for determin-
ing the ionization energy (E) is
where Z is the atomic number, k is
2.18 10
18
J, and n is the energy level. What is
the energy required to eject an electron from a
hydrogen atom when the electron is in the
ground state (n 1)? In the second energy level?
How much energy is required to eject a ground
state electron from the species Li
?
76. The energy (E) of a photon absorbed or emitted
by a body is proportional to its frequency ().
E h
The constant h equals 6.63 10
34
Js. What is
the energy of a photon of microwave radiation
with a frequency of 3.20 10
11
s
1
?
Energy of
photon (J)
Frequency
(s
1
)
Wavelength
(cm)
3.45 10
21 a.
5.77 10
3
2.92 10
20
b. 6.82 10
4
6.29 10
20
c. 3.16 10
4
1.13 10
19
d. 1.76 10
4
1.46 10
19
e. 1.36 10
4
3.11 10
19
f. 6.38 10
5
E Z
2
k
n
2
152 Chapter 5
ASSESSMENT
continued
CHAPTER
5
77. a and b are heterogeneous; c is
homogeneous
78. Answers will vary but could
include, water is lost as steam and
burned meat gives off carbon
dioxide.
79. A compound has constant compo-
sition; the composition of a mix-
ture can vary.
80. a heterogeneous mixture
81. 7.7 10
-5
m
82. 18.9 cm
3
83. the piece of lead
84. a. 3.9 10
-5
kg
b. 7.84 10
2
L
c. 8.30 10
-2
g
d. 9.7 10
6
ng
85. a and b are exact
86. Mass remains the same; weight
decreases because gravity on
moon is less than gravity on earth.
87. 8.92 g/cm
3
88. 154 g, 1.54 10
-1
kg
89. Helium gas is less dense than the
nitrogen gas and oxygen gas in
the air.
90. a. 55 protons, 55 electrons
b. 47 protons, 47 electrons
c. 48 protons, 48 electrons
d. 34 protons, 34 electrons
91. Accuracy is a measure of how close
the value is to the true value; preci-
sion is a measure of how close a
series of measurements are to one
another.
92. a
93. Neon-20 has 10 neutrons; neon-21
has 11 neutrons.
94. The value 35.453 amu is a
weighted average. Its calculation is
based on the percentage natural
abundance of two isotopes, chlo-
rine-35 and chlorine-37.
152 Chapter 5
CHAPTER
5 Assessment continued
77. Classify each of the following as homogeneous
or heterogeneous (Chapter 2)
a. a page of this textbook
b. a banana split
c. the water in bottled water
78. Hamburger undergoes a chemical change when
cooked on a grill. All chemical changes are sub-
ject to the law of conservation of mass. Yet a
cooked hamburger will invariably weigh less than
the uncooked meat patty. Explain. (Chapter 2)
79. Homogeneous mixtures and compounds are
both composed of two or more elements. How
do you distinguish between a homogeneous
mixture and a compound? (Chapter 2)
80. The photo shows a magnied view of a small
piece of granite. Is granite a substance or a
mixture? (Chapter 2)
81. The diameter of a carbon atom is 77 pm. Express
this measurement in m. (Chapter 3)
82. A silver bar has a mass of 368 g. What is the
volume, in cm
3
, of the bar? The density of silver
is 19.5 g/cm
3
. (Chapter 3)
83. Which has more mass, a 28.0-cm
3
piece of lead
or a 16.0cm
3
piece of gold? The density of lead
is 11.4 g/cm
3
; the density of gold is 19.3 g/cm
3
.
(Chapter 3)
84. Express the following measurements in scien-
tic notation. (Chapter 3)
a. 0.000 039 kg b. 784 L
c. 0.0830 g d. 9 700 000 ng
85. Which of these quantities or relationships are
exact? (Chapter 3)
a. 10 cm 1 dm
b. 9 baseball players on the eld
c. a diamond has a mass of 12.4 g
d. the temperature is 21 C
86. A one-kilogram steel bar is brought to the moon.
How are its mass and its weight each affected by
this change in location? Explain. (Chapter 3)
87. When a piece of copper with a mass of 36.4 g is
placed into a graduated cylinder containing
20.00 mL of water, the water level rises to
24.08 mL. What is the density of copper?
(Chapter 3)
88. The density of gold is 19.3 g/cm
3
. What is the
mass, in grams, of a cube of gold 2.00 cm on each
edge? In kilograms? (Chapter 3)
89. A balloon lled with helium will rise upward
when released. What does this show about the
relative density of helium and air? Explain.
(Chapter 3)
90. Give the number of protons and electrons in
each of the following. (Chapter 4)
a. Cs b. Ag
c. Cd d. Se
91. Explain the difference between the accuracy of a
measurement and the precision of a measurement.
92. Which of these was an essential part of Daltons
atomic model? (Chapter 4)
a. indivisible atoms
b. electrons
c. atomic nuclei
d. neutrons
93. How do neon-20 and neon-21 differ from each
other? (Chapter 4)
94. The mass of an atom should be very nearly the
sum of the masses of its protons and neutrons.
The mass of a proton and the mass of a neutron
are each very close to 1 amu. Why is the atomic
mass of chlorine, 35.453 amu, so far from a
whole number? (Chapter 4)
Cumulative Review
Electrons in Atoms 153
Standardized Test Prep
1. d
2. b
3. a
4. c
5. d
6. (E)
7. (B)
8. (C)
9. (A)
10. (C)
11. c
12. b
13. d
14. a
15. c
16. a
17. a
18. According to the aufbau principle,
electrons enter orbitals of lowest
energy rst. According to the Pauli
exclusion principle, an orbital may
contain at most two electrons.
According to Hunds rule, one elec-
tron will enter each orbital of equal
energy before electrons begin to
pair up.
Standardized Test Prep 153
Test-Taking Tip
Standardized Test Prep
Eliminate wrong answers If you dont know which
response is correct, start by eliminating those you
know are wrong. If you can rule out some choices,
youll have fewer left to consider and youll increase
your chances of choosing the correct answer.
Select the choice that best answers each question or
completes each statement.
1. Select the correct electron conguration for sili-
con, atomic number 14.
a. 1s
2
2s
2
2p
2
3s
2
3p
2
3d
2
4s
2
b. 1s
2
2s
2
2p
4
3s
2
3p
4
c. 1s
2
2s
6
2p
6
d. 1s
2
2s
2
2p
6
3s
2
3p
2
2. Which pair of orbitals has the same shape?
a. 2s and 2p b. 2s and 3s
c. 3p and 3d d. More than one is correct.
3. Which of these statements characterize the
nucleus of every atom?
I. It has a positive charge.
II. It is very dense.
III. It is composed of protons, electrons, and
neutrons.
a. I and II only b. II and III only
c. I and III only d. I, II, and III
4. As the wavelength of light increases,
a. the frequency increases.
b. the speed of light increases.
c. the energy decreases.
d. the intensity increases.
5. In the third energy level,
a. there are two energy sublevels.
b. the f sublevel has 7 orbitals.
c. there are three s orbitals.
d. a maximum of 18 electrons are allowed.
The lettered choices below refer to Questions 610. A
lettered choice may be used once, more than once, or
not at all.
(A) s
2
p
6
(B) s
2
p
2
(C) s
2
(D) s
4
p
1
(E) s
2
p
4
Which configuration is the outer shell electron config-
uration for each of these elements?
6. sulfur
7. germanium
8. beryllium
9. krypton
10. strontium
Use the atomic models to answer Questions 1113.
11. Which model of the atom most closely resem-
bles a planetary model?
12. Which model was proposed based on data from
Rutherfords gold foil experiment?
13. In which model are electrons in orbitals?
Use the drawings to answer Questions 1417. Each
drawing represents an electromagnetic wave.
14. The wave has the longest wavelength.
15. The wave has the highest energy,
16. The wave has the lowest frequency.
17. The wave has the highest amplitude.
Write a short essay to answer Question 18.
18. Explain the rules that determine how electrons
are arranged around the nuclei of atoms.
a.
c.
b.
d.
a.
b.
c.
Waves
You might also like
- Chemical Bonding IPEDocument37 pagesChemical Bonding IPEAdiChemAdi100% (1)
- Some Basic Concepts in Chemistry: TopicDocument8 pagesSome Basic Concepts in Chemistry: TopicRishabh RanjanNo ratings yet
- Formulas, Masses and MolesDocument23 pagesFormulas, Masses and MolesAjay0% (1)
- Chap 8 Reaction Kinetics 1415FARRADocument129 pagesChap 8 Reaction Kinetics 1415FARRA黄麒安No ratings yet
- KD Engine Specs and Systems GuideDocument72 pagesKD Engine Specs and Systems Guidemoises valenzuela janampaNo ratings yet
- q801 Junior Engineer Part IIDocument28 pagesq801 Junior Engineer Part IIRohan ChaudharyNo ratings yet
- We n5523521 v7 Hendrix Covered ConductorDocument55 pagesWe n5523521 v7 Hendrix Covered ConductorJ. Mauricio A. BejaranoNo ratings yet
- Method of Statement-RTDocument7 pagesMethod of Statement-RTbuddhikasat50% (2)
- Free Online Science Education ResourcesDocument17 pagesFree Online Science Education ResourcesDIONYSUS100% (1)
- Astm G154Document11 pagesAstm G154jesoneliteNo ratings yet
- Atoms, Molecules and IonsDocument58 pagesAtoms, Molecules and IonsJunaid Alam100% (1)
- Ch. 15 - Science Notebook Sec. 2Document4 pagesCh. 15 - Science Notebook Sec. 2Savannah MontelongoNo ratings yet
- Paper 3 Acid Dew Point Corrosion in HRSGsDocument83 pagesPaper 3 Acid Dew Point Corrosion in HRSGsKarna2504100% (1)
- Compressor Station MallnowDocument8 pagesCompressor Station MallnowMANIU RADU-GEORGIANNo ratings yet
- EXPANDED INSPECTION CHECKLISTDocument2 pagesEXPANDED INSPECTION CHECKLISTfredy2212100% (1)
- Regents Chemistry Periodic Table Practice Test ADocument7 pagesRegents Chemistry Periodic Table Practice Test Achandro57100% (1)
- Organic Chemistry NomenclatureDocument8 pagesOrganic Chemistry NomenclaturetasneemNo ratings yet
- Practice estimating to find the best answer.: Δt 5 x rate = -Δ (B) 5 x 0.0243 M/s = - Δ (B) -0.12125~ - 0.122 M/sDocument10 pagesPractice estimating to find the best answer.: Δt 5 x rate = -Δ (B) 5 x 0.0243 M/s = - Δ (B) -0.12125~ - 0.122 M/sjeffrey XiaoNo ratings yet
- 3-day CertificateDocument25 pages3-day Certificateganesh kondikire100% (8)
- Chap15 Periodic TableDocument32 pagesChap15 Periodic TableSanthiya MadhavanNo ratings yet
- Organizing Elements Int - Reader - Study - Guide PDFDocument4 pagesOrganizing Elements Int - Reader - Study - Guide PDFMiguel RuizNo ratings yet
- CHM271 - Chapter 3 Ionic Equilibrium PDFDocument72 pagesCHM271 - Chapter 3 Ionic Equilibrium PDFMuhd AmirNo ratings yet
- Periodicity (Chemistry) PDFDocument13 pagesPeriodicity (Chemistry) PDFMarga AsuncionNo ratings yet
- Chem Office Enterprise 2006Document402 pagesChem Office Enterprise 2006HalimatulJulkapliNo ratings yet
- Stoichiometry Manual PDFDocument32 pagesStoichiometry Manual PDFAbdi Putra RamadhanNo ratings yet
- Qualitative Analysis Guide SheetDocument19 pagesQualitative Analysis Guide SheetMr. Swai W.JNo ratings yet
- Chem Int CC CH 12 - Stoichiometry - Answers (09.15)Document7 pagesChem Int CC CH 12 - Stoichiometry - Answers (09.15)Emma GillesNo ratings yet
- Introduction to Chemical Reactions and EquationsDocument6 pagesIntroduction to Chemical Reactions and EquationsayanNo ratings yet
- Margolis, Emil - J. - Formulation and Stoichiometry PDFDocument230 pagesMargolis, Emil - J. - Formulation and Stoichiometry PDFOscar Perez RosalesNo ratings yet
- Chapter 6 TestDocument5 pagesChapter 6 TesthelloblargNo ratings yet
- JC1 Atomic Structure NotesDocument35 pagesJC1 Atomic Structure NotesLeng RyanNo ratings yet
- Test-2-10 Science Chemical Reactions and Equations Test 02Document2 pagesTest-2-10 Science Chemical Reactions and Equations Test 02Ramesh MuthusamyNo ratings yet
- Gas Laws Practice Test GuideDocument10 pagesGas Laws Practice Test GuideTAHA GABRNo ratings yet
- Characteristics of Chemical EquilibriumDocument43 pagesCharacteristics of Chemical Equilibriumpimpin1No ratings yet
- Chemical Reactions and EquationsDocument2 pagesChemical Reactions and EquationsGENERAL COCNo ratings yet
- Periyava 50 Quotes in EnglishDocument55 pagesPeriyava 50 Quotes in EnglishvseenuNo ratings yet
- Rates Practice Exam QuestionsDocument18 pagesRates Practice Exam QuestionsisheanesuNo ratings yet
- Redox WKSHTDocument4 pagesRedox WKSHTMarco ConopioNo ratings yet
- UNIT 1 - Assignment 7 - Harder Balancing Problems - Answer KeyDocument2 pagesUNIT 1 - Assignment 7 - Harder Balancing Problems - Answer KeyAayush ChoudharyNo ratings yet
- Chemistry - Periodic TableDocument19 pagesChemistry - Periodic Tablesgw67No ratings yet
- Chemical Reactions and EquationsDocument8 pagesChemical Reactions and Equationsapi-246793885No ratings yet
- Equivalent Concept & Titration ExplainedDocument13 pagesEquivalent Concept & Titration ExplainedDIPESH100% (1)
- Dynamic Equilibrium Reactions Reach Constant ConcentrationsDocument22 pagesDynamic Equilibrium Reactions Reach Constant ConcentrationsAN NGUYENNo ratings yet
- Test-2-Key-10 Science Chemical Reactions and Equations Test 02 Answer 0n4sDocument2 pagesTest-2-Key-10 Science Chemical Reactions and Equations Test 02 Answer 0n4sRamesh MuthusamyNo ratings yet
- Acid Base Reactions WorksheetDocument5 pagesAcid Base Reactions WorksheetOmar IjazNo ratings yet
- Chapter 7 Ionic and Metallic BondingDocument56 pagesChapter 7 Ionic and Metallic BondingCharles GibbsNo ratings yet
- Understanding Oxidation Numbers Through Electronegativity and Partial ChargesDocument14 pagesUnderstanding Oxidation Numbers Through Electronegativity and Partial ChargesEricNo ratings yet
- Empirical Versus Molecular FormulasDocument5 pagesEmpirical Versus Molecular FormulasJaz SantosNo ratings yet
- Chemical Kinetics Part - IDocument43 pagesChemical Kinetics Part - ISanskar BhattacharyaNo ratings yet
- C1 - Basic Concepts of Chemistry - Solutions (v18) - HD - CLDocument20 pagesC1 - Basic Concepts of Chemistry - Solutions (v18) - HD - CLAashish DubeyNo ratings yet
- Back TitrateDocument16 pagesBack Titratepicket1019No ratings yet
- IB Chemistry Objectives - KineticsDocument1 pageIB Chemistry Objectives - KineticslizarrdoNo ratings yet
- Unit 15 - Reaction Rates and EquilibriumDocument68 pagesUnit 15 - Reaction Rates and EquilibriumGarett Berumen-RoqueNo ratings yet
- Day 2 - Introduction To Stoichiometry Guided Notes AssignmentDocument15 pagesDay 2 - Introduction To Stoichiometry Guided Notes AssignmentDaveNo ratings yet
- Chapter 12 Student NotesDocument8 pagesChapter 12 Student Notesapi-307565882No ratings yet
- Chemical Bonding and Molecular Geometry: Chapter OutlineDocument68 pagesChemical Bonding and Molecular Geometry: Chapter OutlineMaden betoNo ratings yet
- Molecular Shapes WorksheetDocument5 pagesMolecular Shapes WorksheetAbdur RehmanNo ratings yet
- Worksheet #3 - Mole ConceptDocument6 pagesWorksheet #3 - Mole Conceptjfkdmfmdf100% (1)
- Chemistry Form 5 Module Organic CompoundDocument24 pagesChemistry Form 5 Module Organic CompoundTiviya Tarini ManiamNo ratings yet
- Assignment Chemical Bonding JH Sir-4163 PDFDocument70 pagesAssignment Chemical Bonding JH Sir-4163 PDFAkhilesh AgrawalNo ratings yet
- Ib PPT 3 SL PDFDocument24 pagesIb PPT 3 SL PDFzarna nirmal rawalNo ratings yet
- Oxford Resources For IB: Structure 3.1 - The Periodic Table: Classification of ElementsDocument19 pagesOxford Resources For IB: Structure 3.1 - The Periodic Table: Classification of ElementsGian Paolo GerzonNo ratings yet
- States of Matter Notes Class 11 Chemistry Chapter 5 Download in PDocument2 pagesStates of Matter Notes Class 11 Chemistry Chapter 5 Download in PisaacNo ratings yet
- Science Notes For Class 10 Chapter 5 Periodic Classification of ElementsDocument4 pagesScience Notes For Class 10 Chapter 5 Periodic Classification of Elementscrazy about readingNo ratings yet
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisFrom EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisRating: 4 out of 5 stars4/5 (2)
- Transition Metal ToxicityFrom EverandTransition Metal ToxicityG. W. RichterNo ratings yet
- Strength Improvement of Loose Sandy Soils Through Cement GroutingDocument56 pagesStrength Improvement of Loose Sandy Soils Through Cement Groutinganilsmg09No ratings yet
- Station Locations in Caloocan, Malabon, Valenzuela & Nearby CitiesDocument15 pagesStation Locations in Caloocan, Malabon, Valenzuela & Nearby CitiesTricia Mae Paras WajeNo ratings yet
- Rotational Motion Engineering Mechanics IIT KanpurDocument67 pagesRotational Motion Engineering Mechanics IIT KanpurNitin SharmaNo ratings yet
- PanasonicDocument15 pagesPanasonicMohd HaniffNo ratings yet
- Molecular Orbital TutorialDocument28 pagesMolecular Orbital TutorialehmedNo ratings yet
- Holec ERM TDRM Restart ModulesDocument6 pagesHolec ERM TDRM Restart Modules0b0biyd,u,uNo ratings yet
- 301 Basic Mechanics Course DescriptionDocument2 pages301 Basic Mechanics Course DescriptionAnonymous q9eCZHMuSNo ratings yet
- Thermochemistry Practice ProblemsDocument3 pagesThermochemistry Practice ProblemsElla BombergerNo ratings yet
- Notes on Heat Transfer Methods and EquationsDocument6 pagesNotes on Heat Transfer Methods and Equationsjme733k9100% (1)
- Pump QuotationsDocument3 pagesPump Quotationsdibyendu65No ratings yet
- V-3111-002-A-711 - 2 Method Statement For Water Well WorkDocument14 pagesV-3111-002-A-711 - 2 Method Statement For Water Well WorkWidya PrasetyaNo ratings yet
- AERMOTORDocument12 pagesAERMOTORSharid PickeringNo ratings yet
- Concept Note For Wind ParkDocument7 pagesConcept Note For Wind ParkArun MehtaNo ratings yet
- Engineering Physics - G. AruldhasDocument100 pagesEngineering Physics - G. AruldhasJaya sankarNo ratings yet
- Compare Energy Sources & Their Environmental EffectsDocument2 pagesCompare Energy Sources & Their Environmental Effectsamit shuklaNo ratings yet
- Plastic Road 35pageDocument35 pagesPlastic Road 35pageME A 04 AvisekNo ratings yet
- Motor Logic™ Solid-State Overload Relays (SSOLRS) : Other FeaturesDocument4 pagesMotor Logic™ Solid-State Overload Relays (SSOLRS) : Other FeaturesSantiago BorjaNo ratings yet
- Kaise Batera Agm 12v 200ahDocument2 pagesKaise Batera Agm 12v 200ahJOHN FREDY IBAÑEZ HERNANDEZNo ratings yet
- Biofuel For Sri LankaDocument0 pagesBiofuel For Sri LankaJaanu SanthiranNo ratings yet
- Data Base Questions PhotosynthesisDocument10 pagesData Base Questions PhotosynthesisValeCalderónNo ratings yet
- Transient Stability Improvement of Power System Using UpfcDocument18 pagesTransient Stability Improvement of Power System Using UpfcMuhammadWaqarNo ratings yet