Professional Documents
Culture Documents
The Biomedical Engineering Handbook: Second Edition
Uploaded by
Karthik Rajv0 ratings0% found this document useful (0 votes)
25 views10 pagesvirtual instrumentation
Original Title
ch088
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentvirtual instrumentation
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
25 views10 pagesThe Biomedical Engineering Handbook: Second Edition
Uploaded by
Karthik Rajvvirtual instrumentation
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 10
Rosow, E., Adam . J . Virtual Instrumentation: Applications in Biomedical Engineering.
The Biomedical Engineering Handbook: Second Edition.
Ed. J oseph D. Bronzino
Boca Raton: CRC Press LLC, 2000
2000 by CRC Press LLC
88
Virtual
Instrumentation:
Applications in
Biomedical Engineering
88.1 Overview
A RevolutionGraphical Programming and Virtual
Instrumentation
88.2 Virtual Instrumentation and Biomedical
Engineering
Example 1 Example 2
88.1 Overview
A RevolutionGraphical Programming and Virtual Instrumentation
Over the last decade, the graphical programming revolution has empowered engineers to develop cus-
tomized systems, the same way the spreadsheet has empowered business managers to analyze nancial
data. This software technology has resulted in another type of revolutionthe virtual instrumentation
revolution, which is rapidly changing the instrumentation industry by driving down costs without
sacricing quality.
Virtual Instrumentation can be dened as:
A layer of software and/or hardware added to a general-purpose computer in such a fashion that users
can interact with the computer as though it were their own custom-designed traditional electronic
instrument.
Today, computers can serve as the engine for instrumentation. Virtual instruments utilize the open
architecture of industry-standard computers to provide the processing, memory, and display capabilities;
while the off-the-shelf, inexpensive interface boards plugged into an open bus, standardized communi-
cations bus provides the vehicle for the instruments capabilities. As a result, the open architecture of
PCs and workstations allow the functionality of virtual instruments to be user dened. In addition, the
processing power of virtual instruments is much greater than stand-alone instruments. This advantage
will continue to accelerate due to the rapid technology evolution of PCs and workstations that results
from the huge investments made in this industry.
The major benets of virtual instrumentation include increased performance and reduced costs. In
addition, because the user controls the technology through software, the exibility of virtual instrumentation
Eric Rosow
Hartford Hospital and Premise
Development Corporation
Joseph Adam
Premise Development Corporation
2000 by CRC Press LLC
is unmatched by traditional instrumentation. The modular, hierarchical programming environment of
virtual instrumentation is inherently reusable and recongurable.
88.2 Virtual Instrumentation and Biomedical Engineering
Virtual Instrumentation applications have encompassed nearly every industry including the telecommu-
nications, automotive, semiconductor, and biomedical industries. In the elds of healthcare and biomed-
ical engineering, virtual instrumentation has empowered developers and end-users to conceive of,
develop, and implement a wide variety of research-based biomedical applications and executive infor-
mation tools. These applications fall into several categories including: clinical research, equipment testing
and quality assurance, data management, and performance improvement.
In a collaborative approach, physicians, researchers, and biomedical and software engineers at Hartford
Hospital (Hartford, CT) and Premise Development Corporation (Avon, CT) have developed various data
acquisition and analysis systems that successfully integrate virtual instrumentation principles in a wide
variety of environments. These include:
The EndoTester, a patented quality assurance system for beroptic endoscopes
a Non-Invasive Pulmonary Diffusion and Cardiac Output Measurement System
a Cardiovascular Pressure-Dimension Analysis System
BioBench, a powerful turnkey application for physiological data acquisition and analysis
PIVIT, a Performance Indicator Virtual Instrument Toolkit to manage and forecast nancial
data
a Virtual Intelligence Program to manage the discrete components within the continuum of care
BabySave, an analysis and feedback system for apnea interruption via vibratory stimulation
This chapter will describe several of these applications and describe how they have allowed clinicians
and researchers to gain new insights, discover relationships that may not have been obvious, and test
and model hypotheses based on acquired data sets. In some cases, these applications have been developed
into commercial products to address test and measurement needs at other healthcare facilities throughout
the world.
Example 1: BioBenchA Virtual Instrument Application for Data
Acquisition and Analysis of Physiological Signals
The biomedical industry is an industry that relies heavily on the ability to acquire, analyze, and display
large quantities of data. Whether researching disease mechanisms and treatments by monitoring and
storing physiological signals, researching the effects of various drugs interactions, or teaching students
in labs where students study physiological signs and symptoms, it was clear that there existed a strong
demand for a exible, easy-to-use, and cost-effective tool. In a collaborative approach, biomedical engi-
neers, software engineers and clinicians, and researchers created a suite of virtual instruments called
BioBench.
BioBench (National Instruments, Austin, TX) is a new software application designed for physiolog-
ical data acquisition and analysis. It was built with LabVIEW, the worlds leading software development
environment for data acquisition, analysis, and presentation.
1
Coupled with National Instruments data
acquisition (DAQ) boards, BioBench integrates the PC with data acquisition for the life sciences market.
Many biologists and physiologists have made major investments over time in data acquisition hardware
built before the advent of modern PCs. While these scientists cannot afford to throw out their investment
in this equipment, they recognize that computers and the concept of virtual instrumentation yield
tremendous benets in terms of data analysis, storage, and presentation. In many cases, traditional
1
BioBench was developed for National Instruments (Austin, TX) by Premise Development Corporation (Avon,
CT).
2000 by CRC Press LLC
medical instrumentation may be too expensive to acquire and/or maintain. As a result, researchers and
scientists are opting to create their own PC-based data monitoring systems in the form of virtual
instruments.
Other life scientists, who are just beginning to assemble laboratory equipment, face the daunting task
of selecting hardware and software needed for their application. Many manufacturers for the life sciences
eld focus their efforts on the acquisition of raw signals and converting these signals into measurable
linear voltages. They do not concentrate on digitizing signals or the analysis and display of data on the
PC. BioBench is a low-cost turnkey package that requires no programming. BioBench is compatible
with any isolation amplier or monitoring instrument that provides an analog output signal. The user
can acquire and analyze data immediately because BioBench automatically recognizes and controls the
National Instruments DAQ hardware, minimizing conguration headaches.
Some of the advantages of PC-Based Data Monitoring include:
Easy-to-use-software applications
Large memory and the PCI bus
Powerful processing capabilities
Simplied customization and development
More data storage and faster data transfer
More efcient data analysis
Figure 88.1 illustrates a typical setup of a data acquisition experiment using BioBench. BioBench also
features pull-down menus through which the user can congure devices. Therefore, those who have
made large capital investments can easily migrate their existing equipment into the computer age.
Integrating a combination of old and new physiological instruments from a variety of manufacturers is
an important and straightforward procedure. In fact, within the clinical and research setting, it is a
common requirement to be able to acquire multiple physiological signals from a variety of medical devices
and instruments which do not necessarily communicate with each other. Often times, this situation is
compounded by the fact that end-users would like to be able to view and analyze an entire waveform
and not just an average value. In order to accomplish this, the end-user must acquire multiple channels
of data at a relatively high sampling rate and have the ability to manage many large data les. BioBench
can collect up to 16 channels simultaneously at a sampling rate of 1000 Hz per channel. Files are stored
in an efcient binary format which signicantly reduces the amount of hard disk and memory require-
ments of the PC. During data acquisition, a number of features are available to the end-user. These
features include:
FIGURE 88.1
A typical biomedical application using BioBench (courtesy of National Instruments).
2000 by CRC Press LLC
Data Logging:
Logging can be enabled prior to or during an acquisition. The application will either
prompt the user for a descriptive lename or it can be congured to automatically assign a lename
for each acquisition. Turning the data logging option on and off creates a log data event record
that can be inspected in any of the analysis views of BioBench.
Event Logging:
The capacity to associate and recognize user commands associated with a data le
may be of signicant value. BioBench has been designed to provide this capability by automatically
logging user-dened events, stimulus events, and le logging events. With user-dened events, the
user can easily enter and associate date and time-stamped notes with user actions or specic
subsets of data. Stimulus events are also data and time-stamped and provide the user information
about whether a stimulus has been turned on or off. File logging events note when data has been
logged to disk. All of these types of events are stored with the raw data when logging data to le
and they can be searched for when analyzing data.
Alarming:
To alert the user about specic data values and thresholds, BioBench incorporates user-
dened alarms for each signal which is displayed. Alarms appear on the user interface during data
acquisition and notify the user that an alarm condition has occurred.
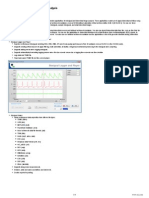
Figure 88.2 is an example of the Data Acquisition mode of BioBench. Once data has been acquired,
BioBench can employ a wide array of easy-to-use analysis features. The user has the choice of importing
recently acquired data or opening a data le that had been previously acquired for comparison or teaching
purposes. Once a data set has been selected and opened, BioBench allows the user to simply select and
highlight a region of interest and choose the analysis options to perform a specic routine.
BioBench implements a wide array of scalar and array analyses. For example, scalar analysis tools will
determine the minimum, maximum, mean, integral, and slope of a selected data set, while the array
analysis tools can employ Fast Fourier Transforms (FFTs), peak detection, histograms, and X vs. Y plots.
The ability to compare multiple data les is very important in analysis and BioBench allows the user
to open an unlimited number of data les for simultaneous comparison and analysis. All data les can
be scanned using BioBenchs search tools in which the user can search for particular events that are
FIGURE 88.2
BioBench acquisition mode with alarms enabled.
2000 by CRC Press LLC
associated with areas of interest. In addition, BioBench allows the user to employ lters and transforma-
tions to their data sets and all logged data can be easily exported to a spreadsheet or database for further
analysis. Finally, any signal acquired with BioBench can be played back, thus taking lab experience into
the classroom. Figure 88.3 illustrates the analysis features of BioBench.
Example 2: A Cardiovascular Pressure-Dimension Analysis System
Introduction
The intrinsic contractility of the heart muscle (myocardium) is the single most important determinant
of prognosis in virtually all diseases affecting the heart (e.g., coronary artery disease, valvular heart disease,
and cardiomyopathy). Furthermore, it is clinically important to be able to evaluate and track myocardial
function in other situations, including chemotherapy (where cardiac dysfunction may be a side effect of
treatment) and liver disease (where cardiac dysfunction may complicate the disease).
The most commonly used measure of cardiac performance is the ejection fraction. Although it does
provide some measure of intrinsic myocardial performance, it is also heavily inuenced by other factors
such as heart rate and loading conditions (i.e., the amount of blood returning to the heart and the
pressure against which the heart ejects blood).
Better indices of myocardial function based on the relationship between pressure and volume through-
out the cardiac cycle (pressurevolume loops) exist. However, these methods have been limited because
they require the ability to track ventricular volume continuously during rapidly changing loading con-
ditions. While there are many techniques to measure volume under steady state situations, or at end-
diastole and end-systole (the basis of ejection fraction determinations), few have the potential to record
volume during changing loading conditions.
Echocardiography can provide online images of the heart with high temporal resolution (typically 30
frames per second). Since echocardiography is radiation-free and has no identiable toxicity, it is ideally
suited to pressurevolume analyses. Until recently however, its use for this purpose has been limited by the
need for manual tracing of
the endocardial borders, an extremely tedious and time-consuming endeavor.
FIGURE 88.3
BioBench analysis mode.
2000 by CRC Press LLC
The System
Biomedical and software engineers at Premise Development Corporation (Avon, CT), in collaboration
with physicians and researchers at Hartford Hospital, have developed a sophisticated research application
called the Cardiovascular Pressure-Dimension Analysis (CPDA) System. The CPDA system acquires
echocardiographic volume and area information from the acoustic quantication (AQ) port, in conjunc-
tion with vetricular pressure(s) and ECG signals to rapidly perform pressurevolume and pressure-area
analyses. This fully automated system allows cardiologists and researchers to perform online pressure-
dimension and stroke work analyses during routine cardiac catheterizations and open-heart surgery. The
system has been designed to work with standard computer hardware. Analog signals for ECG, pressure,
and area/volume (AQ) are connected to a standard BNC terminal board. Automated calibration routines
ensure that each signal is properly scaled and allows the user to immediately collect and analyze pressure-
dimension relationships.
The CPDA can acquire up to 16 channels of data simultaneously. Typically, only three physiological
parameters, ECG, pressure, and the AQ signals are collected using standard data acquisition hardware.
In addition, the software is capable of running on multiple operating systems including Macintosh,
Windows 95/98/NT, and Solaris. The CPDA also takes advantage of the latest hardware developments
and form-factors and can be used with either a desktop or a laptop computer.
The development of an automated, online method of tracing endocardial borders (Hewlett Packards
AQ Technology) (Hewlett-Packard Medical Products Group, Andover, MA) has provided a method for
rapid online area and volume determinations. Figure 88.4 illustrates this AQ signal from a Hewlett
Packard Sonos Ultrasound Machine. This signal is available as an analog voltage (1 to +1 volts) through
the Sonos Dataport option (BNC connector).
Data Acquisition and Analysis
Upon launching this application, the user is presented with a dialog box that reviews the license agreement
and limited warranty. Next, the Main Menu is displayed, allowing the user to select from one of six
options as shown in Fig. 88.5.
Clinical Signicance
Several important relationships can be derived from this system. Specically, a parameter called the
End-
Systolic Pressure-Volume Relationship
(
ESPVR
)
describes the line of best t through the peak-ratio (max-
imum pressure with respect to minimum volume) coordinates from a series of pressurevolume loops
FIGURE 88.4
The Acoustic Quantication (AQ) signal (Hewlett Packard).
2000 by CRC Press LLC
generated under varying loading conditions. The slope of this line has been shown to be a sensitive index
of myocardial contractility that is independent of loading conditions. In addition, several other analyses,
including
time varying elastance
(
E
max
)
and
stroke work,
are calculated.
Time-varying elastance is measured
by determining the maximum slope of a regression line through a series of isochronic pressurevolume
coordinates. Stroke work is calculated by quantifying the area of each pressurevolume loop. Statistical
parameters are also calculated and displayed for each set of data. Figure 88.7 illustrates the pressure-
dimension loops and each of the calculated parameters along with the various analysis options. Finally,
the user has the ability to export data sets into spreadsheet and database les and export graphs and
indicators into third-party presentation software packages such as Microsoft PowerPoint.
FIGURE 88.5
Cardiovascular pressure-dimension analysis main menu.
FIGURE 88.6
The data selection front panel.
2000 by CRC Press LLC
Summary
Virtual Instrumentation allows the development and implementation of innovative and cost-effective
biomedical applications and information management solutions. As the healthcare industry continues
to respond to the growing trends of managed care and capitation, it is imperative for clinically useful,
cost-effective technologies to be developed and utilized. As application needs will surely continue to
change, virtual instrumentation systems will continue to offer users exible and powerful solutions
without requiring new equipment or traditional instruments.
References
1. Fisher, J.P., Mikan, J.S., Rosow, E., Nagle, J., Fram, D.B., Maffucci, L.M., McKay, R.G., and Gillam,
L.D., Pressure-Dimension Analysis of Regional Left Ventricular Performance Using Echocardio-
graphic Automatic Boundary Detection: Validation in an Animal Model of Inotropic Modulation,
J. Am. College of Cardiol.,
19(3), 262A, 1992.
2. Fisher, J.P., McKay, R.G., Mikan, J.S., Rosow, E., Nagle, J., Mitchel, J.F., Kiernan, F.J., Hirst, J.A.,
Primiano, C.A., Fram, D.B., Gillam, L.D., Hartford Hospital and University of Connecticut, Hart-
ford, CT,
Human Left Ventricular Pressure-Area and Pressure-Volume Analysis Using Echocardio-
graphic Automatic Boundary Detection.
65th Scientic Session of the American Heart Association
(11/92).
3. Fisher, J.P., Mitchel, J.F., Rosow, E., Mikan, J.S., Nagle, J., Kiernan, F.J., Hirst, J.A., Primiano, Gillam,
L.D., Hartford Hospital and University of Connecticut, Hartford, CT,
Evaluation of Left Ventricular
Diastolic Pressure-Area Relations with Echocardiographic Automatic Boundary Detection,
65th Sci-
entic Session of the American Heart Association (11/92).
4. Fisher, J.P., McKay, R.G., Mikan, J.S., Rosow, E., Nagle, J., Mitchel, J.F., Fram, D.B., Gillam, L.D.,
Hartford Hospital,
A Comparison of Echocardiographic Methods of Evaluating Regional LV Systolic
Function: Fractional Area Change vs. the End-Systolic Pressure-Area Relation.
65th Scientic Session
of the American Heart Association (11/92).
FIGURE 88.7
The cardiac cycle analysis front panel.
2000 by CRC Press LLC
5. Fisher, J.P., McKay, R.G., Rosow, E. Mikan, J. Nagle, J. Hirst, J.A., Fram, D.B., Gillam, L.D.,
On-
Line Derivation of Human Left Ventricular Pressure-Volume Loops and Load Independent Indices of
Contractility Using Echocardiography with Automatic Boundary Detection-A Clinical Reality.
Cir-
culation 88, I-304, 1993.
6. Fisher, J.P., Chen, C., Krupowies, N., Li Li, Kiernan, F.J., Fram, D.B., Rosow, E., Adam, J., Gillam,
L.D.,
Comparison of Mechanical and Pharmacologic Methods of Altering Loading Conditions to
Determine End-Systolic Indices of Left Ventricle Function.
Circulation 90 (II), 1-494, 1994.
7.
Fisher, JP, Martin, J., Day FP, Rosow, E., Adam J, Chen C, Gillam LD. Validation of a Less Invasive
Method for Determining Preload Recruitable Stroke Work Derived with Echocardiographic Auto-
matic Boundary Detection. Circulation 92, 1-278, 1995.
8. Fontes ML. Adam J, Rosow E, Mathew J, DeGraff AC. Non-Invasive Cardiopulmonary Function
Assessment System, J Clin Monit 13, 413, 1997.
9. Johnson, GW, LabVIEW Graphical Programming: Practical Applications in Instrumentation and
Control, 2nd ed., McGraw-Hill, New York, 1997.
10. Mathew JP, Adam J, Rosow E, Fontes ML, Davis L, Barash PG, Gillam L., Cardiovascular Pres-
sure-Dimension Analysis System, J Clin Monit 13, 423, 1997.
11. National Instruments 1999 Measurement and Automation Catalog, National Instruments, Austin,
TX.
12. Rosow, E. Technology and Sensors, Presented at the United States Olympic Committees Sports
Equipment and Technology Conference, Colorado Springs, CO; November 19-23, 1992.
13. Rosow, E. Biomedical Applications using LabVIEW, Presented at the New England Society of
Clinical Engineering, Sturbridge, MA; November 14, 1993.
14. Rosow, E., Adam, J.S., Nagle, J., Fisher, J.P. and Gillam, L.D.; Premise Development Corporation,
Avon, CT and Hartford Hospital, Hartford, CT, A Cardiac Analysis System: LabVIEW and Acoustic
Quantication (AQ), Presented at the Association for the Advancement of Medical Instrumenta-
tion in Washington, D.C. May 21-25, 1994.
You might also like
- Sensors: Breathing Analysis Using Thermal and Depth Imaging Camera Video RecordsDocument10 pagesSensors: Breathing Analysis Using Thermal and Depth Imaging Camera Video RecordsKarthik RajvNo ratings yet
- Sensors: Absorbance Based Light Emitting Diode Optical Sensors and Sensing DevicesDocument27 pagesSensors: Absorbance Based Light Emitting Diode Optical Sensors and Sensing DevicesKarthik RajvNo ratings yet
- Non Contact Measurement Vital ParametersDocument108 pagesNon Contact Measurement Vital ParametersKarthik RajvNo ratings yet
- Assignment 1Document2 pagesAssignment 1Karthik RajvNo ratings yet
- Manual ElectrodiagnosticsAndElectrotherapyDocument5 pagesManual ElectrodiagnosticsAndElectrotherapyKarthik RajvNo ratings yet
- Very Imp ConceptDocument4 pagesVery Imp ConceptKarthik RajvNo ratings yet
- 7.014 Problem Set 4Document9 pages7.014 Problem Set 4Karthik RajvNo ratings yet
- Ultrasound in Gynecology N ObstetricDocument9 pagesUltrasound in Gynecology N ObstetricKarthik RajvNo ratings yet
- NI Tutorial 9037 enDocument4 pagesNI Tutorial 9037 enKarthik RajvNo ratings yet
- Section11 AkDocument6 pagesSection11 AkKarthik RajvNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Fu Well 250605Document2 pagesFu Well 250605Play ChapNo ratings yet
- Zenith Z-200 PC Owners ManualDocument160 pagesZenith Z-200 PC Owners ManualWolfgangNo ratings yet
- Satellite A215-S5818 PDFDocument3 pagesSatellite A215-S5818 PDFviewwareNo ratings yet
- Misfortune Cookie DemystifiedDocument12 pagesMisfortune Cookie DemystifiedchuiyewleongNo ratings yet
- Pinnacle DV500 User GuideDocument188 pagesPinnacle DV500 User GuideDarko BelevNo ratings yet
- SXS Memory Card / Hardware Compatibility ChartDocument1 pageSXS Memory Card / Hardware Compatibility ChartSonyProUSANo ratings yet
- m220 Service ManualDocument49 pagesm220 Service ManualDave SharpNo ratings yet
- Laptop & Desktop Hardware Level 1 Repairing - NYX TechnologiesDocument4 pagesLaptop & Desktop Hardware Level 1 Repairing - NYX TechnologiesRamalingam Rathinasabapathy EllappanNo ratings yet
- Metrologic MS7120ETXSTX PDFDocument184 pagesMetrologic MS7120ETXSTX PDFLuis SAnNo ratings yet
- Model Small Stack Data msg1 DB "Enter First Number $" msg2 DB "Enter Second Number: $" msg3 DB "Difference BTW Two Numbers: $" Num1 DB ? Num2 DB ? Code Main Proc, @data,, msg1Document10 pagesModel Small Stack Data msg1 DB "Enter First Number $" msg2 DB "Enter Second Number: $" msg3 DB "Difference BTW Two Numbers: $" Num1 DB ? Num2 DB ? Code Main Proc, @data,, msg1Test CaseNo ratings yet
- BCM54282 Block DiagramDocument1 pageBCM54282 Block DiagramMohammad Sajjad Jahan PanahNo ratings yet
- Performing Mensuration and Calculations QuizDocument2 pagesPerforming Mensuration and Calculations QuizArt Dollosa100% (4)
- MPMC Rejinpaul (Other Univ) NotesDocument187 pagesMPMC Rejinpaul (Other Univ) NotesSheraaz0% (1)
- Pic 18 Microcontroller NotesDocument15 pagesPic 18 Microcontroller NotesMOTILAL SUTHAR215291No ratings yet
- Manual ASRock P4i65GVDocument36 pagesManual ASRock P4i65GVInfonova RuteNo ratings yet
- Scheme Toshiba A70 La 2301Document47 pagesScheme Toshiba A70 La 2301Жељко ГелићNo ratings yet
- Partnering With Dell Technologies Services SPDocument41 pagesPartnering With Dell Technologies Services SPPaulo Victor SilvaNo ratings yet
- ISA 2 Regular Solution-1Document4 pagesISA 2 Regular Solution-1mqtmqyNo ratings yet
- 5.3.3.5 Packet Tracer - Configure Layer 3 Switches InstructionsDocument2 pages5.3.3.5 Packet Tracer - Configure Layer 3 Switches InstructionsPriyoHadi SuryoNo ratings yet
- Gpiozero PDFDocument239 pagesGpiozero PDFSanthosh V PillaiNo ratings yet
- Spider2 - Bus Comunicacion PDFDocument27 pagesSpider2 - Bus Comunicacion PDFJavierSupoNo ratings yet
- Display Unit FHT 6020: Technical Specification ZT-091-0708EDocument12 pagesDisplay Unit FHT 6020: Technical Specification ZT-091-0708EHamzeh AbualrobNo ratings yet
- Computer Basics Lesson 1-What Is A Computer (Answer Key)Document3 pagesComputer Basics Lesson 1-What Is A Computer (Answer Key)Radha RamineniNo ratings yet
- Cimplicity - System Sentry Operation ManualDocument89 pagesCimplicity - System Sentry Operation ManualEduardo DiazNo ratings yet
- DC17 Ch03Document48 pagesDC17 Ch03Usman SiddiquiNo ratings yet
- 3eb4c Quanta r09, R09a R3a February 20 2012 SchematicsDocument58 pages3eb4c Quanta r09, R09a R3a February 20 2012 SchematicsalshameNo ratings yet
- TS932 1Document1 pageTS932 1Manuel HTNo ratings yet
- Colibri Carrier Board Design GuideDocument47 pagesColibri Carrier Board Design GuideshahbazNo ratings yet
- 8th Sem NotesDocument8 pages8th Sem NotesRanjith Shetty .cover.No ratings yet
- MODULE 1: Parts and Functions of Computers Basic Parts of ComputerDocument31 pagesMODULE 1: Parts and Functions of Computers Basic Parts of ComputerSophia Shannon D. DeiparineNo ratings yet