Professional Documents
Culture Documents
Cold Acclimation in Eucalyptus Hybrids
Uploaded by
marianarias0 ratings0% found this document useful (0 votes)
12 views12 pagesSeedlings of half-sib families of Eucalyptus and hybrids were exposed daily for 56 days to a 9-h photoperiod at 14. "C, followed by 15 h in a dark cold room maintained at 2. "C to cold harden the seedlings. Both hardened and unhardened plants of hybrid families were more cold tolerant than E. Globulus.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSeedlings of half-sib families of Eucalyptus and hybrids were exposed daily for 56 days to a 9-h photoperiod at 14. "C, followed by 15 h in a dark cold room maintained at 2. "C to cold harden the seedlings. Both hardened and unhardened plants of hybrid families were more cold tolerant than E. Globulus.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views12 pagesCold Acclimation in Eucalyptus Hybrids
Uploaded by
marianariasSeedlings of half-sib families of Eucalyptus and hybrids were exposed daily for 56 days to a 9-h photoperiod at 14. "C, followed by 15 h in a dark cold room maintained at 2. "C to cold harden the seedlings. Both hardened and unhardened plants of hybrid families were more cold tolerant than E. Globulus.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 12
Tree Physiology 14,921-932
0 1994 Heron Publishing--Victoria, Canada
Cold acclimation in eucalypt hybrids
M. H. ALMEIDA, M. M. CHAVES* and J. C. SILVA3
Departamento Eng. Florestal, Institute Superior de Agronomia, 1399 Lisboa Codex, Portugal
2 Department of Botany, Institute Superior de Agronomia, 1399 Lisboa Codex, Portugal
3 Royal Veterinary and Agricultural University, Arboretum, DK-2970 H@rsholm, Denmark
Received October 8, 1993
Summary
We evaluated cold resistance and the capacity for cold acclimation of different Eucalyptus genotypes.
Seedlings of half-sib families of E. globulus and hybrids E. gunnii x glob&s, E. viminalis x globulus
and E. cypellocarpa x glob&us were exposed daily for 56 days to a 9-h photoperiod at 14.7 C, followed
by 15 h in a dark cold room maintained at 2.5 C with the root system maintained at 8 C to cold harden
the seedlings. Unhardened seedlings were maintained at about 16 C during the dark period. Cold
acclimation occurred in all families with decreases in the temperature causing 50% mortality (LTsa) of
between 1.5 and 3 C. Both hardened and unhardened plants of hybrid families were more cold tolerant
than E. globulus. A significant correlation between LTsa and leaf osmotic pressure was observed; the
increase in osmotic pressure in hardened plants was predominantly a result of an increase in the
concentration of soluble sugars. Exotherm peaks were similar in hardened and unhardened plants. These
results indicate that cold hardening increased the ability of eucalypts to endure extracellular ice
formation. The maintenance of photosynthetic capacity in cold-hardened plants may also play a role in
their response to freezing.
Keywords: cold hardening, Eucalyptus, leaf water potential, osmotic pressure, photosynthetic
capacity, soluble sugars.
Introduction
The episodic occurrence of below zero temperatures is a major factor limiting the
expansion of eucalypt plantations in southern Europe. Within the Eucalyptus genus,
there is large variability in the susceptibility to cold injury (Boden 1958, Harwood
1980, Evans 1986, Cauvin 1988, Tibbits et al. 1991). Cold acclimation decreases the
lower temperature limit causing leaf damage, thereby increasing the length of the
growing period and enabling higher productivity in acclimated plants than in non-
acclimated plants.
Cold hardiness varies with season (Levitt 1980). The importance of the tempera-
ture regime for cold hardening in Eucalyptus species was recognized by Eldridge
(1969). Short photoperiods are not required for cold hardening to occur, provided
that night temperatures are maintained between 0 and 4 C (Eldridge 1969, Harwood
1980, Tibbits and Reid 1987~).
Based on the large differences in cold tolerance among Eucalyptus species, Pryor
(1957) suggested that natural and artificially produced hybrids should be used for
breeding eucalypts for freezing resistance. Resistance to freezing is inherited in a
predominantly additive manner in interspecific hybrids. Tibbits et al. (1991) found a
922 ALMEIDA. CHAVES AND SILVA
partial dominance toward the more sensitive species in several combinations, e.g.,
E. gunnii x E. glob&us.
We have compared the cold tolerance and acclimation potential of two half-sib
families of E. glob&us with two full-sib families of each of the hybrids E. gunnii x
glob&s, E. viminalis x globulus and E. cypellocarpa x glob&us. We chose these
hybrids because they may (combine the wood quality and fast-growing ability of
E. glob&s with the cold hardiness of the other Eucalyptus species. We tried to relate
differences in cold hardiness following the cold hardening period with changes in
growth and physiology. We also evaluated the influence of low soil temperatures
(around 2 C) on water status and growth of potted eucalypts.
Material and methods
Experiment A
To evaluate the effects of low soil temperature on growth and water status of
eucalypts, we used 27 seedlings of a half-sib family of E. glob&us from the
Bogalheira clonal seed orchard (39lO N, 904 W, altitude 90 m). The seedlings,
which were seven months old with a mean height of about 53 cm, were grown in
2500-cm3 pots filled with al 3/1/l mixture of loam, sand and peat, and kept well
watered throughout the experiment. All seedlings were exposed to a 9-h photoperiod
outdoors (average temperature 23 C ) and during the night, the seedlings were kept
either (1) at 20 C in a controlled-environment room (controls), (2) in a cold room at
2.5 C with soil heating, or (3) in a cold room at 2.5 C without soil heating. The
experiment lasted for 16 days and nine seedlings were randomly assigned to each
treatment. Soil temperature in the pots was about 20 C in the controls, and about 8
and 2.5 C in the treatments with and without soil heating, respectively. The soil
heating system consisted of a wooden structure covered with polystyrene with an
aluminum floor heated by an electrical system controlled through a sensor placed
near the pots. The pots were covered with insulating plastic foil and polystyrene.
We recorded the plant height, number of new leaves produced on the main stem
and stomata1 conductance (with an LI-1600 steady-state porometer Li-Cor, Inc.,
Lincoln, NE) of all seedlings at the beginning, middle and end of the experiment. On
the last day of the experiment, water potential (Y) was measured with a Schiilander
pressure chamber at predawn (before plants were transferred outdoors at 0800 h) and
at midday in three randomly chosen seedlings of each treatment. Leaf stomata1
conductance was measured in all seedlings at 0800, 1200 and 1600 h on the last day
of the experiment.
Experiment B
We compared the degree of cold acclimation in seedlings of two full-sib families of
the hybrids E. gunnii x globulus, E. viminalis x globulus and E. cypellocarpa x
glob&s with that of two half-sib families of E. glob&us. The E. viminalis x globulus
families share the same female parent, and the male parents are the Fo generation of
COLD ACCLIMATION IN EUCALYPT HYBRIDS 923
E. globulus Families 1 and 2. The Ft hybrids E. cypellocarpa x glob&s (Family 3)
and E. gunnii x globulus (Family 3) share the same male parent. E. cypellocarpa X
globulus (Family 1) has the same male parent as E. viminalis x globulus (Family 1).
Hardening procedure Eight to eleven seedlings of each family, taken at random,
were subjected to a 9-h photoperiod outdoors at an average temperature of 14.7 C,
followed by 15 h in a dark cold room at an air temperature of 2.5 C with the root
system heated (average soil temperature 8 C). These plants were designated har-
dened plants. Another eight to eleven plants of each family were subjected to similar
conditions except that the night air and soil temperatures were kept at 16 C. These
plants were designated unhardened plants. The hardening period was 56 days
(beginning in October).
Cold acclimation measurements Photosynthetic capacity and quantum yield of non-
cyclic electron transport (Genty et al. 1989) were measured in three plants per
treatment of E. viminalis x globulus (Family l), E. cypellocarpa x globulus (Family
1) and E. globulus (Family 1). The measurements were made at the end of the
experiment on one leaf disc of 1 cm2, taken from the bottom of the second newly
expanded leaf pair. Photosynthetic capacity was assessed at saturating CO2 (5%) and
light (1650-1800 pm01 m-* s-l) with an oxygen electrode (LD-2 Hansatech Ltd.,
King s Lynn, U.K.). Quantum yield of noncyclic electron transport (FL - F,/F,,,)
measurements were made at an irradiance of 190 pmol me2 s-l with a modulated
fluorometer (H. Walz, Effeltrich, Germany)
At the end of the 56-day hardening period, the first and second leaf pairs of six
hardened and six unhardened seedlings, randomly chosen from each family, were
collected and immediately placed in liquid nitrogen. A sample of the combined
material from each family was used. Osmotic pressure was measured with a hygrom-
eter (HR-33 T-Wescor). Soluble sugars, starch and proline concentrations were
determined according to Sumner (1925), Stitt et al. (1989) and Bates (1973),
respectively. The contribution of the various solutes to the total osmotic pressure was
calculated with the vant Hoff equation.
Height growth and the number of new leaves produced on the main stem were
determined in all plants, every two weeks.
Frost resistance measurements In a preliminary experiment with 54 potted euca-
lypts, we compared the effects of cold treatments in whole seedlings and detached
leaves, and observed that the percentage of death was not significantly different
(Figure 1). Therefore, we used detached leaves to evaluate the temperature that causes
50% mortality (LTso). To estimate LT50, all hardened and unhardened plants were
subjected first to -7.3 C, and subsequently, according to the survival rates at this
temperature, to three of the following temperatures: -4.1, -5.3, -6.0, -7.9, -9.4,
-9.8 and -11.2 C.
To determine cold tolerance, one leaf was detached from the third or fourth newly
expanded leaf pair of each hardened or unhardened plant, wrapped in aluminum foil
and subjected to freezing temperatures in a controlled temperature chamber
924 ALMEIDA, CHAVES AND SILVA
-6.6-6-4.6-4-3.6-3-2.5-2-1.6-1-0.60
Temperature (C)
Figure 1. Comparison of percentage mortality evaluated in detached leaves and whole seedlings of
54 potted eucalypts subjected to different cold treatments.
(Bioclima 750E, Aralab, Oeiras, Portugal). The temperature in the chamber was
lowered to 2 C at a rate of 0..3 C min- and thereafter at approximately 0.1 C min-
to the desired temperature, which was held constant for 2 h. Warming was done at
the same rates as cooling. Air and leaf temperatures were recorded during the frost
treatments with 12 copper clonstantan thermocouples, and the air temperature varia-
tion among the different thermocouples never exceeded 0.4 C once the desired frost
temperature was reached. Exotherm peaks were identified from the cooling curves
of the leaves.
After the cold treatment, seven discs (6 mm in diameter) from each leaf were
carefully cut and immediately placed in a Pyrex vial containing 1.5 ml of distilled
deionized water. The vials were capped and placed in a water bath at 25 C. After
24 h, the conductivity of the liquid in the vial (CI) was determined with a conductiv-
ity meter (Consort k220) to the nearest l,tS cm-. The vials were then autoclaved at
105 C for 10 min before being returned to the water bath at 25 C. After 24 h,
conductivity of the dead tissue (CT) was determined. Relative conductivity (CR) was
calculated as CR = CI/CT (%) (Hallam and Tibbits 1988). The lethal temperature,
defined as the temperature: resulting in 50% loss of cellular electrolytes (7s,&
estimated from linear interpolation as described by Tibbits and Reid (1987a), was
used as the index of frost tolerance. The CR data were used to define the lethal
temperature because the CR. values for each seedling were directly correlated (r2 =
0.98) with the degree of le,af damage (Hallam and Tibbits 1988, Almeida 1993).
Relative conductivity was also well correlated with photochemical quenching of
fluorescence of chlorophyll a in eucalypt leaves (Chaves et al. 1990). This fluores-
cence parameter is a good indicator of cold resistance in several species (Havaux
1987).
Statistical analysis
The x2 test was used to evaluate the effects of the cold treatments (-5.3, -7.3 and
-9.4 C) on cold acclimation, family and their interaction. Analysis of variance, with
COLD ACCLIMATION IN EUCALYIT HYBRIDS 925
a factorial fixed effects model, was performed to compare the cold hardening and
family effects on LTsO and osmotic pressure as well as the effects of root temperature
on leaf conductance and leaf water potential. The effects of root temperature,
hardening and genotype on height and leaf production rate were subjected to analysis
of covariance with initial height and initial number of leaves used as covariates.
Comparisons between treatment means were made with Duncans multiple range
test.
Results
Experiment A
Predawn leaf water potentials measured after 16 nights of cold treatment were
significantly lower in plants without soil heating than in control plants and plants
with soil heating (Figure 2). The differences between controls and plants with soil
heating were not statistically significant (P > 0.05). By midday, however, control
plants had the lowest Y, presumably as a result of higher stomata1 conductances.
Plants without soil heating showed lower stomata1 conductances than both control
plants and plants with soil heating (significant differences at P < 0.05) (Table 1).
Seedlings subjected to an air temperature of 2.5 C during the night, with or
without soil heating, showed lower height increment than the controls (Table 2). On
the other hand, the number of new leaves was only significantly reduced (P < 0.05)
in plants without soil heating.
Experiment B
The hybrids showed intermediate morphological characteristics when compared
O-
-0%
-1.4-
-1.6'
PWd.W Ylddl
Figure 2. Leaf water potential in control plants (kept at 23/20 C, day/night temperatures), and plants
(kept at 23/2.5 C, day/night temperatures) with and without soil heating, measured at predawn and
midday at the end of the 16-day experiment. The values correspond to the means of three replicates. Bars
represent the standard errors of the mean.
926 ALMEIDA, CHAVES AND SILVA
Table 1. Values of mean leaf stomata1 conductance (mmol m -2 s-) measured in controls and plants with
and without soil heating at predawn, midday and afternoon on the last day of the experiment. The values
(k SE) correspond to the means of nine replicates.
Treatment Predawn
Control 466 + 48
With soil heating 3119 * 58
Without soil heating 199 * 25
Midday
513k99
494 + 125
199+66
Afternoon
256 k 36
225 * 39
168k38
Table 2. Height increment and number of new leaves on the main stem measured at the beginning and at
the end of the experiment in controls and plants with and without soil heating. The values (k SE)
correspond to the means of nine replicates. Mean values for height increment designated by the same
letters (a, b) and values for number of new leaves with the same letters (c, d) are not significantly different
(P > 0.05).
Treatment Height increment (cm) Number of new leaves
-
Control
With soil heating
Without soil heating
5.2 +0.3a
3.4 k 0.3b
2.6 IL 0.4b
1.8 k 0.2~
1.6 f 0.4~
0.2 k 0.2d
with the pure species in lboth unhardened and hardened seedlings. During the
experiment, the leaves of all hardened seedlings progressively displayed a reddish
coloration.
The increment in height and the number of new leaves on the main stem was
reduced in hardened seedlings of all families (Figure 3). Analysis of covariance of
seedling height at the end of the hardening period showed significant differences
-z
23
E
is
i?!2
E
.-
E
ml
5
I
0
GGJ GG4
d
GG3 GG4
Figure 3. (a) Height increment (and (b) number of new leaves on the main stem of hardened and
unhardened plants of E. globulus Family 1 (Gl), E. globulus Family 2 (G2), E. viminalis x globulus
Family 1 (VGl), E. viminalis x globulus Family 2 (VG2), E. cypellocarpu x globulus Family 1 (CGl),
E. cypellocarpa x globulus Family 3 (CG3), E. gunnii x globulus Family 3 (GG3) and E. gunnii x
globulus Family 4 (GG4) at the enld of the 56-day hardening period. The values correspond to the means
of eight replicates. Bars represent the standard errors of the mean.
COLD ACCLIMATION IN EUCALYPT HYBRIDS 921
between hardened and unhardened plants (P < O.OOl), and among families (P =
0.001). Seedlings of E. viminalis x glohulus (both families) and E. globulus (Fam-
ily 2) had the highest mean height. Duncans multiple range test showed that these
families were not significantly different from each other (P > 0.005), but were
significantly different (P < 0.05) from the other families. By the end of the 56-day
hardening period, unhardened plants had produced significantly more leaves than
hardened plants (P < 0.001). Seedlings of families E. viminalis x glohulus and E.
globulus produced significantly more new leaves than seedlings of the other families.
Differences in photosynthetic capacity and quantum yield of noncyclic electron
transport between hardened and unhardened seedlings were not significant (P > 0.05)
(Figure 4).
For all families, the exotherm peaks were not significantly different in hardened
and unhardened plants (Table 3). The LTso of hardened plants was significantly lower
than that of unhardened plants (P < 0.001). Some differences in cold resistance
among families were apparent, but were not statistically significant (data not shown).
The lowest values of LT50 were associated with high osmotic pressures (Figure 5).
Osmotic pressure was significantly higher (P < 0.001) in hardened plants than in
unhardened plants, and differences among families were also significant (P < 0.001).
Eucalyptus viminalis x globulus (Family 1) and E. cypellocarpa x globulus (Fam-
ily 3) had the highest osmotic pressures and the lowest LT50 values (Table 3).
In most families, the increase in leaf osmotic pressure following hardening was
predominantly the result of an increase in the concentration of soluble sugars
(Figure 6). No differences in proline concentration were observed between hardened
and unhardened plants, except in E. cypellocarpa x globulus (Family 3) (Table 4).
The increase in sugar concentration after hardening was paralleled by a decrease in
starch concentration in all families except E. viminalis x glob&us (Family 2) (Table 4
and Figure 6). Proline contribution to osmotic pressure was negligible (Figure 6).
25 0.6
0
YGI
0
Gl CO1 Gl VGl CGl
0 Unhardeed q Hardened cl hardened ITi Hardened
Figure 4. (a) Photosynthetic capacity (A) and (b) quantum yield of noncyclic electron transport (0,) in
hardened and unhardened seedlings of E. glohulus Family 1 (Cl), E. viminalis x globulus Family 1
(VGl) and E. cypellocarpa x globulus Family 1 (CGl). The values correspond to the means of three
replicates. Bars represent the standard errors of the mean.
928 ALMEIDA, CHAVES AND SILVA
Table 3. Frost tolerance (C) of the different hybrids and E. glohulus families evaluated by LTsa in eight
hardened and eight unhardened plants. Exotherm peak (E-peak) values correspond to the means of three
replicates.
Family Unhardened
E-peak LTso
Hardened
E-peak LTso
E. globulus Family 1
E. glohulus Family 2
E. viminalis x globulus Family 1
E. viminali.~ x globulus Family 2
E. cypello x globulus Family I
E. cypello x globulus Family 3
E. gunnii x globulus Family 3
E. gunnii x globulus Family 3
-6.1 * 0.4 -5.3
-5.1 * 0.4 -5.6
-5.1 * 0.4 -6.0
-4.6 * 0.6 -6.5
-5.4 -4.9
-4.9 * 0.1 -6.3
-5.5 -6.5
-6.2 k 1 .O -7.0
-6.3 0.4 -7.8
-6.1 f 1.1 -7.6
-5.5 ZtI 0.4 -9.4
-5.5 * 0.4 -7.9
-5.9 k 0.4 -7.9
-5.9 IL 0.4 -9.5
-6.0 5~ 0.2 -7.8
-6.3 k 0.7 -8.6
Only one observation.
-101
0.6 0.9 1 1.1 1.2 1.3 1.4 1.6 1.6
Osmotic Pressure (MPa)
* Hardened n Unhardened
Figure 5. Values of LTso plotted against leaf osmotic pressure in hardened and unhardened plants of all
families.
Discussion
Low soil temperature can impose a direct limitation on forest productivity by
affecting plant water status., leaf gas exchange (Kozlowski et al. 1991), and root and
shoot growth (Kaufmann 1975, DeLucia 1986). Species differences have been
detected in this respect (Kramer 1942). Because, under natural conditions, the
decrease in soil temperature is slower and less pronounced than the decrease in air
temperature, it may be important to protect the root system against cooling in plants
subjected to artificial cold acclimation to prevent experimental artifacts. We con-
clude that the seedlings were sensitive to low soil temperature, because plants
exposed to cold without soil heating exhibited lower predawn water potentials and
leaf stomata1 conductances than plants with soil heating (Figure 2 and Table 1). The
decrease in water potential suggests that the effects of cold soil in these plants may
COLD ACCLIMATION IN EUCALYPT HYBRIDS 929
g1.6
z.1.4
0 Others
LZ Proline*
0 SoLSugar
Gl G2 VGI VG2 CG1 CG3
Figure 6. Components of the osmotic pressure in leaves of hardened and unhardened plants ofB. globulus
Family 1 (Gl), E. globulus Family 2 (G2), E. viminalis x globulus Family 1 (VGl), E. viminalis x
globulus Family 2 (VG2), E. cypellocarpa x globulus Family 1 (CGl), E. cypellocarpa x globulus
Family 3 (CG3), E. gunnii x globulus Family 3 (GG3) and E. gunnii x glohulus Family 4 (GG4). A mean
sample from six seedlings of each family was used.
Table 4. Starch and proline concentrations at the end of the hardening period in six hardened and six
unhardened seedlings of E. globulus and hybrids. Values correspond to mean samples.
Family Starch (mg gdwml)
Unhardened Hardened
E. globulus Family 1 2.26 0.66
E. globulus Family 2 1.49 0.69
E. viminalis x globulus Family 1 8.79 1.17
E. viminulis x globulus Family 2 0.76 2.12
E. cypello x globulus Family 1 1.78 1.13
E. cypello x globulus Family 3 2.15 1.46
PrOhe (kg gdw-I)
Unhardened Hardened
396.4 315
382 309.7
145.8 115.5
140.5 76.2
88.7 101
53.9 331.3
involve inhibition of water uptake by the roots, presumably as a result of decreased
root permeability or increased water viscosity (cf. Kaufmann 1975, Lawrence and
Oechel 1983). Height growth and the production of new leaves were also less in
seedlings in the cold soil than in seedlings in the warm soil (Table 2).
After cold hardening, all of the eucalypt families exhibited a reduction in growth;
however, the correlation between the ability to harden and the degree of growth
inhibition was low (r2 = 0.2).
Cold acclimation occurred in all families with decreases in LTsa between 1.5 and
3 C. These values are of the same order of magnitude found by Tibbits and Reid
(1987b) and Scarascia-Mugnozza et al. (1989), but differ greatly from the values
reported for pure subalpine species (Scarascia-Mugnozza et al. 1989). We observed
930 ALMEIDA, CHAVES AND SILVA
a marginal increase in the ability to cold harden in the hybrids compared with
E. glohulus, but we did not detect a superior ability to cold harden in the hybrids of
E. gunnii x glohulus as observed by Marien (1979). Differences in ability to cold
harden may be related to the high intraspecific variability of the F, generation
(Tibbits et al. 1991) or to the small number of hybrid families used in the experiment.
Nevertheless, we observed that, at -5 C, unhardened seedlings of E. gunnii x
globulus suffered the 1owe;st mortality of the families examined.
There was a significant correlation between LT~o and leaf osmotic pressure. The
increase in osmotic pressure in hardened plants was predominantly a result of an
increase in the concentration of soluble sugars, although the decrease in leaf starch
concentration may also be partially responsible for the increase in osmoticum (cf.
Valentini et al. 1990). Proline concentration did not increase in response to cold in
any families with the exception of E. cypellocarpa x glohulus (Family 3). The
observed increase in osmotic pressure was not enough to produce a significant
decrease in the temperature of ice formation. We estimate, based on Raoults law,
that the maximal decrease .in the freezing point was around 0.2 C. This is consistent
with the finding that cold hardening induced a decrease in lethal temperature while
maintaining the temperature of ice formation. This means that cold hardening
increased the ability of the seedlings to endure extracellular ice formation. The
differences between LT50 and exothenn peaks were between 1.5 and 3.5 C, indicat-
ing a modest capacity to withstand equilibrium freezing and the progressive dehy-
dration of tissues, which is in accordance with data presented by Steponkus (1984)
and Scarascia-Mugnozza et al. (1989).
We conclude that the increase in cold resistance in eucalypts is not associated with
a decrease in the exotherm peaks but may be related to an increase in the concentra-
tion of sugars. It is known that the concentration of soluble sugars is strongly
correlated with freezing .tolerance (Levitt 1980, Steponkus 1990). Recent data
suggest that low molecular weight carbohydrates, in addition to their role in stabiliz-
ing cellular membranes on freeze-dehydration (Santarius 1982), potentiate the sys-
tem by making available photosynthetic intermediates such as hexose phosphates,
thereby enhancing the rate of photosynthesis at low temperatures (Labate and
Leegood 1990). Additionally, ijquist et al. (1993) have shown that freezing tolerance
in cereals is related to the capacity to increase or maintain photosynthesis after cold
hardening. However, rather than a mechanistic link between photosynthesis and cold
hardening, these authors suggest that the maintenance of photosynthetic activity is
important in cold acclimation because it enables the leaf to acquire the energy
necessary for the cellular changes that are required for the induction of cold hardi-
ness. This hypothesis is in accordance with the evidence that changes occurring in
plasma membranes play a key role in plant defense against freezing temperatures
(Steponkus 1990). We observed that, following a 56-day hardening period, photo-
synthetic capacity and quantum yield were not significantly affected (Figure 4), as
observed in other species exposed to low night air temperatures (DeLucia and Smith
1987). This indicates that feedback inhibition of photosynthesis did not occur in the
leaves following cold hardlening, despite carbohydrate accumulation. The results are
COLD ACCLIMATION IN EUCALYPT HYBRIDS 931
in contrast with those obtained with conifers, where the capacity for light-saturated
photosynthesis is suppressed during cold hardening (oquist et al. 1980).
Acknowledgments
We are grateful to Dr. M. Lucilia Rodrigues for support in the measurements and calculations of osmotic
pressure and its components, and to Prof. J.S. Pereira for valuable discussions and critical reading of the
manuscript. This work was supported by the EEC project-MA 2B-CT91-0030 (SSMA). We also
acknowledge the seeds from CELBI and financial support from SOPORCEL.
References
Almeida, M.H. 1993. Estudo da variabilidade geogrtiica em Eucalyptus globulus Labill. Tese de
Doutoramento, Institute Superior de Agronomia (Abstract in English).
Bates, L.S. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39:205-207.
Boden, N.B. 1958. Differential frost resistance within one Eucalyptus species. Aust. .I. Sci. 21:84-86.
Cauvin, B. 1988. Eucalyptus: les tests de rtsistance au froid. AM. Afocel 1987:161-195.
Chaves, M.M., M.H. Almeida, J.C. Silva and J.S. Pereira. 1990. Tolerance to low temperatures in
E. globulus. In Biomass for Energy and Industry. Eds. E. Grassi, G. Gosse and G. dos Santos. Elsevier
Applied Sciences, London, 1,164-l. 170.
DeLucia, E.H. 1986. Effect of low root temperature on net photosynthesis, stomata1 conductance and
carbohydrate concentration in Engelmann spruce (Picea engelmanni Parry ex Engelm.) seedlings.
Tree Physiol. 2:143-154.
DeLucia, E. and W.K. Smith. 1987. Air and soil temperature on photosynthesis in Engelmann spruce
during summer. Can. J. For. Res. 17:527-533.
Eldridge, K.G. 1969. Altitudinal variation in Eucalyptus regnuns F. Muell. Ph.D. Thesis, Australian
National University, Canberra, 195 p.
Evans, J. 1986. A reassessment of cold hardy eucalypts in Great Britain. Forestry 59:223-242.
Genty, B., J.M. Briantais and N.R. Baker. 1989. The relationship between the quantum yield of
photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta
990:87-92.
Hallam, P.M. and W.N. Tibbits. 1988. Determination of frost hardiness of Eucalyptus using the electrical
conductivity of diffusate in conjunction with a freezing chamber. Can. J. For. Res. 18:595-600.
Harwood, C.E. 1980. Frost resistance of subalpine Eucalyptus species. I. Experiments using a radiation
frost room. Aust. J. Bot. 28:587-599.
Havaux, M. 1987. Effects of chilling on the redox state of the primary electron acceptor QA of
photosystem II in chilling-sensitive and resistant plant species. Plant Physiol. Biochem. 25:735-743.
Kaufmann, M.R. 1975. Leaf water stress in Engelmann spruce: influence of the root and shoot
environments. Plant Physiol. .56:841-844.
Kozlowski, T.T., P. Kramer and S.G. Pallardy. 1991. The physiological ecology of woody plants.
Academic Press, London, 237 p.
Kramer, P.J. 1942. Species differences with respect to water absorption at low soil temperature. Am. J.
Bot. 29:828-832.
Labate, C.A. and R.C. Leegood. 1990. Factors influencing the capacity for photosynthetic carbon
assimilation in barley leaves at low temperatures. Planta 182:492-5(X).
Lawrence, W.T. and W.C. Oechel. 1983. Effects of soil temperature on the carbon exchange of taiga
seedlings. II. Photosynthesis, respiration and conductance. Can. J. For. Res. 13:850-859.
Levitt, J. 1980. Responses of plants to environmental stress. Vol. I. Chilling, freezing, and high
temperature stresses. 2nd Edn. Academic Press, London, 497 p.
Marien, J.N. 1979. La sClection juvtnil des eucalyptus pour leur rksistance au froid. AM. Afocel
1979:225-253.
oquist, G., L. Brunes, J.-E. Hallgren, K. Gezelius, M. Hallen and G. Malmberg. 1980. Effects of
artificial frost hardening and winter stress on photosynthesis, photosynthetic electron transport and
RuBP carboxylase activity in seedlings of Pinus sylvestris. Physiol. Plant. 48:526-531.
932 ALMEIDA, CHAVES AND SILVA
Gquist, G., V.H. Hurry and NRA. Hurter. 1993. Low-temperature effects on photosynthesis and
correlation with freezing tolerance in spring and winter cultivars of wheat and rye. Plant Physiol.
101:245-250.
Potts, B.M., W.C. Potts and B. C,auvin. 1987. Inbreeding and interspecific hybridization in Eucalyptus
gunnii. Silvae Genet. 36:543-562.
Pryor, L.D. 1957. Selecting and breeding for cold resistance in Eucalyptus. Silvae Genet. 6:98-109.
Santarius, K.A. 1982. The mechanism of cryoprotection of biomembrane systems by carbohydrates. In
Plant Cold Hardiness and Freezing Stress. Vol. II. Ed. P.H. Li and A. Sakai. Academic Press, London,
New York, pp 475-486.
Scarascia-Mugnozza, G., R. Valentini, E. Kuzminsky and E. Giordano. 1989. Freezing mechanisms,
acclimation processes and cold injury in Eucalyptus species planted in the Mediterranean region. For.
Ecol. Manage. 29:81-94.
Steponkus, PL. 1984. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev.
Plant Physiol. 35:543-584.
Steponkus, PL. 1990. Cold acclimation and freezing injury from a perspective of the plasma membrane.
In Environmental Injury to Plants. Ed. F. Katterman. Academic Press, San Diego, pp l-15.
Stitt, M., R. Mcilley, R. Gerhardt and H.W. Heldt. 1989. Determination of metabolite levels in specific
cells and sub-cellular compartments of leaves. Methods Enzymol. 174518-552.
Sumner, J.B. 1925. A more specific reagent for the determination of sugar in urine. Science 3:501-5 13.
Tibbits, W.N. and J.B. Reid. 1987~. Frost resistance in Eucalyptus nitens (Deane & Maiden) Maiden:
physiological aspects of hardiness. Aust. J. Bot. 35:235-250.
Tibbits, W.N. and J.B. Reid. 1987h. Frost resistance in Eucalyptus nitens (Deane & Maiden) Maiden:
genetic and seasonal aspects of variation. Aust. For. Res. 17:29-47.
Tibbits,W.N., B.M. Potts and M.H. Sava. 1991. Inheritance of freezing resistance in interspecific FI
hybrids of Eucalyptus. Theor. Appl. Genet. 83: 126-l 35.
Valentini, R., G. Scarascia-Mugnozza and E. Kuzminsky. 1990. Influence of cold hardening on water
relations of three Eucalyptus species. Tree Physiol. 6: I-10.
You might also like
- Gaspar 2004Document4 pagesGaspar 2004marianariasNo ratings yet
- Navarro 2010Document14 pagesNavarro 2010marianariasNo ratings yet
- A Rapid Method For Extraction of CottonDocument7 pagesA Rapid Method For Extraction of CottonmarianariasNo ratings yet
- Molecular genetics of plant cold acclimationDocument33 pagesMolecular genetics of plant cold acclimationmarianariasNo ratings yet
- Cold Acclimation and Freezing Stress Tolerance: Role of Protein MetabolismDocument37 pagesCold Acclimation and Freezing Stress Tolerance: Role of Protein MetabolismmarianariasNo ratings yet
- Plant Resistance To Cold Stress: Mechanisms and Environmental Signals Triggering Frost Hardening and DehardeningDocument11 pagesPlant Resistance To Cold Stress: Mechanisms and Environmental Signals Triggering Frost Hardening and DehardeningmarianariasNo ratings yet
- American Association For The Advancement of Science ScienceDocument11 pagesAmerican Association For The Advancement of Science SciencemarianariasNo ratings yet
- Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory MechanismsDocument30 pagesPlant Cold Acclimation: Freezing Tolerance Genes and Regulatory MechanismsmarianariasNo ratings yet
- Riederer 2001 Protegiendo La Perdida de Agua Por La Cuticula PDFDocument10 pagesRiederer 2001 Protegiendo La Perdida de Agua Por La Cuticula PDFmarianariasNo ratings yet
- AntunezDocument12 pagesAntunezmarianariasNo ratings yet
- Characteristics Bearing Species1: of Cold Acclimation and Deacclimation in Tuber-SolanumDocument3 pagesCharacteristics Bearing Species1: of Cold Acclimation and Deacclimation in Tuber-SolanummarianariasNo ratings yet
- Deficiencia de Agua PDFDocument12 pagesDeficiencia de Agua PDFmarianariasNo ratings yet
- Relative Quantitation of Gene ExpressionDocument60 pagesRelative Quantitation of Gene ExpressionmarianariasNo ratings yet
- DNeasy® Mericon® Food HandbookDocument32 pagesDNeasy® Mericon® Food HandbookmarianariasNo ratings yet
- How Do I Make A Science News Story For RadioDocument5 pagesHow Do I Make A Science News Story For RadiomarianariasNo ratings yet
- Expresion de Genes CutinaDocument4 pagesExpresion de Genes CutinamarianariasNo ratings yet
- Sistema de Separacion de Betalainas PDFDocument8 pagesSistema de Separacion de Betalainas PDFmarianariasNo ratings yet
- RAPD and Freezing Resistance in GlobulusDocument7 pagesRAPD and Freezing Resistance in GlobulusmarianariasNo ratings yet
- Efecto AntioxidanteDocument5 pagesEfecto AntioxidantemarianariasNo ratings yet
- Monomeros de Cutina y SuberinaDocument4 pagesMonomeros de Cutina y SuberinamarianariasNo ratings yet
- Gradiente de Saturacion Lignina CutinaDocument12 pagesGradiente de Saturacion Lignina CutinamarianariasNo ratings yet
- Alcohol y Nicotina en RatasDocument13 pagesAlcohol y Nicotina en RatasmarianariasNo ratings yet
- Epignetica y Productividad de Los CultivosDocument8 pagesEpignetica y Productividad de Los CultivosmarianariasNo ratings yet
- Monomeros de Cutina y SuberinaDocument4 pagesMonomeros de Cutina y SuberinamarianariasNo ratings yet
- Caracterizacion Molecular de CutinaDocument6 pagesCaracterizacion Molecular de CutinamarianariasNo ratings yet
- Cutina y Suberina en ArabidopsisDocument16 pagesCutina y Suberina en ArabidopsismarianariasNo ratings yet
- Calcio y Magnesio en El FrutoDocument5 pagesCalcio y Magnesio en El FrutomarianariasNo ratings yet
- Comparacion de Plantas Medicinales de Peru y BoliviaDocument28 pagesComparacion de Plantas Medicinales de Peru y BoliviamarianariasNo ratings yet
- Aceites EsencialesDocument8 pagesAceites EsencialesmarianariasNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Fundamentals of Human Resource Management: Decenzo and RobbinsDocument16 pagesFundamentals of Human Resource Management: Decenzo and RobbinsshafianikNo ratings yet
- WEEK 7 Center of MassDocument10 pagesWEEK 7 Center of Massmaria1345No ratings yet
- Consumer Behaviour Ppt-Freudian TheoriesDocument21 pagesConsumer Behaviour Ppt-Freudian TheoriesAnNo ratings yet
- Sensors 21 02453Document18 pagesSensors 21 02453Sérgio CustódioNo ratings yet
- Detailed Math Lesson Plan on Sets and OperationsDocument9 pagesDetailed Math Lesson Plan on Sets and OperationsAYYA CAMPONGNo ratings yet
- 1 s2.0 S0950061815006923 MainDocument20 pages1 s2.0 S0950061815006923 MainTobias RibeiroNo ratings yet
- Preview: Walden UniversityDocument24 pagesPreview: Walden UniversityHodan HaruriNo ratings yet
- Supersonic Air-to-Air Ejector: Created in COMSOL Multiphysics 5.3aDocument24 pagesSupersonic Air-to-Air Ejector: Created in COMSOL Multiphysics 5.3aWill SoNo ratings yet
- Rheology Modifier: Zhejiang Fenghong New Material Co., LTDDocument2 pagesRheology Modifier: Zhejiang Fenghong New Material Co., LTDdamiendamNo ratings yet
- ETI-MAX 3000 BrochureDocument4 pagesETI-MAX 3000 BrochureVÍCTOR LÓPEZ SÁNCHEZNo ratings yet
- New Microsoft Office Word DocumentDocument4 pagesNew Microsoft Office Word DocumentTejpalNo ratings yet
- Weekly Home Learning Plan DRAFTING 8 Week 1 3Document6 pagesWeekly Home Learning Plan DRAFTING 8 Week 1 3John B. BataraNo ratings yet
- YTO Maths Centre Primary 1 Question PaperDocument5 pagesYTO Maths Centre Primary 1 Question PaperEngclassesby Hlh100% (3)
- Trends ExamDocument6 pagesTrends ExamKristel Anne SadangNo ratings yet
- Techniques For Indoor Location-Based On Bluetooth Fingerprinting Using Artificial Intelligence AlgorithmsDocument10 pagesTechniques For Indoor Location-Based On Bluetooth Fingerprinting Using Artificial Intelligence AlgorithmsTJPRC PublicationsNo ratings yet
- Life Sciences p2 EngDocument12 pagesLife Sciences p2 Engapi-202349222100% (2)
- FIRST QUARTER RESEARCH TOPICSDocument9 pagesFIRST QUARTER RESEARCH TOPICSDennisNo ratings yet
- Introduction To Polymer - Chapter 9Document39 pagesIntroduction To Polymer - Chapter 9Abrham HaileNo ratings yet
- St. Therese College Parent OrientationDocument36 pagesSt. Therese College Parent OrientationMc BuenconsejoNo ratings yet
- Artificial Intelligence: Not a threat to legal professionDocument5 pagesArtificial Intelligence: Not a threat to legal professionYouCuber SaaraswataNo ratings yet
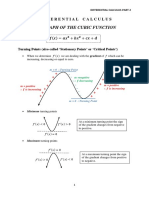
- Grade 9 Add Maths Notes On Cubic FunctionsDocument13 pagesGrade 9 Add Maths Notes On Cubic FunctionsMohammad Abdul OhabNo ratings yet
- Lube Oil Analysis ReportDocument11 pagesLube Oil Analysis ReportPhilic RohitNo ratings yet
- AN18f - Power Gain Stages For Monolithic AmplifiersDocument16 pagesAN18f - Power Gain Stages For Monolithic AmplifiersMallickarjuna A.SNo ratings yet
- Vitruvian Man Student WorksheetDocument7 pagesVitruvian Man Student WorksheetstewartciscoNo ratings yet
- Paracetamol Overdose: BW 40 KG Without Fluid RestrictionDocument2 pagesParacetamol Overdose: BW 40 KG Without Fluid RestrictionAndy TanNo ratings yet
- The Warlord Class - GM BinderDocument16 pagesThe Warlord Class - GM BinderАнтон Дюран0% (1)
- Mid Test Revision Revision Lecture: AF301 Theories of Regulation 1Document5 pagesMid Test Revision Revision Lecture: AF301 Theories of Regulation 1Shivneel Naidu100% (1)
- 3.3. Phase and Amplitude Errors of 1-D Advection EquationDocument52 pages3.3. Phase and Amplitude Errors of 1-D Advection EquationSamKtkNo ratings yet
- Nursing Care Plan - Acute PainDocument6 pagesNursing Care Plan - Acute PainAJ Tuban Compelio100% (1)
- New Cover Letter of Jaspreet SinghDocument4 pagesNew Cover Letter of Jaspreet SinghBrar BrarNo ratings yet