Professional Documents
Culture Documents
Chemical Shrinkage of Cement Pastes With Plasticizing
Uploaded by
Ahmed TahaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical Shrinkage of Cement Pastes With Plasticizing
Uploaded by
Ahmed TahaCopyright:
Available Formats
CHEMICAL SHRINKAGE OF CEMENT PASTES WITH PLASTICIZING
ADMIXTURES
Harald Justnes, Dr. Ing., Chief Scientist, SINTEF Civil and Environmental Engineering,
Cement and Concrete, N-7465 Trondheim, NORWAY
Erik J. Sellevold, Ph.D., Professor, Dept. of Structural Engineering, The Norwegian
University of Science and Technology, N-7034 Trondheim, NORWAY
Bert Reyniers, M. Sc., Dirk Van Loo, M. Sc., Arne Van Gemert, M. Sc., Frank Verboven,
M. Sc. and Dionys Van Gemert, Ph.D., Prof., Katholieke Universiteit te Leuven, Faculteit
Toegepaste Wetenschappen, Departement Burgerlijke Bouwkunde, B-3001 Heverlee,
BELGIUM
Abstract
Total and external chemical shrinkage have been followed for a number of cement pastes
until 48 h. Total chemical shrinkage is believed to roughly be proportional to the degree
of hydration, while the differences in external chemical shrinkage give an impression on
how prone the mixture may be to micro-cracking. The difference between total and
external shrinkage results in contraction pores. The study is part of a larger on-going
study focusing on the early volume change of binders of high performance concrete.
Variables in the present part of the study have been 5 cement types, plasticizer (sodium
lignosulphonate denoted LS) and 2 super-plasticizer types (sodium salts of sulphonated
melamine - formaldehyde condensate named SMF and naphthalene sulphonate -
formaldehyde condensate called SNF) admixture dosage (0.0, 0.7, 1.0 and 1.4 %) and
water-to-cement ratio (0.30 and 0.40). However, only 25 of the 100 possible
combinations were made.
Total chemical shrinkage was used to monitor the retardation of cement hydration by e.g.
LS and the acceleration of hydration rate thereafter. LS retard early hydration (until 10 h)
more than the super-plasticizers SNF and SMF, which accelerated hydration for some
cements for the whole period of measurement. LS often accelerated hydration from 24 to
48 h, probably due to improved dispersion of cement grains. The flattening-out-level of
the external chemical shrinkage was independent of admixture type, and the same as the
reference without admixtures, when the samples were rotated (undisturbed by
bleeding).
The admixtures did not directly influence the chemical shrinkage in a way that should
indicate a higher risk of cracking. However, the retardation of early hydration and setting
of cement by lignosulphonate could allow a longer period of evaporation of water from
floors and bridge decks in practice. For this reason mixes with lignosulphonate might be
more prone to form drying shrinkage cracks.
Keywords: Cement paste, Chemical shrinkage, plasticizers, super-plasticizers, and w/c.
1. INTRODUCTION
Practical experience has shown that high performance concrete is sensitive to cracking at
early ages (from placing to a few hours after finishing), even when great care is taken to
avoid evaporation from the surface that initiates plastic shrinkage /1, 2/. The main reason
for this early shrinkage is considered to be early volume change producing stresses under
restraint conditions, coupled with low stress/strain capacity of the concrete at this stage.
The present study is part of a larger work. Earlier papers have been focusing on the
influence of cement type /3/, water-to-cement ratio /4/ and mineral additives /5, 6, 7/ on
chemical shrinkage. The influence of the last component, the plasticizers, is treated at
present and comparison is done for a number of Portland cements. A second paper on the
effect of plasticizers at different w/c on chemical shrinkage is recently published /8/.
The total chemical shrinkage during the hydration of cement is caused by the smaller
volume of products (e.g. calcium silicate hydrate gel and calcium hydroxide) compared with
the reactants (e.g. alite and water). As a rule of thumb, the total chemical shrinkage at 100 %
hydration is about 6.25 ml/100 g cement (i.e. 25 % of the chemical bound water to cement
ratio of 0.25) /9/. The total chemical shrinkage equals the external chemical shrinkage until
the net-work of hydration products bridging the unreacted cement grains is strong enough to
resist the contracting forces. At this point (5-9 h depending on cement composition,
fineness, w/c etc.), the external chemical shrinkage rate slows down drastically and the
shrinkage vs. time curve flattens out. Thereafter, the second manifestation of total chemical
shrinkage; the formation of internal contraction pores, is dominating. The relationship
between the two is considered important in the cracking problem. Both quantities are
measured in parallel in the present work.
The terminology in the present paper is total chemical shrinkage, which is the sum of
external chemical shrinkage and the volume of empty contraction pores at all stages. In
literature, there is a terminology confusion: Total chemical shrinkage may be named
chemical shrinkage, water absorption, volume contraction, or Le Chatelier shrinkage after
the first scientist who examined the shrinkage of cement paste. External chemical shrinkage
is also named external volume change, bulk shrinkage and autogeneous shrinkage.
The methods for measuring total chemical shrinkage all have in common that the sample has
to be kept water saturated and that the water needed to replace the volume decrease is
measured, while the common methods for measurement of external chemical shrinkage are
characterised by sealed curing of the cement paste. There are mainly three techniques for
measuring chemical shrinkage; 1) dilatometry, 2) gravimetry and 3) pycnometry.
Dilatometry is based on direct measurements of length or volume change.
The most common dilatometry technique for total chemical shrinkage measurement is to put
the paste in a recipient (e.g. glass tube), filling the rest of the recipient with water and
plugging the recipient with a stopper with a water filled pipette stuck through. Reading the
fall of the water level in the pipette versus time gives the total volume change. Knudsen and
Geiker /10/ have pioneered a variety of applications for this method since the 1980's. This
method is used in the present paper.
A dilatometry technique for external shrinkage measurement has been utilised by measuring
the uniaxial length change of a flexible, sealed tube by inductive sensors /11/.
Gravimetry is based on indirect measurement of volume change by recording reduced
buoyancy under water by weighing (i.e. the law of Archimedes). If the weight change of a
container filled with paste and excess water having at least one flexible wall is recorded, the
total chemical shrinkage is measured /12/. If the weight change of a sealed elastic bag filled
with paste only is registered, the external chemical shrinkage is measured. The latter method
is used in the present paper. Note that the samples for measurement of external chemical
shrinkage should be continuously rotated in order to avoid artificially high values caused
by imbibition of "bleeding" water /13/.
Pycnometry is only applicable for total chemical shrinkage measurements, and is carried out
by filling a pycnometer with paste and topping it with water. Water is added to refill the
pycnometer at different ages, and the weight increase relates to the total volume change.
2. EXPERIMENTAL
2.1 Chemicals
The cements were all Portland cements produced by Norcem A/S, Norway, over a period of
time. The cement characteristics are given in Table 1.
P30 = Standard Portland cement produced at the Dalen plant until 1993.
P30c = Standard Portland cement produced at the Dalen plant after 1993.
P30k = Standard Portland cement produced at the Kjpsvik plant.
SR = Sulphate Resistant Portland cement produced at the Dalen plant.
G = API Class G Oil well Cement produced at the Dalen Plant.
The mixing water was distilled before use.
The plasticizer sodium lignosulphonate (LS) was of the type Borresperse Na from
Borregaard, Sarpsborg, Norway, a solution of 40% solids with some minor antifoaming
agent.
The super-plasticizer sodium naphthalene sulphonate - formaldehyde condensate (SNF)
was of the type Mighty 150 produced by Kao, Japan, a solution with 42 % solids.
The super-plasticizer sodium salt of sulphonated melamine - formaldehyde condensate
was of the type Melment from SKW, Germany, a solution with 40 % solids.
2.2 Methods
2.2.1 Mixing procedure
Cement and water was mixed in a Hobart mixer of 5 litre capacity. Mixing times were 2 min
at gear 1 and 1 min at gear 2. The bowl with paste was put on a vibrating table to remove
most of the entrained air. The starting time of the experiments was the first contact between
cement and water (time = 0), and the first measurements started at 1 h.
Table 1 Cement characteristics
Cement P30 P30c P30k SR G
Oxides (%):
CaO (C)
SiO
2
(S)
Al
2
O
3
(A)
Fe
2
O
3
(F)
MgO (M)
SO
3
()
Alkalis
63.16
20.28
4.89
3.61
2.21
2.98
1.13
63.44
20.92
4.60
3.54
1.80
3.06
0.94
63.61
20.84
5.04
3.30
-
2.70
1.06
64.62
21.98
3.48
4.88
1.45
2.18
0.69
64.82
22.0
3.53
4.78
1.42
1.75
0.60
Blaine (m
2
/kg) 309 358 361 290 303
Minerals (%)
(Bogue)
C
3
S
C
2
S
C
3
A
C
4
AF
C
57
16
6.9
11.0
5.1
55
19
6.2
10.8
5.2
54
19
7.8
10.0
4.6
60
18
1.0
14.9
3.7
61
17
1.3
14.6
3.0
2.2.2 Total chemical shrinkage
Cement paste was put into three Erlenmeyer flasks for parallel experiments. The weights of
the empty and filled recipients were measured in order to determine the amount of paste.
The recipients were then carefully filled with distilled water at room temperature in a
manner to avoid turbulence. A silicon rubber stopper was used to plug each recipient, taking
care not to enclose any air bubbles. A pipette was filled with water and stuck through a hole
in the stopper. A graded pipette of 0.2, 0.5 or 1 ml was chosen depending on the expected
volume change. The recipients were put in a water bath at 201C. Every hour until 48 h,
the position of the meniscus in the pipettes was read. The decrease of the water column in
ml is directly the total chemical shrinkage (as a drop of liquid paraffin on top prevented
evaporation), which were expressed as ml/100 g cement after a calculation of the mean
value from the three parallel measurements with a typical standard deviation of 2%. The
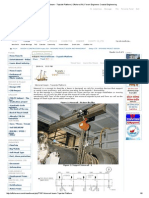
method relies on the assumption that all contraction pores are filled with water. A photo of
the experimental set-up is reproduced in Fig. 1.
Fig. 1. Picture showing experimental
set-up for measuring total chemical
shrinkage. Erlenmeyer flasks are filled
with cement paste and excess water.
The pipette on top is to read the total
chemical shrinkage. The flasks are
placed in a tray with water in a
temperature controlled room or
alternatively in a temperature regulated
water bath.
2.2.3 External chemical shrinkage
Three elastic rubber bags (i.e. condoms) were filled with cement slurry while the condoms
were inserted in a 100 mm plastic tube of 50 mm inner diameter. Each condom was closed
by twisting the upper part and tying it with a thin copper wire, and sealed by spraying
silicon glue into the open end. The excess end part was cut off and the total mass
determined.
The filled and sealed condoms in their tubes were kept in a water bath of 201C after the
tubes had been turned perpendicularly to their axes in order to let all air bubbles escape.
The tubes were kept on an ordinary rotating table modified to function under water (i.e. a
chain transfer between the motor and the rollers placed under water). During the first 10 h,
the condom was weighed every hour under water. According to Archimedes principle, an
external shrinkage will lead to a reduction in buoyancy, which will be registered as a
weight increase. Each condom was weighed in a basket under water hanging on a scale in a
separate water bath with no stirring to avoid turbulence. The transfer between the bath for
rotation and the bath for weighing took place under water at all times by placing the tube in
a water filled glass under water in one bath and taking it out of the glass under water in the
second bath. This was done to avoid trapping of any air bubbles in the transfer process.
After the last weighing under water at 48 h, the condoms were wiped dry and weighed in
air. Finally, the condom, including copper wire and silicon glue, were stripped off and
weighed in order to calculate the net weight of the cement slurry after subtracting the
weight of the tube. The external shrinkage is presented as the mean value of three parallel
measurements and given in ml/100 g cement with a standard deviation of about 5%. In the
present study, the samples with w/c = 0.30 were not rotated since they were not prone to
bleeding. In such a case the tube in the preceding procedure was excluded and the
condoms carefully weighed as such.
Photos of the rotating table with cement filled condoms in tubes under water and the
weighing arrangement with a cement filled condom in a tube are reproduced in Fig. 2.
Fig. 2 Upper photo illustrate three parallels of cement filled condoms in tubes on a rotating
table under water as described in the procedure for external chemical shrinkage in
the preceding text. The lower photo shows one of the cement filled condoms in its
tube in the weighing basket under water attached to a scale. The reduced buoyancy
as a function of time is proportional to the external volume reduction of the condom
according to the principle of Archimedes.
3. RESULTS AND DISCUSSION
The total and external chemical shrinkage versus time profiles of P30 cement paste with w/c
= 0.30 are shown in Figs. 3 and 4, respectively. The effect of 0.7 % additions of the
plasticizer LS (lignosulphonate), super-plasticizer SNF (naphthalene sulphonate
formaldehyde condensate) and super-plasticizer SMF (sulphonated melamine -
formaldehyde condensate) are compared with the reference without admixtures. It is evident
from the total chemical shrinkage that all admixtures retard the early hydration (4 12 h) of
the cement in the increasing order SMF < SNF < LS, assuming that chemical shrinkage
approximately is proportional with degree of hydration, . From 24 to 48 h (last data point),
the degree of hydration is higher than the reference for all admixtures and in fact the highest
for LS. The reason for this effect is probably better dispersion and breaking of possible
aggregates of cement grains. The external chemical shrinkage curves in Fig. 4 show the
same trend of early hydration retardation as in Fig. 3. The knee-points leading into the
flattening out levels (FOL) is quite gradual (6-8 h) for all pastes but the one with LS (rather
sharp transition at 9 h). The FOLs are equal for all pastes, except for the paste with 0.7 % LS
reaching a significantly higher FOL ( + 30%). LS is known to interact strongly with the
cement mineral C
3
A, otherwise known to form ettringite in reaction with gypsum. If a more
amorphous C
3
A-LS complex is formed on the expense of needle shaped ettringite crystals, it
is possible that more hydration is needed to make a strong enough hydration network to
resist the contraction forces. This could explain the higher FOL. Since the samples for
external chemical shrinkage is not rotated in this study, any tendency of separation
(bleeding) will lead to a higher FOL when the bleeding water is sucked into the sample
after contraction pores are formed. However, it is unlikely that LS should lead to more
bleeding than SNF or SMF for the P30 cement.
Fig. 3 Total chemical shrinkage versus time curves for P30 cement paste with w/c = 0.30
and 0.7 % LS, SNF and SMF as compared with the reference without admixtures.
P30 cement, w/c = 0.30
0
0.5
1
1.5
2
2.5
3
3.5
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
0.7% LS
0.7% SNF
0.7% SMF
Fig. 4 External chemical shrinkage versus time curves for P30 cement paste with w/c =
0.30 and 0.7 % LS, SNF and SMF as compared with the reference without
admixtures. Samples are not rotated.
The total and external shrinkage versus time profiles for SR cement pastes with w/c = 0.30
are plotted in Figs. 5 and 6, respectively, for the different admixtures. Unlike for the P30
cement, only 0.7% LS weakly retard the hydration (as seen by comparing Figs. 3 and 5),
while 0.7% SNF or SMF actually accelerates the hydration all the way to 48 h compared
with the reference. The degree of hydration for the paste with 0.7% LS is also higher than
the reference between 24 and 48 h, which again may be subscribed to a better dispersion of
cement grains and breaking of possible agglomerates.
The flattening out levels (FOLs) for the external chemical shrinkage curves for the SR
cement pastes in Fig. 6 are very different with an increasing level in the order SNF > SMF >
reference > LS. SR cement is more prone to bleeding than the P30 cement, even though
the surface is only marginally lower (290 versus 309 m
2
/kg), as demonstrated by loss of
hydrostatic pressure during separation /1/. This may be due to the low C
3
A content of SR
cement and thereby lower water binding by ettringite formation on the surface. The reason
why paste with 0.7% LS actually has a lower FOL than the reference paste may be
explained by a weak bleeding tendency of the reference that is absent when LS is added
since LS causes a gelling (i.e. thixotropy) of the SR paste. This gelling tendency increases
with decreasing C
3
A content of different cements /14/.
The total and external chemical shrinkage versus time curves for P30k cement paste with
w/c = 0.40 are shown for 0.7 % LS, SNF and SMF in Figs. 7 and 8, respectively, and for 1.0
% LS, SNF and SMF together with 1.4 % SMF in Figs. 9 and 10, respectively. All are
compared with the curves for reference paste without admixtures. Note that all the samples
in the w/c = 0.40 series are rotated for the external shrinkage measurements.
P30 cement, w/c = 0.30, static
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
0.7% LS
0.7% SNF
0.7% SMF
Fig. 5 Total chemical shrinkage versus time curves for SR cement paste with w/c = 0.30
and 0.7 % LS, SNF and SMF as compared with the reference without admixtures.
Fig. 6 External chemical shrinkage versus time curves for SR cement paste with w/c = 0.30
and 0.7 % LS, SNF and SMF as compared with the reference without admixtures.
Samples not rotated.
SR-cement, w/c = 0.30, static
0
0.2
0.4
0.6
0.8
1
1.2
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
0.7% LS
0.7% SNF
0.7% SMF
SR-cement, w/c = 0.3
0
0.5
1
1.5
2
2.5
3
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref. Rep.
0.7% LS
0.7% SNF
0.7% SMF
Fig. 7 Total chemical shrinkage versus time curves for P30k cement paste with w/c = 0.40
and 0.7 % LS, SNF and SMF as compared with the reference without admixtures.
Fig. 8 External chemical shrinkage versus time curves for P30k cement paste with w/c =
0.40 and 0.7 % LS, SNF and SMF as compared with the reference without
admixtures.
P30k cement, w/c = 0.40
0
0.5
1
1.5
2
2.5
3
3.5
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
0.7% LS
0.7% SNF
0.7% SMF
P30k cement, w/c = 0.40
0
0.2
0.4
0.6
0.8
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
0.7% LS
0.7% SNF
0.7% SMF
Fig.9 Total chemical shrinkage versus time curves for P30k cement paste with w/c = 0.40
and 1.0 % LS, SNF and SMF, and 1.4 % SMF, as compared with the reference
without admixtures.
Fig. 10 External chemical shrinkage versus time curves for P30k cement paste with w/c =
0.40 and 1.0 % LS, SNF and SMF, and 1.4 % SMF, as compared with the reference
without admixtures.
P30k cement, w/c = 0.40
0
0.5
1
1.5
2
2.5
3
3.5
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
1% LS
1% SNF
1% SMF
1.4% SMF
P30k cement, w/c = 0.40
0
0.2
0.4
0.6
0.8
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
1% LS
1% SNF
1% SMF
1.4% SMF
The results in Fig. 7 show that 0.7 % SNF and SMF have equal influence on hydration and
that both accelerate the hydration for the whole 48 h period of measurement compared with
the reference. 0.7% LS marginally retards the hydration in the early period from 4 - 10 h, but
are otherwise equal to the reference. The external chemical shrinkage curves, including the
FOLs, are for all practical purposes equal (Fig. 8) when admixtures are dosed 0.7 % of
cement weight. Fig. 9 reveals that even with a 1 % dosage the SNF and SMF accelerate
hydration between 10 and 48 h (about equal to reference before 10 h), and that 1.4 % SMF
behaves about equal (marginally extra retardation between 4 8 h) to 1.0 % SMF. The
hydration acceleration by SNF and SMF is less at 1.0 % than at 0.7 % dosage. LS retards
somewhat more in the early period from 4 to 10 h when dosed at 1.0 % compared with 0.7
%, but the degree of hydration after 24 h is higher for the highest dosage. The external
chemical shrinkage curves for the higher dosages in Fig. 10 is about equal until the
flattening out level, which is marginally increasing in the order Ref. 1 % SMF < 1.4 %
SMF 1 % SNF < 1 % LS. The difference between the highest and lowest FOL is however
not more than 0.2 ml/100 g cement.
The total and external shrinkage versus time profiles for P30c cement pastes with w/c =
0.40 are plotted in Figs. 11 and 12, respectively, for the different admixtures at 1 %
dosage. 1 % LS retards the P30c cement hydration in the period 5 - 8 h, while it is
marginally accelerated by the other admixtures in the same period. The degree of
hydration is higher than the reference and equal for the three admixtures in the period 24
- 48 h. The external shrinkage curves in Fig. 12 for all three admixtures changes
gradually from plastic to rigid state and reaches an equal flattening out level (about 0.7
ml/100 g cement) that is much lower than the reference (about 1.2 ml/100 g cement). The
latter difference is probably due to bleeding since the reference sample was rotated by
hand (180 every 15 min, which was probably insufficient).
The total and external shrinkage versus time profiles for G cement pastes with w/c = 0.40
are plotted in Figs. 13 and 14, respectively, for the different admixtures at 1 % dosage.
Fig. 13 shows that the hydration of G cement is virtually unaffected by the admixtures
until 7 h. Thereafter the G cement hydration is retarded by LS and SNF, but accelerated
by SMF. However, at 48 h the hydration with LS is equal to the reference. The external
chemical shrinkage profiles for G cement in Fig. 14 are equal for 1% SNF and SMF until
the flattening out level, which is marginally higher for SNF (+ 0.1 ml/100 g cement). The
curve for 1 % LS is retarded relative to the others, but reaches the same FOL as 1% SNF.
Fig. 11 Total chemical shrinkage versus time curves for P30C cement paste with w/c = 0.40
and 1.0 % LS, SNF and SMF as compared with the reference without admixtures.
Fig. 12 External chemical shrinkage versus time curves for P30c cement paste with w/c =
0.40 and 1.0 % LS, SNF and SMF as compared with the reference without
admixtures. Reference rotated by hand.
P30c cement, w/c = 0.40
0
0.5
1
1.5
2
2.5
3
3.5
4
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
1% LS
1% SNF
1% SMF
P30c cement, w/c = 0.40
0
0.2
0.4
0.6
0.8
1
1.2
1.4
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
k
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
1% LS
1% SNF
1% SMF
Fig. 13 Total chemical shrinkage versus time curves for G cement paste with w/c = 0.40 and
1.0 % LS, SNF and SMF as compared with the reference without admixtures.
Fig. 14 External chemical shrinkage versus time curves for G cement paste with w/c = 0.40
and 1.0 % LS, SNF and SMF as compared with the reference without admixtures.
Rotated reference not measured.
G cement, w/c = 0.40
0
0.5
1
1.5
2
2.5
1 10 100
Time (h)
T
o
t
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
Ref.
1% LS
1% SNF
1% SMF
G cement, w/c = 0.40
0
0.2
0.4
0.6
0.8
1 10 100
Time (h)
E
x
t
e
r
n
a
l
c
h
e
m
i
c
a
l
s
h
r
i
n
k
a
g
e
(
m
l
/
h
g
c
e
m
e
n
t
)
1% LS
1% SNF
1% SMF
4. CONCLUSION
As expected, LS (lignosulphonate) retards early hydration (until 10 h) more than the
super-plasticizers SNF (naphthalene sulphonate - formaldehyde condensate) and SMF
(sulphonated melamine - formaldehyde condensate).
In spite of retardation of early hydration and setting, LS may increase cement hydration
in the period 24 to 48 h, probably due to improved dispersion and breaking of possible
cement agglomerates.
SNF and SMF accelerated hydration for some cements for the whole period of
measurement (48 h).
The flattening-out-level of the external chemical shrinkage is independent of admixture
type, and the same as the reference without admixtures, when the samples were rotated
(undisturbed by bleeding).
The admixtures did not directly influence the chemical shrinkage in a way that should
indicate a higher risk of cracking. However, the retardation of early hydration and setting
of cement by lignosulphonate could allow a longer period of evaporation of water from
floors and bridge decks in practice. For this reason mixes with lignosulphonate might be
more prone to form drying shrinkage cracks.
5. REFERENCES
/1/ Sellevold E.J., Bjntegaard, ., Justnes, H. and Dahl, P.A. "High Performance
Concrete: Early Volume Change and Cracking Tendency" Proceedings of the
International RILEM Symposium "Thermal Cracking in Concrete at Early Ages",
Munich, October 10-12th, 1994, pp. 229-236, Editor R Springenschmid, Pub. E&FN
Spon, London, UK.
/2/ Kompen, R. "High Performance Concrete: Field Observations of Cracking Tendency
at Early Age" Proceedings of the International RILEM Symposium "Thermal
Cracking in Concrete at Early Ages", Munich, October 10-12th, 1994, pp. 229-236,
Editor R Springenschmid, Pub. E&FN Spon, London, UK.
/3/ Justnes, H., Sellevold, E.J., Reyniers, B., Van Loo, D, Van Gemert, A., Verboven, F.,
Van Gemert, D.: The Influence of Cement Characteristics on Chemical Shrinkage,
Proceedings of the International Workshop on Autogeneous Shrinkage of Concrete,
Hiroshima, Japan, June 13-14, 1998, pp. 67-76.
/4/ Justnes, H., Van Gemert, A., Verboven, A. and Sellevold, E.J.: "Total and External
Chemical Shrinkage of Low W/C-ratio Cement Pastes", Advances in Cement Research,
Vol. 8, No. 31, 1996, pp. 121-126.
/5/ Justnes, H., B. Ardoullie, E. Hendrix, E.J. Sellevold, D. van Gemert: "The Chemical
Shrinkage of Pozzolanic Reaction Products", Proceedings of the 6th International
Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolana in Concrete, Bangkok,
Thailand, May 31-June 5, 1998, ACI SP 179-11, pp. 191-205.
/6/ Justnes, H., Hammer, T.A., Ardoullie, B., Hendrix, E., Van Gemert, D., Overmeer, K.
and Sellevold, E.J.: Chemical Shrinkage of Cement Paste, Mortar and Concrete,
Proceedings of the International Workshop on Autogeneous Shrinkage of Concrete,
Hiroshima, Japan, June 13-14, 1998, pp. 201-211.
/7/ Justnes, H., Sellevold, E.J., Reyniers, B., Van Loo, D., Van Gemert, A., Verboven, F.
and Van Gemert, D.: Chemical Shrinkage of Cementitious Pastes with Mineral
Additives, Proceedings of Second International Research Seminar on Self-desiccation
and its Importance in Concrete Technology, Lund, Sweden, June 18, 1999, pp. 73-84.
/8/ Justnes, H., Van Gemert, A., Van Gemert, D., Verboven, F., Sellevold, E.J. :
Influence of Platicizers and Super-plasticizers on Chemical Shrinkage of Cement,
6
th
CANMET/ACI Int. Conf. on Super-plasticizers and Other Chemical Admixtures in
Concrete, October 10-13, 2000, Plaza Concorde Hotel, Nice, France, 10 pp.
/9/ Powers, T.C.: "Structure and Physical Properties of Hardened Portland Cement
Paste", J. Am. Ceramic. Soc., Vol. 41, No. 1, 1958, pp 1-6.
/10/ Knudsen, T. & Geiker, M.: "Chemical Shrinkage as an Indicator of the Stage of
Hardening", Proceedings of the International RILEM Conference on Concrete of
Early Ages, Vol. I, Session V: "Methods of Indicating the Stage of Hardening",
Ecole National des Pouts et Chaussees, Paris, April 6-8, 1982, pp 163-167.
/11/ Jensen, O.M.: "Autogen Deformation og RF-ndring - selvudtrring og
selvudtrringssvind", Dr. Thesis TR 284/93 (ISSN 0907-7073), The Technical
University of Denmark, Department of Civil Engineering, Building Materials
Laboratory, May 1993, 117 pp (in Danish).
/12/ Paulini, P.: "Reaction Mechanisms of Concrete Admixtures", Cement and Concrete
Research, Vol. 20, 1990, pp. 910-918.
/13/ Justnes, H., Van Gemert, A., Verboven, F., Sellevold, E.J., Van Gemert, D.:
"Influence of Measuring Method on Bleeding and Chemical Shrinkage Values of
Cement Pastes", Proceedings of 10th International Congress on the Chemistry of
Cement, Gothenburg, Sweden, 2-6 June, 1997, paper 2ii069, 8 pp.
/14/ Justnes, H., Koren, J., Lundevall, G., Meland, I., Skjeggerud, K., Totland, A. and
Wallevik, O.: "Materialutvikling Hyfast Betong, DP3 - Reologi, 3.6 Konsistenstap",
SINTEF Report STF70 A92034, 1992-01-20, 54 pp (In Norwegian).
You might also like
- Applsci 13 01463 v4Document22 pagesApplsci 13 01463 v4Ahmed TahaNo ratings yet
- Design Optimization of GFRP Pole Structures Using Finite Element Analysis PDFDocument8 pagesDesign Optimization of GFRP Pole Structures Using Finite Element Analysis PDFAhmed TahaNo ratings yet
- Hardware Catalog No Prices (PTHW0208.1C) PDFDocument11 pagesHardware Catalog No Prices (PTHW0208.1C) PDFAhmed TahaNo ratings yet
- RUS Adds 9 More PUPI Crossarms to Accepted Materials ListDocument1 pageRUS Adds 9 More PUPI Crossarms to Accepted Materials ListAhmed TahaNo ratings yet
- Only. This File Is Illegal.: Design of Four-Axis Winding Machine For Tapered Composite Pole Using Heated-Mandrel CuringDocument8 pagesOnly. This File Is Illegal.: Design of Four-Axis Winding Machine For Tapered Composite Pole Using Heated-Mandrel CuringAhmed TahaNo ratings yet
- Comparison of Loads Timber Vs Jerol - Signature PDFDocument1 pageComparison of Loads Timber Vs Jerol - Signature PDFAhmed TahaNo ratings yet
- PL010 PUPI Technical Information REV01.2016 PDFDocument16 pagesPL010 PUPI Technical Information REV01.2016 PDFAhmed TahaNo ratings yet
- Structural Assessment of Fiber-Reinforced Polymer Composite Electric PolesDocument8 pagesStructural Assessment of Fiber-Reinforced Polymer Composite Electric PolesAhmed TahaNo ratings yet
- Durable Fiberglass Cross Arms for UtilitiesDocument3 pagesDurable Fiberglass Cross Arms for UtilitiesAhmed TahaNo ratings yet
- 5th Percentile Strength Development Values R1 PDFDocument12 pages5th Percentile Strength Development Values R1 PDFAhmed TahaNo ratings yet
- Only. This File Is Illegal.: Design of Four-Axis Winding Machine For Tapered Composite Pole Using Heated-Mandrel CuringDocument8 pagesOnly. This File Is Illegal.: Design of Four-Axis Winding Machine For Tapered Composite Pole Using Heated-Mandrel CuringAhmed TahaNo ratings yet
- Case Study: Composite Power Pole Benefits Out-Weigh Those of Wood and ConcreteDocument1 pageCase Study: Composite Power Pole Benefits Out-Weigh Those of Wood and ConcreteAhmed TahaNo ratings yet
- 2016 Seismic Assessment of Guyed Towers A Casestudy Combining Eld Measurements and Pushover Analysis PDFDocument7 pages2016 Seismic Assessment of Guyed Towers A Casestudy Combining Eld Measurements and Pushover Analysis PDFAhmed TahaNo ratings yet
- 494-13-09 Composite Fiberglass Reinforced Polymer Utility Poles PDFDocument4 pages494-13-09 Composite Fiberglass Reinforced Polymer Utility Poles PDFAhmed TahaNo ratings yet
- 5th Percentile Strength Development Values R1 PDFDocument12 pages5th Percentile Strength Development Values R1 PDFAhmed TahaNo ratings yet
- Assessing Structural Capacity of Power Towers Under WindDocument4 pagesAssessing Structural Capacity of Power Towers Under WindAhmed TahaNo ratings yet
- Structural Assessment of Fiber-Reinforced Polymer Composite Electric PolesDocument8 pagesStructural Assessment of Fiber-Reinforced Polymer Composite Electric PolesAhmed TahaNo ratings yet
- RUS Adds 9 More PUPI Crossarms to Accepted Materials ListDocument1 pageRUS Adds 9 More PUPI Crossarms to Accepted Materials ListAhmed TahaNo ratings yet
- Analysis and Design of Four Legged Transmission Tower: Archana R, Aswathy S KumarDocument5 pagesAnalysis and Design of Four Legged Transmission Tower: Archana R, Aswathy S Kumaranon_108261563No ratings yet
- 2013 Capacity - Assessment - of - A - Transmission - Tower - Under - Wind - Loading PDFDocument192 pages2013 Capacity - Assessment - of - A - Transmission - Tower - Under - Wind - Loading PDFAhmed TahaNo ratings yet
- Pushover Analysis For Seismic Assessment and Design of StructuresDocument287 pagesPushover Analysis For Seismic Assessment and Design of Structuresapirakq100% (2)
- 2014 In-Plane Buckling and Semi-Rigid Joints of Tubular High Strength Steel Trusses PDFDocument169 pages2014 In-Plane Buckling and Semi-Rigid Joints of Tubular High Strength Steel Trusses PDFAhmed TahaNo ratings yet
- Joint Slippage Effects On Mechanical Behavior of A New Anti-Icing TowerDocument5 pagesJoint Slippage Effects On Mechanical Behavior of A New Anti-Icing TowerAhmed TahaNo ratings yet
- Assessing Structural Capacity of Power Towers Under WindDocument4 pagesAssessing Structural Capacity of Power Towers Under WindAhmed TahaNo ratings yet
- 2016 Cigre Iec 068 PDFDocument9 pages2016 Cigre Iec 068 PDFAhmed TahaNo ratings yet
- X-Tech DeckCoat CP Top Coat PDFDocument2 pagesX-Tech DeckCoat CP Top Coat PDFAhmed TahaNo ratings yet
- 2012 Pushover Analysis For Cold Formed Storage Rack Structures PDFDocument12 pages2012 Pushover Analysis For Cold Formed Storage Rack Structures PDFAhmed TahaNo ratings yet
- X-Tech DeckCoat CP (Anti Skid Coat) PDFDocument2 pagesX-Tech DeckCoat CP (Anti Skid Coat) PDFAhmed TahaNo ratings yet
- X-Tech DeckCoat CP - Method Statment PDFDocument4 pagesX-Tech DeckCoat CP - Method Statment PDFAhmed TahaNo ratings yet
- X-Shield SF Primer PDFDocument2 pagesX-Shield SF Primer PDFAhmed TahaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- OpenBoard AM335xDocument2 pagesOpenBoard AM335xvedanttaNo ratings yet
- Supersafari - 2 Activity BookDocument99 pagesSupersafari - 2 Activity BookShwe Yee Thet paingNo ratings yet
- Sap Fi Accounts ReceivableDocument66 pagesSap Fi Accounts ReceivableNikola100% (1)
- Field Data: Item Description Symbol Unit Value Step 1 Rectangle-1 (GIS Hall)Document2 pagesField Data: Item Description Symbol Unit Value Step 1 Rectangle-1 (GIS Hall)MELVINNo ratings yet
- Turbine Heat Rate CalculationDocument2 pagesTurbine Heat Rate Calculationkaruna34650% (2)
- Chrysler Neon 99 - Immobiliser SystemDocument4 pagesChrysler Neon 99 - Immobiliser SystemeephantomNo ratings yet
- DIN3-Luis Chen Yi-21PBD09429-ABBE1033Document12 pagesDIN3-Luis Chen Yi-21PBD09429-ABBE1033WeiHang NgNo ratings yet
- 1.overview Construction N DevelopmentDocument27 pages1.overview Construction N Development_ain_No ratings yet
- ATR72 Maintenance Manual Zones & FramesDocument10 pagesATR72 Maintenance Manual Zones & FramesRonald OngNo ratings yet
- Supersot Sot23 NPN Silicon Power (Switching) Transistors: Fmmt617 Fmmt618 Fmmt619 Fmmt624 Fmmt625Document4 pagesSupersot Sot23 NPN Silicon Power (Switching) Transistors: Fmmt617 Fmmt618 Fmmt619 Fmmt624 Fmmt625BBFulNo ratings yet
- DTFT Analysis in MATLABDocument7 pagesDTFT Analysis in MATLABRUTUJA MADHURENo ratings yet
- LS VCB Mec PDFDocument100 pagesLS VCB Mec PDFDarrell Wilson100% (2)
- The Aesthetics of Interactive Music Systems: Robert RoweDocument5 pagesThe Aesthetics of Interactive Music Systems: Robert Rowesertimone0% (1)
- Gigabyte Sandy Bridge Overclocking GuideDocument27 pagesGigabyte Sandy Bridge Overclocking GuideGIGABYTE UK100% (2)
- Atp CucmDocument26 pagesAtp CucmnoumanNo ratings yet
- Haynes 230 Alloy: Principal FeaturesDocument28 pagesHaynes 230 Alloy: Principal FeaturesMatheus DominguesNo ratings yet
- Data MigrationDocument6 pagesData MigrationsivasivasapNo ratings yet
- CadburyDocument4 pagesCadburyWong Kai WeiNo ratings yet
- HIS 770B Series-User ManualDocument33 pagesHIS 770B Series-User ManualVishwas MaritronicsNo ratings yet
- Igrid SV LV 3kva Split Phase Hybrid Solar Inverter: 110V-120V Grid Tie Solar Inverter With Energy StorageDocument1 pageIgrid SV LV 3kva Split Phase Hybrid Solar Inverter: 110V-120V Grid Tie Solar Inverter With Energy Storagejccl2No ratings yet
- PTCL's service problems and proposed solutionsDocument3 pagesPTCL's service problems and proposed solutionsSohail AhmadNo ratings yet
- Bornemann Twin Screw Pumps Series W V U and TDocument16 pagesBornemann Twin Screw Pumps Series W V U and TFelipeNo ratings yet
- SPE 00 301120 40mah en 1.0verDocument10 pagesSPE 00 301120 40mah en 1.0verAndreea FilipNo ratings yet
- Cyber Security and Reliability in A Digital CloudDocument95 pagesCyber Security and Reliability in A Digital CloudBob GourleyNo ratings yet
- BP Solar Bp275Document2 pagesBP Solar Bp275NandoMoralesNo ratings yet
- Risk-MaPP, ICH Q9, ASTM 2500 in Action Project Advantages of Practical QualityDocument7 pagesRisk-MaPP, ICH Q9, ASTM 2500 in Action Project Advantages of Practical Qualitybo.ratchadapornNo ratings yet
- Hands On PythonDocument240 pagesHands On PythonAmol MunaseNo ratings yet
- Troubleshooting Polyacrylamide Gel Electrophoresis (PAGE)Document9 pagesTroubleshooting Polyacrylamide Gel Electrophoresis (PAGE)sangeetsamratNo ratings yet
- Wind Load Calculations For PV Arrays: Solar America Board For Codes and StandardsDocument24 pagesWind Load Calculations For PV Arrays: Solar America Board For Codes and StandardsSalman KhanNo ratings yet
- Monorail Beam - Topside Platform - OffshoreDocument6 pagesMonorail Beam - Topside Platform - OffshoreBolarinwaNo ratings yet