Professional Documents
Culture Documents
Monitoring Granulation Drying Using Near-Infrared Spectros
Uploaded by
niknenad0 ratings0% found this document useful (0 votes)
46 views6 pagesPAT granulation
Original Title
http___www.foss-nirsystems.com_doc.aspx_name=pdf_applications_Monitoring%20Granulation%20Drying
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentPAT granulation
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
46 views6 pagesMonitoring Granulation Drying Using Near-Infrared Spectros
Uploaded by
niknenadPAT granulation
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 6
Monitoring Granulation Drying
Using Near-Infrared Spectroscopy
for In Situ Analysis of Residual Moisture and Methanol
Robert A. Mattes,* Rudolf Schroeder, Vinny Dhopeshwarkar, Robert Kowal, and William Randolph
ear-infrared (NIR) spectroscopy is an analytical tech-
nique based on absorption measured between the visi-
ble and mid-infrared electromagnetic spectral region.
Strong fundamental absorption bands of moieties occur
in the mid-IR region and frequently require mulls or dilutions to
bring the absorbances within the detectors linear range. The over-
tone absorptions enable direct measurement without sample
preparation because the absorption is relatively weak. Economic
fiber-optic cables can be used to measure processes that are lo-
cated far from the analyzer by inserting probes into processing
equipment.
For more than a decade, NIR spectroscopy and fiber-optic
probes have been used for real-time process monitoring (1, 2).
The turbulent flow of powder granules, however, poses a chal-
lenge for high-shear granulator moisture analysis monitoring.
For example, the turbulence constantly and randomly changes
the apparent sample density in front of the NIR probe window,
making accurate analysis impossible. When the sample density
change is brought within an acceptable narrow range, NIR analy-
sis can be performed by multivariate methods that account for
density variance as well as moisture variance. This paper dis-
cusses a novel probe design to reduce the powder-sample den-
sity variance such that moisture and solvent levels can be ac-
curately modeled and predicted.
Determining a formulations moisture level is essential for
subsequent process capability and product stability. Over-
drying wastes energy, ties-up critical facilities, and can damage
the formulation because of hydration changes in some active
ingredients and excipients (3, 4).
Typically, moisture content is monitored off-line with Karl
Fischer volumetric titrimetry or at-line with thermogravimet-
ric loss-on-drying (LOD) methodology (5, 6). Karl Fischer and
LOD analyses usually require the operator to stop the process,
use a sample thief to collect a sample, and then analyze the sam-
ple while the drying process continues. It is not possible to ob-
tain real-time moisture level trends. In addition, Karl Fischer
analysis is costly, time-consuming, and uses chemical reagents
that require purchase and proper disposal. LOD analysis is not
L
.
B
.
B
O
H
L
E
N
A novel probe design can reduce the powder-sample density
variance such that moisture and solvent levels can be accurately
modeled and predicted in process.
The single-pot high-shear granulator (L.B. Bohle, Warminster, PA) used
in the study.
Robert A. Mattes is an instrumentation
scientist at FOSS NIRSystems, Inc., 7703
Montpelier Road, Laurel, MD 20723
tel. 301.680.7251, fax 301.236.0134,
rmattes@foss-nirsystems.com. Rudolf
Schroeder, PhD, is a pharmaceutical
scientist at L.B. Bohle (Ennigerloh, Germany).
Vinny Dhopeshwarkar, PhD, is a
principal scientist in the Technical
Development group, Robert Kowal,
RPh, is a manager of technical operations,
and William Randolph, PhD, is a senior
director of techical operations, all at PSGA.
A Division of Ortho-McNeil Pharmaceutical
(Titusville, NJ).
*To whom all correspondence should be addressed.
www. phar mt ech. com
eration (approximately two complete scans
of 32 co-added scans per minute) result-
ing in 150 spectra per batch. Three test
batches and three calibration batches were
run. All spectral data collection and analy-
ses were performed with Vision
TM
soft-
ware (FOSS NIRSystems).
Results and discussion
Moisture analysis. Figure 1 shows spectra
with no math treatment (raw spectra) of
the raw materials used in the granulation.
Figure 2 illustrates the second-derivative
spectra of the raw materials. The second
derivitive is commonly used in NIR spec-
troscopy to remove the baseline offset
caused by scattering and to en-
hance absorbance peaks (11). The
second-derivative spectral peaks
appear inverted with respect to
the raw spectra (12). Figure 3
shows the samples raw spectra
collected during the drying cycle.
Water absorbs strongly in the
1400 and 1900-nm NIR range,
as evidenced by the peaks in those
regions. Figure 4 shows an en-
larged view of a region used to
model the samples moisture and
methanol content. Because of the
second-derivative math treatment, the
moisture content increases in the down-
ward direction in this region. The LOD re-
sults taken for the raw materials and the
gravimetric percent added during granu-
lation were used as the initial value for
modeling the moisture during the drying
cycle. At least four degrees of freedom were
modeled in this study: methanol content,
water content, temperature variance, and
moisture specific because all volatiles con-
tribute to the thermogravimetric loss.
In addition, monitoring the removal of
granulation liquids such as methanol is
important for product purity. Typically,
methanol level is measured by taking a
sample with a sample thief and sending it
to the laboratory for high-performance
liquid chromatography (HPLC) analysis.
HPLC requires long elution times, uses
solvents that must be disposed of prop-
erly, and requires calibration samples to
be run frequently.
NIR spectroscopy is a rapid, non-
destructive technique that can provide
real-time analysis without sample prepa-
ration and can store data automatically.
NIR spectroscopy is well-suited for the
process analytical technology (PAT) ini-
tiative proposed by the Food and Drug
Administration (710). NIR can moni-
tor low levels of residual moisture, alco-
hol, and other process constituents to yield
better process control and quality man-
agement.
Experiment
A process instrument (FOSS NIRSystems,
Laurel, MD) was set up and connected to
a fiber-optic probe with a specially de-
signed tip, which was installed into a sin-
gle-pot high-shear granulator (VMA 70,
L.B. Bohle, Warminster, PA).
The probe (FOSS NIRSys-
tems) has an angled face
that diverts the powder
sample in a quasilaminar
flow over the measurement
window. This design main-
tains a constant sample den-
sity at the window interface.
A 24.0-kg charge of 91%
lactose anhydrous and 9%
starch 1500 was prepared
and loaded into the gran-
ulator. The granulator
blended the dry mixture to
homogeneity for 10 min.
Water, methanol, and yel-
low dye (FD&C #6) were
sprayed into the mixture
and blended. The yellow
dye measured in the parts-
per-billion range and was
not a source of interference
with NIR measurements.
NIR spectra were collected
continuously during the blending and
granulation operations. Samples for LOD
analysis were withdrawn at specific inter-
vals. Each NIR spectrum consisted of 32
co-added sample and reference scans in
the 8002100-nm NIR range.
After the moisture and methanol were
added and blended, an agglomeration
cycle was run for 6 min. The heated jacket
was energized, and a vacuum was pulled
to 200 mbar to start the drying opera-
tion. The drying operation was uniform
and gradual over a period of 43 min with
an agitator speed of 200 rpm and a nitro-
gen purge rate of 1.0 NM
3
/h at standard
temperature and pressure. NIR spectra
were collected continuously during the en-
tire blending, granulation, and drying op-
Table I: LOD samples and NIR predictions.
MeOH
H
2
O% LOD H
2
O% MeOH%
Blend Sample LOD% NIR NIR NIR
4 7 1.22 1.23
4 67 10.1 1.73 8.37 8.22
4 104 5.3 1.32 3.98 3.64
4 137 2.10 0.89 1.21 0.32*
4 176 1.10 0.70 0.40 0.75
4 204 1.27 0.53 0.74 0.35
5 206 1.35 1.38
5 315 9.90 1.70 8.20 7.82
5 346 4.95 1.43 3.52 4.44
5 381 1.97 0.97 1.00 0.45
5 410 1.25 0.77 0.48 1.07
5 432 1.07 0.64 0.43 0.49
6 468 1.36 1.32
6 563 9.42 1.75 7.67 7.77
6 604 3.42 1.28 2.14 1.94
6 631 1.90 0.99 0.91 0.71
6 663 1.34 0.76 0.58 0.93
6 693 0.96 0.63 0.33 0.12
* indicates that sample did not pass library qualification
For the study, the standard temperature probe
was replaced with the angled NIR probe.
A novel probe (FOSS NIRSystems) with an angled sample
interface.
density variance.
Figure 5 shows the
NIR-predicted samples
versus the values calcu-
lated from LOD values.
The moisture levels with
time were not precisely
known because the LOD
accounts for moisture
and methanol losses. For
example, Table I shows
that blend 4 initially had
1.22% moisture before
wet granulation; 0.5%
moisture was added dur-
ing granulation resulting
in 0.53% after drying.
This method was re-
peated for blends 5 and
6. These values and the
linearly interpolated in-
termediate values were
applied to the spectra ac-
quired at corresponding
times. A three-factor par-
tial least squares (PLS)
regression model was de-
veloped with the 18 data
points in Table I. The
second derivative inten-
sity and the standard
normal variate (SNV)
(6) for scatter correction
over the 11002100-nm range were used to develop the model
with multiple correlation coefficient (R
2
) value of 0.9541 and
a standard error of calibration (SEC) of 0.0966%. The standard
error of cross-validation was 0.1835, which is not unreasonable
for a small sample set.
The spectra from the drying cycle used to develop the pre-
diction model were used to develop a library to qualify samples
for quantification. This library and the prediction model were
then used in a routine analysis function to predict on the aver-
age of five consecutive samples. The results are plotted in Fig-
ure 6. Some initial samples were not predicted when the aver-
age spectrum did not meet library qualification because of high
rpm and turbulence during the blending cycle. Although the
true values are partly theoretical, the predictions are reasonable
even through the granulation cycle (not modeled).
Methanol analysis. Figure 4 shows the second-derivative spec-
tra of blend 5s analytical region as an example of the spectra
used for predicting methanol levels in the lactose anhydrous
and starch 1500 blend. To estimate the methanol level the NIR-
predicted moisture values were subtracted from the LOD val-
ues. A model was made from the resulting 17 adjusted values
(see Table I). According to the qualification library, one spec-
trum was an outlier and was not used in the model. As shown
in Figure 7, a PLS regression model was developed using the
second
derivative intensity over the 18002100-nm range and resulted
in an R
2
value of 0.9823, and an SEC of 0.4427%. The standard
error of cross-validation was 0.6224. These samples are not well
spaced because of the faster initial methanol removal. The water
2.9136
2.6222
2.3308
2.0395
1.7481
1.4568
1.1654
0.8741
0.5827
0.2914
0.0000
A
b
s
o
r
b
a
n
c
e
Wavelength (nm)
8
0
0
9
0
0
1
0
0
0
1
1
0
0
1
2
0
0
1
3
0
0
1
4
0
0
1
5
0
0
1
6
0
0
1
7
0
0
1
8
0
0
1
9
0
0
2
0
0
0
2
1
0
0
Figure 1: Raw spectra of raw materials.
0.7899
0.7108
0.6316
0.5525
0.4734
0.3942
0.3151
0.2360
0.1568
0.0777
-0.0014
A
b
s
o
r
b
a
n
c
e
Wavelength (nm)
8
0
0
9
0
0
1
0
0
0
1
1
0
0
1
2
0
0
1
3
0
0
1
4
0
0
1
5
0
0
1
6
0
0
1
7
0
0
1
8
0
0
1
9
0
0
2
0
0
0
2
1
0
0
Figure 3: Raw spectra of drying samples.
0.0120
0.0087
0.0053
0.0020
-0.0014
-0.0047
-0.0081
-0.0115
-0.0148
-0.0182
-0.0215
A
b
s
o
r
b
a
n
c
e
Wavelength (nm)
1
8
5
0
1
8
6
1
1
8
7
1
1
8
8
2
1
8
9
3
1
9
0
4
1
9
1
4
1
9
2
5
1
9
3
6
1
9
4
6
1
9
5
7
1
9
6
8
1
9
7
9
1
9
8
9
Increasing
H
2
O
Figure 4: Second-derivative spectra in the analytical
region.
0.2925
0.2309
0.1694
0.1079
0.0464
-0.0151
-0.0766
-0.1382
-0.1997
-0.2612
-0.3227
A
b
s
o
r
b
a
n
c
e
Wavelength (nm)
8
0
0
9
0
0
1
0
0
0
1
1
0
0
1
2
0
0
1
3
0
0
1
4
0
0
1
5
0
0
1
6
0
0
1
7
0
0
1
8
0
0
1
9
0
0
2
0
0
0
2
1
0
0
Figure 2: Second-derivative spectra of
raw materials.
lactose anhydrous
starch 1500
methanol
water
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
N
I
R
-
p
r
e
d
i
c
t
e
d
(
%
)
Theoretical moisture (%)
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
Figure 5: Moisture Calibration set: NIR predicted values versus those
calculated from LOD data in Table I. R
2
value is 0.9541 and SEC is
0.0966%.
was removed in a quasi-linear function of time. The methanol
level, however, was nonlinear, thus leaving less data in the mid-
level. To develop a robust model, more data must be taken ini-
tially. The use of HPLC as a reference method provides more
accurate results.
Figure 8 is the process trend chart from routine analysis for
the granulation and drying operation. These spectra were ana-
lyzed in routine analysis, averaging five spectra for each predic-
tion, and tested against a qualification library. The methanol
level of some initial samples was not predicted by the routine analy-
sis when the average spectrum did not meet library qualification be-
cause the granulator and turbulence had a high rpm during the blend-
ing cycle. The methanol level reached a minimum and then increased
slightly before reaching the final minimum. This effect was most
likely caused by the removal of free methanol, the fracturing of
granules, and the release of additional interstitial methanol. Again,
although the true values are partly theoretical, the predictions are
smooth and reasonable throughout the granulation cycle that was
not modeled.
Conclusion
The in situ NIR spectroscopic method predicted water and methanol
loss in samples of lactose anhydrous and starch 1500 during the
blending, granulation, and drying operations. Although the accu-
racy is not known, the trended predictions appear smooth and rea-
sonable from known initial and end-point data. The single-pot gran-
ulator trial indicates that an NIR process instrument with the novel
angled probe can measure a moving sample in turbulent flow when
it is placed in the sample medium in a manner that provides suffi-
ciently constant sample density. Proper probe placement and design
in process equipment is essential for successful implementation of in
situ measurements.
With HPLC reference data for the methanol and Karl Fischer ref-
erence data for the moisture, the accuracy and precision of the model
predictions should be improved. It should be possible to model par-
ticle size, blend uniformity, and temperature with reasonable preci-
sion if proper reference data are provided.
Acknowledgments
The authors wish to thank Karl Norris and Katherine Bakeev for
their review and valued comments on this manuscript.
References
1. J.B. Callis, D.L. Illman, and B.K. Kowalski, Process Analytical Chemistry,
Anal. Chem. 59 (9), 624A635A (1987).
2. K.A. Bakeev, Near-Infrared Spectroscopy as a Process Analytical Tool: At-
Line and On-Line Applications and Implementation Strategies, Spectroscopy
19 (1), 3942 (2004).
3. S.M. Maggard, D.E. Root, and M. Duell, On-line Monitoring of Pharma-
ceutical Dryers, J. Process Anal. Chem. 7 (1) (2002).
4. R.M. Leasure and M.K. Gangwer, Near-Infrared Spectroscopy for In-Process
Moisture Determination of a Potent Active Pharmaceutical Ingredient,
Am. Pharm. Rev. 5 (1), 103109 (2002).
5. E.W. Ciurczak and J.K. Drennen III, Pharmaceutical and Medical Applica-
tions of Near-Infrared Spectroscopy (Marcel Dekker, New York, NY, 2002).
6. M.B. Hicks et al., In Situ Moisture Determination of a Cytotoxic Com-
pound During Process Optimization, J. Pharm. Sci. 92 (3), 529535 (2003).
7. M.L. Balboni, Process Analytical Technology Concepts and Principles,
Pharm. Technol. 27 (10), 5467 (2003).
8. FDA Draft Guidance PATA Framework for Innovative Pharmaceutical
Manufacturing and Quality Assurance, August 2003,
http://www.fda.gov/cder/OPS/PAT.htm.
9. H. Forcinio, Pharmaceutical Industry Embraces NIR Technology, Spec-
troscopy 18 (9), 1624 (2003).
10. R.C. Lyon et al., Exploring Pharmaceutical Applications of Near-Infrared
Technology, Am. Pharm. Rev. 6 (3), 6270 (2003).
11. T.C. OHaver and T. Begley, Signal-to-Noise Ratio in Higher-Order De-
rivative Spectrometry, Anal. Chem. 53 (12), 18761878 (1981).
12. H. Mark and J. Workman, Jr., Derivatives in Spectroscopy: Part I, The Be-
havior of the Derivative, Spectroscopy 18 (4), 3237 (2003). PT
1.6
1.4
1.2
1
0.8
0.6
0.4
0.2
0
M
e
t
h
a
n
o
l
(
%
)
Methanol
5:09:36 5:16:48 5:24:00 5:31:12 5:38:24 5:45:36 5:52:48 6:00:00 6:07:12 6:14:24 6:21:36
1.8
Figure 6: The moisture process trend chart for the granulation and
drying operations for blend 4.
9.0
8.0
7.0
6.0
5.0
4.0
3.0
2.0
1.0
0.0
-1.0
N
I
R
-
p
r
e
d
i
c
t
e
d
m
e
t
h
a
n
o
l
(
%
)
Theoretical methanol (%)
-1.0 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0
Figure 7: Methanol Calibration set: NIR predicted values versus
those calculated from LOD data in Table I. R
2
value is 0.9823 and
SEC is 0.4427%.
8
7
6
5
4
3
2
1
0
M
e
O
H
(
%
)
Time
5:09:36 5:16:48 5:24:00 5:31:12 5:38:24 5:45:36 5:52:48 6:00:00 6:07:12 6:14:24 6:21:36
9
Figure 8: The methanol process trend chart for the granulation and
drying operations for blend 4.
Reprinted from PHARMACEUTICAL TECHNOLOGY, September 2004 Printed in U.S.A.
7703 Montpelier Road
Laurel, MD 20723
U. S. A.
T: 301.680.9600
F: 301.236.0134
E: info@foss-nirsystems.com
www.foss.dk
You might also like
- On Bottom Stability of PipelinesDocument62 pagesOn Bottom Stability of PipelinesVictor DaggersNo ratings yet
- Experiment 6 PhosphorusDocument4 pagesExperiment 6 PhosphorusMia Domini Juan Loa100% (1)
- 10-Lab-10Spectrophotometric Determination of PhosphatDocument4 pages10-Lab-10Spectrophotometric Determination of PhosphatHoang Huong Tra33% (3)
- The Application of Green Solvents in Separation ProcessesFrom EverandThe Application of Green Solvents in Separation ProcessesFrancisco Pena-PereiraRating: 4 out of 5 stars4/5 (6)
- Beam Deflection - Moment Area Method PDFDocument10 pagesBeam Deflection - Moment Area Method PDFنور عليNo ratings yet
- Determination of Nitrate in Municipal Waste Water by UV SpectrosDocument7 pagesDetermination of Nitrate in Municipal Waste Water by UV SpectrosNeal CuaNo ratings yet
- Experiment 10 (Chemistry)Document10 pagesExperiment 10 (Chemistry)nikenantha100% (1)
- 15.ISCA RJCS 2012 057 - DoneDocument4 pages15.ISCA RJCS 2012 057 - DoneSaurav BhattacharjeeNo ratings yet
- CH Oel Edited Uv Vis SpectrosDocument13 pagesCH Oel Edited Uv Vis SpectrosSultan Ali WaryamNo ratings yet
- CE6611 Environmental Engineering Lab ManualDocument44 pagesCE6611 Environmental Engineering Lab ManualArun Pugal100% (3)
- Gravimetric Sampling Procedure For Aqueous Ozone CDocument5 pagesGravimetric Sampling Procedure For Aqueous Ozone CAlejandro VerdugoNo ratings yet
- Particle Size Analysis by Laser Diffraction: Geological Society, London, Special PublicationsDocument12 pagesParticle Size Analysis by Laser Diffraction: Geological Society, London, Special PublicationsDanielle CruzNo ratings yet
- Recent Advances in Trace Quantity Sample Identification and Determination of Absolute Stereochemistry by NMRDocument60 pagesRecent Advances in Trace Quantity Sample Identification and Determination of Absolute Stereochemistry by NMRDharmendra TiwariNo ratings yet
- s00216 005 3416 9Document7 pagess00216 005 3416 9LINQA LINQANo ratings yet
- Short Report: Colorimetric Detection of Glucose in Biological Fluids Using Toner-Based Microzone PlatesDocument5 pagesShort Report: Colorimetric Detection of Glucose in Biological Fluids Using Toner-Based Microzone PlatesBruno LuccaNo ratings yet
- PharmaceuticalDocument7 pagesPharmaceuticallunarNo ratings yet
- Análisis de Trazas ArtículoDocument31 pagesAnálisis de Trazas ArtículoOmar BojórquezNo ratings yet
- Drying SolventsDocument4 pagesDrying Solventsalchemist90No ratings yet
- Art 23Document3 pagesArt 23Fahra Aqilla AzzurahNo ratings yet
- 1995, Barnabas, Experimental Design Approach For The Extraction of Polycyclic Aromatic Hydrocarbons From Soil Using Supercritical Carbon DioxideDocument6 pages1995, Barnabas, Experimental Design Approach For The Extraction of Polycyclic Aromatic Hydrocarbons From Soil Using Supercritical Carbon DioxideBhupendra SuryawanshiNo ratings yet
- USEPA m-07d PDFDocument7 pagesUSEPA m-07d PDFmulmulmulNo ratings yet
- Combination of Physico-Chemical Treatment and Nanofiltration To Reuse Wastewater of A Printing, Dyeing and Finishing Textile IndustryDocument8 pagesCombination of Physico-Chemical Treatment and Nanofiltration To Reuse Wastewater of A Printing, Dyeing and Finishing Textile IndustryChittaranjan SahooNo ratings yet
- Preparation and Study of Pdms Material: SciencedirectDocument7 pagesPreparation and Study of Pdms Material: SciencedirectgpaivNo ratings yet
- Dry Mass Zeiller Et AlDocument8 pagesDry Mass Zeiller Et AlMeluti ArguelloNo ratings yet
- Solvent DryingDocument10 pagesSolvent DryingEvs GoudNo ratings yet
- Infra R RojoDocument4 pagesInfra R RojoRonaldo JanglinNo ratings yet
- Analysis of Water in Food by Near Infrared SpectroscopyDocument16 pagesAnalysis of Water in Food by Near Infrared SpectroscopyAlexis Valdez ValdezNo ratings yet
- Effect of Varying Concentration On AbsorbanceDocument5 pagesEffect of Varying Concentration On AbsorbanceAriel ChenNo ratings yet
- A1 TarwiDocument67 pagesA1 TarwiMafer Mabel GalarzaNo ratings yet
- Analysis of Total Chloride Content in Concrete: RecommendationDocument3 pagesAnalysis of Total Chloride Content in Concrete: RecommendationKhalid JavedNo ratings yet
- A Colorimetric Method For Ammonia in Natural WatersDocument7 pagesA Colorimetric Method For Ammonia in Natural WatersDiễn Đàn Hóa HọcNo ratings yet
- Combination of Physico-Chemical Treatment and Nanofiltration To Reuse Wastewater of A Printing, Dyeing and Finishing Textile IndustryDocument8 pagesCombination of Physico-Chemical Treatment and Nanofiltration To Reuse Wastewater of A Printing, Dyeing and Finishing Textile IndustrywaqaskhanNo ratings yet
- Environmental Engg LabDocument72 pagesEnvironmental Engg LabRajjan SinghNo ratings yet
- 2222 PDFDocument13 pages2222 PDFhshobeyriNo ratings yet
- Mccomb 1997Document7 pagesMccomb 1997wad elshaikhNo ratings yet
- Tanery Effluents PDFDocument6 pagesTanery Effluents PDFAditya RahmatNo ratings yet
- UPLCDocument33 pagesUPLCrat001No ratings yet
- Mdhs 102Document6 pagesMdhs 102José Luís TrigoNo ratings yet
- Hydrogen Sulfide in Workplace AtmospheresDocument13 pagesHydrogen Sulfide in Workplace AtmospheresnayakyaNo ratings yet
- Yanina Corrotea, Karen Sánchez, M. Angélica Rubio, Pablo RichterDocument4 pagesYanina Corrotea, Karen Sánchez, M. Angélica Rubio, Pablo RichterridermateNo ratings yet
- Car Do SoDocument16 pagesCar Do SoMichael Gergis HannaNo ratings yet
- A New All-Season Passive Sampling System For Monitoring NO2 in AirDocument8 pagesA New All-Season Passive Sampling System For Monitoring NO2 in AirCicero EscobarNo ratings yet
- Research Article: Highly Sensitive Filter Paper Substrate For SERS Trace Explosives DetectionDocument8 pagesResearch Article: Highly Sensitive Filter Paper Substrate For SERS Trace Explosives DetectionSha W ShankNo ratings yet
- Doehlert Ii PDFDocument4 pagesDoehlert Ii PDFAna María TorresNo ratings yet
- Automatic Multicommutation Ow System For Wide Range Spectrophotometric Calcium DeterminationDocument9 pagesAutomatic Multicommutation Ow System For Wide Range Spectrophotometric Calcium DeterminationRajan PandaNo ratings yet
- Analysis of Phosphate in Wastewater Using An Autonomous Microfluidics Based AnalyserDocument4 pagesAnalysis of Phosphate in Wastewater Using An Autonomous Microfluidics Based AnalyserRonaldo Kazuyoshi SatomiNo ratings yet
- Fuller & Griffiths (1978) KMDocument5 pagesFuller & Griffiths (1978) KMpchin13No ratings yet
- Surfactant AnalysisDocument5 pagesSurfactant Analysisjuli_radNo ratings yet
- Experiment 7Document8 pagesExperiment 7Shinichi KudoNo ratings yet
- Expt. 1 CMB Lab Written-LATESTDocument7 pagesExpt. 1 CMB Lab Written-LATESTTimothy John BautistaNo ratings yet
- 4 HydrometerDocument9 pages4 HydrometerHawkar IBRAHIMNo ratings yet
- Elements by Icp 7300 (Nitric/Perchloric Acid Ashing) : MW: Table 1 CAS: Table 2 RTECS: Table 2Document8 pagesElements by Icp 7300 (Nitric/Perchloric Acid Ashing) : MW: Table 1 CAS: Table 2 RTECS: Table 2Juli Cardenas BarzolaNo ratings yet
- Food Chemistry: Andrea Felgner, Regina Schlink, Peter Kirschenbu Hler, Birgit Faas, Heinz-Dieter IsengardDocument6 pagesFood Chemistry: Andrea Felgner, Regina Schlink, Peter Kirschenbu Hler, Birgit Faas, Heinz-Dieter IsengardNicolas EstebanNo ratings yet
- Journal of Food Engineering: G. Romano, D. Argyropoulos, M. Nagle, M.T. Khan, J. MüllerDocument11 pagesJournal of Food Engineering: G. Romano, D. Argyropoulos, M. Nagle, M.T. Khan, J. MüllerMathes WaranNo ratings yet
- HF (2) HCL (3) H Po MW: Table 1 Cas: Table 1 Rtecs: Table 1 (4) HBR (5) Hno (6) H SoDocument6 pagesHF (2) HCL (3) H Po MW: Table 1 Cas: Table 1 Rtecs: Table 1 (4) HBR (5) Hno (6) H SoJesus de la HozNo ratings yet
- Experiment 1 BDocument5 pagesExperiment 1 BWNo ratings yet
- Kayali Sayadi2000Document5 pagesKayali Sayadi2000Andreea CristinaNo ratings yet
- Mathematical Model On Thin Layer Drying of Finger Millet (Eluesine Coracana)Document5 pagesMathematical Model On Thin Layer Drying of Finger Millet (Eluesine Coracana)Radhikagb16No ratings yet
- Nitrification DenitrificationDocument4 pagesNitrification DenitrificationDon Javier HubbleNo ratings yet
- Ultrafiltration of Aqueous Solutions Containing DextranDocument11 pagesUltrafiltration of Aqueous Solutions Containing DextranJulio TovarNo ratings yet
- Kappa Number DeterminationDocument12 pagesKappa Number Determinationnedian_2006No ratings yet
- Handbook of Infrared Standards II: with Spectral Coverage betweenFrom EverandHandbook of Infrared Standards II: with Spectral Coverage betweenNo ratings yet
- Interphex Manesty PAT TechnologyDocument33 pagesInterphex Manesty PAT TechnologyniknenadNo ratings yet
- Kollicoat Smartseal 30 D ShortDocument39 pagesKollicoat Smartseal 30 D ShortniknenadNo ratings yet
- PC-11994 Aquarius Color ChartDocument2 pagesPC-11994 Aquarius Color ChartniknenadNo ratings yet
- Spraying Systems CatalogueDocument116 pagesSpraying Systems Catalogueniknenad50% (2)
- PC-11642 Cavamax Native CyclodextrinsDocument4 pagesPC-11642 Cavamax Native CyclodextrinsniknenadNo ratings yet
- PC-11792 Polyplasdone Ultra Ultra10Document2 pagesPC-11792 Polyplasdone Ultra Ultra10niknenadNo ratings yet
- Kollicoat Smartseal 30 D ShortDocument39 pagesKollicoat Smartseal 30 D ShortniknenadNo ratings yet
- HTTP WWW - Dow.com PublishedLiterature DH 0081 0901b80380081505.pdf Filepath Polyox Pdfs Noreg 326-00013 PDFDocument12 pagesHTTP WWW - Dow.com PublishedLiterature DH 0081 0901b80380081505.pdf Filepath Polyox Pdfs Noreg 326-00013 PDFniknenadNo ratings yet
- PTR 037Document8 pagesPTR 037niknenadNo ratings yet
- 24 Laktoza MeggleDocument3 pages24 Laktoza MeggleniknenadNo ratings yet
- FMEA Training GuideDocument10 pagesFMEA Training GuideniknenadNo ratings yet
- 250 42aECDocument35 pages250 42aECniknenadNo ratings yet
- Manesty Coating Presentation - 2009Document16 pagesManesty Coating Presentation - 2009niknenadNo ratings yet
- FMC Coating EquipmentDocument26 pagesFMC Coating EquipmentniknenadNo ratings yet
- Experiment # 6: PurposeDocument5 pagesExperiment # 6: PurposeHamza HussainNo ratings yet
- Taylor Introms11GE PPT 01Document27 pagesTaylor Introms11GE PPT 01hddankerNo ratings yet
- NDVI Index Forecasting Using A Layer Recurrent Neural Network Coupled With Stepwise Regression and The PCADocument7 pagesNDVI Index Forecasting Using A Layer Recurrent Neural Network Coupled With Stepwise Regression and The PCALuis ManterolaNo ratings yet
- MODULE 3 and 4Document6 pagesMODULE 3 and 4Jellene Jem RonarioNo ratings yet
- Liquid Holdup in PackedDocument11 pagesLiquid Holdup in PackedVictor VazquezNo ratings yet
- Gomory's CutsDocument7 pagesGomory's Cutsgladiator001No ratings yet
- Math 139-1 Lesson 6 and 7Document52 pagesMath 139-1 Lesson 6 and 7jenny teresa50% (2)
- MSC - Patran Results PrimerDocument138 pagesMSC - Patran Results PrimeralfredaoudeNo ratings yet
- DepressionnnDocument5 pagesDepressionnnMilkie MangaoilNo ratings yet
- Advertising Appeal and Tone - Implications For Creative Strategy in TV CommercialsDocument16 pagesAdvertising Appeal and Tone - Implications For Creative Strategy in TV CommercialsCapitan SwankNo ratings yet
- Let Us C SolutionsDocument81 pagesLet Us C Solutionsneonav100% (2)
- PTM Phy F.4.Ch.2.4Document13 pagesPTM Phy F.4.Ch.2.4Bazil BoliaNo ratings yet
- Basic ProbDocument12 pagesBasic ProbChaaaNo ratings yet
- Sari, AliDocument13 pagesSari, AliMustafa ShabanNo ratings yet
- Chapter One Part 1Document20 pagesChapter One Part 1enedaylalu bassieNo ratings yet
- One Sample Z TestDocument10 pagesOne Sample Z TestCornelius DuraiNo ratings yet
- Cost-Volume-Profit Relationships: © 2010 The Mcgraw-Hill Companies, IncDocument97 pagesCost-Volume-Profit Relationships: © 2010 The Mcgraw-Hill Companies, IncInga ApseNo ratings yet
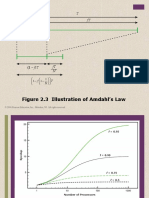
- Figure 2.3 Illustration of Amdahl's Law: © 2016 Pearson Education, Inc., Hoboken, NJ. All Rights ReservedDocument10 pagesFigure 2.3 Illustration of Amdahl's Law: © 2016 Pearson Education, Inc., Hoboken, NJ. All Rights ReservedAhmed AyazNo ratings yet
- Impact of Cash Conversion Cycle On Firm's ProfitabilityDocument3 pagesImpact of Cash Conversion Cycle On Firm's ProfitabilityAnonymous 7eO9enzNo ratings yet
- Modeling of Dissolution DataDocument39 pagesModeling of Dissolution DataanupnakatNo ratings yet
- Assignment Brief and Guidance:: P Standard Atmospheric Pressure (101.3 Kpa) R Gas Constant Its Value For Air Is 286.9Document7 pagesAssignment Brief and Guidance:: P Standard Atmospheric Pressure (101.3 Kpa) R Gas Constant Its Value For Air Is 286.9Khanur AysahNo ratings yet
- NCERT Solutions For Class 6 Maths Chapter 8 DecimalsDocument23 pagesNCERT Solutions For Class 6 Maths Chapter 8 DecimalsABYAN ShaikNo ratings yet
- ERT2016 Lab Sheet 20112012 MechanicsDocument7 pagesERT2016 Lab Sheet 20112012 MechanicsKesava ShankarNo ratings yet
- Inductive and Deductive ReasoningDocument37 pagesInductive and Deductive ReasoningGadela KevinNo ratings yet
- Queen's University Belfast: Phy3012 Solid State PhysicsDocument131 pagesQueen's University Belfast: Phy3012 Solid State PhysicsOğuz Alp KurucuNo ratings yet
- 5 - RegressionDocument63 pages5 - RegressionMarcello RossiNo ratings yet
- MTH302 Solved MCQs Alotof Solved MCQsof MTH302 InonefDocument26 pagesMTH302 Solved MCQs Alotof Solved MCQsof MTH302 InonefiftiniaziNo ratings yet