Professional Documents
Culture Documents
Research Paper
Uploaded by
Shafaet Ara Moon0 ratings0% found this document useful (0 votes)
37 views12 pagespran frooto
Original Title
Research Paper (1)
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentpran frooto
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
37 views12 pagesResearch Paper
Uploaded by
Shafaet Ara Moonpran frooto
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 12
ORIGINAL RESEARCH PAPER
TITLE:- A study on In Vitro Interaction of Cephradine ith !an"o #uice at $oer pH
%ony &a$$i'(
)epart!ent of Phar!acy* +GC Trust ,ni-ersity +an"$adesh* Chitta"on"
E!ai$: #ony!a$$i'.y!ai$/co!
&o0i$e No/ 12234253466676
A+STRACT
Cephradine is a first generation semi-synthetic cephalosporin antibiotic, is widely used in clinics for its activity
against both Gram-positive and Gram-negative bacteria. It is indicated for the treatment of urinary tract
infections, skin and skin structure infections, respiratory tract infections and otitis media. Interaction of
cephradine with mango juice were investigated by U-spectrophotometer in simulated gastric juice !p"
#.$%&.$'. In this research work, cephradine capsules were collected from drug shops. (he samples were
analy)ed according to *ritish +harmacopoeia !*+' method &. In this work in vitro dissolution studies were
carried out in ,--ml of acidic buffer !p" #.$%&.$' in a dissolution tester with a speed of .# rpm at &/0-..1C for
si2 hours. (he absorbance was measured by using U-spectrophotometer at a 3ma2 of $.4nm. Compare with
the absorbance of drug and the drug in presence of mango juice simulated gastric juice !p" #.$%&.$'. (he drug
release kinetics was also measured. It is observed from the drug release profile that, there is no significant
difference in the drug release curve of p" #.$%&.$.
Key Words:- Cephradine, U-spectrophotometer, 5issolution, 6ango juice.
INTRO),CTION
7oods and therapeutic products are both used for well defined purposes. In simple terms food provides energy for
sustenance, while therapeutic products are taken for managing ailments $.
"owever, over the years roles of foods have changed considerably. 8ow, food no longer is seen as simply the
provider of energy, but it is e2pected to provide physiological benefits for good health and productive lifestyles.
9ell managed combination of foods and therapeutic products plays important role in the prevention and
treatment of many diseases, including a number of chronic diseases such as cancer, diabetes, hypertension,
obesity. 6ost often food is combined with medicine to enhance the benefits of medicine - an additive and:or
synergistic effect; food-therapeutic product synergism. <t the most basic level, food is a comple2 mi2ture of
chemicals with many functional groups= hence, they not only confer positive effects, but may also make negative
contributions.
Cephradine is in a group of drugs called cephalosporin antibiotics. Cephradine fights bacteria in the body.
Cephradine is used to treat infections caused by bacteria, including upper respiratory infections, ear infections,
skin infections, and urinary tract infections >. Cephradine may also be used for other purposes not listed in this
medication guide. Cephradine is the most commonly used antibiotic for prophyla2is in orthopaedic patients as it
is safe and effective. 9e report a case of severe anaphylactic reaction to Cephradine in an elderly patient who had
no history of allergic reactions to any drugs until then.
?+@<8A is currently the most well known household name among the millions of people in *angladesh and
abroad also. Bince its inception in #,C-, +@<8 Group has grown up in stature and became the largest fruit and
vegetable processor in *angladesh. It also has the distinction of achieving prestigious certificate like IBD
,--#;$---, and being the largest e2porter of processed agro products . +@<8 is the pioneer in *angladesh to be
involved in contract farming and procures raw material directly from the farmers and processes through state of
the art machinery at our several factories into hygienically packed food and drinks products. (he brand ?+@<8A
has established itself in every category of food and beverage industry and can boost a product range from Euices,
Carbonated 5rinks, Confectionery, Bnacks, and Bpices. 4
8i"ure 4: Le0ac9 :Cephradine; Capsu$e 8i"ure 7: &an"o %uice :8rooto;
&ATERIALS < ÐO)S
8eature of Cephradine standard
Cephradine !compacted powder'
+otency; ,,.#4F
GD5- 4 F! 86('
Drigin; China
Collected from; +harmik Gaboratories Gtd.
Rea"ents used in this or'
#. "ydrochloric acid,
$. Bodium hydro2ide,
&. Citric acid,
4. +ottasium chloride,
.. 5i-sodium hydrogen orthophosphate,
>. +otassium di-hydrogen orthophosphate.
(hey were all of analytical grade. Dne brands of cephradine capsule and the innovator brands with labeled
contents of .--mg each were obtained from retail pharmacies in Chittagong city. (he samples were checked
for their production and e2piry dates before purchasing.
Instru!ent ,sed in this ðod
#. Hlectric balance
$. U- Bpectrophotometer
&. 5issolution <pparatus
4. (hermometer
.. p" 6eter
)ISSOL,TION RATE )ETER&INATION
(his was determined using the +harma (est 5issolution @ate (esting <pparatus !6odel 5->&.#$, "ainburg'.
(hese studies were conducted at &/0-..
-
C on an UB+ specification dissolution rate test type II apparatus !+addle
apparatus' with si2 section assembly according to the UB+ IIIII procedure with minor modification :,SP ==II
and N8 =>II* 4??@;/
7or in vitro dissolution studies simulated gastric medium !p" #.$ % &.$' and simulated intestinal medium !p"
>.C' were reJuired.
Preparation of si!u$ated "astric !ediu!:pH 4/7;
#---ml buffer solution with p" #.$ $.- ml of -.# 6 "Cl solution was taken in #---ml beaker and .--ml -.#6
"Cl solution were added into the #---ml beaker. <djust the p" #.$ with adding distilled % demineralised water
respectively. <fter adjusting the p" of the buffer solution the buffer solution were taken into#---ml volumetric
flask.
Preparation of si!u$ated "astric !ediu!:pH 5/7;
#---ml buffer solution with p" &.$ $.- ml of -.# 6 "Cl solution was taken in #---ml beaker and .--ml -.#6
"Cl solution were added into the #---ml beaker. <djust the p" &.$ with adding distilled % demineralised water
respectively. <fter adjusting the p" of the buffer solution the buffer solution were taken into#---ml volumetric
flask.
)isso$ution study state!ent
(he dissolution study of Cephradine!GebacK' were investigated firstly in the presence of tap water and then
same investigation were performed in the presence of buffer solution p" #.$ prepared by using both distilled and
demineralised water. (he dissolution study were investigated in the presence of buffer solution p" &.$ prepared
by using demineralised water.
(he dissolution study of Cephradine!Gebac' were investigated in the presence of $.-ml mango juice!+ran
7rooto' and >.-ml of buffer solution p" #.$. <gain dissolution study of Cephradine !GebacK' were investigated
in the presence of $.-ml mango juice >.-ml of buffer solution p" &.$.
)isso$ution study description
<ccording to the statement every time two capsules are places in the baskets, where one basket contain the the
drug !cephradine capsule form' and another basket contain the drug with mango juice. 7irst time drug was placed
in tap water and demineralised water. "ere one drug was placed in tap water and another was in demineralised
water. 8e2t time a drug was placed in buffer with p" #.$ and another was in combined solution of $.-ml mango
juice and >.-ml buffer p" #.$.again a drug was placed in buffer with p" &.$ and another was in combined
solution of $.-ml mango juice and >.-ml buffer p" &.$.again. (he operation in the acid stages were carried out
for > hours.(han the dissolution apparatus are switched on and the temperature was &/LC and the rpm was .#.<t
every time interval .ml solution were taken into test tube and the volume adjust by fresh media.(he time interval
were followings;-
-min, .min, #-min, $-min, &-min, 4.min, >-min, ,-min, #&.min, #,.min, $C.,min, &>-min !upto > hours'.
7rom the test tube of each,#ml were taken into #--ml volumetric flask and it is diluted to #--ml with buffer.
(han it was filtered, taken into cell and the released drug was assayed by using U spectrophotometer at $.4nm.
RES,LTS < )ISC,SSION
(he absorbances of standard Cephradine solution under a concentration range of # to #-Mg:ml !-.--# to
-.-# mg:ml' where the average of Concentration : <bsorbance were also calculated to determine the release
kinetics. 5ata are shown at Ta0$e 4 /
Ta0$e 4:
Conc :!"A!$; A0sor0ance Conc AA0sor0ance A-era"e
-.--# -.-/$ -.-#&CCCCC,
-.--$ -.#-$ -.-#,>-/C4&
-.--& -.#C$ -.-#>4C&.#>
-.--4 -.$>4 -.-#.#.#.#.
-.--. -.&#. -.-#.C/&-#>
-.--> -.&C/ -.-#..-&C/> -.-$##C$>->
-.--/ -.4$& -.-#>.4C4>&
-.--C -.4,C -.-#>->4$./
-.--, -..4& -.-#>./4.C>
-.-# -.>## -.-#>&>>>#$
8i"ure 5 : Btandard Curve of Cephradine !blue line' represents the measured absorbance were plotted
against the respective concentrations of the standard solutions which give a straight line in the
concentration range of # to #-Mg:ml !-.--#--.-# mg:ml'.
)isso$ution test of Cephradine in presence of #uice:7@3!$; in PH 4/7:B@3!$;
(he dissolution test of cephradine in presence of juice were conducted % collect the absorbance of the
dissolute solution after every . minutes. <ll the respected value are shown at Ta0$e 7
Ta0$e 7.
Ti!e:!inutes; A0sor0ance Ct C re$ease C Re!ain
$o" of C
re!ain
- -.-#$ -.---C#C. #.4/&&/C& ,C..$>>$#>4 #.,,&..&.,$
. -.-$# -.--#4&$4 $../C4#$# ,/.4$#.C/C/ #.,CC>..$-4
#- -.-& -.--$-4>& &.>C&44., ,>.&#>..4# #.,C&/--,&>
$- -.-4. -.--&->,. ...$.#>CC ,4.4/4C&##4 #.,/.&#>#$4
&- -.-># -.--4#>-,& /.4C,>/&&& ,$..#-&$>>> #.,>>#,-$#4
4. -.-C -.--.4.>,. ,.C$$.$$4 ,-.#//4//., #.,..-,C-C&
>- -.#.> -.-#->4#- #,.#.&,#C C-.C4>-C#& #.,-/>.C,/4
,- -.#.$ -.-#-&>C$# #C.>>$/,$ C#.&&/$-/4$ #.,#-$C,$.C
#&. -.##$ -.--/>&,/ #&./.#.&# C>.$4C4>C>$ #.,&./.#&,&
#,. -.-/> -.--.#C4# ,.&&#&,>$ ,-.>>C>-&/# #.,./4.>,$C
$C. -.-,C -.-->>C4/ #$.-&$.C, C/.,>/4#--. #.,44&$#C->
&>- -.#C, -.-#$C,$-># $&.$-./-,$ />./,4$,-C #.CC.&$C,&4
7orm the table it is found that percent release of the drug is increased upto ,- minutes and than decreased
again by time. <s well as the concentration of drug in e2perimental medium. "ence the percent remain and
log of percent remain decreased. (he graphical presentation of rate kinetics are shown at 8i"ure 6 * @ * B
respectively.
8i"ure 6 : 8i"ure @ :
Nero order plot of release kinetics of Cephradine 7irst order plot of release kinetics of Cephradine
8i"ure B: "iguchi plot of release kinetics
Re$ease para!eters of cephradine capsu$es in presence of #uice:7@3!$; in PH 4/7:B@3!$;
Para!eters Dero order 8irst order Hi"uchi
RE
3/@6@754B5F 3/6FF2FF4?7 3/2757BBFB
(he @-sJuared value is highest in case of "iguchi release kinetics
)isso$ution test of Cephradine in presence of #uice:7@3!$; in PH 5/7:B@3!$;
(he dissolution test of cephradine in presence of juice were conducted % collect the absorbance of the
dissolute solution after every . minutes. <ll the respected value are shown at Ta0$e 5
Ta0$e 5
Ti!e:!inutes; A0sor0ance Ct C re$ease C Re!ain
$o" of C
re!ain
- -.--4 -.---$/$C4 -.4,##$>#$# ,,..-CC/&CC #.,,/C>#C##
. -.-#C -.--#$$/C# $.$#-->/.4$ ,/./C,,&$4> #.,,-$,4#4>
#- -.-$/ -.--#C4#/ &.&#.#-# ,>.>C4C,C>, #.,C.&.C>4>
$- -.-&, -.--$>>-$> 4./CC4/,>/. ,..$##.$-&$ #.,/C>C,.
&- -.-.C -.--&,.>$, /.#$#&$C/4C ,$.C/C>/#$. #.,>/,#.,,4
4. -.->/ -.--4./-$- C.$$>&>$.#, ,#.//&>&/4C #.,>$/#/,4.
>- -.-C# -.--..$.#> ,.,4.&-&,4# ,-.-.4>,>-> #.,.4.->&>.
,- -.#-# -.-->CC,4- #$.4--,&4.4 C/..,,->.4> #.,4$4,,4/&
#&. -.#$4 -.--C4.C$C #..$$4,-,/4 C4.//.-,-$> #.,$C$>C$>#
#,. -.#4C -.-#--,.& #C.#/#>>>4> C#.C$C&&&.4 #.,#$,-&/-/
$C. -.#>$ -.-##-.-&& #,.C,->-/CC C-.#-,&,$#$ #.,-&>C&4&>
&>- -.#/$ -.-##/&$4. $#.##C4$&#C /C.CC#./>C$ #.C,>,/..C&
7orm the table it is found that percent release of the drug is increased by time. <s well as the concentration
of drug in e2perimental medium. "ence the percent remain and log of percent remain decreased.
8i"ure F : 8i"ure 2 :
Nero order plot of release kinetics of cephradine 7irst order plot of release kinetics of cephradine
8i"ure ?: "iguchi plot of release kinetics
Re$ease para!eters of cephradine capsu$es at in presence of #uice:7@3!$; in PH 5/7:B@3!$;
Para!eters Dero order 8irst order Hi"uchi
RE
3/2B3723F?5 3/5FBF2F@2 3/??342274
(he @-sJuared value is highest in case of higuchi release kinetics
)eter!ination of re$ease !echanis! fro! corre$ation coefficients :R
7
; :
7rom the drug release data of cephradine in presence of mango juice were treated in different kinetics orders
such as Nero Drder +lot, 7irst Drder +lot and "iguchi +lot and their correlation coefficients were determined
to identify their release mechanism.
Ta0$e 6 : Corre$ation coefficients deter!ination data for pH 4/7
Sa!p$e corre$ation coefficients :R
7
;
Nero order 7irst order "iguchi
Cephradine -.,>#,,#.>4 -.&>/&.-C&C -.,$.>.$,,$
Cephradine1&an"o #uice -..4.$&#>&/ -.4//C//#,$ -.C$&$>>/>
7rom ta0$e 6 it is seen that Cephradine in presence of 6ango juice at p" #.$.Indicates that the Correlation
Coefficients was close to # in case of "iguchi plot than Nero Drder and 7irst Drder Oinetics. Bo "iguchi
release kinetics predominates in simulated gastric medium of p" #.$.
Ta0$e @ : Corre$ation Coefficients deter!ination data for pH 5/7
Sa!p$e corre$ation coefficients :R
7
;
Nero order 7irst order "iguchi
Cephradine -.C.>&$#-C& -.#/.>&->-# -.,>>4,&.&&
Cephradine1&an"o
#uice
-.C>-$C-/,& -.&/>/C/.C -.,,-#CC$#
7rom ta0$e @ it is seen that Cephradine in presence of 6ango juice in simulated Gastric medium at p" &.$
indicates that the Correlation Coefficients is close to # in case of "iguchi plot that Nero Drder and 7irst
Drder kinetics. "iguchi release kinetics predominates in simulated gastric medium of p" &.$.
CONCL,SION
(he percent release data suggest that, in the simulated gastric medium !p"#.$ % &.$', the percent release of
Cephradine not increased significantly. It is also seen that in different p" the percent release neither
increased nor decreased when Cephradine is taken with the 6ango juice. 7rom the Correlation coefficients
determination data it is seen that, Correlation Coefficients !@
$
' is close to # in case of "iguchi plot. Bo
"iguchi release kinetics predominates in simulated gastric medium of p" #.$ % &.$.
It is also observed from the release kinetics profile !Nero order, 7irst order, "iguchi', both of the line are
close to each other and there is no significant distance between two line. *oth of the line appear in between
--$- percent of drug release. "ence, we can say that on the basis of our present study if the patient take
Cephradine and 6ango juice at a time, no harmful effect will occur.
ACGNOHLE)GE&ENT
I am greatful to the +harmik Gaboratories Gtd. for providing the Btandard sample of Cephradine for my research
work
RE8ERENCES:
#.<meer *, 9eintraub @<. 5rug interactions with grapefruit juice. Clin Pharmacokinet #,,/=&;#-&-$#.
$. P<dvisory Btatement. <ntibiotic +rophyla2is for 5ental +atients 9ith (otal Eoint @eplacements. <merican
5ental <ssociation= <merican <cademy of Drthopedic Burgeons,P J Am Dent Assoc, #,,/, #$C!/';#--4-C.
3. Bamberger, D. M. & Dahl, S. L. (1!". #m$act o% &ol'ntar( &s en%orce) com$liance o% thir)*generation
ce$halos$orin 'se in a teaching hos$ital. Archi&es o% #nternal Me)icine 1+!, ++,-..
4. *587!*angladesh 8ational 7ormulary',+ublished by; 5irectorate of 5rug <dministration,&
rd
edition, +age
no. #>,#/,#C
..*arcina Q, <lcalde <I, Ilundain <, Garralde E. Hffect of cephale2in and tetracycline on galactose absorption in
rat small intestine. 5rug 8utr Interact #,C>=4;$,,-&-/.
>.*ailey 5G, 6alcom E, <rnold <, Bpence E5. Grapefruit juice-drug interactions. Br J Clin Pharmacol
#,CC=4>;#-#-#-.
/.Creed, @ichard !$-#---,--.'. P@elative Dbscurity; ariations of antigodlin growP. /inston*Salem Jo'rnal.
@etrieved $-#---,-->.
R)ea) linkS
C.5aly <O, *rockmoller E, *roly 7, et al. 8omenclature for human CQ+$5> alleles. +harmacogenetics #,,>= >;
#,&-$-#.
,. 5.<rcy +7. 8utrient-drug interactions. A)&erse Dr'g 0eact 1o2icol 0e& #,,.=#4;$&&-.4.
#-. 5ebnam CB, (homson H(. Hffects of neomycin on galactose absorption across rat jejunum. *r E +harmacol
#,C4=C$; >/&->.
##. 5ie)-Bampedro <, Urdaneta H, Gostao 6+, *arber <.Galactose transport inhibition by cytochalasin H in rat
intestine in vitro. Can E +hys +harmacol #,,,=//;,>-#-#.
#$. 5rug T 5rug Interactions *y +rofessor Ghada "ashem, 5epartment of +harmacology, 7aculty of
6edicine,Cairo University $--.
#&. Goshman G, 7ish E, @oller O.; Clinically significant cytochrome +4.- drug interactions. +harmacotherapy
!9isconsin' #,,,= 6ay:Eune; $&-&C.!6H(<*DGIB6'
#4 "ansten +5, "orn E@. "ansten and "ornUs 5rug interactions analysis and management. Bt. Gouis, 6D; 7acts
and Comparisons, $---.
#.. Idoate I, 6endi)abal 6, Urdaneta H, Garralde E. Interactions of cephradine and cefaclor with the intestinal
absorption of 5-galactose. E +harm +harmacol #,,>=4C; >4.-.-.
#>. Eedele B, "au <6, von Dppen 6. <n analysis of the world market for mangoes and its importance for
developing countries. Conference on International <gricultural @esearch for 5evelopment, $--& R#S
#/. Oarchmer <9 !#,,.'. Ce$halos$orins, In. 6andell, 5ouglas and *ennettA. Princi$les an) Practice o%
#n%ectio's Diseases, 4th edition, Churchill Givingstone, 8ew Qork, pp.$4/-$>&.
#C. Gieber CB. 6echanisms of ethanol-drug-nutrition interactions. J 1o2icol Clin 1o2icol #,,4=&$;>&#-C#.
#,. 6ichalets HG. Update; Clinically significant Cytochrome +-4.- drug interaction, +harmacotherapy #,,C=
#C; C4-##$.
!3. Moellering, 0. C. (1!". 4mergence o% 4nterococc's as a signi%icant $athogen. Clinical #n%ectio's
Diseases 1,, 11.3-5.
$#. 9ichman O, ed. 8ew drugs:drug news; 5rug interactions with grapefruit juice. +harmaCQ Connection
#,,,= >!4';iiTiv.
!!. 6nite) State Pharmaco$eia, 77# 0e&., 1he 8ational 9orm'lar(, 7:#th 4)., (he United Btate +harmacopeial
Convention Inc., 8ew Qork, #,C..
You might also like
- Indigenous Fermented Foods of Southeast Asia - J. David OwensDocument442 pagesIndigenous Fermented Foods of Southeast Asia - J. David OwensDiego GieroNo ratings yet
- Bracketing MethodsDocument13 pagesBracketing Methodsasd dsa100% (1)
- FIN 1050 - Final ExamDocument6 pagesFIN 1050 - Final ExamKathi100% (1)
- Food Sample Test For Procedure Observation InferenceDocument2 pagesFood Sample Test For Procedure Observation InferencefadzreenshahiraNo ratings yet
- Karakteristik Antioksidan Ekstrak Kasar Dari Rumput Laut Gracilaria Lichenoides Terhadap PH Dan SuhuDocument8 pagesKarakteristik Antioksidan Ekstrak Kasar Dari Rumput Laut Gracilaria Lichenoides Terhadap PH Dan SuhuRinto Felly HartanaNo ratings yet
- Analysis of pH in Popular Cold DrinksDocument20 pagesAnalysis of pH in Popular Cold DrinkskunwarjeetNo ratings yet
- Study The Rate of Fermentation of Fruits and Vegetables JuicesDocument9 pagesStudy The Rate of Fermentation of Fruits and Vegetables JuicesYogesh ZopeNo ratings yet
- 3711 MethodDocument25 pages3711 Methodkaira musahariNo ratings yet
- Global Yield of The Supercritical CO2 Extraction From Cordia Verbenacea DC - Anticancer and Antimycobacterial ActivitiesDocument8 pagesGlobal Yield of The Supercritical CO2 Extraction From Cordia Verbenacea DC - Anticancer and Antimycobacterial ActivitiesSocrates QuispeNo ratings yet
- Trabajo Deinvestigacion de Pepe 1Document62 pagesTrabajo Deinvestigacion de Pepe 1Jesus Ibañez JimenezNo ratings yet
- HistologyDocument7 pagesHistologyمحمدأميندماجNo ratings yet
- Literature Review On Citric Acid Production by Aspergillus NigerDocument6 pagesLiterature Review On Citric Acid Production by Aspergillus Nigern0nijitynum3No ratings yet
- Aspirin ProductionDocument11 pagesAspirin ProductionRiska PurwantiNo ratings yet
- Determination of vitamin C levels in commercial products and fresh foods using redox titrationDocument8 pagesDetermination of vitamin C levels in commercial products and fresh foods using redox titrationSyahirrashahar100% (1)
- The Ball Blue Book 1938Document60 pagesThe Ball Blue Book 1938debbiebrummerNo ratings yet
- Experiments Enzymes: Science Technology ActionDocument2 pagesExperiments Enzymes: Science Technology ActionHartini HassanNo ratings yet
- Bignay Local LitDocument8 pagesBignay Local LitJean FlorencondiaNo ratings yet
- ANTIOXIDANT ACTIVITY OF AROMATIC PLANTSDocument15 pagesANTIOXIDANT ACTIVITY OF AROMATIC PLANTSFariza Fauzia Purnama PurnamaNo ratings yet
- Strawberry Dna Extraction LabDocument11 pagesStrawberry Dna Extraction Labapi-237946379No ratings yet
- Over-the-Counter Painkillers: Aspirin vs Paracetamol SynthesisDocument12 pagesOver-the-Counter Painkillers: Aspirin vs Paracetamol SynthesisAditya SinghNo ratings yet
- Journal Homepage: - : IntroductionDocument10 pagesJournal Homepage: - : IntroductionIJAR JOURNALNo ratings yet
- Study oxalate ion guava fruitDocument19 pagesStudy oxalate ion guava fruitAditya Soni100% (3)
- Article Links: Conditions of UseDocument8 pagesArticle Links: Conditions of UsePutriYuriandiniYulsamNo ratings yet
- Chemistry Investigatory ProjectDocument19 pagesChemistry Investigatory Projectmr.priyanshu2005No ratings yet
- Lime HerbsDocument2 pagesLime HerbsnepretipNo ratings yet
- Rund ThesiseDocument81 pagesRund ThesisenomanhaimourNo ratings yet
- Submitted By:: Juber KhanDocument33 pagesSubmitted By:: Juber KhanPushpendra KumarNo ratings yet
- MP Agus Triyono - 27 DesemberDocument5 pagesMP Agus Triyono - 27 DesembernajibrendraNo ratings yet
- Pepsi: Analysis of Pepsi Household ProgrammeDocument26 pagesPepsi: Analysis of Pepsi Household ProgrammeAnshul SharmaNo ratings yet
- Submitted By:: Lavendra TayalDocument35 pagesSubmitted By:: Lavendra TayalPushpendra KumarNo ratings yet
- Research Journal of Pharmaceutical, Biological and Chemical SciencesDocument7 pagesResearch Journal of Pharmaceutical, Biological and Chemical Sciencesprift2No ratings yet
- Hybrid Rice Breeding Seed ProductionDocument41 pagesHybrid Rice Breeding Seed ProductionGENIUS1507No ratings yet
- Indigenous Fermented Foods Southeast Asia: J. David OwensDocument44 pagesIndigenous Fermented Foods Southeast Asia: J. David OwensFelipe AndrésNo ratings yet
- Enzyme Lab: Factors Affecting ActivityDocument5 pagesEnzyme Lab: Factors Affecting ActivityhunarsandhuNo ratings yet
- Abstract 1996Document31 pagesAbstract 1996Civ NortubNo ratings yet
- AyanDocument11 pagesAyanPushpa BaruaNo ratings yet
- Yeast GrowthDocument13 pagesYeast GrowthSmartPurdyNo ratings yet
- OF A.H.M. Khurshid Alam: Curriculum VitaeDocument7 pagesOF A.H.M. Khurshid Alam: Curriculum VitaeRocky RoyNo ratings yet
- Literatura i kvalitet kajmakaDocument4 pagesLiteratura i kvalitet kajmakaDragan Fića FilipovićNo ratings yet
- Laboratory/Kitchen ReportDocument4 pagesLaboratory/Kitchen ReportBabyruth BalderamaNo ratings yet
- Fermented Orange Juice: Source of Higher Carotenoid and Flavanone ContentsDocument10 pagesFermented Orange Juice: Source of Higher Carotenoid and Flavanone ContentsTrúc NguyễnNo ratings yet
- Determination of Level Sodium Benzoate in Different Packed Soft Drinks and Juices in Addis Ababa Market Using HPLC TechniquDocument13 pagesDetermination of Level Sodium Benzoate in Different Packed Soft Drinks and Juices in Addis Ababa Market Using HPLC TechniquTadesse Gebregiyorgis TakeleNo ratings yet
- XCXXDocument4 pagesXCXXchamaldsNo ratings yet
- Brjexppathol00255 0037 PDFDocument13 pagesBrjexppathol00255 0037 PDFAndresPimentelAlvarezNo ratings yet
- Lab Procedure For Osmosis AdvancedDocument4 pagesLab Procedure For Osmosis Advancedapi-264668182No ratings yet
- Project File on Galvanometer and its IntroductionDocument14 pagesProject File on Galvanometer and its IntroductionAnkit SharmaNo ratings yet
- Intel Science Research Format DepedDocument126 pagesIntel Science Research Format DepedCris Esteban100% (6)
- Antiulcer and Antioxidant Effect of Enteric Coated Sodium Alginate Beads of Substituted Benzimidazole Proton Pump InhibitorDocument7 pagesAntiulcer and Antioxidant Effect of Enteric Coated Sodium Alginate Beads of Substituted Benzimidazole Proton Pump InhibitorkavitaNo ratings yet
- Physico-Chemical and Shelf Life Evaluation of Pasteurized Coconut MilkDocument9 pagesPhysico-Chemical and Shelf Life Evaluation of Pasteurized Coconut Milkzub12345678No ratings yet
- Journal Homepage: - : IntroductionDocument16 pagesJournal Homepage: - : IntroductionIJAR JOURNALNo ratings yet
- Chemical Composition and Properties of Coconut WaterDocument8 pagesChemical Composition and Properties of Coconut WaterAmandeep Singh0% (1)
- ChemDocument10 pagesChemreadingchallenge jnvsklmNo ratings yet
- IJPPR, Vol 7, Issue 4, Article 23Document8 pagesIJPPR, Vol 7, Issue 4, Article 23madhujayarajNo ratings yet
- 4.4 Antibiotics IV To Oral Switch Guidelines For Pharmacists Southern HealthDocument5 pages4.4 Antibiotics IV To Oral Switch Guidelines For Pharmacists Southern HealthditaokkyNo ratings yet
- Bioequivalence Study of Carbamazepine Tablets: in Vitro/in Vivo CorrelationDocument8 pagesBioequivalence Study of Carbamazepine Tablets: in Vitro/in Vivo CorrelationFahmy SanjayaNo ratings yet
- Detection of 1-Hydroxypyrene Glucoronide Using ElisaDocument9 pagesDetection of 1-Hydroxypyrene Glucoronide Using ElisaPopoola OpeyemiNo ratings yet
- Effect of Sodium Metabisulphite On Blood Metabolic Status of Wistar RatsDocument10 pagesEffect of Sodium Metabisulphite On Blood Metabolic Status of Wistar Ratsام محمدNo ratings yet
- CHEMISTRYDocument14 pagesCHEMISTRYruhani.bhagat2020No ratings yet
- Acyclovir CreamDocument6 pagesAcyclovir CreamMuqeet KazmiNo ratings yet
- Aquaponics Systems, Fish. Volume 5: Sistemas de acuaponíaFrom EverandAquaponics Systems, Fish. Volume 5: Sistemas de acuaponíaNo ratings yet
- Aquaponics For Everyone: The complete guide to easy aquaponic gardening at home!From EverandAquaponics For Everyone: The complete guide to easy aquaponic gardening at home!Rating: 5 out of 5 stars5/5 (1)
- Aquaponics for Homeowners: Setup, Water Quality, Plant and Fish Selection, System Maintenance, and Organic Food Production: Sustainable Living and Gardening, #4From EverandAquaponics for Homeowners: Setup, Water Quality, Plant and Fish Selection, System Maintenance, and Organic Food Production: Sustainable Living and Gardening, #4No ratings yet
- Capital BudgetingDocument18 pagesCapital BudgetingShafaet Ara MoonNo ratings yet
- Dekha Na Dekha by Humayun AhmedDocument66 pagesDekha Na Dekha by Humayun AhmedAkidulIslamNo ratings yet
- Chayabithi by Humayun AhmedDocument122 pagesChayabithi by Humayun AhmedRayhanMohamedNo ratings yet
- Basanta Bilap - Humayun Ahmed (Dobd99) PDFDocument158 pagesBasanta Bilap - Humayun Ahmed (Dobd99) PDFShafaet Ara MoonNo ratings yet
- Amader Shada Bari by Humayun AhmedDocument41 pagesAmader Shada Bari by Humayun Ahmedjahid069No ratings yet
- Aaj Dupure Tomar Nimantron by Humayun Ahmed (2009) PDFDocument110 pagesAaj Dupure Tomar Nimantron by Humayun Ahmed (2009) PDFShamsul ArefinNo ratings yet
- Humayun Ahmed's Novel AnyodinDocument33 pagesHumayun Ahmed's Novel AnyodinJesanKamalNo ratings yet
- Brishti O Meghomala by Humayun AhmedDocument115 pagesBrishti O Meghomala by Humayun AhmedShafaet Ara MoonNo ratings yet
- General Manager Marketing and Communications April 2013Document7 pagesGeneral Manager Marketing and Communications April 2013Shafaet Ara MoonNo ratings yet
- Bangladesh DisastersDocument25 pagesBangladesh DisastersShafaet Ara MoonNo ratings yet
- HTTP WWW - BdbanglaDocument2 pagesHTTP WWW - BdbanglaShafaet Ara MoonNo ratings yet
- Krittika Takiar PDFDocument2 pagesKrittika Takiar PDFSudhakar TomarNo ratings yet
- DC Rebirth Reading Order 20180704Document43 pagesDC Rebirth Reading Order 20180704Michael MillerNo ratings yet
- 04 - JTC Template On Project ProposalDocument10 pages04 - JTC Template On Project Proposalbakelm alqamisNo ratings yet
- How Should Management Be Structured British English StudentDocument7 pagesHow Should Management Be Structured British English Studentr i s uNo ratings yet
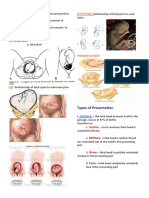
- The 4Ps of Labor: Passenger, Passageway, Powers, and PlacentaDocument4 pagesThe 4Ps of Labor: Passenger, Passageway, Powers, and PlacentaMENDIETA, JACQUELINE V.No ratings yet
- Module 1 in Contemporary Arts First MonthDocument12 pagesModule 1 in Contemporary Arts First MonthMiles Bugtong CagalpinNo ratings yet
- State of Indiana, County of Marion, SS: Probable Cause AffidavitDocument1 pageState of Indiana, County of Marion, SS: Probable Cause AffidavitIndiana Public Media NewsNo ratings yet
- Ecomix RevolutionDocument4 pagesEcomix RevolutionkrissNo ratings yet
- RQQDocument3 pagesRQQRazerrdooNo ratings yet
- Analysis of Cocoyam Utilisation by Rural Households in Owerri West Local Government Area of Imo StateDocument11 pagesAnalysis of Cocoyam Utilisation by Rural Households in Owerri West Local Government Area of Imo StatePORI ENTERPRISESNo ratings yet
- AIA Layer Standards PDFDocument47 pagesAIA Layer Standards PDFdanielNo ratings yet
- Management of Dyspnoea - DR Yeat Choi LingDocument40 pagesManagement of Dyspnoea - DR Yeat Choi Lingmalaysianhospicecouncil6240No ratings yet
- Expectations for Students and ParentsDocument15 pagesExpectations for Students and ParentsJasmine TaourtiNo ratings yet
- Introduction To ResearchDocument5 pagesIntroduction To Researchapi-385504653No ratings yet
- North South University: AssignmentDocument14 pagesNorth South University: AssignmentRakib HasanNo ratings yet
- Entrepreneurship - Quarter 2 - Week 1-3 - 4 M's of Production and - Business ModelDocument6 pagesEntrepreneurship - Quarter 2 - Week 1-3 - 4 M's of Production and - Business ModelJude Del RosarioNo ratings yet
- B767 WikipediaDocument18 pagesB767 WikipediaxXxJaspiexXx100% (1)
- WritingSubmission OET20 SUMMARIZE SUBTEST WRITING ASSESSMENT 726787 40065 PDFDocument6 pagesWritingSubmission OET20 SUMMARIZE SUBTEST WRITING ASSESSMENT 726787 40065 PDFLeannaNo ratings yet
- Keyboard notes to Fur Elise melodyDocument2 pagesKeyboard notes to Fur Elise melodyReji SarsalejoNo ratings yet
- IkannnDocument7 pagesIkannnarya saNo ratings yet
- Instafin LogbookDocument4 pagesInstafin LogbookAnonymous gV9BmXXHNo ratings yet
- Polystylism in The Context of Postmodern Music. Alfred Schnittke's Concerti GrossiDocument17 pagesPolystylism in The Context of Postmodern Music. Alfred Schnittke's Concerti Grossiwei wuNo ratings yet
- Drama For Kids A Mini Unit On Emotions and Character F 2Document5 pagesDrama For Kids A Mini Unit On Emotions and Character F 2api-355762287No ratings yet
- Mobile Services Tax Invoice for Dr Reddys LaboratoriesDocument3 pagesMobile Services Tax Invoice for Dr Reddys LaboratoriesK Sree RamNo ratings yet
- Budgetary Control NumericalDocument8 pagesBudgetary Control NumericalPuja AgarwalNo ratings yet
- Zeal Study 10th English Synonym Unit 1 - 7Document24 pagesZeal Study 10th English Synonym Unit 1 - 7viaanenterprises2008No ratings yet
- KINGS OF TURKS - TURKISH ROYALTY Descent-LinesDocument8 pagesKINGS OF TURKS - TURKISH ROYALTY Descent-Linesaykutovski100% (1)
- Eng 188 Change in Ap, Ae, and ErrorsDocument5 pagesEng 188 Change in Ap, Ae, and Errorsmkrisnaharq99No ratings yet