Professional Documents
Culture Documents
CDPCEUT00038
Uploaded by
Siddhant Yadav0 ratings0% found this document useful (0 votes)
144 views156 pagesmiconazole emulgel
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentmiconazole emulgel
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
144 views156 pagesCDPCEUT00038
Uploaded by
Siddhant Yadavmiconazole emulgel
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 156
i
STUDIES ON LECITHIN-MICROEMULSION BASED
ORGANOGELS AS CARRIERS FOR TOPICAL
DELIVERY OF MICONAZOLE NITRATE
By
MD.MUQTADAR AHMED
Reg. No. 04PU254
Dissertation Submitted to the
Rajiv Gandhi University of Health Sciences, Karnataka, Bangalore
In partial fulfillment
of the requirements for the degree of
MASTER OF PHARMACY
in
PHARMACEUTICS
Under the Guidance of
Dr.MOHAMED HASSAN DEHGHAN
M.Pharm., Ph.D.
DEPARTMENT OF PHARMACEUTICS
LUQMAN COLLEGE OF PHARMACY,
GULBARGA-585 102
APRIL 2006
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
ii
RAJIV GANDHI UNIVERSITY OF HEALTH SCIENCES,
KARNATAKA, BANGALORE
Declaration By The Candidate
I hereby declare that this dissertation/ thesis
entitled STUDIES ON LECITHIN-MICROEMULSION
BASED ORGANOGELS AS CARRIERS FOR TOPICAL
DELIVERY OF MICONAZOLE NITRATE is a bonafide
and genuine research work carried out by me under the
guidance of Dr.Mohamed Hassan Dehghan.
Date:
Place: GULBARGA MD.MUQTADAR AHMED
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
iii
Certificate By The Guide
This is to certify that the dissertation entitled
STUDIES ON LECITHIN-MICROEMULSION BASED
ORGANOGELS AS CARRIERS FOR TOPICAL
DELIVERY OF MICONAZOLE NITRATE is a bonafide
research work done by Mr.MD.MUQTADAR AHMED
in partial fulfillment of the requirement for the degree of
MASTER OF PHARMACY in PHARMACEUTICS.
Date:
Place: GULBARGA Dr.Mohamed Hassan Dehghan
M.Pharm. Ph.D.
Hon. Research Guide
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
iv
RAJIV GANDHI UNIVERSITY OF HEALTH SCIENCES,
KARNATAKA, BANGALORE
ENDORSEMENT BY THE HOD, PRINCIPAL/
HEAD OF THE INSTITUTION
This is to certify that the dissertation entitled
STUDIES ON LECITHIN-MICROEMULSION BASED
ORGANOGELS AS CARRIERS FOR TOPICAL
DELIVERY OF MICONAZOLE NITRATE is a bonafide
research work done by Mr.MD.MUQTADAR AHMED under
the guidance of DR.MOHAMED HASSAN DEHGHAN.
Date:
Place: GULBARGA Prof.Syed Sanaullah
Principal,
Luqman College of Pharmacy,
Gulbarga-585102
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
v
COPYRIGHT
Declaration By The Candidate
I here by declare that the Rajiv Gandhi University of
Health Sciences, Karnataka shall have the rights to
preserve, use and disseminate this dissertation/ thesis in
print or electronic format for academic/ research purpose.
Date:
Place: GULBARGA Mr.MD.MUQTADAR AHMED
Rajiv Gandhi University of Health Sciences, Karnataka
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
vi
ACKNOWLEDGEMENT
I am especially deeply indebted to my honourable research guide
Dr.Mohammed Hassan Dehghan for the constant guidance,
encouragement and support, which I have received from him whole-
heartedly. I substituted for the feeling of gratitude, indebtedness and
appreciation for him who brought me from darkness to lightness in my
life, which I would never forge Thank You Sir.
It is my privilege to express my heartfelt thanks to Prof.Syed Sanaullah,
Principal, Luqman College of Pharmacy for allowing me to use all the
facilities of the college and support me like a pillar for constant
inspiration and guidance as environment required.
I am most thankful to Dr.Syed Rahmatullah, General Secretary,
Vocational Education Society, Gulbarga for his silent encouragement.
My sincere thanks to Dr.Mujeeb and Mr.Abdul Majeed, President,
Vocational Education Society, Gulbarga for providing me all the facilities.
I honestly acknowledge Prof.M.A.Saleem, Prof.S.S.Bushetti, Prof.Syeda
Humera, Prof.Divakar, Mr.M.H.Hugar, Mr.Jafar, Mr.Najmuddin,
Prof.Satyanandam and Mr.Omar Khan and other teaching staff of
Luqman College of Pharmacy, Gulbarga for their timely encouragement
during my entire P.G. course.
A very special thanks to Mr.Zahid, Lecturer, Y.B.Chauhan College of
Pharmacy, Aurangabad.
My heartfelt gratitude and sincere thanks to my teachers Mr.Rajesh
Patwari, Mr.Shaikh Bahadur, Dr.Mazhar Farooqui and Mr.Sadat Ali
for their timely suggestions and perspective guidance in enriching my
knowledge.
I place on record a respectful thanks to Dr.M.G.Purohit, Department of
Pharmaceutical Chemistry, Gulbarga University, Gulbarga for his
guidance in my analytical work.
A special thanks to M/s.Adelphi A/c Sigma Labs, Goa for providing drug
sample of Miconazole Nitrate.
I also thanks Mr.Sridhar, Sipra Labs, Hyderabad for IR analytis of all
formulations, the Librarians of NIN, O.U., IICT, Hyderabad for their kind
help during literature survey.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
vii
I could never forget (Mrs).Ayesha, (Mrs).Florence for the inspiration,
encouragement and moral support during the course of my studies.
It gives me immense pleasure to record my sincere thanks to my
colleagues and friends Abdul Muqtadir, Asif, Aleem, Vinod Singh,
Taher, Imran, Anant Kulkarni, Nagashesha R, Mohan VK, Masood,
Rizwan, Saleem, Mir Imran, Sajid, Ilyaz, Vijay, Imtiyaz, Manish Kumar
Mital, Fazil, Shad, Noor, Shahid, Sarim, Abhisek, Wali, Areefulla H. for
helping me in carrying out this work.
It gives me immense pleasure to record my thanks to my seniors Faisal,
Azim, Riyaz, Ismail Mauzam for their guidance during work.
I express my thnaks to non-teaching staff Asad, Pasha, Naveed, Narender,
Hassan and Librarian Rubina Anjum and Pratibha for their help and
cooperation.
I am thankful to Micro Computers, Gulbarga for their cooperation during
the time of typing of this research work.
In closing I have reserved my special and most grateful thanks to one who
has not only initiated my interest in this work but has also led me through
the dark alley and abyssess to the brighten path. I am referring to none
other than my seniors Mr.Abdullah Khan and Mr.Md.Jafar who worked
for my success thank you.
The presence of the Almighty God was felt by me during my research
work. I am thankful to the Supreme energy for manifesting Himself
through the various helpful people, I came across in my life. I bow my
head to Him and ask for His blessings to be with me forever.
And above all words fails to express my feelings to my Parents and my
Family whose initiation, constant source of inspiration and
encouragement throughout my life.
Date:
Place: Gulbarga Md.Muqtadar Ahmed
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
viii
LIST OF ABBREVIATIONS USED
..................... micro
%.................... Percentage
BP................... British Pharmacopoeia
cm................... centimeter
CPs................. centipoise
F
127
................. Pluronic
fig................... figure
gm.................. gram
hrs................... hour
ICH................. International Conference for Hormonization
IP.................... Indian Pharmacopoeia
IPM................. Isopropyl myristate
IR.................... infrared
Km.................. weight ratios
LOs................. Lecithin organogels
MBGs............ Microemulsion based organogels
mcg/g............ microgram(s)
mg.................. milligram(s)
min................. minute(s)
ml ................... milliliter
mol-wt............ Molecular weight
nm.................. nanometer
NTU ............... Nephaloturbidity unit
PLO................ Pluronic lecithin organogels
rpm................. Revolution per minute
sec.................. Second(s)
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
ix
Sq-cm............. Square centimeter
Std.................. Standard(s)
USP................ United States Pharmacopoeia
UV.................. Ultraviolet
w/w................. Weight per weight
Wt................... Weight
P..................... Penicillin chrysogenum
C..................... Candida albicans
A..................... Aspergillus niger
T..................... AM
1
Formulation
F..................... FM
1
Formulation
I ...................... SR
1
Formulation
P..................... MN
1
Formulation
Mkd................ Marketed
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
x
LIST OF FORMULATION CODE
AM
1
....................Lecithin 7.54%, tween-80 3.77%, isopropyl myristate 30.16%,
water 56.56%, miconazole nitrate 2%
AM
2
....................Lecithin 20.63%, tween-80 10.31%, isopropyl myristate 30.95%,
water 36.11%, miconazole nitrate 2%
AM
3
....................Lecithin 35.43%, tween-80 1.77%, isopropyl myristate 11.81%,
water 21.26%, miconazole nitrate 2%
AM
4
....................Lecithin 56.02%, tween-80 28.01%, isopropyl myristate 14.00%,
water 28.01%, miconazole nitrate 2%
FM
1
.....................Lecithin 7.54%, isopropyl myristate 30.16%, pluronic 30% in
water 60.32, miconazole nitrate 2%
FM
2
.....................Lecithin 20.63%, isopropyl myristate 26.73%, pluronic 30% in
water 53.47%, miconazole nitrate 2%
FM
3
.....................Lecithin 35.43%, isopropyl myristate 24.50%, pluronic 30% in
water 36.76%, miconazole nitrate 2%
FM
4
.....................Lecithin 56.02%, isopropyl myristate 14.00%, pluronic 30% in
water 28.01%, miconazole nitrate 2%
SR
1
......................Lecithin 8.9%, IPM 35.1%, water 53.5%, miconazole 2%
SR
2
......................Lecithin 26.22%, IPM 30.33%, water 47.5%, miconazole 2%
SR
3
......................Lecithin 43.8%, IPM 28.8%, water 25.95%, miconazole 2%
SR
4
......................Lecithin 60.33%, IPM 15.08%, water 22.63%, miconazole 2%
MN
1
....................Lecithin 13.43%, paraffin 53.78%, water 30.88%, miconazole 2%
MN
2
....................Lecithin 31.6%, paraffin 47.4%, water 18.99%, miconazole 2%
MN
3
....................Lecithin 49.0%, paraffin 32.67%, water 16.3%, miconazole 2%
MN
4
....................Lecithin 67.61%, paraffin 16.90%, water 13.52%, miconazole 2%
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xi
ABSTRACT
In the present study lecithin-microemulsion based organogels were formulated as
topical carrier for miconazole nitrate, an antifungal drug practically insoluble in
water. The organogels were prepared by two different methods employing
lecithin, lecithin in combination with Tween-80 and lecithin with pluronic,
isopropyl myristate(IPM) was used as organic solvent, whereas gelation was
achieved on addition of aqeuous phase. Organogels using lecithin in liquid
paraffin and water were also prepared by hot-melt method. Increase in the
amount of lecithin resulted in organogels with higher viscosity and lower
spreadability. MN
4
formulation showed maximum viscosity 22868 CPs, whereas
AM
1
formulation containing Tween-80 (3.77%) was least viscous 15726 CPs.
Organogels with higher viscosity were found to be more stable. Organogels
containing lecithin and Tween-80 were the most stable. AM
4
showed highest gel
life of 220 hours at 25C. 28.5 gm% of water was required to produce maximum
gelation in SR
1
organogels containing lecithin and IPM. Organogels produced by
hot melt method required least amount of water for maximum gelation 9.09 gm%.
In vitro release of miconazole nitrate from organogels showed that organogels
containing lecithin and Tween-80 in IPM (AM
1
) gave highest release. The order
of release for the best releasing formulation prepared by different methods was
AM
1
>FM
1
>SR
1
>MN
1
. In vitro antifungal activity of miconazole nitrate
organogels against Candida albicans, P.chrysogenum, A.niger was in the order
AM
1
>FM
1
Mkd>SR
1
>MN
1
. In vitro antifungal activity significantly correlated
with in vitro release of miconazole nitrate. Lecithin microemulsion based
organogels have good potential as carrier for topical delivery of antifungal agent
such as miconazole nitrate.
Keywords: Lecithin organogels; Miconazole nitrate; In vitro release, In vitro anti-fungal
activity.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xii
TABLE OF CONTENTS
LIST OF TABLES........................................................... xiv-xv
LIST OF FIGURES.............................................................. xvi
CHAPTER-1 INTRODUCTION............................................................ 01-29
1.1 Conventional Technologies.......................................... 01
1.2 Newer Technologies.................................................... 02
1.3 Topical Dosage Forms................................................. 04
1.4 Historical Overview..................................................... 06
1.5 Skin as a Route of Topical and Transdermal Drug
Delivery System........................................................... 07
1.6 Fungal Infection........................................................... 11
1.7 Rational Approach to Drug Delivery to and Via
Skin............................................................................. 14
1.8 Utilization of Different Gels as Topical Vehicles......... 15
1.9 Emulsion Gels as Topical Formulations....................... 15
1.10 Organogels................................................................... 16
1.11 Lecithin Organogels An Overview............................ 19
1.12 Method of Preparation................................................. 23
1.13 Characterization of Organogels.................................... 24
1.14 In Vitro Drug Release.................................................. 25
1.15 Topical Drug Delivery Applications of Lecithin
Organogel Based System........................................... 26
1.16 Safety and Skin Compatibility...................................... 28
1.17 Future Prospects........................................................... 28
CHAPTER-2 OBJECTIVES .................................................................. 30-32
2.1 Need for the Study....................................................... 30
2.2 Objective of the Study.................................................. 31
2.3 Scheme of the Work..................................................... 31
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xiii
CHAPTER-3 REVIEW OF LITERATURE.......................................... 33-58
3.1 Review of Literature.................................................... 33
3.2 Drug Profile................................................................. 42
3.3 Polymer Profile............................................................ 50
CHAPTER-4 METHODOLOGY........................................................... 59-69
4.1 Raw Material Characterization..................................... 61
4.2 Preparation of Miconazole Nitrate Loaded Lecithin
Microemulsion based Organogels................................ 61
4.3 Construction of Calibration Curve of Miconazole
Nitrate.......................................................................... 64
4.4 Evaluation of Lecithin-Microemulsion based
Organogels................................................................... 65
CHAPTER-5 RESULTS ....................................................................... 70-115
5.1 Standardization of Raw Materials............................ 70-79
5.2 Preparation of Lecithin based Organogels.................... 80
5.3 Construction of Curve of Miconazole Nitrate............... 80
5.4 Evaluation of Lecithin-Microemulsion based
Organogels............................................................ 82-115
CHAPTER-6 DISCUSSION ............................................................... 116-119
CHAPTER-7 CONCLUSION............................................................. 120-121
CHAPTER-8 SUMMARY.................................................................. 122-124
BIBLIOGRAPHY......................................................... 125-134
ANNEXURE
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xiv
LIST OF TABLES
Sl.
No.
Table
No.
Title
Page
No.
1. 1.1 Major Lipids of the Stratum Corneum 10
2. 4.2.1 Preparation of Organogels Loaded with Miconazole Nitrate 62
3. 4.2.2 Preparation of Pluronic Lecithin Organogels 63
4. 4.2.3 Preparation of Miconazole Nitrate Loaded Organogels 63
5. 4.2.4 Preparation of Hot Melt Type Organogels 64
6. 5.1.1 Standardization of Miconazole Nitrate 70
7. 5.1.2a Standardization of Lecithin 72
8. 5.1.2b Standardization of tween 80 72
9. 5.1.2c Standardization of pluronic (poloxames) 75
10. 5.1.2d Standardization of Isopropyl myristate 75
11. 5.1.2e Standardization of Lipid Paraffin 78
12. 5.3 Calibration curve of Miconazole Nitrate 80
13. 5.4 Physical Evaluation of Formulations 82
14. 5.4.1a Gelation Kinetics AM
1
83
15. 5.4.1b Gelation Kinetics AM
2
84
16. 5.4.1c Gelation Kinetics AM
3
85
17. 5.4.1d Gelation Kinetics AM
4
86
18. 5.4.2a Gelation Kinetics FM
1
88
19. 5.4.2b Gelation Kinetics FM
2
89
20. 5.4.2c Gelation Kinetics FM
3
90
21. 5.4.2d Gelation Kinetics FM
4
91
22. 5.4.3a Gelation Kinetics SR
1
93
23. 5.4.3b Gelation Kinetics SR
2
94
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xv
Sl.
No.
Table
No.
Title
Page
No.
24. 5.4.3c Gelation Kinetics SR
3
95
25. 5.4.3d Gelation Kinetics SR
4
96
26. 5.4.4a Gelation Kinetics MN
1
98
27. 5.4.4b Gelation Kinetics MN
2
98
28. 5.4.4c Gelation Kinetics MN
3
99
29. 5.4.4d Gelation Kinetics MN
4
99
30. 5.4.5 Gelatin Kinetics Data 101
31. 5.4.6 Percentage drug contents of Organogels 102
32. 5.4.7a In vitro percentage drug release of formulations AM
1
, AM
2
,
AM
3
and AM
4
103
33. 5.4.7b In vitro percentage drug release of formulations FM
1
, FM
2
, FM
3
and FM
4
105
34. 5.4.7c In vitro percentage drug release of formulations SR
1
, SR
2
, SR
3
and SR
4
107
35. 5.4.7d In vitro percentage drug release of formulations MN
1
, MN
2
,
MN
3
and MN
4
109
36. 5.4.8 In Vitro Antifungal Activity of Miconazole Nitrate Organogels 111
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xvi
LIST OF FIGURES
Sl.
No.
Figure
No.
Title
Page
No.
1. 1.1 Structure of Skin 8
2. 5.1.1 IR Spectra of Miconazole Nitrate 71
3. 5.1.2a IR Spectra of Lecithin 73
4. 5.1.2b IR Spectra of Tween-80 74
5. 5.1.2c IR Spectra of Pluronic 76
6. 5.1.2d IR Spectra of Isopropyl Myristate 77
7. 5.1.2e IR Spectra of Liquid Paraffin 79
8. 5.3 Calibration curve of miconazole nitrate 81
9. 5.4.1 Effect of addition of water on turbidity of microemulsion based
organogel of formulations AM
1
, AM
2
, AM
3
& AM
4
87
10. 5.4.2 Effect of addition of water on turbidity of microemulsion based
organogel of formulations FM
1
, FM
2
, FM
3
& FM
4
92
11. 5.4.3 Effect of addition of water on turbidity of microemulsion based
organogel of formulations SR
1
, SR
2
, SR
3
& SR
4
97
12. 5.4.4 Effect of addition of water on turbidity of microemulsion based
organogel of formulations MN
1
, MN
2
, MN
3
& MN
4
100
13. 5.4.7a In vitro percentage drug release of formulations AM
1
, AM
2
,
AM
3
and AM
4
104
14. 5.4.7b In vitro percentage drug release of formulations FM
1
, FM
2
, FM
3
and FM
4
106
15. 5.4.7c In vitro percentage drug release of formulations SR
1
, SR
2
, SR
3
and SR
4
108
16. 5.4.7d In vitro percentage drug release of formulations MN
1
, MN
2
,
MN
3
and MN
4
110
17. 5.4.8 Comparative Antifungal Activity of Selected Miconazole
Nitrate Organogel Formulations with Control
111
18. 5.4.9a IR Spectra of AM
1
Organogel 112
19. 5.4.9b IR Spectra of FM
1
Organogel 113
20. 5.4.9c IR Spectra of SR
1
Organogel 114
21. 5.4.9d IR Spectra of MN
1
Organogel 115
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
xvii
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
1
CHAPTER1
INTRODUCTION
With the advent of high throughput screening techniques the discovery of
biologically active molecules is taking place at a pace never seen before. Most of the
chemical entities that are being discovered are lipophilic in nature and have poor
aqueous solubility, there by posing problems in their formulation into delivery
systems. Because of their low aqueous solubility and high permeability, dissolution
and/or release rate from the delivery system forms the rate-limiting step in their
absorption and systemic availability. More than 60% of potential drug products
suffer from poor water solubility. This frequently results in potentially important
products not reaching the market or not achieving their full potential. Pharmaceutical
industry is quick in realizing the importance of solubility and dissolution rate in
bioavailability and good deal of research has been done in this area. Currently a
number of technologies are available to address the poor solubility, dissolution rate
and bioavailability of insoluble drugs
1
.
1.1 CONVENTIONAL TECHNOLOGIES
Conventionally used techniques
2
based on Noyes-Whitney equation
3
for
enhancing solubility, dissolution rate and thereby bioavailability of insoluble drugs
include buffered tablets, use of salts, solvates and hydrates, polymorphic forms,
complexation, prodrugs, micronisation, solid dispersions and solvent deposited
systems.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
2
1.2 NEWER TECHNOLOGIES
Newer and novel drug delivery technologies developed in recent years for
bioavailability enhancement of insoluble drugs are described below.
1.2.1 LIPID BASED DELIVERY SYSTEMS
1. Lipid emulsion technology
4
.
2. Self-emulsifying drug delivery system
5
.
3. Micro emulsion media as novel drug delivery system
6
.
1.2.1.1 MICRO EMULSION SYSTEM
6, 7
:
Microemulsions are four component mixtures composing of an oil phase, a
water phase surfactant/s and a co-surfactant. The tendency towards formation of w/o
or o/w microemulsions is dependent on the properties of the oil and the surfactant, the
water-to-oil-ratio and the temperature. When a mixture of surfactant and co-
surfactant is added to a biphasic oil-water system, a thermodynamically stable,
optically transparent or translucent, low viscosity and isotropic mixture spontaneously
forms. The transparency of these systems arises from their small droplets
diameter(10-100 nm). Such small droplets produce only weak scattering of visible
light when compared with that from the coarse droplets (0.5-100 m) of traditional or
standard macroemulsions such as emollient liquids, cream, lotions, etc., Structurally,
microemulsions have normal micellar solutions, reverse micelles, cores or droplets of
water or oil, and, for some systems, even bicontinuous structures could solubilize
large amounts of both oil and water soluble drugs within microemulsions.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
3
There is rather confusing situation in the medical literatures, where the term
microemulsion is indifferently used to indicate systems of presumably unlike
structure (true microemulsions and miniemulsions). Some studies have compared
the performance of different emulsified systems (macroemulsions, microemulsion,
multiple emulsion and gel-emulsions) prepared with similar oils and surfactants for
applications such as controlled drug release or drug protection.
The surfactants used to stabilized such systems may be (i) Non-ionic (ii)
Zwitterionic (iii) Cationic (iv) Anionic surfactants. Combinations of these,
particularly ionic and non-ionic, can be very effective at increasing the extent of the
microemulsion region.
Advantages:
Advantages associated with microemulsions include their thermodynamic
stability, optical clarity and ease of preparation.
Applications
6
:
a. Oral delivery
b. Parentral delivery
c. Pulmonary delivery
d. Ocular delivery
e. Topical delivery
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
4
1.3 TOPICAL DOSAGE FORM:
Topical dosage forms are those which are applied to the skin. These
preparation are applied to the skin either for their physical effects, that is for their
ability to act as skin protectants, lubricants, emollients, drying agents, etc. or for their
specific effect of medicinal agents present. Preparations sold over the country
frequently contain mixtures of medicinal substance used in the treatment of such
condition as minor skin infection, itching, bruise, acne, psoriasis and eczema. Skin
application, which require a prescription generally contain a single medicinal agent
intended to counter a specific diagnosed condition
8
.
Topical dosage forms have been used since very ancient times. The
application of medicinal substance to skin or to various body orifices is a concept as
old as humanity. Various ointments, creams, gels, lotions, pastes, powders and
plasters have been used for many years
9
.
The primary topical drug delivery systems (TDDS) is that they could provide
controlled constant administration of a medicament by simple application to the skin
surface.
1.3.1 Advantages of Topical Systems
10
:
1. They are of least therapeutic interest but of practical relevance is good patient
compliance. The systems are easy to apply and remove. It avoids risks and
inconveniences associated with intravenous therapy.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
5
2. They eliminate the variables, which influences gastrointestinal absorption
such as food intake, stomach emptying, intestinal motility and transit time.
3. Produces sustained and controlled level of drug in plasma thus reduces the
chance of over or under-dosing.
4. Reduces frequency of drug dosing.
5. Topical systems are easily retractable thereby termination of drug input, if
toxic effects are observed.
6. Offers an alternative route when oral therapy is not possible as in case of
nausea and vomiting.
7. Helps in achievement of more constant blood levels with lower dosage of drug
by continuous drug input and by by-passing hepatic first-pass metabolism and
consequent degradation.
8. In certain circumstances, enzymatic transformation within epidermis may be
used to improve permeability of certain hydrophilic drugs when applied to the
skin in the form of prodrug.
1.3.2 Limitations of Topical Systems
11
:
1. Drugs with reasonable partition coefficient and possessing solubility both in
oil and water are most ideal, as drug must diffuse through lipophilic stratum
corneum and hydrophilic viable epidermis to reach the systemic circulation.
Only drugs, which are effectively absorbed by the percutaneous routes as such
or by using penetration promoters, can be considered.
2. The route is not suitable for drugs that irritate or sensitize the skin.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
6
3. The route is restricted by the surface area of delivery system and the dose that
needs to be administered in the chronic state of disease.
4. Topical drug delivery systems are relatively expensive compared to
conventional dosage forms. They may contain a large amount of drug, of
which only a small percentage may be used during the application period.
Apart from these limitations other problems include pharmacokinetics and
pharmacodynamic restrictions. Thus clinical need has to be examined carefully
before developing a TDDS.
1.4 HISTORICAL OVERVIEW:
With the advent of scientific medicine in the last half of the 19
th
century, this
route of administration fell out of favour. In 1877, Fliescher declared that the skin
was totally impermeable. This extreme view could not hold for long. By the turn of
the century, Schwenkenbecker perceived that the skin would admit some substances
much better than others. In 1957, Monash proved a superficially located barrier in
skin as an obstacle to penetration. Later Stoughton used simple methods to explore
the permeability behaviour of human skin. He brought to light many important facts
by applying substances, which cause some rapid physiologic display such as
blanching, reddening or sweating.
The most important efforts in establishing a theoretical foundation were from
works by Ireger, Blank and Scheuplein.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
7
These pioneering works were followed by extensive research ultimately
proving that the stratum corneum was the main barrier to percutaneous absorption and
that although it allows no substance to penetrate easily, it does allow all substances to
enter slightly
12
.
1.5 SKIN AS ROUTE OF TOPICAL AND TRANSDERMAL DRUG
DELIVERY:
The transdermal permeation of a chemical involves partitioning into and
transport through the cutaneous layers, namely the stratum corneum, the viable
epidermis (stratum basale) and the upper dermis
10
. A topical product is designed to
deliver the drug into the skin to treat dermal disorders and therefore skin is the target
organ. Non steady state transport generally characterizes a topical product. The skin
is a barrier to topically administered drugs
13,14
. Topical formulations usually contain
several excipients, which also partition into the skin according to their
physicochemical properties. Certain excipients change the integrity of stratum
corneum. Stratum corneum can exhibit swelling by water. Thus, the permeability of
drugs depends on the degree of hydration. Cosolvents may later the barrier properties
of the skin
3,15
. Some substances having considerable polarities also enhance the
permeability of the horney layer. It is known that use of oleaginous vehicles
enhances the skin permeation
10
.
Topical preparation applied to the skin may be designed for surface, local or
systemic offers. In order to understand these effects, a brief review of the skin
structure is provided
16,17
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
8
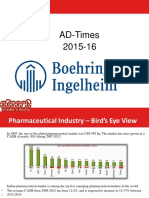
Figure-1.1: Structure of Skin
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
9
1.5.1 Relevant Anatomy and Microstructure:
The skin is one of the most extensive and readily accessible organs of the
human body. The skin of average adult covers over 20,000 cm of surface area and
receives overall of all blood circulation through the body. It is a multilayered organ
18
.
The skin is composed of three layers: the outer most is the epidermis, next is the
dermis and the innermost layer is the subcutaneous. The epidermis itself is composed
of the stratum corneum, horny layer (about 10 m thick), which is a layer of
compressed, overlapping keratinized cells that form a flexible, tough, coherent
membrane. This layer contain dead cells with keratin filaments in a matrix of
proteins with lipids and water-soluble substances. The epidermis is more resistant to
the diffusion of chemicals than other layers of the skin, infact, it forms the protective
barrier for the layer.
Below the epidermis is the dermis, a matrix of connective tissue,
approximately 4 mm thick, woven from fibrous proteins, which are embedded in an
amorphous ground substance of mucopolysaccharide. Nerves, blood vessel and
lymphatics are contained in the dermis. The innermost layer of skin is the
subcutaneous tissue, which contains adipose cells and collagen fibers. The eccrine
sweat glands produce sweat, empty on the skin surface and function to control heat
exchange
16
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
10
1.5.2 Biochemical Makeup of the Stratum Corneum:
Along with cellular maturation of the keratonocytes, there is also a remarkable
shaft of the lipid composition of the epidermal layers. In humans, the extracellular
motor consists of a structural complex containing several groups of lipids.
Table-1.1: Major lipids of the stratum corneum
17
Lipid type
Amount (weight
percent)
Polar lipids (phosphotidyl serine, choline, ethanol,
sphigmyelin)
4.9
Neutral lipids 64.6
Free sterols 14.0
Sterol esters 6.1
Free fatty acids 19.3
Triglycerides 25.2
Sphigolipids 18.1
Glycosphingolipids 2.6
Ceramides 15.5
*Abdominal region
1.5.3 Immunological Functions of Cells found in the Skin:
As the skin is increasingly understood as an immunological organ, the
immunologic functions of the skin has recently become an issue of considerable
concern. Termination of drug through the skin may be associated with immunologic
side effect
19
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
11
The immune response could have serious implications. In addition to contact
dermatitis, the immune response could also neutralize the drug activity.
1.6 FUNGI:
Although fungi causes varity of diseases to the skin few common diseases
along with their causitive organism are listed below.
Fungi and yeast constitute the eumycetes. They are eukaryotes with a
differentiated nucleous and rigid chitinous cell wall were formerly regarded as plant
without chlorophyll or differentiation of root stem and leaves. They are now regarded
as neither plant nor animals and are grouped with protozoa, slime moulds and most
algae on higher protista. They may be unicellular and multicellular. The cell show
various degree of specialization
21
.
Classification of Fungi
22
Fungi can be classified in two ways. Depending upon the cell morphology,
fungi can be divided into 4 classes viz., yeast, yeast like fungi< moulds and
dimorphic fungi.
Yeast:
Yeast are unicellular fungi, which occur as spherical or ellipsoidal cells are
reproduce by simple budding. On culture they form smooth, creamy, colonies. The
only pathogenic yeast is cryptococcus neoformus.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
12
Yeast like Fungi:
They grow partly as yeast and partly as elongated cells resembling hyphae.
The latter form as pseudomycelium, candida albicans in a pathogenic yeast like
fungus. On solid media most creamy coloured colonies are produced
23
.
Moulds (Filamentous mycelial fungi):
They grow as long as filamentus or hyphae, which branch and interlace to
form a meshwork or mycelium, the vegetative mycelium grows on and penetrate into
substrate absorbing nutrient for growth. This may become powdery on its surface due
to the abundant formation of spores e.g. ring worm fungi.
The Dimorphic Fungi:
They either as filamentus or as yeast, according to the culture condition.
Growth usually take place in the mycelial form on culture media at 22C and in the
soil but in the yeast form on media at 37C and in the animal body, Histoplasma
capsulatum is the most important of them.
Fungal Infection
23
:
Fungal infections are termed mycoses and is general can be divided into
superficial infections (affecting skin, nails, scalp or mucous membranes) and systemic
infections (affecting deeper tissue and organ).
Superficial fungal infections can be classified into the dermatomycoses and
candidiasis. Dermatomycoses are infection of the skin, hair and nails caused by
dermatophytes. The commonest are due to the tinea organism, which cause various
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
13
types of ring worm, tenia capitis affect the scalp. Tinea pedis causes atheletes foot.
In superficial candiasis, yeast like organism infect the mucous membrane of the
mouth, vagina or skin.
Some of the fungal infections are as follows:
Pityriasis versicolor:
Pityriasis versicolor (Tenia versicolor) is a chronic usually asymptomatic
involvement of stratum corneum characterized by multiple scalum in children discrete
or confluent macular areas of discoloration or depigmentation of the skin. The areas
involved are mainly the chest, abdomen, upper limb and back. Facial involvement is
common. The causative agent is a lipophilic yeast like fungus pityrosporum
orbiculare (Malassezia furfur).
Tinea Nigra:
Tinea nigra is localized infection of stratum corneum, particularly of palm,
producing black or brown macular lesion. It is found mainly in the tropics and is
caused by cladosporium wernickii (now designated as exophiala wernickii).
Piedra:
Piedra is a fungus infection of the hair, characterized by the appearance of
firm, irregular nodules along the hair shaft. Nodules are composed of fungus
filaments, cemented together on the hair. Two varieties of piedra are recognized
black piedra caused by piedra kortai and white piedra caused by trichosporon
beigelli.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
14
Candidiasis:
Candidiasis is an infection of the skin, mucosa and rarely of the internal organ
caused by a yeast like fungus candida albicans normally present in the mouth,
intestine and vagina
21
.
Vulvovaginal candidiasis is the infection with Candida albicans.
Approximately 75% of women have a vaginal infection with Candida strains during
their life and about 40-50% of them suffer a second one, and a small percentage
shows chronic cause
24
.
Sporotrichosis:
Sporotrichosis is caused by fungus sporothrix schenckii and is characterized
by the development of the skin in subcutaneous tissue and lymph node, of nodules
which soften and breakdown to form indolent ulcers.
Rhinosporidiosis:
Rhinosporidiosis is a chronic granulomatus disease characterized by the
development of friable polyps usually confined to nose, mouth and eye but rarely
seen in the genitalia or other mucous membrane. The causative fungus is
Rhinosporidium seebri.
1.7 RATIONAL APPROACH TO DRUG DELIVERY TO & VIA SKIN
20
:
There are three main ways to solve the problem of formulating a successful
topical dosage formulation:
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
15
1. We can manipulate the barrier function of the skin e.g., topical antibiotics and
antibacterials help a damaged barrier to ward-off infection, sunscreen agents and
the horny layer protect the viable tissue from ultraviolet radiation and emollient
preparations restore palatability to a desiccated horny layer.
2. We can direct drug to the viable skin tissue without using oral, systemic or other
route of therapy.
3. The third approach uses skin delivery for systemic treatment. For example,
topical drug delivery systems provide systemic therapy for conditions such as
motion sickness, angina and pain.
Dermatologists aim at five main target regionskin surface, horny layers,
viable epidermis and upper dermis, skin glands and systemic circulation.
1.8 UTILISATION OF DIFFERENT GELS AT TOPICAL VEHICLES
17
:
Gels have a variety of applications in the administration of medications orally,
topically, intranasally, vaginally and rectally.
1.9 EMULSION-GELS AS TOPICAL FORMULATIONS
17
:
Transdermal and topical formulations are becoming increasingly important
and their use in therapy is becoming more widespread. But the skin acts as a barrier
to topically administered drugs. Attempts have been made to circumvent the skin
barrier by several means, emulsion-gels being one such promising technique.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
16
1.10 ORGANOGELS:
The topical administration of drugs in order to achieve optimal cutaneous and
percutaneous drug delivery has recently gained an importance because of various
advantages such as ease of administration, non-invasive, better tolerated and
compliance, local enhanced transdermal delivery, avoidance of local gastrointestinal
toxicity, avoidance of first pass metabolism.
In search of a vehicle to deliver the medicament into the skin layer (cutaneous
delivery) or through the skin and into systemic circulation (percutaneous absorption)
and to target the skin, varied kind of formulation systems and strategies have been
evolved.
Amongst the many, the lipid-based formulations have been in use since
decades. Pharmaceutically, lipid emulsions may allow the sustained release of drugs
by sink mechanism
7
.
The importance of lipids has especially increased after realizing the utility of
phospholipids. The natural bio-friendly molecules which in collaboration with water
can form diverse type of polymolecular/ super molecular structure with retardant
release in sustained release formulation
25
.
The topical delivery has been attempted and made successful using a number
of lipid based systems viz., vesicular systems
26
, lipid microsphere, lipid
nanoparticles
1
, lipid emulsion
4
, polymeric gels
27
. In a recent development,
phospholipids in conjunction with some other additives have been shown to provide a
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
17
very promising topical drug delivery vehicle i.e., lecithin organogel. Lecithin
organogels (LOs) are thermodynamically stable, clear, viscoelastic, biocompatible
and isotropic gels composed of phospholipids (lecithin), appropriate organic solvent
and a polar solvent
28
. Lecithin organogel, the jelly like phase consists of three
dimensional network of entangled reverse cylindrical (polymer like) micelle, which
immobilize the continuous or macroscopic external organic phase, thus turning liquid
into a gel
25
. The formation of three-dimensional network in the organogel is the
result of transition at the micellar level in a low viscous network liquid consisting of
lecithin cause micelles in non-polar organic liquid
29
. This spherical reverse micellar
state of lipid aggregates, twins on to form elongated tubular micelles with the addition
of water, and subsequently entangle to form a temporal three dimensional network in
the solution bulk. The latter serves to immobilize the external organic phase, thus
producing a gel form or the jelly like state of the initial non-viscous solution.
However, the transparency and optical isotropy of the organogel remain as before.
For this reason, these systems are often called as polymer like micelles and are also
termed as living or equilibrium polymer, worm like or thread like micelles
25
.
1.10.1 Advantages of Organogels
28,30,31
:
Template vehicle: Lecithin organogels provide opportunities for incorporation of
wide range of substances with diverse physicochemical characters viz., chemical
nature, solubility, molecular weight, and size etc.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
18
Process Benefits: Spontaneity of organogel formation by virtue of self-assembled
supramolecular arrangement of surfactant molecules, makes the process very simple
and easy to handle.
Structural/ Physical Stability: Being thermodynamically stable, the structural
integrity of lecithin oranogels is maintained for longer time periods.
Chemical Stability: Lecithin organogels are moisture insensitive and being organic
in character also resist microbial contamination.
Topical Delivery Potential:
Being well balanced in hydrophilic and lipophilic character, they can efficiently
partition with the skin and therefore enhance the skin penetration and transport of
the molecules.
Lecithin organogels also provide the desired hydration of skin in a lipid-enriched
environment so as to maintain the bioactive state of skin.
Lecithin might influence the structure of the skin by disorganizing the lipid layer
in the stratum.
Safety: Use of biocompatible, biodegradable and non-immunogenic materials makes
them safe for long-term applications.
1.10.2 Limitations of Organogels:
In the lecithin organogels, the lecithin should be pure otherwise no gelling will
occur.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
19
Lecithin is most costly.
Lecithin is not available on large scale.
Should be stored in a proper condition.
The organogel has greasy property.
Less stable to temperature.
1.11 LECITHIN ORGANOGEL AN OVERVIEW:
The first description of lecithin organogels was given in an article published
by Scartazzini and Luisi in the year 1988
28
. In this study, water was added to various
organic solutions of purified soyabean lecithin. It was observed that addition of soy-
lecithin caused an abrupt rise in the viscosity (1010
4
times)
30
, producing a transition
of the initial non-viscous solution into gel of jelly like state. The amount of water
required to produce the gel was found to be critical. The phenomenon was observed
with various non-polar media and the list includes more than fifty solvents
28
.
By now, lecithin organogels have been studied extensively in many
laboratories worldwide with regard to their varied aspects such as formulation
component, formation and gelling mechanism, physicochemical properties, etc. and
have also been proposed as a matrix for topical drug delivery.
1.11.1 Organogelling Composition:
The organogel matrix chiefly consists of surfactant (lecithin) as gelation
molecules, a non-polar organic solvent as external or continuous phase and polar
agent, usually water. Lecithin is a trivial name for 1,2-diacyl-Sn-3-phosphocholine.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
20
It belongs to a biological essential class of substance termed phosphoglycerides or
phospholipids. The latter form the lipid matrix of biological membrane and also play
a key role in the cellular metabolism.
As a biocompatible surfactant, it is widely used in every day life including
human and animal food, medicine, cosmetics and manifold industrial applications
32
.
Synthetic lecithin containing residues of saturated fatty acids failed to form
organogel
28,30,33
. However, it has been established that unsaturation in phospholipid
molecules is a desired property for the formation of lecithin organogels.
Besides lecithin as gelation molecules, the role of organic solvent in providing
the desired solvent action into the gelatin molecules is much emphasized. A large
variety of organic solvent are able to form gel in the presence of lecithin. Among
them are linear, branched and cyclic alkenes, ethers and esters, fatty acids and
amines. Specific examples includes ethyl laurates, ethyl myristate, isopropyl
myristate (IPM), isopropyl palmitate (IPP), cyclopentane, cycloclane, trans-decalin,
trans-pinane, n-pentane, n-hexane, n-hexadecane nd tripropylamine
28
.
Amongst the above, the fatty acid esters i.e., application of lecithin
organogels. This has been attributed to their skin penetration enhancing property
besides their biocompatible and biodegradable nature
32,34
.
The third component of polar agent acts as a structure forming and stabilizing
agent and has a very crucial role to play in the process of gelling. Water is the most
commonly employed polar agent although some other polar solvents such as glycerol,
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
21
ethylene glycol and formamide have also been found to possess the capability of
transferring an initial non-viscous lecithin solution into jelly like state on organogel
25
.
As described earlier, the major limitation in formation of lecithin organogels
is the requirement of high purity lecithin, the high purity grade lecithin is not only
expensive but also difficult to obtain in large quantities. However, recent reports
indicates the incorporation of synthetic polymers i.e., pluoronic in lecithin
organogels, for their usefulness as cosurfactant and stabilizer
35
. It has been shown
that the inclusion of pluronic as cosurfactant makes the organogelling feasible with
lecithin of relatively lesser purity
36
. The term pluronic refers to series of non-ionic
closely related block copolymers of ethylene oxide and propylene oxide
32
. Also
known as poloxamers, poloxamer polyols or Lutrol
. These are primarily used in
pharmaceutical formulations as co-surfactants, emulsifier, solubilizers, suspending
agents and stabilizers. These pluronic containing lecithin organogels have been
termed as pluronic lecithin organogels, poloxamer organogels, pluronic organogels,
PLO gel or simply PLOs.
1.11.2 Phase-behavior of organogels:
Sameles containing different weight ratios (k
m
) of lecithin/IPM (20:80)
(40:60) (60:40) (80:20) were prepared, phase studies were carried out by adding
water while stirring. After each addition of 1 liter of aqueous phase of pure water to
the lecithin solutions, the resulting systems were examined for clarity and viscosity.
The course of each addition was monitored through cross polaroids in order to
determine the boundaries of any organogel and briefringent liquid crystalline
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
22
domains. The endpoint of the organogel domain at a given k
m
was determined when
the system became turbid after the addition of a specific amount of water. The phase
behavior of the systems was mapped on phase diagrams with the top apex
representing the lecithin and the other apices representing IPM and water solution.
The transparent, homogeneous, nonbirefringent area enclosed by the line connecting
the endpoints was considered as microemulsion based organogel
37
.
1.11.3 Organogel structure and mechanism of organogelling
37
:
The initially spherical reverse micelles that are formed by lecithin molecules
in a nonpolar organic solvent transform into cylindrical micelles, once water is added.
This was established with the help of light scattering and small angle neutron
scattering techniques. This one dimensional growth of micelles is caused by the
formation of hydrogen bonds between water molecules and phosphate groups of
lecithin molecules so that two adjacent lecithin molecules are bridged together by one
water molecule IR and NMR spectroscopic methods have revealed that water
molecules could interact simultaneously with phosphate groups of neighboring lipid
molecules via hydrogen bonding, acting as a bridge between them. In this case
solvent molecules and lecithin phosphate groups can arrange in such a way that a
hydrogen-bonded network will be formed. The increase in the amount of water
results in the formation of long tubular and flexible micelles. These micelles can be
entangled and therefore build up a transient three-dimensional network, that is
responsible for the viscoelastic properties of the lecithin organogels. At a critical
concentration of water, network shrinks and phase separation occurs. At still higher
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
23
contents of water a transformation to a solid, nontransparent precipitate can be
observed. This diluted solution is composed of rod-like micelles, which their length
is not enough to overlap and form a three-dimensional network. The existence of
microdomains of different polarity within the same single-phase solution enables
water-soluble and oil-soluble drugs to be incorporated. Results have shown that for a
given system, as k
m
increases, the incorporation capacity increases. This could be
attributed either to the increase in the number of cylindrical micelles or to the further
growth of the cylindrical micelles or both, leading to the increase in the solubilizing
capacity.
1.12 METHOD OF PREPARATION:
The oil-surfactant mixture was heated at 60C to obtain a clear solution which
on cooling forms organogels
38
. Based on the phase diagrams constructed, lecithin
solutions were prepared by first dissolving lecithins in an organic solvents with the
aid of magnetic stirrer. Formation of organogels takes place on addition of water
with the help of micropiopette syringe. Sometime heat is applied for complete
solubilization of drug
29
.
The oil phase is prepared by mixing lecithin and organic solvent, the mixture
is allowed to stand overnight to ensure complete dissolution. The aqueous (polar)
phase is prepared by adding pluronic to ice cold water, the mixture is agitated to
ensure complete dissolution. The prepare PLO, the oil phase is mixed with aqueous
phase of pluronic using a high shear mixing method by magnetic stirrer
35
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
24
1.13 CHARACTERIZATION OF ORGANOGELS:
In contrast to the ease of preparation, characterization of LOs is relatively
complicated on account of their interior structural design build up on the self-
associated supramolecules. These microstructures, the resultant of varied polar non-
polar interactions, are highly sensitive and pose difficulties in the investigative
studies. However, different characterization studies are extremely useful while
investigating the potential applications of organogel systems as a topical vehicle. For
instance, it has been reported that many of the physicochemical properties of Los viz.
Rheological behavior, physical and mechanical stability, and drug release behavior
are dependent upon how do molecules arrange themselves to provide the specific
structural network within the organogel system
25,38
.
1.13.1 Rheological behavior
For any vehicle to be used for topical drug delivery applications, it is essential
to study its rheological behavior. The latter is important for it efficacy in delivering
the molecules onto or across the skin site. The critical parameters like spreadibility,
adhesiveness (property related to bioadhesion on skin site), cohesiveness (which
indicates structural reformation following application of shear stress, and consistency
need to be modified in a favorable manner. Lecithin organogels (LOs) have been
studied extensively for their rheological attributes and determined to be viscoelastic
in nature
25
.
At higher lecithin concentrations, there is more extensive entanglement of
long cylindrical micelles with each other, forming a network-like structure with a
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
25
very high viscosity. The entrapment of the drug within this network lowers the
amount of free drug available for release, causing a decrease in the release across the
membrane
29
.
1.13.2 Determination of gelation temperature
39
:
Formulations were enclosed in glass tubes (2 mm inside diameter) and
observed over a temperature range of 4-5C. The change from solution to gel ov
vice-versa was determined by inverting the tube. The temperature was changed at a
rate of 5C h and the temperature at which the physical state of the formulation was
changed was regarded as the gelation temperature. In all cases the gelation
temperature was reproducible to within 0.1C. The gel melted completely within a
0.2 0.3 C range.
1.13.3 Gelation Kinetics
40
:
The gelation properties of organogels were investigated in the presence of
various solvents. Gel-sol and sol-gel transitions were evaluated by the inverse
method and gelation kinetics were determined by turbidimetry.
1.14 IN VITRO DRUG RELEASE
29,41
:
The permeation apparatus designed as described by Chowdary et al was
employed to study the release profile of drugs from the semisolid formulations.
Phosphate buffer 6.4 used as receptor fluid.
The release/ permeation of drugs from lecithin gels through various membrnes
was determined using Franz diffusion cell.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
26
1.15 TOPICAL DRUG DELIVERY APPLICATIONS OF LECITHIN
ORGANOGEL-BASED SYSTEMS:
Organogel formulation Major findings
Lecithin (200mM) IPP gel of broxaterol
and scopolamine
42
.
Transdermal delivery of compounds
Phosphatidylcholine (PC) gel in
isopropyl palmitate (IPP) or
cyclooctane
30
.
Investigated for transdermal transport of
various drugs along with aminoacids and
peptides
IPP-lecithin gel of diclofenac and
indomethacin
43
Enhanced efficacy of NSAIDs
administered through topical route
Phytosphingosine or sphingosine lecithin
organogel comprising soy PC, IPP,
ethanol and water
44
Treatment of scars
Soya lecithin-isopropyl myristate (IPM)
organogel containing ketamine
hydrochloride and amitryptiline
hydrochloride
45
Enhance skin penetration and partitioning
of the drugs into the skin layers
Nicardipine lecithin-IPM organogel
46
Enhanced skin permeation across guinea
pig and human skin
Methimazole in LO gel
31
Significant percutaneous absorption of
methimazole
LO gel of cardiac glycoside digoxin
47
Topial administration of the compound in
LO gel was found to be effective for the
treatment of muscle spasm
Cyclobenzaprin in lecithin organogel
48
(lecithin 10-30%, IPM 10-30%, water 30-
60%)
Topical formulation for bauxism.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
27
1.15.2 Topical delivery of therapeutic substances in pluronic lecithin organogels:
PLO gel formulation Applications
Ketoprofen PLO gel
30
Administration of ketoprofen in PLO gel offered
convenience, produced less side effects and
alleviated pain in a specific location
PLO gel of Diclofenac,
Ibuprofen, Ketamine
48
Randomized, placebo controlled study on
lateral epicondylites employing diclofenac in PLO
gel reduced pain and increased functional status
Preparation also found to be effective treatment
for osteoarthritis
Lecithin organogjel in
combination of Pluronic F-127
(poloxamer 407) solution/
Cyclobenzaprin
36
Effective formulation for topical treatment of
carpal tunnel syndrome
Lecithin (20-40% v/v) in
isopropyl palmitate or isopropyl
myristate containing suitable
amount of pluronic and water
with or without short chain
alcohol
44
.
The components of PLO gel provide desired
hydration state to the skin, thus effective in the
treatement of eczema or psoriasis
1.15.3 Commercially available pluronic lecithin organogels
49
:
Therapeutic category Therapeutic agents
Antiemetics Dexamethasone, Dimenhydrate, Scopolamine
Muscle relaxants Cyclobenzaprine, Baclofen, Buspirone
Neuropathy drugs Clonidine, Capsaicin, Amitryptilne, Gabapentin, Phenytoin,
NSAIDs Diclofenac, Ibuprofen, Ketoprofen, Indomethacin,
Systemic analgesics Acetaminophen, Hydromorphone, Morphine sulphate
Systemic hormones Progestrone, Testosterone
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
28
1.16 SAFETY AND SKIN COMPATABILITY STUDIES:
Lecithin-based organogel system i.e., LO or PLO gels are composed of
pharmaceutically approved (non-immunogenic and biocompatible) excipient.
However, the level of surfactant and organic solvents in lecithin organogels is faily
high. Therefore, it is important to consider the safety and irritancy of the formulation
on prolong use. In this context, skin (human skin) compatibility of the gels have been
evaluated employing various techniques before and after applications with either IPP
alone or with 200 mM-IPP gel. No significant alteration in the skin were apparent
after three days and stratum corneum was still intact. The irritation potential of LOs
has been assessed by Dreher et al by carrying out human skin irritation study
50
.
Result indicated a very low cumulative skin irritation potential of LOs. That supports
the stability of LO based gels as topical vehicle for long-term application.
1.17 FUTURE PROSPECTS:
In the field of topical drug delivery, LOs have emerged as one of the most
potential carrier systems. In contrast to other lipid-based system such as vesicular
system (liposomes and niosomes) lecithin-organogel systems may prove to have an
edge in term of efficacy, stability and most importantly, the technological feasibility.
Morevoer, the topical drug delivery of new biotech generated proteinaceous
molecules in the protective non-polar microenvironment of these systems may help
protect these sensitive macromolecules from and degradation, while their transport to
the desired site
48
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
29
Thus, amidst the increasing opportunities and challenges, the LOs may prove
to be highly promising system in realizing the drug delivery objectives while
scientists are desperately trying for more viable alternative viz-a-viz existing carrier
system.
PLO is probably due to financial constrains as well as the industry focusing on
area such as biotechnology and genomics. However, the great interest in PLO in the
US has led to formulation of a second generation lecithin organogel premium, lecithin
organogel base by Xenex Labs and Max Pharmaceuticals, USA
35
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
30
CHAPTER2
OBJECTIVES
2.1 NEED FOR THE STUDY:
A gel can be described as the cross-linked material that retains a large amount
of solvent inside its medium and if the solvent retained in organic one, such material
is known as organogel. Traditionally organogel type systems are applied topically
when the active agent is oil soluble one or if we need the sustained release of the drug
into the deep skin layers
51
. A polar organic solvents, soya bean lecithin can form
thermo-reversible, isotropic, non-birefrigerant gel like system so called micro-
emulsion based organogels, characterized by high viscosity and optical
transparency
13
.
In the present study, various polymers such as soya bean lecithin, isopropyl
myristate, poly sorbate 80, mineral oil (liquid paraffin), pluronic F-127, have been
employed for organogels
29,52,53
.
Miconazole nitrate is a synthetic imidazole derivative with molecular formula
C
18
H
14
C
14
N
2
CHNO
3
and practically insoluble in water has the antifungal activity with
a broad spectrum activity against pathogenic fungi (including yeast and
dermatophytes and gram positive Candida albicans, Staphylococcus and
Streptococcus)
54
. It may act by interfering with permeability by inhibiting the fungal
cytochrome P-450 enzyme responsible for the synthesis of ergosterol the main sterol
in the fungal cell membrane
55
. Miconazole is topically active drug and only rarely
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
31
administered parenterally due to its extensive first pass metabolism and severe
toxicity
56
. Miconazole on oral administration causes nausea, vomiting and diarrhea
57
.
Miconazole readily penetrate the stratium corneum of the skin
58
, but less than 1% in
blood. Irritation and burning are rare after cutaneous application. It is also used in
the treatment of several systemic fungal infections including Candidiasis.
Organogels may be formulated to enhance the release and to provide more
sustained topical antifungal effect of miconazole nitrate.
2.2 OBJECTIVES OF THE STUDY:
1. Formulation of lecithin based organogels of miconazole nitrate.
2. Evaluation of in vitro antifungal activity of optimized miconazole
nitrate organogels.
3. To evaluate the influence of formulation variables on the release rate
of miconazole nitrate.
2.3 SCHEME OF WORK:
Phase-I:
1. Extensive literature survey.
2. Procurement of materials.
Phase-II:
Standardization of Materials:
a) Standardization of miconazole nitrate
b) Standardization of polymers
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
32
1. Lecithin
2. Tween-80
3. Isopropyl myristate
4. Pluoronic F-127
5. Liquid paraffin.
Phase-III:
1. Formulation of lecithin based organogels
2. Preliminary evaluation of organogels.
3. Selection of best composite based on preliminary evaluation.
Phase-IV:
1. Incorporation of drug
2. Preparation of standard calibration curve of miconazole nitrate
3. Evaluation of Organogels.
a) pH
b) Spreadibility
c) Viscosity
d) Gelation kinetics
e) Gel life (stability)
Phase-V:
1. Drug content uniformity
2. In vitro drug diffusion
3. In vitro antifungal activity
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
33
CHAPTER3
REVIEW OF LITERATURE
3.1 REVIEW OF LITERATURE
Rajani V and Verma PRP (1993) determine the diffusion studies of ibuprofen
from ointment bases using cellophase membrane. Data reveals that percent drug
release were concentration dependent. Similar observations have been made with
respect to the release of benzocaine, sorbic acid, salicyclic acid and benzocaine acid.
The diffusion rate was higher in the first hour and therefore decline. The general rank
of order of the drug release was found to be water soluble >water miscible >
hydrophilic >oleaginous. The slowest release was found with a water-oil emulsion.
Release was generally dependent on the drug concentration
59
.
Shoba Rani R Hiremath, et al
60
had carried out the permeation studies of
marketed clotrimazole creams. The drug release was less than 20% with all the
formulations tested at the end of 8 hours and hence isopropyl myristate was chosen
next as a medium because of its bipolar properties.
Gondaliya DP and Pundarika Kshudu K
61
had carried the investigation-
examined preparation and evaluation of nimesulide clear aqueous gels and emulgel
using acrypol 940p. A 3 factorial design was adopted for the optimization of aqeous
gel formulation. Propylene glycol and polyethylene glycol 400 were chosen as
independent variable to study their effects as cosolvents. The clear aqueous gel
formulation containing 15% w/w ethanol, 20% w/w propylene glycol and 30% w/w
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
34
PEG-400 showed maximum drug penetration (18.68%) in 5 hours in in-vitro
diffusion study. Drug diffusion was increased by addition of chromophore EL, a
lipophilic penetration enhancer.
Ilango R et al
62
developed transdermal preparation of nimesulide gel. The
effect of polymer-concentration on in vitro nimesulide release from carbpol 940
HPMC gel and effect of permeation enhancer like Tween-80 and SLS at different
concentration on drug release were studied.
Magdy C Mohammed
63
have studied optimization of chlorphenesin emulgel
formulation. He prepared emulgel using different polymers and evaluate emulgel for
various parameter like rheological study, in vitro study release, antifungal activity and
stability studies.
Miconazole nitrate used in the treatment of severe systemic fungal infection,
chronic muco cutaneous candidasis, fungal meningitis, vulvo vaginal candidiasis,
tinea infection, otomicosis, cutaneous candidiasis and rarely given as i.v for system
mycosis
58
.
Mucoadhesive buccal patches of miconazole nitrate was prepared and its in
vitro / in vivo performance were evaluated by Wafee and Ismail
64
. The patches were
prepared with ionic polymers sodium corboxy methylcellulose and chitosan and non
ionic like hydroxy ethyl cellulose and hydroxy propyl methyl cellulose. Convenient
bio adhesion, acceptable elasticity, swelling and surface pH were obtained patches
exhibited sustained release over more than 5 generally enhanced the release rate.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
35
Dibiase MD and Rhodes CT
65
had carried out the work on pluronic F-27 gel
and evaluated as a potential topical vehicle for epidermal growth factor delivery. The
chemical stability of the polypeptide within the gel matrix was investigated using
HPLC. Thermal stability studies was performed on the base gel formulation.
Modifications to the formulation was made to improve physical characteristics and
chemical stability. Humectants and antioxidants was investigated as potential
formulation additives and the microbial status of the product was also evaluated.
Minghetti Patel
66
reported dermal patches of miconazole were evaluated for
their technological characters by Minghetti et al for the treatment of tinea argium
infection. Artificial silk used as backing layer. Eudragit and plastoid were used
which provided release of at least 24 hours.
Khurana and Ahuja
67
prepared and evaluated mucoadhesive tablets of
miconazole nitrate for the treatment of oral candidiasis. It produced satisfactory drug
release.
David H et al
68
investigation of two hundred eighty (280) patients with
symptomatic vulvovaginal candidiasis were randomly assigned to treatment with
either miconazole nitrate 4% vaginal cream for 3 days, followed by placebo for 4
days, or Monistat-7 (miconazole nitrate 2%) vaginal cream for 7 days in this double-
blind, parallel-group, outpatient study. Sixteen US centers participated. Patients
were seen on admission and then at 8 to 10 days and 30 to 35 days after completion
of treatment. Clinical, microbiologic, and therapeutic efficacy was assessed. This
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
36
study compared the safety and efficacy of a new cream formulation of 5g of
miconazole nitrate 4% administered once daily for 3 days with that of 5g of
miconazole nitrate 2% vaginal cream, the currently marketed product, administered
for 7 days. Although not significantly clinically different, cure rates were slightly
higher with the 3-day treatment. Relapse rates were low in both treatment groups and
symptom relief was also comparable. The most frequent adverse experiences were
genital (itching, burning, irritation, and discharge), as well as headache and
respiratory congestion; reports of adverse experiences were similar in the two
treatment groups. Miconazole nitrate 4% vaginal cream administered for 3 days was
found to be promising new candidate for over-the-counter treatment of vulvovaginal
candidiasis.
Chandia Valenta et al
34
studied the soya-lecithin aggregates prepared by a
technique using compressed gas to formulate new dermal preparations. Ketoprofen
(KP) a Non-steroidal anti-inflammatory drug (NSAID) is included as a model drug.
The novel soya-lecithin aggregates are promising candidates for new drug delivery
system in dermatology and cosmotology. Lecithin aggregates loaded with drugs are
multifunctional causes that also act as penetration enhancers. The improvement in
skin permeation is related to both the solubilizing effect of lecithin matrix and
penetration enhancing effect of lecithin itself.
Murda S
35
reported the great interest in pluronic lecithin organogel in the US
has led to the formulation of a second generation lecithin organogel, premium lecithin
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
37
organogel base by Xenex laboratories and has non-greasy, non-tacky and improved
stability to temperature as compared with original pluronic lecithin organogels.
Reza Aboofazeli et al
69
stated that the partial phase diagrams were constructed
with soybean lecithin water, sodium salicylate, alcohol and isopropyl myristate.
Phase diagrams showed the area of existence of a stable isotropic region along the
surfactant / oil axis (i.e., reverse microemulsion area).
Shilpa K et al
75
showed that the microemulsion based organogel are useful in
iontophoretic drug delivery vehicles include their potential to increase the maximum
loading of a water soluble agent and the drug ability may be improved especially in
comparison to the use of hydrogels where the presence of an aqueous continuous
phase may allow degrative processes to occurs.
Singh R et al
70
investigation reveals that the antifungal activity measured as
zone of inhibition of lecithin organogel ketoconazole as a model drug was better as
compared to the activity recorded for hydrogel base.
William N et al
30
concludes that lecithin gels may be efficient vehicles for the
transdermal transport of various drugs. The presence of lecithin in organic solvent
result with increase in drug solubility as compared with heat solvent the transport rate
is 10 times higher that with the aqueous solution of drug. Lecithin organogel can be
prepared easily and rapidly and can be obtained with biocompatible components.
They are stable for a long time, can incorporate sizeable amount of different chemical
as guest molecules and fulfill the cosmetics and pharmacological application.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
38
Shelke VB et al
38
research result shows that the organogels can be used for all
type of drug molecule, controlled release increased resistance to microbial
contamination and reduced risk of toxicity.
Meiying Ning et al
24
reveals vulvovaginal candidiasis is the infection with
Candida albicans. Approximately 75% of women have a vaginal infection with
candida strain during their life and about 40 to 50% of them suffer a second one and
small percentage shows a chronic cause. The entrapment of drug in vesicles may
help in the localized delivery of the drug and an improved solubility and availability
of the drug at the site may reduce the dose and systemic side effects.
Hoffmann G et al
31
showed that lecithin organogels were developed for
transdermal transport of drugs and consists of a spaghetti like network of lecithin
micelles, which can host various guest molecules by solubilization in their
transdermal methimazole treatment research work to treat cat hyperthyroidism.
Marco Antonio M et al
71
evaluate the system containing water lecithin/
polysorbate 80/ isopropyl myristate the results showed high stability, very low
toxicity for the parentral use.
Vitonial M, Bentley M et al
39
studies showed that in vitro permeation of a
model drug triamcinolone acetonide was decreased when the lecithin concentration
was increased. The presence of lecithin in the poloxamer (pluronic) gel improved the
characteristics for topical drug delivery.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
39
Gelatin containing microemulsion based organogels (MBGs) have been
formulated using pharmaceutical acceptable surfactant and oils such as tween-85 and
isopropyl myristate. MBGs are clinically conducting employed for ionotphoretic
delivery of a model drug. MGBs also appear to offer improved microbial resistance
in comparison to aqueous solution or hydrogels. This work is done by Kantaria S et
al
72
.
Scartazzini R, Luisi R
28
showed that the interest in lecithins as basic
components for gel materials lies in biomimetic chemistry. This is vague term and
one that should be used sparingly. The point can be made that lecithin gels may be
related more closely than others to gel like lipidic aggregates that exist in vivo.
Luisi PL et al
30
suggested organogels can be used to solubilize a variety of
drug candidates therefore it is widely applied in chemical, pharmaceutical, cosmetic
applications.
Dreher F et al
43
in investigation to estimate the function of the gel as a
potential transdermal penetration enhancer system performed the interaction of
lecithin, isopropyl palmitate with the human stratum corneum using DSC and FTIR
and found more significant irritancy. Lecithins and isopropyl palmitate affects the
stratium corneum lipid organization.
Murdan S et al
77
determined the sorbitan monostearate organogels can be used
as delivery vehicles for hydrophilic and hydrophobic drug and vaccines. The gels
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
40
may also provide sustained release of appropriate active entity after intramuscular and
subcutaneous administration.
In short communication, Murdan S et al
26
showed that sorbitan monostearate
organogels are opaque, thermo reversible, semisolid whose microstructure consists of
surfactant tubules dispersed in organic continuous phase. Inverse toroidal vesicles are
the precursors of the surfactant tubules. The toroids are thought to be analogues to
other well known vesicles, liposomes and niosomes except for their toroidal (rather
than spherical) shape and their inverse nature.
Murdan S et al
76
suggested in their studies that the sorbitan stearate and
isopropyl pulmitate organogels may have potential applications as delivery vehicles
for drugs and antigens.
Desai A and Kadam V et al
73
noted that, mother nature has gifted India with
great variety of flora and fauna. Since centuries man has made an effective use of
materials from natural origins in the medical and pharmaceutical field, natural
materials have been used in controlled release drug delivery system. The natural
release retardants include phospholipids (lecithins).
Kavitha K et al
74
reported, a topical drug delivery system localizing the drug
at skin will be much favourable for the treatment of skin infection.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
41
Rajiv Kumar and Om Prakash Katare
25
determined enhanced skin penetration
and site specific delivery of bioactive into the deeper layer of skin has been achieved
employing organogels as a topical vehicles.
Sudaxshina Murdan
35
showed that, in man the in vivo studies in man, where
pluronic lecithin organogels was applied using diclofenac as a model drug may be
beneficial as a delivery vehicle for local action during treatment. Patient feels less
pain.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
42
3.2 DRUG PROFILE
Miconazole Nitrate is included in National List of Essential Medicines,
2003
78
.
Miconazole Nitrate:
Azoles: Azoles are group of synthetic fungistatic agents with a brand spectrum
activity.
Mechanism of action: Miconazole nitrate inhibit fungal cytochrome P-450 enzyme
responsible for the synthesis of ergosterol the main sterol in the fungal cell
membrane.
The resulting depletion of ergosterol alter the fluidity of the membrane and
this interfers with the action of membrane associated enzymes the overall effect is
inhibition of replication. A further repercussion is the inhibition of the transformation
of candidal yeast cells into hyphae-the invasive pathogenic form of the parasite
23
.
The requirement for IV administration and toxicity of the older antifungal
agents created a need for antifungal agents with a better therapeutic profile. The
relatively non toxic oral azole medications represents the 1
st
major advance in this
direction since their introduction in 1980s played important role in systemic therapy
of fungal diseases.
According to number of nitrogen atom azoles are classified as imidazoles and
triazoles.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
43
Imidazoles: Ketoconazole, Miconazole and Clotrimazole.
Triazoles: Itroconazole and Fluconazole.
Miconazole nitrate
Brand Names: Canticid, Gyno-Dartarin, Micogel, Candistat, Daktarin, Decanazole,
Fungitop.
Chemical Structure
58
:
Molecular formula
79
Micronazole nitrate C
18
H
14
C
14
N
2
CHNo
3
Molecular weight Miconazole nitrate 479.15
Properties:
Colour White to pale cream
Form Crystalline or microcrystalline powder
Description Odourless or almost odourless
Solubility Practically insoluble in water
1 in 9.5 of alcohol, 1 in 15 of ether, 1 in 4 of isopropylalchol
1 in 5.3 of methyl alcohol, 1 in 9 of propylene glycol
Cl OCH
2
Cl CH CH
2
N
N
Cl
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
44
Melting point - 179 180C
Shelf life - at least 5 years
Storage condition - Store in a well closed container protect from light
Indications:
Administered by intravenous infusion in the treatment of severe systemic
fungal infections including candidiasis, coccidioidomycosis, crypto-coccossis, para
coccidomycosis and infections due to pseudeliescheria boydii, has been given
prophylactically to patients at high risk of opportunistic fungal infections.
It is administered by intravenous infusion in the treatment of severe fungal
infections including Candidiasis, Cryptococcosis
58
.
Used for vulvovaginal candidiasis.
Used for dermatophytic infections including tinea corpis (ring worm), tinea
pendis (atheletes foot) and tinea crucis.
Therapeutic doses:
Adult
Oral
Oral and intestinal candioliasis.
125 to 250 mg as tablets or gel 4 times a day
Parenteral: I.V. dose 02. To 1.2 g 3 times daily
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
45
In fungal meningitis intravenous treatment may be supplemented by a single
daily dose of 20mg given by intrathecal injection every 3 to 7 days.
Skin: Once or twice a day as 2% gel, cream lotion or powder.
Vaginal: 5-10 g of a 2% cream once daily for 7-14 days or tampons containing
100mg twice a day for 5 days.
Children:
Oral: Oral and intestinal candidiasis 125 mg 4 times dialy.
Contraindications:
Should not be used in patients known to be hypertensive to this drug.
First trimester of pregnancy.
Routes of Entry:
Oral for oral and intestinal candidiasis.
Dermal: For treatment of fungal infections of the skin and nails.
Eye: Solution of miconazole as topical.
Parenteral: As intravenous infusion in the treatment of severe systemic fungal
infections
79
.
Adverse Reactions
79
:
Cardiac arrhythmias (IV administration), tachycardia, haematological alterations
aggregation of erythrocytes, gastrointestinal disturbancesanorexia, nausea,
vomiting, diarrhea, local irritation and clinical effects. Vulvovaginal burning,
itching, irritation, pelvic cramps, cutaneous pruritis, flushes.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
46
Kinetics
Absorption by route of exposure
Oral: After oral administration, miconozole is incompletely absorbed from the
gastrointestinal tract; peak plasma concentrations of about 1 mcg/mL have been
achieved 4 hours after a dose of 1g
79
.
Parenteral: By intravenous infusion, doses above 9 mg/kg body-weight usually
produce plasma concentration above 1mcg per mL.
Topical: There is little absorption through skin or mucous membranes when
miconazole nitrate is applied topically
79
.
Following intravaginal administration of miconazole nitrate, small amounts
are absorbed
81
.
Administration of a single dose of miconazole nitrate suppositories (100 mg)
to healthy subjects resulted in a total recovery from the urine and faeces of 0.85%
(+0.43%) of the administered dose
82
.
Distribution by route of exposure: Data available indicates that over 90% of
miconazole is bound to plasma proteins. Penetration into the cerebrospinal fluid and
sputum is poor but miconazole diffuses well into infected joints. Animal studies
indicate that the drug crossed the placenta but it is unknown whether micronazole
nitrate is excreted in breast milk
79
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
47
Biological half-life by route of exposure:
Oral: The information available indicates that miconazole orally administered has a
half-life of about 24 hours.
Parenteral: The decrease of plasma concentrations after an intravenous perfusion is
biphasic, with half-life of 30 minutes (t 1/2a) and 24 hours (t 1/2b).
Metabolism: Micronazole is metabolised in the liver to inactive metabolites.
Miconazole has an important first pass effect. Hepatic metabolism of miconazole is
by O-dealkylation and oxidative N-dealkylation.
Elimination by route of exposure:
Oral: Approximately 50% of an oral dose may be excreted mainly unchanged in the
faeces. From 10 to 20% of an oral dose is excreted in the urine, mainly an
metabolites, within 6 days.
Parenteral: From 10 to 20% of an intravenous dose is excreted in the urine, mainly
as metabolites, within 6 days. After an intravenous dose of miconazole, 40% of the
radioactivity is found in faeces and 18% in urine. About 14 to 22% of the dose is
excreted via the kidneys, mainly as inactive metabolites.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
48
Pharmacology and toxicology:
Mode of action:
Toxicodynamics: Since composition of fungal and mammalian cells membrane is
different, miconazole does not affect the human cells. Many adverse effects have
been associated with the injection vehicle, which contains Cremophor EL.
Pharmacodynamics: The pharmacological mode of action of miconazole is
unknown. In-vitro studies suggest that imidazoles impair the synthesis of ergosterol,
which is a vital component of the fungi cell membranes. In-vitro miconazole inhibited
the ability of neutrophils to reach the site of infection proptly (chemotaxis) and it
demonstrates marked immunosuppressive properties. The clinical significance of
these data is unknown.
Interactions
81
:
Miconazole given systemically may enhance the activity of anticoagulant or
suphonylurea hypoglycaemic drugs. The combination of amphotericin and
miconazole appeared to be less effective when either drug used alone. Miconazole
enhanced the activity of clomipramine, carbamazepine and phenytoin.
The base contained in certain suppository formulations may interact with
some latex products, such as that used in vaginal contraceptive diaphragms.
Since concomitant administration of rifampin and ketoconazole, reduces the
blood levels of the latter, the concurrent administration of miconazole intravenously
and rifampicin should be avoided.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
49
Ketoconazole increases the blood level of cyclosporin a, therefore, there is the
possibility of a drug interaction involving cyclosporin a and intravenous miconazole;
blood levels of cyclosporin a should be monitored if the two drugs are given
concomitantly.
Preliminary studies in-vitro and in a few patients indicate that systemic use of
both amphotericin B and miconazole may result in antagonistic effect rather than
additive antifungal effect.
Several patients have developed an enhanced hypoprothrombinemic response
to warferin following miconazole therapy.
Management:
One should be alert for an altered hypoprothrombinemic response to oral
anticoagulants if miconazole therapy is initiated or discontinued, adjust oral
anticoagulant dose as necessary
80,83
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
50
3.3 POLYMER PROFILE
3.3.1 Lecithin
30,32
:
Nonproprietary Names: USPNF: Lecithin
Synonyms: E322; egg lecithin, Epikuron; Espholip; LSC; mixed soybean
phosphatides; ovolecithin; Ovothin; soybean lecithin; soybean phospholipids;
vegetable lecithin.
Structural Formula:
-phosphatidycholine
where R
1
and R
2
are fatty acids which may be different or identical.
Lecithin is a complex mixture of materials. The structure above shows
phosphatidylcholine, the principal component of egg lecithin, in its -form. In the -
form the phosphorus containing group and the R
2
group exchange positions.
Functional Category: Emolient emulsifying agent; solubilizing agents.
Applications in Pharmaceutical Formulation or Technology:
Lecithins are used in a wide variety of pharmaceutical applications. They are
also used in cosmetics and food products. Lecithins are mainly used in
pharmaceutical products as dispersing, emulsifying and stabilizing agents and are
included in intramuscular and intravenous injections, parenteral nutrition
formulations and topical products, such as creams and ointments. Lecithins are also
CH
2
O C R
1
CH
2
O P OCH
2
CH
2
N
+
(CH
3
)
3
O
O
O
CH O C R
2
O
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
51
used in suppository bases, to reduce the brittleness of suppositories and have been
investigated for their absorption enhancing properties in an intranasal insulin
component of enteral and parenteral nutrition formulations. Liposomes in which
lecithin is included as a component of the bilayer have been used to encapsulate drug
substances and their potential as novel delivery systems has been investigated.
Lecithin as a naturally biocompatible surfactant is capable of producing
balance microemulsions in the presence of short chain alcohols, as cosurfactants
alcohol can decrease the polarity of the polar medium or incorporate into the ligand
layer and change the critical packing parameters (CPP) of lecithin molecule. This
effect in turn promote micellization of the microemulsion. Lecithin also deorganize
the skin organelles and helps in drug permeation.
Description: Lecithin vary greatly in their physical form, from viscous semiliquids to
powders, depending upon the free fatty acid content. They may also vary in color
from brown to light yellow, depending upon whether they are bleached or
unbleached. Lecithins have practically no odor. Those derived from vegetable
sources have a bland or nut like taste, similar to soybean oil.
Method of Manufacture:
Lecithins are essential components of cell membranes and may thus in
principle be obtained from a wide variety of living matter. In practice however,
lecithins are usually obtained from vegetable products such as soybean, peanut,
cottonseed, sunflower, rapeseed, corn or groundnut oil, Soybean lecithin is the most
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
52
commercially important vegetable lecithin. Lecithin obtained from eggs is also
commercially important and was the first lecithin to be discovered.
Vegetable lecithins are obtained as a byproduct in the vegetable oil refining
process. Polar lipids are extracted with hexane and after removal of the solvent a
crude vegetable oil obtained. Lecithin is then removed from the crude oil by water
extraction. Following drying the lecithin may then be further purified.
With egg lecithin, a different manufacturing process must be used since the
lecithin in egg yolks is more tightly bound to proteins than in vegetable sources. Egg
lecithin is thus obtained by solvent extraction from liquid egg yolks using acetone or
from freeze dried egg yolks using ethanol. Synthetic lecithins may also be produced.
Safety:
Lecithin is a component of cell membranes and is therefore consumed as a
normal part of the diet. Although excessive consumption may be harmful, oral doses
of up to 80 g daily have been used therapeutically in the treatment of tardive
dyskinesia. When used in topical formulations lecithin is generally regarded as a
non-irritant and non-sensitizing materials.
3.3.2 Polyoxyethylene Sorbitan Fatty Acid Esters
32,71
:
Nonproprietry Names:
BP: Polysorbates 80
Ph Eur: Polysorbatum 80
USPNF: Polysorbates 80
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
53
Synonyms: Polysorbate 80: Armotan PMO 20, Capmul POE-O; Crillett 4; Crillet 50;
E433; Glycosperse 0-20; Hodg PSMO-20, Liposorb O-20; Liposorb O-20K,
Montanox 80; polyoxyethylene 20 oleate; Protasorb O-20, Tween 80.
Functional Category: Emulsifying agent; nonionic surfactant, solubilizing agent;
wetting agent.
Applications in Pharmaceutical Formulation or Technology:
Polysorbates are hydrophilic nonionic surfactants used widely as emulsifying
agents in the preparation of stable oil-in-water pharmaceutical emulsions. They may
also be used as solubilizing agents for a variety of substnces including essential oils
and oil soluble vitamins, and as wetting agents in the formulation of oral and
parenteral suspensions.
Description: The color is yellow, oil liquid at 25C.
Stability and Storage: Polysorbates are stable to electrolytes and weak acids and
bases; gradual saponification occurs with strong acids and bases. The oleic acid
esters are sensitive to oxidation. Polysorbates should be stored in a well-closed
container, protected from light, in a cool, dry place.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
54
3.3.3 Isopropyl Myristate
32,71
:
Nonproprietary Names:
BP: Isopropyl myristate
Ph Eur: Isopropylis myristas
USPNF: Isopropyl myristate.
Synonyms: Bisomel; Crodamol IPM; Deltyl Extra; Emocol-IM; Emerest 2314;
Estergel; Estol 1514; Isomysr; Isopropyl ester of myristic acid; Ja-Fa IPM; Kessco
IPM 95; Kesscomir; Plymouth IPM; Starfal IPM; Unimate IPM, etc.
Structural Formula: CH
3
(CH
2
)
12
COOCH(CH
3
)
2
Functional Category: Emollient, oleaginous vehicle; skin penetrant; solvent.
Applications in Pharmaceutical Formulation or Technology:
Isopropyl myristate is a non-oleaginous emollient that is absorbed readily by
the skin. It is used as a component of semisolid bases and as a solvent for many
substances applied topically. Applications in topical pharmaceutical and cosmetic
formulations include; bath oils; make-up; hair and nail care products; creams; lotions;
lip products; shaving products; suntan preparations; skin lubricants; deodorants; otic
suspensions and vaginal creams.
Description:
Isorpopyl myristate is a clear, colorless, practically odorless mobile liquid
with a bland taste. It consists of esters of propan-2-ol and saturated high molecular
weight fatty acids, principally myristic acid.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
55
Stability and Storage Conditions:
Isopropyl myristate is resistant to oxidation and hydrolysis and does not
become rancid. It should be stored in a well closed container in a cool, dry, place and
protected from light.
Incompatibilities:
When isopropyl myristate comes into contact with rubber, there is a drop in
viscosity with concomitant swelling and partial dissolution of the rubber; contact with
plastics, e.g., nylon and polyethylene, results in swelling. Isopropyl myristate is
incompatible with hard paraffin, producing a granular mixture. Also incompatible
with strong oxidizing agents.
Regulatory Status:
Included in the FDA inactive ingredients guide (otic, topical and vaginal
preparations). Used in non-parenteral medicines licensed in the UK.
3.3.4 Pluronic
32
:
Nonproprietary Names: USPNF: Poloxamer
Synonyms: Monolan, poloxalkol; polyethylene-propylene,glycol, copolymer,
polyoxyethylene-polyoxypropyle, cepolymer, supronic; Symperonic.
Chemical Name and CAS Registry Number: -hydro--hydroxypoly(oxyethylene)
poly (oxypropylene) poly (oxyethylene) block copolymer.
Functional Category: Emulsifying agent; solubilizing agent; wetting agent.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
56
Applications in Pharmaceutical Formulation or Technology:
Poloxamers are nonionic polyoxyethylene-polyoxypropylene copolymers used
primarily in pharmaceutical formulations as emulsifying or solubiling agents. The
polyoxyethylene segment is hydrophilic whilst the polyoxypropylene segment is
hydrophobic. Poloxamers are used as emulsifying agents in intravenous fat
emulsions, and as solubilizing and stabilizing agents to maintain the clarity of elixiers
and syrups. Poloxamers may also be used as wetting agents, in ointments,
suppository bases, gels and as tablet binders and coatings.
Poloxamer 188 has also been used as an emulsifying agent for fluorocarbons
used as artificially blood substitutes.
Description:
Poloamers generally occurs as white-colored, waxy, free flowing prilled
granules or as cast solids. They are practically odorless and tasteless. At room
temperature, poloxamer 124 occurs as a colorless liquid.
Stability and Storage Conditions:
Poloxamers are stable materials. Aqueous solutions are stable in the presence
of acids, alkalis and metal ions. However, aqueous solutions do support mold
growth. The bulk material should be stored in a well-closed container in a cool dry,
place.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
57
3.3.5 Liquid Paraffin
32
:
Nonproprietary Names:
BP: Liquid paraffin
Ph Eur: Parrafinum liquidum
USP: Mineral oil.
Synonyms: 905 (mineral hydrocarbons), Avatech; Citation; heavy liquid petrolatum;
heavy mineral oil, liquid petrolatum; paraffin oil; white mineral oil.
Functional Category: Emmolient, solvent, tablet and capsule lubricant; therapeutic
agent; oleaginous vehicle.
Applications is Pharmaceutical Formulation or Technology:
Mineral oil is used primarily as an excipient in topical pharmaceutical
formulations where its emollient properties are exploited as an ingredient in ointment
bases. It is additionally used in oil-in-water emulsions, as a solvent.
Description:
Mineral oil is a transparent, colorless, viscous liquid, free from fluorescence in
daylight. It is practically tasteless and odorless when cold, and has a faint odor when
heated.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
58
Stability and Storage Conditions:
Mineral oil undergoes oxidation when exposed to heat and light. Oxidation
begins with the formation of peroxides, exhibiting an induction period. Under
ordinary conditions, the induction period may take months or years. Mineral oil
should be stored in an airtight container, protected from light, in a cool, dry place.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
59
CHAPTER4
METHODOLOGY
MATERIALS AND METHODS:
A] Materials and Source:
Sl.
No.
Material Source
1. Miconazole nitrate Gift Sample Adelphi A/C Sigma Lab, Tivium, Goa
Date 05.03.2005
Batch No. 2004-05/ GRL/SPR/00013/ MZN/
16/03/04
2. Isopropyl myristate Loba Chemicals Pvt. Ltd., Mumbai
Batch No.
3. Soya bean lecithin Hi-media, Mumbai
Batch No. RM-637
4. Pluronic (F-127)
5. Tween-80 SD Fine Chem Ltd., Mumbai
6. Paraffin Liquid SD Fine Chem Ltd., Mumbai
7. Cellophane Membrane Hi-media, Mumbai
8. Sodium dihydrogen phosphate SD Fine Chem Ltd., Mumbai
9. Disodium hydrogen phosphate SD Fine Chem Ltd., Mumbai
10. Sodium hydroxide SD Fine Chem Ltd., Mumbai
11. Sodium chloride SD Fine Chem Ltd., Mumbai
All other chemicals and reagents used were of analytical grade.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
60
Equipment:
Sl.
No.
Equipment Source
1. UV-visible spectrophotometer Shimadzu Corporation, Japan
Model 1700
2. Brookfield DV-II+programmable
viscometer
Sanjay Technologies, Mumbai
Model M-97/164-E-0102
3. Digital Balance Shimadzu Corporation, Japan
Model BL-220II
4. Thermostatic Hot plate with
magnetic stirrer
Remi Udyog, Mumbai
Model DW M51102
5. Digital pH meter (pen type) Hanna Instruments, Italy
6. Keshary-Chein type diffusion cell Fabricated by Sai Enterprises, Pune
7. Autoclave Oswal Companies
8. Hot air oven Sheetal Scientific Industries, Mumbai
9. Incubator Rotex Instrument
10. Micropipette Scientex Pvt Ltd.
11. Digital Stirrer Remi Motors, Mumbai
Model RQ-121/D
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
61
4.1 RAW MATERIAL CHARACTERIZATION:
4.1.1 Standardization of Drug
84
:
Tests were carried out on the sample of miconazole nitrate to establish its
identify and purity as per IP 1996.
4.1.2 Standardization of Polymers:
The polymers used in the study were tested as per pharmacopoeial or official
manufacturers standards.
4.1.2a Lecithin was characterized as per the USP XXV limits
85
.
4.1.2b Tween 80 was tested as per the USP XXV limits
85
.
4.1.2c Pluronic was tested for the compliance with USP XXV specification
85
.
4.1.2d Isopropyl myristate as per the USP XXV limits
85
.
4.1.2e Liquid paraffin was tested for compliance with BP limits
86
.
4.2 PREPARATION OF MICONAZOLE NITRATE LOADED LECITHIN
MICROEMULSION BASED ORGANOGEL:
4.2.1. Preparation of Organogels loaded with Miconazole Nitrate
29
:
Lecithin organogels based systems were prepared by lecithin and tween-80 in
different composition were dissolved in isopropyl myristate (IPM). After
incorporation of drug, gelation was achieved on addition of the aqueous phase.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
62
Table-4.2.1: Preparation of Organogels Loaded with Miconazole Nitrate
Sl.
No.
Formulation
Code
Lecithin
% w/w
Tween-
80
% w/w
IPM
% w/w
Water
% w/w
Drug
% w/w
1. AM
1
7.54 3.77 30.16 56.56 2
2. AM
2
20.63 10.31 30.95 36.11 2
3. AM
3
35.43 1.77 11.81 21.26 2
4. AM
4
56.02 28.01 14.00 28.01 2
4.2.2 Preparation of Pluronic Lecithin Organogel
35
:
The oil phase is prepared by mixing lecithin and isopropyl myristate and
allowing the mixture to stand overnight to ensure complete dissolution. The aqueous
phase is prepared by adding pluoronic F-127 to ice cold water, placing the mixture in
refrigerator and agitating periodically to ensure complete dissolution.
To prepare PLO, the oil phase containing 2% drug miconazole nitrate mixed
with the aqueous phase using a high shear mixing method by magnetic stirrer/ digital
stirrer. It is important that the aqueous phase is cool before mixing because an
aqueous solution of pluoronic F-127 is in the liquid state at a low temperature and
gels at a higher temperature.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
63
Table-4.2.2: Preparation of Pluronic Lecithin Organogels (PLO)
Sl.
No.
Formulation
code
Lecithin
% w/w
IPM
% w/w
30%
Pluronic
in water
% w/w
Drug
% w/w
1. FM
1
7.54 30.16 60.33 2.0
2. FM
2
17.85 26.73 53.47 2.0
3. FM
3
36.76 24.50 36.76 2.0
4. FM
4
56.02 14.00 28.01 2.0
4.2.3 Preparation of Miconazole Nitrate Loaded Organogels
29
:
Based on the different composition ration, the lecithin solutions were prepared
by first dissolving lecithin in the organic solvent isopropyl myristate (IPM) with the
aid of a magnetic stirrer and then while still stirring the required 2% miconazole
nitrate is added followed by necessary amount of water (double distilled) into the
mixture to obtain a clear gel. Formation of clear, homogenous and non-birefringent
gels, after addition of water by a micropipette syringe.
Table-4.2.3: Preparation of Miconazole Nitrate Loaded Organogels
Sl.
No.
Formulation
Code
Lecithin
% w/w
IPM
% w/w
Water
% w/w
Drug
% w/w
1. SR
1
8.90 35.70 53.50 2.0
2. SR
2
20.22 30.33 47.50 2.0
3. SR
3
43.2 28.8 25.95 2.0
4. SR
4
63.33 15.08 22.63 2.0
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
64
4.2.4 Hot Melt Type Organogel
87
:
Lecithin in various concentrations was used as a gelator and paraffin as the oil
medium, the material was heated with stirring on hot plate stirrer and water is added
with micropipette. Upon cooling of the melted disperse system, gellation was
achieved.
Table-4.2.4: Preparation by Hot Melt Type Organogels
Sl.
No.
Formulation
Code
Lecithin
% w/w
Paraffin
% w/w
Water
% w/w
Drug
% w/w
1. MN
1
13.43 53.72 30.82 2.0
2. MN
2
31.60 47.40 18.97 2.0
3. MN
3
49.00 34.67 16.33 2.0
4. MN
4
67.61 16.90 13.52 2.0
4.3 CONSTRUCTION OF CALIBRATION CURVE OF MICONAZOLE NITRATE
88
:
40 mg Of miconazole nitrate accurately weighed & added in a mixture of
10ml of 0.1M Hcl & 50 ml of iso propanol (propan-2ol). The volume is made 100ml
with iso propranol. The UV absorption maximum is 272 nm. The Beer-Lamberts
range used is 4g-32g. Previous studies have shown that miconazole has relatively
low absorption in the UV range and are difficult to analyze in the low concentration.
Therefore, the method adopted by Agarwal and Katare was followed to second the
results.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
65
4.4 EVALUATION OF LECITHIN-MICROEMULSION BASED
ORGANOGELS:
4.4.1 pH
74
:
Digital pen pH meter was used to measure the pH of gels at constant
temperature. Prior to this pH meter was calibrated using buffer solution of pH 7.0
and 9.2 then the electrode was wasted with demineralized water. The electrode was
inserted in to the sample 10 min prior to taking the readings.
4.4.2 Spreadability
17,41
:
Introduction: One of the criteria for gels to meet the ideal qualities is that it should
possess good spreadability. Spreadability is a term express to denote the extent of
area to which the gel readily spreads on application to skin or the affected parts. The
therapeutic efficiency of formulation also depends upon its spreading value. Hence,
determination of spreadability is very important in evaluating gel characteristics.
Spreadability of the formulations was determined by an apparatus suggested
by Mutimer et al, which was suitably modified in the laboratory and used for the
study. It consists of a wooden block, which was provided by a pulley at one end. A
rectangular ground glass plate was fixed on the block. An excess of gels (about 2
gm) under study was placed on this ground plate. The gel was then sandwiched
between this plate and another glass plate having the dimensions of the fixed ground
plate and provided with the hook. A 300 gm weight was placed on the tip of two
plates for five minutes to expel air and to provide a uniform film of the gel between
the plate. Excess of the gel was scrapped off from the edges. The top plate was then
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
66
subjected to pull of 300 gm. With the help of a string attached to the hook and the
time (in seconds) required by the top plate to cover a distance of 10 cms be noted. A
shorter time interval indicates better spreadability.
The spreadability was determined by special apparatus and it was calculated
using the formula:
S =
t
ml
Where,
S = spreadability
m = weight tied to the upper slide
l = length of the glass slide
t = time taken in seconds.
4.4.3 Determination of Viscosity
74
:
The viscosity of formulated organogels were determined. The viscosity was
determined using a Brookfield viscometer. The sample holder taken for the viscosity
measurement was filled with the samples and then inserted into a flow jacket
mounted on the viscometer. The samples adaptor (spindle), rotated at an optimum
speed was used to measure the viscosity of the preparation, the samples was allowed
to settle for five minutes prior to taking the readings.
4.4.4 Stability studies
38,71,89
:
The stability of the formulations kept in the containers was assessed at
different temperatures.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
67
Samples were kept at constant temperature 25C for their physical stability,
organoleptic properties and macroscopically appearance of the microemulsion.
4.4.5 Gelation Kinetics
40
:
Gelation kinetic was determined by turbidimetric method using
Nepheloturbidometer. In this method, the sample cell containing formulation
components without water was placed in the cuvette chamber and meter is set to 0
NTU. Then water is added drop by drop using micropipette. Until the meter shows
maximum NTU i.e., 100 NTU and the water quantity is measured of which turbidity
is maximum.
4.4.6 Determination of Miconazole Nitrate Content
74
:
An accurately weighed amount of each preparation was dissolved in
phosphate buffer pH 6.4. The content of miconazole nitrate was determined spectro-
photometrically at 272 nm using Shimadzu UV-visible spectrophotometer.
4.4.7 In vitro Diffusion Study
74
:
The apparatus consists of a cylindrical glass tube (with 22 mm internal
diameter and 76 mm height), which was opened at both the ends. One gram of (1
gm) of gel formulation containing 20 mg of miconazole was spread uniformly in the
surface of cellophane membrane (previously soaked in receptor medium for overnight
24 hours) and was fixed to the one end of tube such that the preparation occupies
inner circumference of the tube. The whole assembly was fixed in such a way that
the lower end of tube containing gel was just touched (1-2 mm deep) the surface of
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
68
the diffusion medium i.e., 45 ml pH 6.4 phosphate buffer present in 50 ml beaker,
which was placed on thermostatic hot plate with magnetic stirrer and maintained at
372C. The cellophane membrane act as barrier between the gel and pH 6.4
phosphate buffer. The contents were stirred using magnetic bar at 50 rpm. A
quantity of 1 ml samples were withdrawn at an interval of hourly and the same
amount is replaced with pH 6.4 phosphate buffer each time.
The released drug was estimated by using Shimadzu UV-visible
spectrophotometer at 272 nm.
4.4.8 In Vitro Antifungal Activity
63,70,74
:
Ditch plate technique: For evaluation of fungistatic mainly for semisolids. Agar
plate were prepared and sterilized as per standard procedure. A ditch is made in the
centre of agar plates (2.5 x 0.50 cm) and the formulation (0.5 gm) were filled in the
ditch. The prepared culture loops (culture fungi) were streaked across the agar at a
right angle from the ditch to the edge of the plate. Control plate of marketed
formulation was also prepared. After incubation of 72 hours at 25C the fungal
growth was observed and the percentage inhibition was measured as follows:
Percentage inhibition =
1
2
L
L
x 100
Where L
1
= total length of the streaked fungal culture
L
2
= length of inhibition.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
69
4.4.9 Drug-Polymer Interaction Studies (Infrared Spectroscopy):
The infrared spectra (IR) of miconazole nitrate, IPM, pluronic, lecithin, tween
80, liquid paraffin and some selected formulation was obtained using FTIR (Perkin-
Elenmeyer 1600 Series).
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
70
CHAPTER5
RESULTS
RAW MATERIAL CHARACTERIZATION:
Table-5.1.1: Standardization of Miconazole Nitrate
Sl.
No.
Characteristic IP Limit Observation
1. Description White or almost white,
crystalline or microcrystalline
powder
White micro
crystalline powder
2. Solubility Freely soluble m method
slightly soluble in ethonol
and m chloroprm, very
slightly soluble m water and
in ether
Meets the
specification
3. Identification A IR spectra of sample must be
identified to that of standard
Complies
4. Identification E Melts between 178 to 184
C
180C
5. Loss on drying Not more than 0.5
determined on 1 g by drying
in in an oven at 105 for two
hrs.
0.3%
6. Assay 98.5% to 101.5% w.r.t. dried
substance
99.6% w/w
7. Clarity and coloured
solution
1% w/v solution in methanol
is clear
Clear
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
71
Figure-5.1.1: IR Spectra of Miconazole Nitrate
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
72
Table-5.1.2a: Standardization of Lecithin
Sl. No. Characteristic USP Limit Observation
1. Description Lecithins from viscous
semiliquid to powders
depending upon the freely
fatty acid content, colour
from brown to light yellow.
Light yellow powder
2. Solubility Lecithins are soluble in
aliphatic and aromatic
hydrocarbons, halogenated
hydrocarbons, mineral oil,
and fatty acid.
Meets the limits
Table-5.1.2b: Standardization of tween 80
Sl.
No.
Characteristic USP Limit Observation
1. Description Polysorbates have a
characterization odor and a
worm, some what bitter taste
the colors.
Yellow
2. Solubility Soluble methanol and water
insoluble in solver mineral
oil and vegetable oil.
Meets the limit
3. PH 6-8 7
4. Viscosity -- 425 (mPas)
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
73
Figure-5.1.2a: IR Spectra of Lecithin
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
74
Figure-5.1.2b: IR Spectra of Tween-80
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
75
Table-5.1.2c: Standardization of pluronic (poloxames)
Sl.
No.
Characteristic USP Limit Observation
1. Description White, almost odourless laste
less waxy flakes
White flakes
2. Solubility Freely soluble in water allows,
chloroform pratically insoluble
in propylane glycol
Meets the limits
3. Melting point -- 52C
4. pH 5.0-7.5 7
Table-5.1.2d: Standardization of Isopropyl myristate
Sl.
No.
Characteristic USP Limit Observation
1. Description Isopropyl myristate is a clear,
colorless, practically odourless
mobile liquid with a bland taste.
Meets the limits
2. Solubility Miscible with acetone
chloroform, ethanol ethylacetate,
fats, fatty alcohol, fixed oils,
liquid hydrocarbons. Practicatly
in soluble in propylene glycol
and water
Meets the limits
3. pH Between 6-7 6.5
4. Residue on ignition - 0.1%
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
76
Figure-5.1.2c: IR Spectra of Pluronic
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
77
Figure-5.1.2d: IR Spectra of Isopropyl Myristate
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
78
Table-5.1.2e: Standardization liquid paraffin
Sl.
No.
Characteristic BP Limit Observation
1. Description Mineral oil is transparent
colorless. Viscus liquid,
odorless.
Transparent viscous
liquid
2. Solubility Practically insoluble in
ethanol, and water soluble in
acetone, benzone, ether and
missalbe with volatile oil and
fixed oils.
Meet the limits
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
79
Figure-5.1.2e: IR Spectra of Liquid Paraffin
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
80
5.2 PREPARATION OF MICONAZOLE NITRATE ORGANOGELS
Organogels were prepared as described in methodology (section-4.2)
5.3 CALIBRATION CURVE OF MICONAZOLE NITRATE:
Date 12
th
Aug. 2005
Drug Miconazole Nitrate
Mobile Phase SPB 6.4
Wavelength 272 nm
Unit of concentration mcg/ml
Slope of Calibration curve 77.4256
Constant of calibration curve 1.2403
R of calibration curve 0.9998
Table-5.3: Calibration curve of Miconazole Nitrate
Concentration
(mcg/ml)
Absorbance (nm)
04 0.068
08 0.117
12 0.175
16 0.22
20 0.276
24 0.324
28 0.38
32 0.428
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
81
Figure-5.3: Calibration curve of miconazole nitrate
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0 5 10 15 20 25 30 35
Concentration (mcg/ ml)
A
b
s
o
r
b
a
n
c
e
(
n
m
)
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
82
Table-5.4: Physical Evaluation of Formulations
Sl.
No.
Formulation
code
Spreadability
gm-cm/Sec.
Viscosity in
CPs
pH
Gel life at
25C
1. AM1 50.80 1572.00 6.2 35.00
2. AM2 6.40 3246.00 6.0 100.00
3. AM3 1.91 6495.00 5.6 160.00
4. AM4 1.29 13835.00 5.6 220.00
5. FM1 73.30 1764.00 7.4 34.00
6. FM2 7.10 3596.00 7.0 60.00
7. FM3 1.50 7269.00 6.8 72.00
8. FM4 1.20 14963.00 6.4 130.00
9. SR1 82.50 1874.00 7.4 33.00
10. SR2 6.80 3751.00 7.4 58.00
11. SR3 2.40 7598.00 7.0 80.00
12. SR4 1.40 15863.00 6.8 114.00
13. MN1 86.00 3294.00 4.1 36.00
14. MN2 11.00 8059.00 4.8 54.00
15. MN3 1.20 10260.00 5.6 110.00
16. MN4 0.57 22868.00 6.1 192.00
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
83
Effect of addition of water on turbidity of microemulsion based organogel of
formulations (Gelation kinetics)
Table-5.4.1a: Gelation Kinetics AM
1
Sl.
No.
Lecithin
% w/w
Tween 80
% w/w
Isopropyle
Myristate
% w/w
Water % w/w NTU
1 17.85 8.92 71.42 1.78 5
2 17.54 8.77 70.17 3.50 5
3 17.24 8.62 68.96 5.17 10
4 16.94 8.47 67.79 6.77 15
5 16.66 8.33 66.66 8.33 15
6 16.39 8.19 65.57 9.83 22
7 16.12 8.06 64.51 11.29 25
8 15.87 7.93 63.49 12.69 32
9 15.62 7.81 62.50 14.06 38
10 15.38 7.69 61.53 15.38 42
11 15.15 7.57 60.60 16.66 50
12 14.92 7.46 59.70 17.91 55
13 14.70 7.35 58.82 19.11 62
14 14.49 7.24 57.97 20.28 68
15 14.28 7.14 57.14 21.42 75
16 14.08 7.04 56.33 22.53 75
17 13.88 6.94 55.55 23.61 80
18 13.69 6.84 54.79 24.65 90
19 13.51 6.75 54.05 25.67 95
20 13.33 6.66 53.33 26.66 100
21 7.69 3.84 30.76 57.69 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
84
Table-5.4.1b: Gelation Kinetics AM
2
Sl.
No.
Lecithin
% w/w
Tween 80
% w/w
Isopropyle
Myristate
% w/w
Water % w/w NTU
1 32.79 16.39 49.18 1.64 5
2 32.26 16.13 48.39 3.23 10
3 31.75 15.87 47.62 4.76 10
4 31.25 15.63 46.88 6.25 10
5 30.77 15.38 46.15 7.69 15
6 30.30 15.15 45.45 9.09 22
7 29.85 14.93 44.78 10.45 25
8 29.41 14.71 44.12 11.76 32
9 28.99 14.49 43.48 13.04 50
10 28.57 14.29 42.86 14.29 58
11 28.17 14.08 42.25 15.49 62
12 27.78 13.89 41.67 16.67 68
13 27.40 13.70 41.10 17.81 70
14 27.03 13.51 40.54 18.92 75
15 26.67 13.33 40.00 20.00 79
16 26.32 13.16 39.47 21.05 86
17 25.97 12.99 38.96 22.08 92
18 25.64 12.82 38.46 23.08 100
19 25.32 12.66 37.97 24.05 100
20 25.00 12.50 37.50 25.00 100
21 21.05 10.53 31.58 36.84 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
85
Table-5.4.1c: Gelation Kinetics AM
3
Sl.
No.
Lecithin
% w/w
Tween 80
% w/w
Isopropyle
Myristate
% w/w
Water % w/w NTU
1 45.45 22.73 30.30 1.52 5
2 44.78 22.39 29.85 2.99 10
3 44.12 22.06 29.41 4.41 15
4 43.48 21.74 28.99 5.80 15
5 42.86 21.43 28.57 7.14 20
6 42.25 21.13 28.17 8.45 32
7 41.67 20.83 27.78 9.72 35
8 41.10 20.55 27.40 10.96 45
9 40.54 20.27 27.03 12.16 55
10 40.00 20.00 26.67 13.33 60
11 39.47 19.74 26.32 14.47 68
12 38.96 19.48 25.97 15.58 72
13 38.46 19.23 25.64 16.67 85
14 37.97 18.99 25.32 17.72 90
15 37.50 18.75 25.00 18.75 96
16 37.04 18.52 24.69 19.75 100
17 36.59 18.29 24.39 20.73 100
18 36.14 18.07 24.10 21.69 100
19 35.71 17.86 23.81 22.62 100
20 35.29 17.65 23.53 23.53 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
86
Table-5.4.1d: Gelation Kinetics AM
4
Sl.
No.
Lecithin
% w/w
Tween 80
% w/w
Isopropyle
Myristate
% w/w
Water % w/w NTU
1 14.08 7.04 56.34 1.41 5
2 13.89 6.94 55.56 2.78 5
3 13.70 6.85 54.79 4.11 20
4 13.51 6.76 54.05 5.41 25
5 13.33 6.67 53.33 6.67 25
6 13.16 6.58 52.63 7.89 25
7 12.99 6.49 51.95 9.09 35
8 12.82 6.41 51.28 10.26 45
9 12.66 6.33 50.63 11.39 60
10 12.50 6.25 50.00 12.50 60
11 12.35 6.17 49.38 13.58 65
12 12.20 6.10 48.78 14.63 70
13 12.05 6.02 48.19 15.66 78
14 11.90 5.95 47.62 16.67 80
15 11.76 5.88 47.06 17.65 86
16 11.63 5.81 46.51 18.60 100
17 11.49 5.75 45.98 19.54 100
18 11.36 5.68 45.45 20.45 100
19 11.24 5.62 44.94 21.35 100
20 11.11 5.56 44.44 22.22 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
87
Figure-5.4.1: Effect of addition of water on turbidity of microemulsion based organogel of
formulations AM
1
, AM
2
, AM
3
& AM
4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
88
Table-5.4.2a: Gelation Kinetics FM
1
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Pluronic
aqueous
solution
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 5
3 18.87 75.47 5.66 15
4 18.52 74.07 7.41 28
5 18.18 72.73 9.09 36
6 17.86 71.43 10.71 50
7 17.54 70.18 12.28 45
8 17.24 68.97 13.79 50
9 16.95 67.80 15.25 57
10 16.67 66.67 16.67 65
11 16.39 65.57 18.03 70
12 16.13 64.52 19.35 85
13 15.87 63.49 20.63 90
14 15.63 62.50 21.88 92
15 15.38 61.54 23.08 100
16 15.15 60.61 24.24 100
17 14.93 59.70 25.37 100
18 14.71 58.82 26.47 100
19 14.49 57.97 27.54 100
20 14.29 57.14 28.57 100
21 7.69 30.77 61.54 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
89
Table-5.4.2b: Gelation Kinetics FM
2
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Pluronic
aqueous
solution
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 15
3 18.87 75.47 5.66 25
4 18.52 74.07 7.41 32
5 18.18 72.73 9.09 55
6 17.86 71.43 10.71 65
7 17.54 70.18 12.28 68
8 17.24 68.97 13.79 86
9 16.95 67.80 15.25 92
10 16.67 66.67 16.67 100
11 16.39 65.57 18.03 100
12 9.09 36.36 54.55 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
90
Table-5.4.2c: Gelation Kinetics FM
3
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Pluronic
aqueous
solution
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 15
3 18.87 75.47 5.66 25
4 18.52 74.07 7.41 40
5 18.18 72.73 9.09 68
6 17.86 71.43 10.71 85
7 17.54 70.18 12.28 85
8 17.24 68.97 13.79 92
9 16.95 67.80 15.25 100
10 16.67 66.67 16.67 100
11 16.39 65.57 18.03 100
12 12.50 50.00 37.50 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
91
Table-5.4.2d: Gelation Kinetics FM
4
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Pluronic
aqueous
solution
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 55
3 18.87 75.47 5.66 60
4 18.52 74.07 7.41 85
5 18.18 72.73 9.09 100
6 17.86 71.43 10.71 100
7 17.54 70.18 12.28 100
8 17.24 68.97 13.79 100
9 16.95 67.80 15.25 100
10 16.67 66.67 16.67 100
11 16.39 65.57 18.03 100
12 14.29 57.14 28.57 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
92
Figure-5.4.2: Effect of addition of water on turbidity of microemulsion based organogel of
formulations FM
1
, FM
2
, FM
3
& FM
4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
93
Table-5.4.3a: Gelation Kinetics SR
1
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Water
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 10
3 18.87 75.47 5.66 15
4 18.52 74.07 7.41 15
5 18.18 72.73 9.09 15
6 17.86 71.43 10.71 25
7 17.54 70.18 12.28 32
8 17.24 68.97 13.79 40
9 16.95 67.80 15.25 48
10 16.67 66.67 16.67 56
11 16.39 65.57 18.03 60
12 16.13 64.52 19.35 72
13 15.87 63.49 20.63 80
14 15.63 62.50 21.88 83
15 15.38 61.54 23.08 89
16 15.15 60.61 24.24 89
17 14.93 59.70 25.37 92
18 14.71 58.82 26.47 92
19 14.49 57.97 27.54 95
20 14.29 57.14 28.57 100
21 9.09 36.36 54.55 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
94
Table-5.4.3b: Gelation Kinetics SR
2
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Water
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 10
3 18.87 75.47 5.66 15
4 18.52 74.07 7.41 22
5 18.18 72.73 9.09 25
6 17.86 71.43 10.71 25
7 17.54 70.18 12.28 30
8 17.24 68.97 13.79 38
9 16.95 67.80 15.25 45
10 16.67 66.67 16.67 52
11 16.39 65.57 18.03 60
12 16.13 64.52 19.35 72
13 15.87 63.49 20.63 80
14 15.63 62.50 21.88 85
15 15.38 61.54 23.08 92
16 15.15 60.61 24.24 100
17 14.93 59.70 25.37 100
18 14.71 58.82 26.47 100
19 14.49 57.97 27.54 100
20 14.29 57.14 28.57 100
21 8.33 33.33 58.33 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
95
Table-5.4.3c: Gelation Kinetics SR
3
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Water
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 15
3 18.87 75.47 5.66 25
4 18.52 74.07 7.41 30
5 18.18 72.73 9.09 30
6 17.86 71.43 10.71 50
7 17.54 70.18 12.28 65
8 17.24 68.97 13.79 76
9 16.95 67.80 15.25 85
10 16.67 66.67 16.67 100
11 14.71 58.82 26.47 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
96
Table-5.4.3d: Gelation Kinetics SR
4
Sl.
No.
Lecithin
%w/w
Isopropyle
Myristate
%w/w
Water
%w/w
NTU
1 19.61 78.43 1.96 5
2 19.23 76.92 3.85 20
3 18.87 75.47 5.66 25
4 18.52 74.07 7.41 30
5 18.18 72.73 9.09 45
6 17.86 71.43 10.71 50
7 17.54 70.18 12.28 65
8 17.24 68.97 13.79 87
9 16.95 67.80 15.25 98
10 16.67 66.67 16.67 100
11 16.39 65.57 18.03 100
12 15.38 61.54 23.08 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
97
Figure-5.4.3: Effect of addition of water on turbidity of microemulsion based organogel of formulations SR
1
, SR
2
, SR
3
& SR
4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
98
Table-5.4.4a: Gelation Kinetics MN
1
Sl.
No.
Lecithin
%w/w
Liquid
Paraffin
%w/w
Water
%w/w
NTU
1 19.60 78.43 1.96 5
2 19.23 76.92 3.84 30
3 18.86 75.47 5.66 65
4 18.51 74.07 7.40 72
5 18.18 72.72 9.09 100
6 13.69 54.79 31.50 100
Table-5.4.4b: Gelation Kinetics MN
2
Sl.
No.
Lecithin
%w/w
Liquid
Paraffin
%w/w
Water
%w/w
NTU
1 19.60 78.43 1.96 10
2 19.23 76.92 3.84 35
3 18.86 75.47 5.66 70
4 18.51 74.07 7.40 85
5 18.18 72.72 9.09 100
6 16.12 64.51 19.35 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
99
Table-5.4.4c: Gelation Kinetics MN
3
Sl.
No.
Lecithin
%w/w
Liquid
Paraffin
%w/w
Water
%w/w
NTU
1 19.60 78.43 1.96 5
2 19.23 76.92 3.84 15
3 18.86 75.47 5.66 35
4 18.51 74.07 7.40 65
5 18.18 72.72 9.09 100
6 16.66 66.66 16.66 100
Table-5.4.4d: Gelation Kinetics MN
4
Sl.
No.
Lecithin
%w/w
Liquid
Paraffin
%w/w
Water
%w/w
NTU
1 19.60 78.43 1.96 5
2 19.23 76.92 3.84 25
3 18.86 75.47 5.66 45
4 18.51 74.07 7.40 65
5 18.18 72.72 9.09 100
6 17.24 68.96 13.79 100
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
100
Figure-5.4.4: Effect of addition of water on turbidity of microemulsion based organogel of
formulations MN
1
, MN
2
, MN
3
& MN
4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
101
Table-5.4.5: Gelation Kinetic Data
Sl. No. Formulation Code
Water gm% at
maximum NTU
1. AM
1
26.60
2. AM
2
23.07
3. AM
3
19.75
4. AM
4
18.60
5. FM
1
23.07
6. FM
2
16.60
7. FM
3
15.25
8. FM
4
9.09
9. SR
1
28.50
10. SR
2
24.20
11. SR
3
16.60
12. SR
4
16.60
13. MN
1
9.09
14. MN
2
9.09
15. MN
3
9.09
16. MN
4
9.09
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
102
Table-5.4.6: Percentage drug contents of Organogels
Sl.
No.
Formulation Absorbence
% Drug
content
1. AM1 0.279 101.00
2. AM2 0.27 97.82
3. AM3 0.275 99.63
4. AM4 0.272 98.55
5. FM1 0.27 97.82
6. FM2 0.275 99.63
7. FM3 0.276 100.00
8. FM4 0.279 101.00
9. SR1 0.272 98.55
10. SR2 0.275 99.63
11. SR3 0.279 101.00
12. SR4 0.276 100.00
13. MN1 0.272 98.55
14. MN2 0.275 99.63
15. MN3 0.27 97.82
16. MN4 0.276 100.00
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
103
5.4.7a In Vitro Percentage Drug Release
Name of the drug Miconazole Nitrate
Loading dose (mg) 20
Total No. of readings including zero time reading 13
Diffusion medium SPB 6.4
RPM 50
Volume of diffusion media (ml) 45
Volume of sample removed (ml) 1
Dilution factor 5
Slope of calibration curve 77.4256
Constant of calibration curve 1.2403
R of calibration curve 0.9998
Table-5.4.7a: In vitro percentage drug release of
formulations AM
1
, AM
2
, AM
3
and AM
4
Average % Drug Release
Sl. No.
Time
(Hours)
AM
1
AM
2
AM
3
AM
4
1 00 0.000 0.000 0.000 0.000
2 01 4.092 1.131 0.002 0.437
3 02 12.981 2.898 1.828 0.163
4 03 20.410 5.314 7.181 1.996
5 04 24.600 10.656 9.518 4.566
6 05 36.104 24.825 17.738 7.280
7 06 43.322 29.722 22.740 9.442
8 07 47.025 34.106 25.667 16.352
9 08 52.967 40.925 30.564 21.757
10 09 67.991 46.573 36.777 25.096
11 10 76.009 53.635 45.815 27.889
12 11 94.800 58.218 47.981 32.126
13 12 96.815 62.357 52.438 35.573
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
104
Figure-5.4.7a: In vitro percentage drug release of
formulations AM
1
, AM
2
, AM
3
and AM
4
0
20
40
60
80
100
120
0 2 4 6 8 10 12
Time (Hrs)
P
e
r
c
e
n
t
D
r
u
g
R
e
l
e
a
s
e
d
AM1 AM2 AM3 AM4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
105
5.4.7b In Vitro Percentage Drug Release
Name of the drug Miconazole Nitrate
Loading dose (mg) 20
Total No. of readings including zero time reading 13
Diffusion medium SPB 6.4
RPM 50
Volume of diffusion media (ml) 45
Volume of sample removed (ml) 1
Dilution factor 5
Slope of calibration curve 77.4256
Constant of calibration curve 1.2403
R of calibration curve 0.9998
Table-5.4.7b: In vitro percentage drug release of
formulations FM
1
, FM
2
, FM
3
and FM
4
Average % Drug Release
Sl. No.
Time
(Hours)
FM
1
FM
2
FM
3
FM
4
1 00 0.000 0.000 0.000 0.000
2 01 1.566 0.434 0.176 0.524
3 02 7.176 4.625 3.827 1.990
4 03 15.522 7.689 6.438 3.690
5 04 22.483 8.902 12.241 6.036
6 05 35.688 10.226 18.257 7.212
7 06 39.419 26.122 23.094 13.290
8 07 45.656 27.732 25.156 16.451
9 08 64.904 38.947 26.991 19.153
10 09 70.448 43.517 29.290 22.517
11 10 75.736 50.871 37.813 24.205
12 11 83.722 70.997 42.679 28.011
13 12 94.988 74.388 45.197 30.670
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
106
Figure-5.4.7b: In vitro percentage release of
formulations FM
1
, FM
2
, FM
3
and FM
4
0
10
20
30
40
50
60
70
80
90
100
0 2 4 6 8 10 12
Time (Hrs)
P
e
r
c
e
n
t
D
r
u
g
R
e
l
e
a
s
e
d
FM1 FM2 FM3 FM4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
107
5.4.7c In Vitro Percentage Drug Release
Name of the drug Miconazole Nitrate
Loading dose (mg) 20
Total No. of readings including zero time reading 13
Diffusion medium SPB 6.4
RPM 50
Volume of diffusion media (ml) 45
Volume of sample removed (ml) 1
Dilution factor 5
Slope of calibration curve 77.4256
Constant of calibration curve 1.2403
R of calibration curve 0.9998
Table-5.4.7c: In vitro percentage drug release of
formulations SR
1
, SR
2
, SR
3
and SR
4
Average % Drug Release
Sl. No.
Time
(Hours)
SR
1
SR
2
SR
3
SR
4
1 00 0.000 0.000 0.000 0.000
2 01 0.260 0.002 0.437 0.524
3 02 2.269 2.873 2.253 1.990
4 03 5.716 4.069 6.223 4.212
5 04 10.546 7.207 8.135 6.918
6 05 22.274 10.214 10.532 9.769
7 06 29.480 23.647 17.382 10.938
8 07 33.316 26.866 20.982 17.965
9 08 41.078 32.149 21.693 21.310
10 09 46.210 36.403 25.458 23.239
11 10 52.829 46.050 27.815 26.334
12 11 55.481 50.233 29.691 28.094
13 12 59.213 55.533 32.118 29.706
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
108
Figure-5.4.7c: In vitro percentage drug release of
formulations SR
1
, SR
2
, SR
3
and SR
4
0
10
20
30
40
50
60
70
0 2 4 6 8 10 12
Time (Hrs)
P
e
r
c
e
n
t
D
r
u
g
R
e
l
e
a
s
e
d
SR1 SR2 SR3 SR4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
109
5.4.7d In Vitro Percentage Drug Release
Name of the drug Miconazole Nitrate
Loading dose (mg) 20
Total No. of readings including zero time reading 13
Diffusion medium SPB 6.4
RPM 50
Volume of diffusion media (ml) 45
Volume of sample removed (ml) 1
Dilution factor 5
Slope of calibration curve 77.4256
Constant of calibration curve 1.2403
R of calibration curve 0.9998
Table-5.4.7d: In vitro percentage drug release of
formulations MN
1
, MN
2
, MN
3
and MN
4
Average % Drug Release
Sl. No.
Time
(Hours)
MN
1
MN
2
MN
3
MN
4
1 00 0.000 0.000 0.000 0.000
2 01 0.089 0.176 0.437 0.524
3 02 1.913 1.301 0.076 0.596
4 03 3.697 3.246 1.558 1.568
5 04 4.214 4.973 3.510 3.171
6 05 5.960 6.126 4.284 5.331
7 06 8.091 11.658 6.380 5.970
8 07 10.005 13.042 8.521 7.752
9 08 19.014 13.755 10.619 9.310
10 09 21.075 15.609 16.070 10.987
11 10 23.261 18.195 18.327 18.096
12 11 27.227 20.396 20.191 19.436
13 12 30.051 23.857 22.000 20.971
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
110
Figure-5.4.7d: In vitro percentage drug release of
formulations MN
1
, MN
2
, MN
3
and MN
4
0
5
10
15
20
25
30
35
0 2 4 6 8 10 12
Time (Hrs)
P
e
r
c
e
n
t
D
r
u
g
R
e
l
e
a
s
e
d
MN1 MN2 MN3 MN4
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
111
Table-5.4.8: In Vitro Antifungal Activity of Miconazole Nitrate Organogels
Zone of Inhibition (mm)
Formulation Code
Candida Albicans P Chysogenum A Niger
Marketed 9.00 11.00 14.00
AM
1
11.00 13.00 15.00
FM
1
10.00 12.00 14.00
SR
1
9.00 11.00 12.00
MN
1
6.00 8.00 10.00
* mean of three readings.
Figure-5.4.8: Comparative Antifungal Activity of Selected Miconazole Nitrate
Organogel Formulations with Control
5.4.9 Drug Polymer Interactions:
The IR spectra of formulations AM
1
, FM
1
, SR
1
, MN
1
are depicted in figure
5.4.9. a,b,c,d.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
112
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
114
Figure-5.4.9a: IR Spectra of AM
1
Organogel
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
115
Figure-5.4.9b: IR Spectra of FM
1
Organogel
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
116
Figure-5.4.9c: IR Spectra of SR
1
Organogel
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
117
Figure-5.4.9d: IR Spectra of MN
1
Organogel
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
118
CHAPTER6
DISCUSSION
Lecithin, a biocompatible material has recently gained wide popularity for
development of better drug delivery system. In present study, lecithin along with
Tween-80, pluronic, isopropyl myristate and liquid paraffin were used for the
development of lecithin-microemulsion based organogels for the topical delivery of
miconazole nitrate. Miconazole nitrate is a synthetic imidazole derivative with
molecular formula C
18
H
14
N
2
CH NO
3
and practically insoluble in water. Miconazole
nitrate was obtained from Adelphi A/c Sigma Lab., Goa and its standards complied
with IP limits (table-5.1.1). Lecithin, pluronic, tween-80 and isopropyl myristate
complied with USP XXV standards (table-5.1.2a, b, c and d). Liquid paraffin
complied with the BP standards (table-5.1.2.e).
Lecithin organogels based systems were prepared by three different methods.
In the first method lecithin and tween-80 in different compositions were dissolved in
isopropyl myristate (IPM) after incorporation of drug gelation was achieved on
addition of the aqueous phase (table-4.2.1).
In the second method, pluronic lecithin organogels (PLOs) were prepared
employing lecithin, isopropyl myristate and pluronic in water (table-4.2.2).
The third method was based on preparation of lecithin solution by employing
isopropyl myristate (IPM) followed by incorporation of drug. Addition of aqueous
phase resulted in the lecithin organogel system (table-4.2.3). Finally hot melt type
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
119
organogels were prepared by using lecithin and liquid paraffin in various
concentration. Water was added and gelation was achieved on cooling (table-4.2.4).
The parameters studied included pH, spreadability, viscosity and gel life
(stability). The results indicated change in pH with change in composition of lecithin
organogel. An increase in percent of lecithin resulted in increase in viscosity and
decrease in the spreadability of lecithin organogels. An organogel (MN
4
) prepared by
hot melt technique was found to possess highest viscosity i.e., 22868 CPs and lowest
spreadability of 0.57 gm-cm/sec, whereas AM
1
lecithin microemulsion based
organogel containing tween-80 (3.77%) showed lowest viscosity 1572 CPs. Gel
stability studies indicated that organogels with higher viscosity were more stable than
less viscous gels. MN
4
hot melt type organogels containing 67.61% of lecithin
produce the stable gel 192 hours at 25 (table-5.4).
The physical stability of an organogel to be used as drug delivery system is
important as the gel should retain a structure during storage for uniform consistency
during use and for reproducible release profile. Lower the amount of lecithin less
stable the organogels were formed. Organogels containing lecithin and Tween-80
were found to be the most stable, tweens with polyoxy ethylene chain precipitate in
tubular aggregates of sorbiton monostearate and thus stabilize the organogel further
AM
4
showed highest gel-life of 220 hours at 25C. The general trend in gel life
observed was AM
4
>MN
4
>FM
4
>SR
4
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
120
Gelation kinetics studied using nephalo-turbidimeter produce interesting
results the amount water required for gelation was found to be less as the amount of
lecithin increased in the organogel system. SR
1
organogel containing lecithin and
IPM showed maximum gelation on addition of 28.5 gm% of water. Organogels
produced by hot melt method were an exceptional. The amount of water required for
maximum gelation was observed to be a constant i.e., 9.09 gm% (table-5.4.5).
Percent drug content of organogels were determined using calibration curve of
miconazole nitrate in phosphate buffer pH 6.4 at 272 nm. Percent drug content was
found to be between 97.82 and 101% (table-5.4.6).
In vitro release of miconazole nitrate from the organogels was determined
using the method suggested by Chowdary et al
41
. Phosphate buffer pH 6.4 was
employed as diffusion medium in the receptor compartment the organogels
containing lecithin and tween-80 showed retarded release with an increase in the
amount of lecithin. The order of release may be given as AM
1
>AM
2
>AM
3
>AM
4
(table-5.4.7a). Highest release is shown by AM
1
formulation. Similar results were
observed for organogels containing lecithin and pluronic FM
1
>FM
2
>FM
3
>FM
4
with
FM
1
showing highest release 94.98% (Table-5.4.7b). Formulation containing only
lecithin showed a comparatively low percent release of miconazole nitrate in the
order SR
1
>SR
2
>SR
3
>SR
4
. SR
1
showing highest release 59.21% (Table-5.4.7c). The
organogels prepared by hot melt method showed the least percent drug release in the
order MN
1
>MN
2
>MN
3
>MN
4
with MN
1
showing highest release of 30.05% (Table-
5.4.7d).
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
121
It may be noted that the organogel formulations with higher viscosity showed
less release of miconazole nitrate. Since the number and dimension of aqueous
region available for diffusion of the drug are reduced with the possible increase in
micro viscosity in the three dimensional structure of the gel.
In vitro antifungal activity of miconazole nitrate organogels were determined
by Ditch plate method using best release formulation from amongst the four different
types of organogels. A marked preparation was used as a control for comparison the
maximum zone of inhibition studies indicated highest antifungal activity for lecithin,
Tween-80 formulation. AM
1
followed by lecithin, pluronic formulation FM
1
, SR
formulation containing only lecithin showed comparatively less antifungal activity
whereas organogel prepared by hot melt method were found to have least antifungal
activity. The antifungal activity against Candida albicans, Penicillin P.chrysogenum,
Aspergillus niger was in the order AM
1
>FM
1
>Marketed>SR
1
>MN
1
. The in vitro
antifungal activity significantly correlated with the in vitro release of miconazole
nitrate from the mentioned formulation (table-5.4.8).
FTIR of miconazole nitrate showed principle peaks at wavelength 1089, 1313,
827, 812 cm
1
. FTIR studies were also done for lecithin, tween-80, pluronic, IPM,
liquid paraffin and formulation AM
1
, FM
1
, SR
1
and MN
1
as shown in figure
respectively.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
122
CHAPTER7
CONCLUSION
Lecithin microemulsion based organogel have currently generated great
interest as topical and transdermal drug delivery vehicle. In the present investigation
lecithin-microemulsion based organogels were formulated using four different
methods employing lecithin and tween-80, pluronic, and also preparing the
organogels using hot melt method where mineral oil (liquid paraffin) was used. From
the studies, the following conclusions can be drawn.
1. Lecithin organogels can be prepared easily and rapidly and can be obtained
with biocompatible components.
2. Change in composition of the lecithin organogels resulted in a change in pH.
3. With an increase in amount of lecithin viscosity of the organogels were found
to increase resulting in decrease spreadability MN
4
formulation showed
maximum viscosity 22868 CPs, whereas AM
1
formulation containing tween-
80 (3.77%) was least viscous 15726 CPs.
4. Organogels with higher viscosity were found to be more stable. Organogel
containing lecithin and tween 80 were the more stable AM
4
showed highest
gel life of 220 hours at 25C.
5. 28.5 gm% of water was required to produce maximum gelation in SR
1
organogels containing lecithin and IPM. Organogels produced by hot melt
method required least amount of water for maximum gelation 9.09 gm%.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
123
6. In vitro release of miconazole nitrate from organogel showed that organogels
containing lecithin and tween 80 in IPM (AM
1
) gave highest release. The
order of release for the best releasing formulation prepared by different
methods was AM
1
>FM
1
>SR
1
>MN
1
.
7. In vitro antifungal activity of miconazole nitrate organogels against Candida
albicans, P.chrysogenum, A. niger was in the order AM
1
>FM
1
> Mkt>
SR
1
>MN
1
.
8. In vitro antifungal activity significantly correlated with in vitro release of
miconazole nitrate.
Thus, finally it may be concluded that lecithin-microemulsion based
organogel have good potential as carrier for topical delivery of antifungal agent such
as miconazole nitrate.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
124
CHAPTER8
SUMMARY
Lecithin organogels are readily obtained by adding a minimal amount of water
to a solution of lecithin in organic solvents. Surprisingly high viscosities can be
achieved in various organic solvent on addition of water. Such systems have
currently generated great interest as topical and transdermal drug delivery carriers.
In the present study, lecithin based microemulsions were formulated as topical
carriers for miconazole nitrate, an antifungal agent, a synthetic imidazole derivative
with molecular formula C
18
H
14
C
14
CHNO
3
and practically insoluble in water.
The need for the study and objective of the present study have been discussed
extensively in chapter-2. The scheme of work was divided into V-phases. Initially
collection of theoretical and technical data by extensive literature survey, review of
literature and drug profile is presented in chapter-3. This was followed by
procurement of material. Miconazole nitrate was obtained rom Adelphi A/c Sigma
Lab, Goa and standardized as per IP. Standardization of all other material were done
and they met the pharmacopoeial and other established standards.
Lecithin based organogel systems were prepared by three different methods
employing lecithin, tween-80, pluronic, isopropyl myristate was used as organic
solvent whereas gelation was achieved on addition of aqueous phase. Organogels
were also prepared by hot melt method using lecithin in liquid paraffin as non-
aqeuous phase.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
125
Various parameters were evaluated such as pH, spreadability, viscosity,
gelation kinetics and gel life.
From the study, it was observed that:
1. Lecithin organogels can be prepared easily and rapidly and can be obtained
with biocompatible components.
2. Change in composition of the lecithin organogels resulted in a change in pH.
3. With an increase in amount of lecithin viscosity of the organogels were found
to increase resulting in decrease spreadability. MN
4
formulation shown
maximum viscosity 22868 CPs whereas AM
1
formulation containing tween-
80 (3.77%) was least viscous 15726 CPs.
4. Organogels with higher viscosity were found to be more stable. Organogels
containing lecithin and tween-80 were the more stable (AM
4
) showed highest
gel life of 220 hours at 25.
5. 28.5 gm% of water was required to produce maximum gelation in SR
1
organogels containing lecithin and IPM. Organogels produced by hot melt
method required least amount of water for maximum gelation 9.09 gm%.
6. In vitro release of miconazole nitrate from organogel showed that organogels
containing lecithin and tween-80 in IPM (AM
1
) gave highest release. The
order of release for the best releasing formulation prepared by different
methods was AM
1
>FM
1
>SR
1
>MN
1
.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
126
7. In vitro antifungal activity of miconazole nitrate organogels against Candida
albicans, P. chrysogenum, A. niger was in the order AM
1
>FM
1
>Mkt>
SR
1
>MN
1
.
8. In vitro antifungal activity significantly correlated with in vitro release of
miconazole nitrate.
Thus, finally it may be concluded that lecithin microemulsion based organogel
have good potential as carrier for topical delivery of antifungal agent such as
miconazole nitrate.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
127
CHAPTER9
BIBLIOGRAPHY
1. Chowdary KPR, Madhavi BLR. Novel drug delivery technologies for
insoluble drugs. Indian Drugs, 2005 Sept; Vol. 42(9): 557-575.
2. Chowdary KPR, Vijayasrinivas S. Biopharmaceutical classification system.
The Indian Pharmacist, 2004; 111: 30, 7.
3. The Theory and Practice of Industrial Pharmacy. 3
rd
Edition by Leon L,
Herbert AL and Joseph LK, Published by Varghese Publishing House,
Bombay, P-221: 1987.
4. Tejal Shah. Lipid emulsions as drug delivery systems An overview. The
Pharma Review, 2005 Jan-Feb: 41-48.
5. Sojan Jose, Kulkarni PK. Self-emulsifying drug delivery system (SEDDS):
Review. Indian J. Pharma. Educ 2002 oct-Dec; 36(4): 184-90.
6. Jayne Lawrence M, Gareth D, Rees. Microemulsion-based media as novel
drug delivery systems. Advanced Drug Delivery Reviews, 2000; 45:
89-121.
7. Tamilvanan S. Oil-in-water lipid emulsion: implications for parenteral and
ocular delivering systems. Progress in Lipid Research 2004; 43: 489-
533.
8. Pharmaceutical Dosage Form and Drug Delivery System. 5
th
Edition edited by
Howard C Ansel Published by Lea & Febiger, London, 307; 1990.
9. Remington The Science and Practice of Pharmacy, 9
th
Edition, Vol. 2;
Mack Publishing Company, Easton, Pennsylvania, P-1577; 1995.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
128
10. Mehta R. Topical and Transdermal Drug Delivery: What a Pharmacist Need
to Know. http://www.inetce.org.
11. Popli N, Sharma SN. Transdermal drug delivery system. The Eastern
Pharmacist, 1990 May; 41-47.
12. Klingman MD. A biological brief on percutaneous absorption. Drug
Development & Industrial Pharmacy, 1983; 9(4): 521-560.
13. Walters KA. Transdermal drug delivery in: Topics in Pharmacy, Routes of
Drug Administration. Fluorence AT, Salole EG (Ed), Wright 78-135:
1990.
14. http://www.xenexlab.com/technical % 20 information.ihtml
15. Aboofazeli R, Mortazavi SA, Khoshnevis P. In vitro release study of sodium
salicylate from lecithin based phospholipid microemulsion. Iranian
Journal of Pharmaceutical Research, 2003; 95-101.
16. Encyclopedia of Pharmaceutical Technology, 2
nd
Edition edited by Graham
Nairn J, Published by Marcel Dekker Inc., New York, Vol. 15; 4-61;
1987.
17. Kulkarni PK and Pradeep Kaatgi. Emulsion-gels as topical drug delivery
vehicle A Review. Indian J. Pharm. Educ 2002 Jul-Sept; 36(6): 119-
123.
18. R Chien YW. Logics of transdermal controlled drug administration. Drug
Development & Industrial Pharmacy 1983; 9(4): 497-520.
19. R Cairns RJ. Anatomy Development & Immunologic of the Skin, In:
Dermatology, Current Concept & Practice, Smith HP, Cairn RJ (Eds.),
3
rd
Edition, Published by Butte Wostber & Co. Ltd., P-1-10: 1981.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
129
20. Aulton ME. Pharmaceutics: The Science of Dosage Form Design, 2
nd
Edition,
Published by Churchill Livingstone, London, P-503: 2002.
21. Medical Microbiology, Mackie R and McCartney. 3
rd
Edition Published by
ELBS Publication, Vol. 1; P-541-544: 1984.
22. Microbiology, edited by Michael J, Pelgar J L, Scham EC, Kring NR, 5
th
Edition Published by Tata McGraw Hill Publishing Co. Ltd., New
Delhi P-345-360.
23. Pharmacology Edited by R Rang HP, Dale MM, Ritter JN, 4
th
Edition, P-723:
1999.
24. Meying Ning, Zhong Wei GU, Nuaizhong Pan, Heining YU and Kai Xiao.
Preparation and in vitro evaluation of liposomes, niosomes delivery
system for antifungal drug clotrimazole. Indian Journal of
Experimental Biology, 2005 Feb; Vol. 43: 150-157.
25. Kumar R, Katare OP. Lecithin organogels as a potential phospholipids
structural system for topical drug delivery A review. AAPS Pharma
Sci. Tech. 2005 April; 1-47, www.aaps.pharm.sci.tech.com.
26. Murdan S, Gregoriadis G, Florescence AT. Inverse toroidal vesicles:
Precursor of tubules in sorbitan monostearate organogels.
International Journal of Pharmaceutics, 1999; 183: 47-49.
27. Lecithin organogels as model carrier of pharmaceutical; Edited by
Zoumpanioti M, Karavas E, Skopelilis C published by Progress in
Colloid and Polymer Science. Springer-Verlag Heidelberg, Vol. 123;
2004.
28. Scartazzini R and Loisi PC. Organogels from lecithin. The Journal of
Physical Chemistry 1988, Vol. 92(3): 829-833.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
130
29. Nasseri AA , Aboofazeli R, Zia H, Thomas E Needham. Lecithin-stabilized
microemulsions- based organogels for topical application of ketorolac
tromethimine II. In vitro release study. Iranian Journal of
Pharmaceutical Research, 2003 May; 117-124. www.ijpr-online.com.
30. William N, Walde P, Luisi L, Gazzaniga A, Stroppolo F. Lecithin organogels
as matrix for transdermal transport of drugs. Journal of
Pharmaceutical Sciences, 1992 Sept., Vol. 81, No. 9: 871-874.
31. Hoffmann G, Marks SL, Taboada J. Hosgood GL, Wolfsheiner KJ.
Transdermal methimazole treatment in cats with hyperthyroidism.
Journal of Feline medicine and surgery, 2003; 5: 27-82.
32. Handbook of Pharmaceutical Excipients, edited by Ainley Wade and Paul J
Waller. 2
nd
Edition, American Pharmaceutical Association,
Washington published by Pharmaceutical Press, London, 257-268,
352, 314, 316, 375: 1994.
33. Lecithin-organogels for topical delivery of drug. www.rx.ugr/ organogel
_413htm.
34. Valenta C, Wanka M, Heidlas J. Evaluation of novel soya-lecithin
formulation for dermal use containing ketoprofen as a model drug.
Journal of Controlled Release, 2000 (63): 165-173.
35. Murdan S. A review of pluronic lecithin organogel as topical and transdermal
drug delivery systems. Hospital Pharmacist 2005 July-Aug: 267-270.
36. Crandall W, Iso T. Topical moisturizing composition and method. US Patent
6 316 428; 2001: www.uspto.orgn.
37. Nasseri AA, Aboofazeli R, Zia H, Thomas E Needham. Lecithin-stabilized
microemulsion: An organogel for topical application of ketorolac
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
131
tromethamine I: Phase behaviour studies. Iranian Journal of
Pharmaceutical Research 2003 Feb. www.ijpr-online.com.
38. Shelke VB, Thopte K, Wawde GM, Pisal SS, Kadam SS. Nasal delivery of
propranolol hydrochloride for sorbitan monostearate organogels:
Preformulation study. Indian J. Pharm. Sci, 2005; 67(2): 200-205.
39. Bentley LB, Marchetti J M, Ricardo N, Ali Abi Z, Collett JH. Influence of
lecithin on some physical-chemical properties of ploxamer gels,
rheological, microscopic and in vitro permeation studies. International
Journal of Pharmaceutics 1999; 193: 49-55.
40. Jean Christopher Leroux. In situ-forming pharmaceutical organogels based on
the self assembly of C-alanine derivatives. www.insitu forming/
organogels. Com.
41. Shrikande BK and Goupale DC. Development and evaluation of anti-
inflammatory oleogels of gurgul and curcumma long, 2001 Dec. Vol.
38(120: 613-616.
42. Williamann HL, Luisi PL. Lecithin organogels as matrix for the transdermal
transport of drugs. Biochemical and Biophysical Research
Communication 1991 June; Vol. 177(3): 897-900.
43. Dreher F, Walde P, Walter P, Wehli E, Interaction of a lecithin microemulsion
gel with stratum corneum and its effect on transdermal transport. J.
Control Release 1997; 95: 131-140.
44. Crandall, Wilson T. Method for topical treatment of scars with protein kinase
C inhibitor. US Patent 6 306 2001. www.uspto.org.
45. Ford Peter R. Topical analgesic composition containing cream type carriers.
US Patent Appl Publ. 2002028789, 2002. www.uspto.org
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
132
46. Aboofazeli RZ, Hossein N, Thomas EN. Transdermal delivery of nicardipine:
An approach to in vitro permeation enhancement. Drug Delivery
2002; 9(4): 239-247.
47. Charles C, Mallhew D. Cardiac glycosides for treating muscle pain and
spasm. US Pat Appl Publ. 20030229029 (2003). www.uspto.org
48. Keith Anderson. Pharm PK Diffusimax-Trans-dermal delivery, 1999 June.
www.david.at.boomer.org.
49. Diffuse.max organogels. USA. www.pharm-max.com.
50. Drher F, Walde P, Luisi PL, Elsner P. Human skin irritation studies of a
lecithin microemulsion gel and liposomes. Skin Pharmcol 1996; 9:
124-129.
51. Jovanovi J, Adnadjevi B, Ostoji S. An investigation of the dehydration of
super absorbing polyacrylic hydrogels. http://0-87849-940-7.
Scientific.net.
52. Murdan S, Gregonidis G, Florance AT. Non-ionic surfactant based
organogels incorporating niosomes. STP Pharma Sciences 1996; 6(1):
44-48.
53. Stephens JS. Flow properties of aluminum soap-hydrocarbon systems.
Journal of Pharmacy & Pharmacology 1971 Oct; 23: 774-780.
54. Martindale, The Complete Drug Reference, 33
rd
Edition, Edited by Sean C
Sweetman Published by Pharmaceutical Press UK; 391: 2002.
55. Principles of Pharmacology, Basic Concept & Clinical Applications, Edited
by Munson Paul and Muller Robert A, Breese George R, Published by
Champman & Hall, New York; P-1242: 1995.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
133
56. Pharmacology, Edited by Mycek Mary J, Harvey Richard A, Champe
Pamalela C, 2
nd
Edition Published By Lippincott Williams & Wilkins,
USA; P-340: 2000.
57. Medicines Compendium, Published by Data Pharm Communication, Britian;
500: 2003.
58. The Pharmacological Basis of Therapeutics, Edited by Joel Hardmann G,
Limbird Lee E, Goodman & Gilmann, 10
th
International Edition
Published by McGraw Hill, New York; P-1811: 2001.
59. Rajani V, Verma PR. In vitro study of ibuprofen from ointment base. Indian
Journal of Pharmaceutical Sciences, 1995; 57(1): 1-6.
60. Rani SH, Kusumadevi V, Sara Sija Suresh, Praveen S and Thomas A.
Formulation and evaluation of clotrimazole creams. Indian Journal of
Pharmaceutical Sciences 1999 April; 113-115.
61. Gondaliya DP, Kakshunduk P. Studies on permeation, characterization and
transdermal permeation of nimesulide from aqueous and emulgel.
Indian Drugs 2002; 39(9): 465-473.
62. Ilango R. Formulation and evaluation of nimesulide gel. The Eastern
Pharmacist 1998 Nov; 123-125.
63. Magdy I Mohammed. Optimization of chlorphensin emulgel formulation.
The AAPS Journals 2004; 3: 1-7.
64. Wafee NA and Ismail FA. Preparation and in vitro evaluation of
mucoadhesive buccal patches of miconazole nitrate. International
Journal of Pharm. 2003 Oct; 264(1-2): 104.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
134
65. Dibase MD, Rhodes CT. Formulation and evaluation of epidermal growth
factor in pluronic F-127 gel. Drug Development & Industrial
Pharmacy, 1996; 22(8): 823-831.
66. Minghetti P, Cilunzo F. Dermal patch for controlled release of miconazole
nitrate influence of drug on the technological characterization. Drug
Development & Industrial Pharmacy 1999; 25(5): 679-684.
67. Khurana R, Ahuja A. Preparation and evaluation of buccoadhesive tablet of
miconazole nitrate. The Eastern Pharmacist 2000; 5: 117-119.
68. David H, Josephine A. A New formulation of miconazole nitrate in the
treatment of vulvovaginal candidiasis. Advances in Therapy 1998; 1-
15: 14-22.
69. Aboofazeli R, Mostazavi SA, Kohohevis P. Phase diagrams of lecithin-base
microemulsion containing sodium salicylate. Iranian Journal of
Pharmaceutical Research 2003 May: 1-9. www.ijpr-online.com.
70. Ranjit Singh and Vyas SP. Lecithin organogels: Potential as topical vehicles.
Indian Drugs 1994 June; 31(9): 449-450.
71. Moreno MA, Ballesterok MP, Frutos P. Lecithin based oil-in-water
microemulsion for parenteral use: Pseudotertiary phase diagram,
characterization and toxicity studies. Journal of Pharmaceutical
Sciences 2003; July; 92(7): 1428-1437.
72. Kantaria S, Gareth D, Rees, Lawrence MJ. Formulation of electrically
conducting microemulsion-based organogels. International Journal of
Pharmaceutics 2003; 250: 65-83. www.elsevier.com/locate/ijpharm.
73. Desai A, Shindhaya S, Malke S and Kadam V. Use of natural release
retardant in drug delivery systems. Indian Drugs, 2005 Sept; 42(9):
565-575.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
135
74. Kavita A, Sivaramkrishna M, Nelinic CN and Nappinnai H. Formulation and
evaluation of topical drug delivery system of fluconazole. Indian
Drugs 2003 Dec; 40(2): 720-723.
75. Kantaria S, Gareth D, Rees, Lawrence MJ. Gelatin-stabilized microemulsion-
based organogels, rheology and application in iontrophoretic
transdermal drug delivery. Journal of Controlled Release 1999; 60:
355-365.
76. Murdan S, Gregordis G, Florence A. Novel sorbitan monostearate
organogels. Journal of Pharmaceutical Sciences (1999) June; 88 (6).
77. Murdan S, Gregoriadis G, Alexander T. Interaction of non-ionic surfactant-
based organogels with aqueous media. International Journal of
pharmaceutics, 1999; 180: 211-214.
78. National List of Essential Medicines 2003, 354 Essential Drugs Prepared by
Government of India as directed by Supreme Court, 2003 July;
Pharma Times, Vol. 35, 2003 Sept., P-36-37.
79. Martindale. The Extra Pharmacopoeia, 29
th
Edition edited by Reynolds J F
Published by The Pharmaceutical Press, London, P-430: 1989.
80. Roger Walker, Clive Edwards. Clinical Pharmacy & Therapeutics, 3
rd
Edition, 2003; 346.
81. A reference guide to fetal and neontal risk drug in pregnancy and lactation,
Edited by Briggs GG, Freeman RK, Xaffee SF. 2
nd
Edition Published
by Williams and Wilkins, Baltimore, P-294.
82. Ordell PJ. Physician Desk Reference, Medical Economics, 43
rd
Edition, P-
1498: 1989.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
136
83. Philip D, Hansten. Drug Interaction Clinical Significance of Drug Drug
Interaction, 5
th
Edition, P-88-203.
84. Indian Pharmacopoeia, Published by the Controller of Publications, Delhi,
Vol. I, P-492; 1996.
85. United States Pharmacopoeia (USP), NF XXV, the Pharmacopoeial
Convention, Rockville, MD, P-2571, 2603,2593, 2568; 2002.
86. British Pharmacopoeia, The Department of Health, Social Services and Public
Safety, London, Vol. I, P-415: 1988.
87. BPenzes T, Csoka I. Rheological analysis of organogels based on glyceryl
monostearate derivatives. www.rheological analysis/ organogels.com.
88. Agarwal RE, Katare OP. Preparation and in vitro evaluation of miconazole
nitrate loaded topical liposomes. Pharmaceutical Technology, 2002:
48-60.
89. http://www.ich.org.
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
137
ANNEXURES
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
138
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
139
Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)
You might also like
- Tousif Thesis 2010Document158 pagesTousif Thesis 2010Kristine Dwi PuspitasariNo ratings yet
- Complete ThesisDocument204 pagesComplete Thesismarcela gomezNo ratings yet
- Srikanth ReddyDocument160 pagesSrikanth ReddyrambhadesiNo ratings yet
- UMI's PROJECTDocument40 pagesUMI's PROJECTIbrahim Muhammad UsmanNo ratings yet
- 36 PDFDocument129 pages36 PDFjsc rajuNo ratings yet
- Thesis MDTDocument136 pagesThesis MDTAzeem Abidi0% (1)
- Vedavathi 2Document168 pagesVedavathi 2lokeshkunchapuNo ratings yet
- Cdpceut00001 PDFDocument257 pagesCdpceut00001 PDFrafaqat aliNo ratings yet
- Instrumental Methods of Analysis ThakurDocument296 pagesInstrumental Methods of Analysis ThakurDivyanshu Aggarwal100% (1)
- Industrial Pharmacy-IIDocument207 pagesIndustrial Pharmacy-IIMuzaffar AliNo ratings yet
- Instrumental Methods of Drug AnalysisFrom EverandInstrumental Methods of Drug AnalysisRating: 3 out of 5 stars3/5 (3)
- Rasakarpoorantimicrobialrs PDFDocument228 pagesRasakarpoorantimicrobialrs PDFravi100% (1)
- Anil MahajanDocument212 pagesAnil MahajanRAVINDRA BABUNo ratings yet
- Thesis Nasal GelDocument113 pagesThesis Nasal GelRishikesh Chakor100% (1)
- Scribd Upload A Document Search Documents Explore Sign Up Log inDocument6 pagesScribd Upload A Document Search Documents Explore Sign Up Log innadakuditi78No ratings yet
- Anees Ahmed Corrected Thesis - RecentDocument135 pagesAnees Ahmed Corrected Thesis - RecentAbdul MunimNo ratings yet
- (SpringerBriefs in Applied Sciences and Technology_ Forensic and Medical Bioinformatics) Mohd Hafiz Arzmi, Anwar P. P. Abdul Majeed, Rabiu Muazu Musa, Mohd Azraai Mohd Razman, Hong-Seng Gan, Ismail MoDocument41 pages(SpringerBriefs in Applied Sciences and Technology_ Forensic and Medical Bioinformatics) Mohd Hafiz Arzmi, Anwar P. P. Abdul Majeed, Rabiu Muazu Musa, Mohd Azraai Mohd Razman, Hong-Seng Gan, Ismail MoAhmed Mohamed CherifNo ratings yet
- Thesis Submitted in Partial Fulfilment of The Requirement For The Degree ofDocument81 pagesThesis Submitted in Partial Fulfilment of The Requirement For The Degree ofNasir MuhammadNo ratings yet
- Practical Applications of Mechanical Ventilation PDFDocument574 pagesPractical Applications of Mechanical Ventilation PDFmladja77No ratings yet
- Health Effects of Indoor Air Pollution: Volume 2: Air Pollution, Human Health, and the EnvironmentFrom EverandHealth Effects of Indoor Air Pollution: Volume 2: Air Pollution, Human Health, and the EnvironmentMohammad Hadi DehghaniNo ratings yet
- Plagiarism - Report SachinDocument46 pagesPlagiarism - Report SachinTahir HussainNo ratings yet
- Certificate and AcknoledgementDocument3 pagesCertificate and AcknoledgementMittul ThekdiNo ratings yet
- Masters ThesisDocument61 pagesMasters ThesissampathdtNo ratings yet
- NIPST Transdermal Patch FormulationDocument3 pagesNIPST Transdermal Patch FormulationVamsi SakhamuriNo ratings yet
- Binder 1Document151 pagesBinder 1Sudhansu Ranjan SwainNo ratings yet
- Brand Loyalty in Bangladesh: Customer Satisfaction, Brand Trust, Social Media Usage in Electronic Home AppliancesFrom EverandBrand Loyalty in Bangladesh: Customer Satisfaction, Brand Trust, Social Media Usage in Electronic Home AppliancesNo ratings yet
- Seroprevalence of Brucellosis in Goats Around HargeisaDocument50 pagesSeroprevalence of Brucellosis in Goats Around HargeisaAbdiNo ratings yet
- Younas Thesis 16 05 2023Document60 pagesYounas Thesis 16 05 2023Malik RohailNo ratings yet
- Survey On Seasonal Incidence and Management of Onion ThripsDocument84 pagesSurvey On Seasonal Incidence and Management of Onion Thripsshreyash pangavhaneNo ratings yet
- Effects of Promotional Strategies On TheDocument84 pagesEffects of Promotional Strategies On TheMandar MagarNo ratings yet
- Wastewater-Based Epidemiology for the Assessment of Human Exposure to Environmental PollutantsFrom EverandWastewater-Based Epidemiology for the Assessment of Human Exposure to Environmental PollutantsMohammad Hadi DehghaniNo ratings yet
- DR ANU Thesis FINAL PDFDocument152 pagesDR ANU Thesis FINAL PDFKrishna Devarayar50% (2)
- The Impact of Coffee Shop'S Design Factors On Users: Georgetown - Penang As A Case StudyDocument13 pagesThe Impact of Coffee Shop'S Design Factors On Users: Georgetown - Penang As A Case StudyCos MosNo ratings yet
- Phytochemical Screening and Pharmacological Investigation On The Leaves of Clitoria TernateaDocument113 pagesPhytochemical Screening and Pharmacological Investigation On The Leaves of Clitoria TernateavimalNo ratings yet
- Study On Clinical Trials and Its ManagementDocument6 pagesStudy On Clinical Trials and Its ManagementK SANDHYANo ratings yet
- Vinay Thesis Front PageDocument13 pagesVinay Thesis Front PagevinaykumarkolheNo ratings yet
- Biogenic Amines: Occurrence and Analytical Methods ReviewDocument7 pagesBiogenic Amines: Occurrence and Analytical Methods ReviewMuhammad WaqasNo ratings yet
- Dr. SharanendraDocument156 pagesDr. SharanendraABhinav GangwarNo ratings yet
- DeclarationDocument359 pagesDeclarationIllogical IdeasNo ratings yet
- Proceeding International Conference July 2015 in Usm PDFDocument432 pagesProceeding International Conference July 2015 in Usm PDFFairil MaulanaNo ratings yet
- Acknowledgement: Mr. C. Velmurugan, M.Pharm.Document3 pagesAcknowledgement: Mr. C. Velmurugan, M.Pharm.Anonymous 22GBLsme1No ratings yet
- Assessment of Knowledge Attitudes Perception and Barriers Tow Ards Pharmacovigilance Activities Among Community Pharmacists and Final Year Phatmacy Students in MalaysiaDocument46 pagesAssessment of Knowledge Attitudes Perception and Barriers Tow Ards Pharmacovigilance Activities Among Community Pharmacists and Final Year Phatmacy Students in MalaysiaAnitha NoronhaNo ratings yet
- REFEREDDocument125 pagesREFEREDSree LathaNo ratings yet
- AcknowledgementDocument1 pageAcknowledgementAhinurNo ratings yet
- Industrial1 TPDocument281 pagesIndustrial1 TPPriyam Soni67% (3)
- C4 Plants Botany2 Project(Kartik's)Document18 pagesC4 Plants Botany2 Project(Kartik's)Chiku ChikuNo ratings yet
- Fundamentals of Clinical Pharmacy PracticeFrom EverandFundamentals of Clinical Pharmacy PracticeRating: 4.5 out of 5 stars4.5/5 (2)
- Green Technologies for the Defluoridation of WaterFrom EverandGreen Technologies for the Defluoridation of WaterMohammad Hadi DehghaniNo ratings yet
- فطريات بشكل عامDocument149 pagesفطريات بشكل عامdhurgham alfarrajiNo ratings yet
- Dihydropyrimidinones as Potent Anticancer Agents: Medicinal Chemistry PerspectiveFrom EverandDihydropyrimidinones as Potent Anticancer Agents: Medicinal Chemistry PerspectiveMashooq Ahmad BhatNo ratings yet
- D652-Kowshik Kumar MDocument89 pagesD652-Kowshik Kumar MDr osama khamisNo ratings yet
- Rasakarpoorantimicrobialrs012gdg 121228221155 Phpapp02 PDFDocument228 pagesRasakarpoorantimicrobialrs012gdg 121228221155 Phpapp02 PDFVaidya NurNo ratings yet
- Divalproex SodiumDocument85 pagesDivalproex SodiumDipak Russia100% (2)
- Please Note: Royal GazetteDocument16 pagesPlease Note: Royal GazetteSiddhant YadavNo ratings yet
- QMS FinalDocument21 pagesQMS FinalSiddhant YadavNo ratings yet
- Miconazole Gel FormulationDocument11 pagesMiconazole Gel FormulationSiddhant YadavNo ratings yet
- Component Screening of Miconazole Nitrate Nanoemulsion: Research ArticleDocument8 pagesComponent Screening of Miconazole Nitrate Nanoemulsion: Research ArticleSiddhant YadavNo ratings yet
- ErpDocument35 pagesErpSiddhant YadavNo ratings yet
- Miconazole Micro Emul PrepDocument7 pagesMiconazole Micro Emul PrepSiddhant YadavNo ratings yet
- Erpproject 151221074659Document10 pagesErpproject 151221074659Siddhant YadavNo ratings yet
- Interim ReportDocument12 pagesInterim ReportSiddhant YadavNo ratings yet
- International Journal of Pharma and Bio Sciences Issn 0975-6299Document13 pagesInternational Journal of Pharma and Bio Sciences Issn 0975-6299Siddhant YadavNo ratings yet
- Sales ManagementDocument202 pagesSales ManagementdsatyNo ratings yet
- M03p - Personal Selling Approaches and ProcessDocument22 pagesM03p - Personal Selling Approaches and ProcessFarhan BadakshaniNo ratings yet
- Mod 6 Case StudiesDocument5 pagesMod 6 Case StudiesSiddhant Yadav0% (3)
- Personal Selling and Sales ManagementDocument19 pagesPersonal Selling and Sales ManagementE Kay MutemiNo ratings yet
- Personal Selling and Sales Promotion: Chapter ObjectivesDocument17 pagesPersonal Selling and Sales Promotion: Chapter ObjectivesNishu GoyalNo ratings yet
- 6e SM Module02Document20 pages6e SM Module02rojabommalaNo ratings yet
- Personal Selling Concept and ProcessDocument7 pagesPersonal Selling Concept and ProcessSiddhant Yadav100% (1)
- Working Paper HansenDocument50 pagesWorking Paper HansenSiddhant YadavNo ratings yet
- SM - Ch3 - S11 PostDocument4 pagesSM - Ch3 - S11 PostSiddhant YadavNo ratings yet
- Whether Merck Should Take Licensing of DavanrikrugDocument19 pagesWhether Merck Should Take Licensing of DavanrikrugratishmayankNo ratings yet
- (Drugs and The Pharmaceutical Sciences) Carstensen, Jens T. - Drug Stability, Third Edition, Revised, and Expanded - Principles and Practices (2000, CRC Press)Document793 pages(Drugs and The Pharmaceutical Sciences) Carstensen, Jens T. - Drug Stability, Third Edition, Revised, and Expanded - Principles and Practices (2000, CRC Press)Catalina Rivera100% (3)
- Design and Characterization of Bilayer Tablet of Rifampicin and Isoniazid For Tuberculosis TherapyDocument5 pagesDesign and Characterization of Bilayer Tablet of Rifampicin and Isoniazid For Tuberculosis TherapyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Phytochemical Pharmacognostical and Physicochemical Standardization of Peperomia Pellucida (L.) Hbk.Document4 pagesPhytochemical Pharmacognostical and Physicochemical Standardization of Peperomia Pellucida (L.) Hbk.ayi-zidaneNo ratings yet
- Drug Shop Dec. 202021Document88 pagesDrug Shop Dec. 202021Endre Shitaye KulkiNo ratings yet
- Lamp 2 Standar Matkes Kri SHSDocument10 pagesLamp 2 Standar Matkes Kri SHSmusmanNo ratings yet
- Pharma Companies 5Document8 pagesPharma Companies 5Devesh JhaNo ratings yet
- Bates, JPS, PH Dependent Disso Rate of Nitrofurantoin From Commercial Suspensions, Tablets and CapsulesDocument3 pagesBates, JPS, PH Dependent Disso Rate of Nitrofurantoin From Commercial Suspensions, Tablets and CapsulesKonkmanNo ratings yet
- DPP-4 inhibitors for managing Type 2 DiabetesDocument25 pagesDPP-4 inhibitors for managing Type 2 DiabetesYuliarni HasanNo ratings yet
- AD-Times - Boehringer IngelheimDocument12 pagesAD-Times - Boehringer IngelheimAkash GoyalNo ratings yet
- Food and Drug Interactions A General ReviewDocument15 pagesFood and Drug Interactions A General ReviewDaniela AndreiNo ratings yet
- The Generics Pharmacy Franchise Information-V2Document1 pageThe Generics Pharmacy Franchise Information-V2nicelim64No ratings yet
- Pharmaceutical Analysis I - TheoryDocument3 pagesPharmaceutical Analysis I - TheoryHarit0% (1)
- QA Director Manager Pharmaceutical in Denver CO Resume Ann DoughertyDocument4 pagesQA Director Manager Pharmaceutical in Denver CO Resume Ann DoughertyAnnDougherty2No ratings yet
- Company Overview of Abbott Healthcare PVTDocument9 pagesCompany Overview of Abbott Healthcare PVTsceneoritaNo ratings yet
- Ers 2015Document358 pagesErs 2015Dejan ŽujovićNo ratings yet
- Syringe Pump ReportDocument6 pagesSyringe Pump ReportzulayieNo ratings yet
- Introduction and Update On ICH Q12 GuidelineDocument16 pagesIntroduction and Update On ICH Q12 GuidelineDimitris PapamatthaiakisNo ratings yet
- Calculate With Confidence 7th Edition Morris Test BankDocument6 pagesCalculate With Confidence 7th Edition Morris Test Bankalmiraelysia3n4y8100% (21)
- Ranbaxy Nigeria Limited Product CatalogDocument2 pagesRanbaxy Nigeria Limited Product CatalogikponmwonsaNo ratings yet
- Bandung pharmacy price list for prescription drugsDocument13 pagesBandung pharmacy price list for prescription drugsJar JarNo ratings yet
- Pharmacy OrientationDocument23 pagesPharmacy OrientationMohan AnwarNo ratings yet
- M. Talha Saleem Mehwish Amin Ramsha Ahmed Qudsia Basri Sadia Ghousia Baig and Maria BaigDocument4 pagesM. Talha Saleem Mehwish Amin Ramsha Ahmed Qudsia Basri Sadia Ghousia Baig and Maria BaigqbNo ratings yet
- Medical Safety InformationDocument4 pagesMedical Safety InformationaymanNo ratings yet
- PsilocybinDocument2 pagesPsilocybinJackson James WoodNo ratings yet
- Classics in Chemical Neuroscience: Diazepam (Valium) : Nicholas E. Calcaterra and James C. BarrowDocument8 pagesClassics in Chemical Neuroscience: Diazepam (Valium) : Nicholas E. Calcaterra and James C. BarrowEga Trikuntianti100% (1)
- Perioral DermatitisDocument12 pagesPerioral Dermatitissanti dwi rahmawatiNo ratings yet
- Biochem Lab 5Document12 pagesBiochem Lab 5Khadijah Ulol AzmiNo ratings yet
- Zambia Health Shop GuidelinesDocument18 pagesZambia Health Shop Guidelinesdavies100% (1)
- 33 Cisplatin A ReviewDocument18 pages33 Cisplatin A Reviewtrifamonika23No ratings yet