Professional Documents
Culture Documents
Stainless Steel For Exterior Applications
Uploaded by
MikeOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stainless Steel For Exterior Applications
Uploaded by
MikeCopyright:
Available Formats
I I M O A A
Stainless steel is one of the most durable materials used in architecture.
However, stainless steel is not just one material; there are many different
types with different properties and, most importantly, different levels of
corrosion resistance. If an appropriate stainless steel, surface finish, and
design are selected and it is properly maintained, its appearance will
remain virtually unchanged over the life of the building, even if that life is
well over 100 years.
If an inappropriate stainless steel and/or finish are used, corrosion can
be a problem. Although it may not adversely affect structural integrity,
corrosion can be aesthetically undesirable and increase maintenance
requirements. This brochure provides guidelines for evaluating a project
and for determining which stainless steel should be specified.
INTERNATIONAL MOLYBDENUM ASSOCIATION
It is instructive to examine nearby stainless steel installations and
determine their maintenance history before using the Site And Design
Evaluation System inside this brochure. It is prudent to confirm the pre-
dictions made with this evaluation system by exposing samples at the
site, because some variables can be difficult to predict. It is suggested
that the sample simulate the proposed design and be exposed for four to
six months. It may be possible to reduce sample exposure to six weeks
in severe marine or industrial locations. Case studies that illustrate the
application of this evaluation system are available from IMOA.
Which Stainless Steel Should Be Specified
for Exterior Applications?
Instructions
The Site And Design Evaluation System was developed
based on practical experience and atmospheric exposure
studies. Evaluating a site and application can be complex.
Nearby sites can have different requirements due to loca l -
ized conditions or microclimates. The score should be
viewed as a guideline that generates an initial evaluation
of stainless steel requirements. It is not a precise scientific
determination.
The Evaluation System has five sections. Read the back-
ground information for each section and then determine
the section score using the adjoining point system. Sum
all of the sections to obtain a Total Score. Compare the
Total Score with Stainless Steel Selection on page 4.
If the design characteristics, maintenance schedules, or
exposures of different components vary significantly, a
score should be determined for each component.
If the presence of corrosive pollution or salt (chlorides) can-
not be determined by site examination alone, a laboratory
could test the surface of an unwashed sample from the
site. The sample must have had long-term exposure with-
out cleaning. Care must be taken during sample collection
and handling to prevent removal of surface deposits.
When do I need a stainless steel corrosion expert?
If there is uncertainty about the project evaluation or if the
location appears to be particularly corrosive, a stainless steel
corrosion expert with architectural experience should evaluate
the site and architects drawings to recommend an appropriate
stainless steel. The Nickel Institute or a stainless steel market
development association can identify an expert (See page 6 for
contact information). Particularly corrosive conditions include:
Occasional or regular seawater or deicing salt splashing,
immersion or salt spray
High industrial or urban pollution levels
Exposure to both coastal and deicing salt
Hot, humid locations with salt or pollution exposure and
very low or no rainfall
Sites and designs producing a Total Score of 5 or higher
Introduction
In architectural applications, aesthetic and performance
requirements must be balanced against budget considera-
tions for cost effective material specification. The guide-
lines in this brochure assume that corrosion staining of
stainless steel is aesthetically unacceptable even if there is
no struc tural deterioration.
Identifying a stainless steel that meets this aesthetic
standard and is cost effective requires evaluation of the
site environment, weather, finish, design and probable
maintenance schedule. The Site And Design Evaluation
System provides an initial evaluation of a projects suscepti -
bility to corrosion and makes designers aware of factors
that influence stainless steel selection
Site and Design Evaluation System
Points
Section 1: Environment (Select the highest applicable score)
Rural
0 Very low or no pollution
Urban Pollution (Light industry, automotive exhaust)
0 Low
2 Moderate
Industrial Pollution (Aggressive gases, iron oxides, chemicals, etc.)
3 Low or moderate
4 High *
0 Temperate or cold climate, occasional heavy rain
0 Tropical or subtropical, wet, regular or seasonal very heavy rain
0
No salt was detected on a sample from the site and no change in exposure
conditions is expected
1 Very low salt exposure ( 10 m to 1 km (33 to 3,280 ft) or 3 to 60 floors) **
0
Traffic and wind levels on nearby roads are too low to carry chlorides to the site and no
deicing salt is used on sidewalks
Deicing Salt Exposure (Distance from road or ground)
1 Low (>1.6 to 16 km (1 to 10 miles) from salt water) **
4 High (<30 m (100 ft) from salt water)
5 Marine (Some salt spray or occasional splashing) *
3 Moderate (30 m to 1.6 km (100 ft to 1 mile) from salt water)
Coastal or Marine Salt Exposure
0 Boldly exposed for easy rain cleaning
0 Vertical surfaces with a vertical or no finish grain
Section 4: Design Considerations (Select all that apply)
Section 3: Local Weather Pattern (Select only one)
Section 5: Maintenance Schedule (Select only one)
-2 Surface finish is pickled, electropolished, or roughness R
a
0.3 m (12 in)
0 Not washed
-1 Washed at least annually
-2 Washed four or more times per year
-3 Washed at least monthly
2 Low salt exposure (< 10 to 500 m (33 to 1600 ft) or 2 to 34 floors) **
3 Moderate salt exposure (< 3 to 100 m (10 to 328 ft) or 1 to 22 floors) **
4 High salt exposure ( 2 to 50 m (6.5 to 164 ft) or 1 to 3 floors) * **
1 Surface finish roughness R
a
0.5 m (20 in) < X R
a
1 m (40 in)
-1 Surface finish roughness R
a
0.3 m (12 in) < X R
a
0.5 m (20 in)
3 High*
Score
Score
Score
Score
Score
Total Score
-1 Temperate or cold climates, regular heavy rain
-1 Hot or cold climates with typical humidity below 50%
2 Hot, humidity above 50%, very low or no rainfall ***
1 Temperate climate, infrequent rain, humidity above 50%
1 Regular very light rain or frequent fog
8 Severe Marine (Continuous splashing) *
10 Severe Marine (Continuous immersion) *
Total Score
2 Surface finish roughness > R
a
1 m (40 in)
1 Sheltered location or unsealed crevices***
1 Horizontal surfaces
1 Horizontal finish grain orientation
* Potentially a highly corrosive location. Have a stainless steel corrosion expert evaluate the site.
** The range shows how far this chloride concentration has been found from small rural and large
high traffic roads. Test surface chloride concentrations.
*** If there is also salt or pollution exposure, have a stainless steel corrosion expert evaluate the site.
Evaluation System
2
Section 2: Coastal or Deicing Salt (Chloride) Exposure (Select the
highest applicable score). If there is exposure to both coastal and
deicing salt, obtain assistance from a stainless steel corrosion expert
Section 2: Coastal or Deicing Salt Exposure
Salt can corrode architectural metals, including some stainless steels. If the
humidity and temperature are high enough or if there is regular light rain or
fog, salt deposits on the surface will absorb moisture and form a highly con-
centrated and corrosive salt solution.
1
A stainless steel corrosion expert should be consulted if a component is
splashed, sprayed or immersed on an occasional or regular basis with sea-
water or a deicing salt. An expert should also be consulted if a location is ex-
posed to both deicing and coastal salt, because the combined salt exposure
can make the location more corrosive than exposure to only one salt source.
Coastal and marine exposure
Local wind patterns determine how far sea salts are carried inland.
Generally, locations within 8 to 16 kilometers (5 to 10 miles) of salt water
are considered coastal. In some locations, salt is carried a relatively
short distance inland, and, in others, it can be carried much farther than
16 kilometers (10 miles). A sample from the site can be tested for salt
deposits. Applications that will be immersed in seawater
or regularly splashed may require a super duplex, super
ferritic, or 6% molybdenum super austenitic stainless
steel.
Deicing salt exposure
Sodium chloride, calcium chloride and magnesium chloride are used for
deicing. Deicing salt accumulations make the environment near roads and
walkways corrosive. Vehicle and wind speeds and traffic levels are the
most important factors in determining how far deicing
salt laden road mist will travel. Salt concentrations are
higher closer to where it is applied to roads. Unfortu-
nately, salt contamination is not always limited to sites
immediately next to roads.
If traffic and wind levels are high, deicing salt laden road mist can be
carried as high as the 59th floor of buildings and as far as 1 km (0.6 miles)
from busy highways.
2
If the adjoining road has low traffic volume or if the
prevailing wind blows in the opposite direction, salt deposits are generally
only found close to the road. Larger city streets and highways are usually
more heavily deiced and generate much higher levels of salt mists and
dry salt dust. The Evaluation System shows the range of distances at
which different deicing salt concentration levels have
been found from small roads to busy highways. Salt
levels can vary significantly with severity of the winter
weather. If there is uncertainty about salt exposure, test
surface areas for chlorides.
If you are uncertain about the pollution levels for a region, data can usually
be obtained through the Internet or by telephone from local or national
governments. The data in the table was issued by the World Bank in 1998
and compares two pollution factors in a sampling of cities. If pollution levels
are suspected to be high, a stainless steel corrosion expert with architecture
experience should be consulted.
Rural
Rural or suburban areas with low population densities and light, non-
polluting industry are included in this category. Migrant air pollution from
industry upwind of the site could change this classification.
Urban sites
Urban sites include residential, commercial, and light industrial locations
with low to moderate pollution from vehicular traffic. High urban pollution
levels are generally found in areas with little or no air pollution controls or
are caused by localized conditions that concentrate pollution.
Section 1: Environment
City Suspended Particulate g/m
3
Sulfur Dioxide g/m
3
Beijing
Calcutta
Helsinki
Los Angeles
Moscow
New York
Paris
Rio de Janeiro
Sydney
Tokyo
Toronto
377
375
40
46
100
23
14
139
54
49
36
90
49
4
9
109
26
14
129
28
18
17
1995 Urban Pollution Levels, World Health Organization
Information on typical temperatures, humidity, total snow and rainfall
levels, and fog days is usually collected by national governments and
can be ob tained on the Internet or by telephone. This information should
be supplemented with local observations for accurate interpretation.
The moisture in fog, light misty rain or high humidity can combine with
corrosive compounds on a surface to activate them and make corrosion
possible. Higher temperatures will increase the corrosion rate. The most
corrosive environments are areas with very little or no rain, high tem -
peratures, salt (chloride) or aggressive pollution exposure that also have
moderate to high humidity or regular fog.
It is important to look at both annual rainfall levels and the type of rain-
storms that occur. Light rain will not remove surface contaminants. Storms
with high rainfall rates or wind driven rain, like thunderstorms, can remove
corrosive deposits from exposed surfaces. If they are frequent enough to
prevent significant deposit or dirt accumulation on surfaces, they can
reduce the risk of corrosion. For example, much of eastern North America
and Northern Europe get enough heavy rain to minimize surface deposits
and would have a -1 rating.
Section 3: Local Weather Pattern
Industrial sites
Sulfur and nitrogen oxides from coal combustion, and gases released from
chemical and process industry plants are typical in industrial sites.
Airborne particles, such as soot from incompletely burned fuel or iron
oxides, increase the sites corrosiveness. High industrial pollution levels
are generally found in areas with little or no air pollution controls or are
caused by localized conditions that concentrate pollution.
3
Surface roughness
Corrosion cannot occur unless corrosive substances adhere to a surface. A smooth
surface finish makes it difficult for contaminants to adhere and makes manual and heavy
rain-washing more effective. A rough surface accumulates more contaminants and is
less easily cleaned. Therefore, a smooth surface finish reduces the risk of corrosion
staining, and a rough surface finish promotes corrosion staining. A finish is considered
smooth if the surface roughness is R
a
0.5 m (microns or micrometers) or 20 in (micro
inches) or less. The typical surface roughness associated with a specific finish can vary
from supplier to supplier and around the world. Ask the surface finish supplier to provide
the typical surface roughness when samples are requested and include surface rough-
ness requirements in project specifications.
Section 4: Design Considerations
Sheltered and horizontal surfaces
There is more dirt and contaminant accumulation on shel-
tered components or horizontal surfaces because rain may
not be able to remove the contaminants. Leaving corrosive
contaminants on the stainless steel for an extended
period of time can lead to corrosion staining. Horizontal or
sheltered surfaces should be avoided unless rain or
manual cleaning is likely. Alternatively, a more corrosion
resistant stainless steel could be used.
Section 5: Maintenance Schedule
Stainless Steel Selection
If a stainless steel is susceptible to corrosion by salt (chlorides) or pollution, they have to
remain on the surface of the stainless steel long enough and in sufficient concentration
for corrosion to start. Frequent cleaning by heavy rain or manual washing prevents corro-
sive deposit accumulation and corrosion. The manual cleaning frequency necessary for a
pristine appearance depends on the site environment, surface finish, design, stainless
steel selected, and potential for rain cleaning. More frequent cleaning will be required if
the stainless steel does not provide adequate corrosion
resistance or if there is a rough finish, horizontal or shel-
tered surfaces, or crevices. If Type 304 is exposed to more
corrosive environments, it may be necessary to clean it
four or more times per year to maintain an attractive
appearance.
How can I reduce the score?
Some design changes that can improve performance and
possibly change material requirements are:
Boldly expose components for better rain washing
Select smooth surface finishes
Use a vertical surface finish grain orientation
Eliminate horizontal surfaces
Eliminate or seal crevices
Design to facilitate and encourage a regular manual
washing schedule
Add natural or artificial barriers to reduce deicing salt road
mist exposure.
0 to 2
Type 304/304L (UNS S30400, EN 1.4301, SUS 304) is generally the most cost-effective
choice.
3
Type 316/316L (UNS S31600, EN 1.4401, SUS 316) or 444 (UNS S44400, EN 1.4521,
SUS 444) is generally the most cost-effective choice.
4
Type 317L (UNS 31703, EN 1.4438, SUS 317L) or a more corrosion resistant stainless
steel is suggested.
5
A more corrosion resistant stainless steel such as 2205 (UNS S32205, EN 1.4462,
SUS 329J3L), 904L (UNS N08904, EN 1.4539, SUS 890L), 317LMN (UNS S31726,
EN 1.4439, SUS 317LN), super duplex, super ferritic, or a 6% molybdenum super
austenitic stainless steel may be needed. These stainless steels provide different
levels of corrosion resistance as shown in Figure 1. If you obtain a score of 5 or
above, a stainless steel corrosion expert with architectural experience should
evaluate the site and design and suggest an appropriate stainless steel.
Total Score
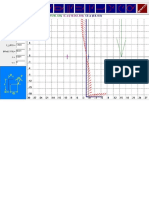
The chart above shows the relation ship between corrosion rate
and surface roughness. The line represents a sur face roughness
of R
a
0.5 m or 20 in. Corrosion rate accelerated rapid ly above
this surface roughness level.
3
C
o
r
r
o
s
i
o
n
R
a
t
e
R
a
micron / micrometer
0,001 0,01 0,1 1 10
0
5
10
15
20
25
4
Finish grain orientation
Finish orientation is important if
there is an obvious grain line
(like No.4, embossed and similar
finishes). Vertical finish grain
orientation makes it easier for
rain to wash the surface and for
water and contaminants to drain
away. A horizontal grain orienta-
tion tends to retain contami -
nants on the surface. Better
corrosion protection is achieved
by installing directional finishes
so that the grain orientation is
vertical.
Crevices
Crevice corrosion can occur when water and corrosive
contaminants remain trapped in narrow gaps and salt
(chloride) is present in the en viron ment (A). Problems can
be avoided by eliminating narrow crevices that can
accumulate moisture or by sealing them with a weld or
sealant (B). Alter natively, specify a more highly alloyed
stainless steel that is not subject to crevice corrosion. For
example, woven stainless
steel has tiny cre vices
where the wires overlap
and corrosion can occur
at each cre vice if there is
salt (chloride) exposure.
Welded mesh is a good
alternative, because it
does not have crevices.
A B
Corrosion Resistance of Stainless Steels
In architectural applications, corrosive environments are defined as locations with deicing or coastal salt exposure and/or areas with industrial or
urban pollution. The constituents that make these environments corrosive include sea or deicing salts (sodium, potassium and calcium chlorides)
and acid rain from exhaust gas condensates generated by power or chemical plants and motor vehicles.
Most stainless steels are classified as austenitic, ferritic or duplex. These stainless steels can generally be hardened by cold work but not by heat-
treatment. Austenitic stainless steels such as Types 304 (UNS S30400, EN 1.4301, JIS SUS 304) and 316 (UNS S31600, EN 1.4401,
SUS 316) contain iron, chromium and nickel. They are non-magnetic and provide very good formability. Ferritic stainless steels, such as 444
(UNS S44400, EN 1.4521, SUS 444), contain iron and chromium. They are magnetic and have limited formability. Duplex stainless steels such as
2205 (UNS 32205, EN 1.4462, SUS 329J3L) contain iron, chromium and an intermediate amount of nickel. They provide higher strength levels than
ferritic or austenitic stainless steels, are magnetic, and have intermediate formability. There are also proprietary lean duplex stainless steels,
which have the corrosion resistance and cost of Types 316 and 317L and the higher strength levels associated with duplex stainless steels. The
most corrosion resistant stainless steels shown in Figure 1 are examples from the stainless steel families known as super ferritic 447 (UNS 44700,
SUS 447J1), super duplex 2507 (UNS S32750, EN 1.4410) and 6% molybdenum super austenitic (UNS S31254, N08926, N08367; EN 1.4547,
1.4529; or SUS 312L), which are resistant to pitting when immersed in seawater. In addition to the basic alloying elements mentioned, molybdenum is
added to all of the stainless steels in Figure 1 expect Type 304 to improve corrosion resistance, particularly resistance to salt (chloride) corrosion.
Figure 1 compares the relative pitting resistance of different stainless steels using a formula to predict relative corrosion resistance, Pitting
Resistance Equivalent (PRE) number.
4
This formula is based on the improvement in stainless steel corrosion resistance associated with increasing
chromium, molybdenum and nitrogen content.
Cold rolling, embossing, and forming operations such as bending or deep drawing change the shape of a metal and thereby cold work or
strengthen it. All three families of stainless steel can be shaped by common architectural forming techniques, however only austenitic stainless
steels are sometimes deliberately strengthened by cold working.
The dominant stainless steels used in architecture are Types 304 and 316. Type 304 is suitable for most interior and exterior applications with
low corrosion risk, even with minimal or no maintenance. In moderately corrosive environments, Type 304 may be acceptable if a smooth finish is
specified and there is regular cleaning. Type 316 or 444 with an appropriate finish can remain attractive in most corrosive applications with little or no
manual cleaning. In very corrosive applications, Type 316 or 444 may require regular cleaning or a more corrosion resistant stainless steel may be
necessary.
If the design includes welded sections that are heavier than about 6 millimeters (0.25 inch), use the low carbon L version of the stainless
steel (e.g. 304L, 316L) to maintain corrosion resistance. Pitting corrosion resistance is improved by specifying that sulfur content should not
exceed 0.005%.
0
5
10
15
20
25
30
35
40
45
50
UNS
EN
304/304L 316/316L 444 317L 2205 317LMN 904L
S30400
S30403
1.4301
1.4307
S31600
S31603
1.4401
1.4404
S44400
1.4521
S31703
1.4438
S32205
S31803
S31726 N08904
1.4439 1.4539 1.4462
I
n
d
e
x
o
f
R
e
l
a
t
i
v
e
C
o
r
r
o
s
i
o
n
R
e
s
i
s
t
a
n
c
e
Most Common Less Commonly Used
JIS
SUS 304 SUS 316 SUS 444 SUS 317L SUS 317LN SUS 890L
447
S44700
S44735
SUS 447J1
2507
S32750
1.4410
6% Mo
S31254
N08926
N08367
1.4547
1.4529
SUS 312L SUS 329J3L
Stainless Steel Corrosion, Fabrication and Resources
Figure 1 This chart of relative pitting resistance is based on the Pitting Resistance Equivalent or PRE number. Austenitic stainless steels are shown in yellow, ferritics in blue, and duplex in gray. The
UNS (Unified Numbering System), EN (European Number), and JIS (Japanese Industrial Standard) designations identify the specific stainless steel chemistries associated with the common names
shown in the bar chart.
5
Fabrication and Installation
Corrosion appearing within a few months of installation is usually the result of improper handling, fabrication, storage or cleaning. If corrosion
staining occurs, it may be possible to restore the finish with cleaning. The cleaning product ingredients should be checked before use even if they
are labeled stainless steel cleaner. Cleaning products that contain chlorides should be avoided or thoroughly washed off surfaces after use to
prevent corrosion. Cleaning products containing Muriatic or hydrochloric acid will cause rapid corrosion of stainless steel and should never be used
on or near it. These products are sometimes used to clean concrete, masonry or tile.
If the surface of the stainless steel has been contaminated with carbon steel or iron, corrosion is rapid and may appear within a few days of exposure
to outside air. Contamination can occur at the job site or during fabrication. Sources include contaminated tools or abrasive media, steel wool
or brushes, airborne steel particles, scratching, and improper shipping or storage. Sometimes the finish can be restored, but it is best to avoid
contamination by using good handling practices.
The corrosion resistance of a weld should be similar to the surrounding material. Often the weld can be blended so that it matches the surrounding
finish and becomes invisible. The surface roughness of the weld should not be greater than that of the surrounding finish.
If a stainless steel with a sulfur level above 0.005% has been used, the surface of the finished component should be passivated with phosphoric or
nitric acid to improve pitting resistance after finishing and fabrication is completed. If the sulfur level is 0.005% or less, this is not necessary.
Where Can I Get More Information?
The International Molybdenum Association (IMOA) has developed case studies on specific architectural applications and environments that can
provide more insight into selecting the correct stainless steel (www.imoa.info). Each case study illustrates the use of the evaluation system
introduced in this brochure. An interactive software version of this brochure was developed to guide users through the stainless steel selection
process. It is free and can be downloaded from the IMOA website. The website also provides links to pollution and wheather data and corrosion
maps.
Additional information about the subjects covered in this brochure is available from IMOA, the Nickel Institute, stainless steel market development
associations, and stainless steel producers around the world. These organizations can provide contact information for stainless steel corrosion
experts who are familiar with architectural applications. Links to stainless steel market development associations can be found at the IMOA
(www.imoa.info) or the Nickel Institute website (www.nickelinstitute.org).
The Nickel Institute publication No. 11 024, Stainless Steels in Architecture, Building and Construction: Guidelines for Corrosion Prevention provides
detailed information about evaluating the environment and selecting an appropriate stainless steel. If a stainless steel with more corrosion resistance
than Type 316 is needed, request the IMOA publication Practical Guidelines for Fabrication of Duplex Stainless Steels and the Nickel Institute
publication 11 021 High Performance Stainless Steels. Examples of buildings and structures that have performed well over time can be found
in the Nickel Institute publication 11 023, Timeless Stainless Architecture and the Euro Inox publication, Stainless Steel Facades, Building Series,
Volume 2 (info@euro-inox.org or www.euro-inox.org). Information on welding, cleaning stainless steel, and post fabrication cleaning is available
from the Nickel Institute, Euro Inox, stainless steel market development associations, and stainless steel producers.
1 Calcium chloride becomes corrosive at 0C (32F) and 45% humidity and sodium chloride becomes corrosive at 10C (50F) and 76% humidity.
Both are found in coastal and deicing salts.
2 Allen L. Williams and Gary J. Stensland, Illinois Department of Transportation, Atmospheric Dispersion Study of Deicing Salt Applied to Roads,
Physical Research Report No. 149, January 2006
3 Surface finishes of stainless steels, Bulletin of the International Dairy Federation No 189, 1985, p3 - 12.
4 Expressed through their Pitting Resistance Equivalent number, PRE = %Cr + 3.3 * %Mo + 16 * %N for austenitic and duplex stainless steels
and PRE = %Cr + 3.3 * %Mo for ferritic stainless steels.
6
International Molybdenum Association
e-mail: info@imoa.info www.imoa.info
The International Molybdenum Association (IMOA) has made every effort to ensure that the information presented is technically correct. However, IMOA does not represent or warrant the accuracy of the information contained in this
case study or its suitability for any general or specific use. The reader is advised that the material contained herein is for information purposes only; it should not be used or relied upon for any specific or general application without first
obtaining competent advice. IMOA, its members, staff and consultants specifically disclaim any and all liability or responsibility of any kind for loss, damage, or injury resulting from the use of the information contained in this publication.
Prepared by IMOA consultant Catherine Houska, TMR Consulting (www.tmrconsulting.com), layout Martina Helzel, circa drei (www.circadrei.de) IMOA ABC 00/09
You might also like
- Salt ContaminationDocument8 pagesSalt Contaminationabdayub100% (1)
- Selection of Stanless Steel For Fluids Containing ChlorideDocument6 pagesSelection of Stanless Steel For Fluids Containing ChlorideADITYA_PATHAK100% (1)
- Leader in Water Purification Systems RougingDocument16 pagesLeader in Water Purification Systems RougingtomcanNo ratings yet
- Guidelines For Alloy Selection For Waters and Waste Water Service PDFDocument6 pagesGuidelines For Alloy Selection For Waters and Waste Water Service PDFssgentisNo ratings yet
- Pickling HandbookDocument20 pagesPickling HandbookRhona100% (1)
- Chlor Rid SlidesDocument47 pagesChlor Rid Slidesmohammed goudaNo ratings yet
- 1498 HydrojettingDocument6 pages1498 HydrojettingjasminneeNo ratings yet
- Surface Contaminations - Salt LevelDocument2 pagesSurface Contaminations - Salt LevelLâm ThanhNo ratings yet
- Pickling HandbookDocument20 pagesPickling HandbookCuteAssadNo ratings yet
- Critical Aspects of The Salt Spray Test (ASTM B117 Altmayer 1985)Document4 pagesCritical Aspects of The Salt Spray Test (ASTM B117 Altmayer 1985)Geowana Yuka PurmanaNo ratings yet
- Preparation of Galvanized Steel Before PaintingDocument6 pagesPreparation of Galvanized Steel Before PaintingjosephNo ratings yet
- Marine Coatings Training Modules 2009Document83 pagesMarine Coatings Training Modules 2009Dejan Vuksan75% (4)
- Stainless Steel Technical PresentationDocument23 pagesStainless Steel Technical PresentationRavi Teja100% (1)
- Hydrostatic and Hydro-Testing in the Oil and Gas FieldFrom EverandHydrostatic and Hydro-Testing in the Oil and Gas FieldRating: 3 out of 5 stars3/5 (2)
- Passivation of SS Closed SystemDocument9 pagesPassivation of SS Closed SystemSin PoulNo ratings yet
- 9 Corrosion Prevention Design Material SelectDocument58 pages9 Corrosion Prevention Design Material SelectJesus De la RosaNo ratings yet
- Corrosion Protection by Design 2014 Balakrishna PalankiDocument14 pagesCorrosion Protection by Design 2014 Balakrishna PalankiBalakrishna PalankiNo ratings yet
- Structural condition assessment of Warwanti Water Treatment PlantDocument11 pagesStructural condition assessment of Warwanti Water Treatment Plantsamirbendre1No ratings yet
- Cleaning & Maintenance Guide To Stainless Steel AUDocument5 pagesCleaning & Maintenance Guide To Stainless Steel AUNarasimha DvlNo ratings yet
- Water-Soluble Salts On Substrate - Bresle MethodDocument5 pagesWater-Soluble Salts On Substrate - Bresle Methodjasminnee100% (1)
- Amtec Guide To Surface Preparation STANDARDDocument19 pagesAmtec Guide To Surface Preparation STANDARDTIẾN LÊ VĂNNo ratings yet
- Information On GI PaintingDocument5 pagesInformation On GI PaintingPavan SharmaNo ratings yet
- Soluble SaltsDocument12 pagesSoluble SaltsIan Naylor100% (1)
- Corrosion Prevention Guide for Steel SurfacesDocument62 pagesCorrosion Prevention Guide for Steel SurfacesbezzelNo ratings yet
- Thames Barrier: Case Study 11Document2 pagesThames Barrier: Case Study 11eugenio.gutenbertNo ratings yet
- Environmental - Cuases of CorrosionDocument12 pagesEnvironmental - Cuases of CorrosionLion ManabatNo ratings yet
- America’s Authority in Membrane Treatment and DesaltingDocument3 pagesAmerica’s Authority in Membrane Treatment and DesaltingRajesh MukkavilliNo ratings yet
- Bresle MethodDocument4 pagesBresle MethodyugandharNo ratings yet
- Maintenance and Repair Issues For Stone Cleaned Sandstone and GraniteDocument7 pagesMaintenance and Repair Issues For Stone Cleaned Sandstone and Granitekazuhiko_aikawaNo ratings yet
- Chemical Surface Preparation For Electroplated and Metallic CoatingsDocument18 pagesChemical Surface Preparation For Electroplated and Metallic CoatingscicerojoiasNo ratings yet
- Coating Presentation TP Bangkok - 23 Jan 2014Document54 pagesCoating Presentation TP Bangkok - 23 Jan 2014thongchai_007100% (1)
- SHELLSDocument6 pagesSHELLStouseef125mNo ratings yet
- Salt Spray TestDocument0 pagesSalt Spray TestRoozbeh PNo ratings yet
- Ngineering ATA: Protective Coating Guide For FansDocument7 pagesNgineering ATA: Protective Coating Guide For FansMohamed TahounNo ratings yet
- PROTECTIVE-COATINGS-SURFACEDocument5 pagesPROTECTIVE-COATINGS-SURFACEkhor_albertNo ratings yet
- Dow Water Solutions Filmtec Reverse Osmosis Membranes Understanding RO Element Salt Rejection Specifications June 2004Document8 pagesDow Water Solutions Filmtec Reverse Osmosis Membranes Understanding RO Element Salt Rejection Specifications June 2004Abdul RazzaqNo ratings yet
- Components and Treatments of Oilfield Produced WaterDocument7 pagesComponents and Treatments of Oilfield Produced WatermoheauNo ratings yet
- Progress Report of The Project EntitledDocument21 pagesProgress Report of The Project EntitledSyed UmairNo ratings yet
- The Importance of Chemical Treatment for Welding Stainless Steel and AluminiumDocument6 pagesThe Importance of Chemical Treatment for Welding Stainless Steel and AluminiumRahul MoottolikandyNo ratings yet
- Korozija U RudnicimaDocument2 pagesKorozija U RudnicimaMiran L HebibovićNo ratings yet
- Surface Preparation: The Essential First Stage Treatment for SteelDocument11 pagesSurface Preparation: The Essential First Stage Treatment for SteelPrabath MadusankaNo ratings yet
- Corrosion, Corrosion Rate Analysis of Steel (Case Study)Document6 pagesCorrosion, Corrosion Rate Analysis of Steel (Case Study)hariskhattakNo ratings yet
- Corrosion Rate AssigmntDocument6 pagesCorrosion Rate AssigmnthariskhattakNo ratings yet
- Design Layout of Solar Salt WorksDocument27 pagesDesign Layout of Solar Salt Worksurmil_2_k100% (1)
- Curing Mechanism of BindersDocument17 pagesCuring Mechanism of BindersSajan MittalNo ratings yet
- Surface Preparation EssentialsDocument18 pagesSurface Preparation EssentialsVincent LecoursNo ratings yet
- Corrosion Control of Marine StructuresDocument35 pagesCorrosion Control of Marine StructuresCong-OanhNguyenNo ratings yet
- Site Safety Handbook for the Petroleum IndustryFrom EverandSite Safety Handbook for the Petroleum IndustryRating: 5 out of 5 stars5/5 (1)
- Corrosion: Corrosion ControlFrom EverandCorrosion: Corrosion ControlL L ShreirRating: 5 out of 5 stars5/5 (1)
- Handbook for Cleaning for Semiconductor Manufacturing: Fundamentals and ApplicationsFrom EverandHandbook for Cleaning for Semiconductor Manufacturing: Fundamentals and ApplicationsKaren A. ReinhardtNo ratings yet
- Case Studies of Material Corrosion Prevention for Oil and Gas ValvesFrom EverandCase Studies of Material Corrosion Prevention for Oil and Gas ValvesNo ratings yet
- Integrated Sand Management For Effective Hydrocarbon Flow AssuranceFrom EverandIntegrated Sand Management For Effective Hydrocarbon Flow AssuranceNo ratings yet
- Corrosion Control in the Oil and Gas IndustryFrom EverandCorrosion Control in the Oil and Gas IndustryRating: 4 out of 5 stars4/5 (12)
- Corrosion Testing for Metal Finishing: Institute of Metal FinishingFrom EverandCorrosion Testing for Metal Finishing: Institute of Metal FinishingNo ratings yet
- Reservoir Characterization of Tight Gas Sandstones: Exploration and DevelopmentFrom EverandReservoir Characterization of Tight Gas Sandstones: Exploration and DevelopmentNo ratings yet
- Designing Amenity Rooftops PDFDocument11 pagesDesigning Amenity Rooftops PDFMikeNo ratings yet
- Otis EscalatorDocument2 pagesOtis Escalatorratnasekhar_g100% (1)
- Meet The Experts - GalvanizingDocument5 pagesMeet The Experts - GalvanizingTraianRus100% (1)
- Tensinews 18-mrs 2010-2Document24 pagesTensinews 18-mrs 2010-2MikeNo ratings yet
- Hot Dip Galvanized Threaded Fasteners - GalvanizersDocument2 pagesHot Dip Galvanized Threaded Fasteners - GalvanizersMikeNo ratings yet
- Paving Systems Over Plaza Waterproofing MembranesDocument7 pagesPaving Systems Over Plaza Waterproofing MembranesMikeNo ratings yet
- 2017 Standard CatalogDocument116 pages2017 Standard CatalogpicottNo ratings yet
- Initial and Maintenance Cost Analysis: Hot-Dip Galvanized Steel PaintDocument2 pagesInitial and Maintenance Cost Analysis: Hot-Dip Galvanized Steel PaintMikeNo ratings yet
- CFD Verification ExampleDocument7 pagesCFD Verification ExampleMikeNo ratings yet
- Morin Roof or Wall Exposed PDFDocument2 pagesMorin Roof or Wall Exposed PDFMikeNo ratings yet
- Hollo-Bolt by Lindapter 960Document8 pagesHollo-Bolt by Lindapter 960Anonymous a5jwqF7tUNo ratings yet
- TecCrete Product Information SheetDocument2 pagesTecCrete Product Information SheetMikeNo ratings yet
- Micropile Design and Construction Guidelines Implementation ManualDocument380 pagesMicropile Design and Construction Guidelines Implementation ManualJose MartinezNo ratings yet
- Catalog - Morin Low Res PDFDocument28 pagesCatalog - Morin Low Res PDFMikeNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument23 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsMikeNo ratings yet
- Morin Roof or Wall Exposed PDFDocument2 pagesMorin Roof or Wall Exposed PDFMikeNo ratings yet
- Micropile Design and Construction Guidelines Implementation ManualDocument380 pagesMicropile Design and Construction Guidelines Implementation ManualJose MartinezNo ratings yet
- Catalog - Morin Low Res PDFDocument28 pagesCatalog - Morin Low Res PDFMikeNo ratings yet
- Heavy Duty Post Shore:: Increase The Load, Decrease The CostDocument2 pagesHeavy Duty Post Shore:: Increase The Load, Decrease The CostJonathan ThomasNo ratings yet
- Brochure Beam-Plywood USADocument4 pagesBrochure Beam-Plywood USAMikeNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument23 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsMikeNo ratings yet
- L&M For 137 Seating OptionsDocument2 pagesL&M For 137 Seating OptionsMikeNo ratings yet
- Flood ZonesDocument1 pageFlood ZonesMikeNo ratings yet
- Corp Catering Fall2018Document12 pagesCorp Catering Fall2018MikeNo ratings yet
- Weld GroupDocument1 pageWeld GroupMikeNo ratings yet
- Design and Behavior of Reinforced Concrete Pile Caps: A Literature ReviewDocument12 pagesDesign and Behavior of Reinforced Concrete Pile Caps: A Literature ReviewMikeNo ratings yet
- Analysis NotesDocument10 pagesAnalysis NotesAhmed SayyidNo ratings yet
- Owner's ManualDocument360 pagesOwner's ManualMikeNo ratings yet
- Owner's ManualDocument360 pagesOwner's ManualMikeNo ratings yet
- Effect of Concentration in Rate of ReactionDocument3 pagesEffect of Concentration in Rate of ReactionblablaNo ratings yet
- A Comparative Study of ZnO-PVP and ZnO-PEG Nanoparticles ActivityDocument8 pagesA Comparative Study of ZnO-PVP and ZnO-PEG Nanoparticles ActivityVũ Hải NamNo ratings yet
- Ats 2 (Csir Net) PDFDocument17 pagesAts 2 (Csir Net) PDFAayushi VermaNo ratings yet
- Crude Oil Tank Mixer Data SheetDocument3 pagesCrude Oil Tank Mixer Data Sheetsiska bedegul100% (1)
- Production Technology Course OutDocument5 pagesProduction Technology Course Outmurjass85No ratings yet
- Development of A Projectile Penetration Theory. Report 1Document101 pagesDevelopment of A Projectile Penetration Theory. Report 1yararaNo ratings yet
- Measuring errors and their classificationDocument6 pagesMeasuring errors and their classificationNarendra Reddy0% (1)
- The High-Latitudude IonospehereDocument639 pagesThe High-Latitudude IonospehereSainath Bharadwaj100% (2)
- Compilation Part 2 (11-19)Document100 pagesCompilation Part 2 (11-19)Joshua Zuniga50% (2)
- Practice Quiz Diffraction 1Document2 pagesPractice Quiz Diffraction 1pauljkt1No ratings yet
- Culverts DesignDocument21 pagesCulverts DesignNani CherryNo ratings yet
- HVAC Validation TestsDocument4 pagesHVAC Validation TestsemonwreNo ratings yet
- XH3-HE User'S Manual: Self-Contained, Single Pump Wellhead Control Panel For Harsh EnvironmentsDocument9 pagesXH3-HE User'S Manual: Self-Contained, Single Pump Wellhead Control Panel For Harsh EnvironmentsprabuNo ratings yet
- Polyaldo PolyglycerolEsters SLSDocument8 pagesPolyaldo PolyglycerolEsters SLSSantos GarciaNo ratings yet
- 442-032 PDF PDFDocument12 pages442-032 PDF PDFCalNo ratings yet
- Glass Ionomer Cement Properties and ApplicationsDocument67 pagesGlass Ionomer Cement Properties and ApplicationsJayalakshmi Preetha100% (1)
- English Literature Syllabus BreakdownDocument35 pagesEnglish Literature Syllabus BreakdownKirti PathakNo ratings yet
- RT Formulas For CalculationsDocument15 pagesRT Formulas For CalculationsAwais Jamil70% (10)
- Inspect F50: Everything Needed For Conventional High Resolution Sample InvestigationDocument4 pagesInspect F50: Everything Needed For Conventional High Resolution Sample InvestigationMiruna PetriaNo ratings yet
- Lecture Planner - Chemistry PDF OnlyDocument1 pageLecture Planner - Chemistry PDF OnlyJai ChandNo ratings yet
- Barett PDFDocument20 pagesBarett PDFlnadolskiNo ratings yet
- Interactive Textbook 5 PDF Elelments 3 1Document5 pagesInteractive Textbook 5 PDF Elelments 3 1api-240094705No ratings yet
- 3RD Quarter Exam Grade 7 ScienceDocument2 pages3RD Quarter Exam Grade 7 Sciencegerald83% (6)
- PHYS 2225 Lab 13 Dispersion and Geometric OpticsDocument6 pagesPHYS 2225 Lab 13 Dispersion and Geometric OpticsT. ArchulettaNo ratings yet
- Coconut Cocos Nucifera As An Alternative To Paraffin FloorwaxDocument7 pagesCoconut Cocos Nucifera As An Alternative To Paraffin FloorwaxMiguel Piquero67% (9)
- 2013 12 Handbok Fittings ENG WebDocument72 pages2013 12 Handbok Fittings ENG WebVictor BiacoloNo ratings yet
- Geology Geophysics in Oil ExplorationDocument70 pagesGeology Geophysics in Oil Explorationberbere68100% (1)
- Quinine Hydrochloride 0018eDocument2 pagesQuinine Hydrochloride 0018eMark GoldbergNo ratings yet
- Sat Vocabulary 6000 Words PDFDocument151 pagesSat Vocabulary 6000 Words PDFUman100% (1)