Professional Documents
Culture Documents
Matter in Our Surroundings
Uploaded by
anjupalCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Matter in Our Surroundings
Uploaded by
anjupalCopyright:
Available Formats
Blog: scienceworkplace.blogspot.
com
Matter In Our Surroundings
Matter Any thing which has mass and occupy space is called matter. Matter is made up of particles. Particles are very small and present in millions in a matter.
Blog: scienceworkplace.blogspot.com
Q. Does the level of water changes when we dissolve salt in water? Ans. No, level of water does not change. When we dissolve salt in water, the particles of salt get into the spaces between particles of water. Q. Discus an activity to prove particles are very tyne and present in millions in a matter.
Ans. We conclude that there must be millions of tiny particles in just one crystal of potassium permanganate, which keep on dividing themselves into smaller and smaller and smaller. Characteristics of Particles of Matter a) Particles of matter have space between them. Order of interstitial spaces is Solids < liquids < gases b) Particles of matter are in continuously moving- Brownian motion and diffusion. Particles possess the kinetic energy. As the temperature rises, particles move faster. c) Particles of matter attract each other. Order of force of attraction solids> liquids > gases
Blog: scienceworkplace.blogspot.com
d)
The intermixing of particles of two different types of matter on their own is called diffusion. We also observe that on heating, diffusion becomes faster.
Blog: scienceworkplace.blogspot.com
States of Matter
Consider the following: a) What about a rubber band. can it change its shape on stretching? Is it a solid? Ans. A rubber band changes shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
4
Blog: scienceworkplace.blogspot.com
(b) What about sugar and salt? When kept in different jars these take the shape of the jar. Are they solid? Ans. The shape of each individual sugar or salt crystal remains fixed, whether we take it in our hand, put it in a plate or in a jar. (c) What about a sponge? It is a solid yet we are able to compress it. Why? Ans. A sponge has minute holes, in which air is trapped, when we press it, the air is expelled out and we are able to compress it. Change in state Solid to liquid ( Fusion) a) On increasing the temperature of solids,the kinetic energy of the particles increases. b) Due to the increase in kinetic energy, the particles start vibrating with greater speed. c) The energy supplied by heat overcomes the forces of attraction between the particles. d) The particles leave their fixed positions and start moving more freely. e) A stage is reached when the solid melts and is converted to a liquid. Melting point The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point. The melting point of ice is 273.16 K . Latent heat of Fusion The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. Liquid to gas ( vaporization ) a) When we supply heat energy to water, particles start moving even faster. b) At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. c) At this temperature the liquid starts changing into gas.
5
Blog: scienceworkplace.blogspot.com
Boiling point The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Latent heat of Vaporisation The amount of heat energy that is required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point is known as the latent heat of vaporisation. Solid to gas ( Sublimation) a) A change of state directly from solid to gas without changing into liquid state (or vice versa) is called sublimation. b) Example; Camphor and Ammonium chloride are sublimate.
Melting and boiling point indicate the strength of intermolecular forces. Volatile liquids like alcohol, petrol, acetone have very weak intermolecular force. Effect of change of pressure The difference in various states of matter is due to the difference in the distance between constituent particles.
6
Blog: scienceworkplace.blogspot.com
Applying pressure and reducing temperature can liquefy gases.
Evaporation In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
Blog: scienceworkplace.blogspot.com
Factors affecting evaporation The rate of evaporation increases with An increase of surface area An increase of temperature: With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state. A decrease in humidity: Humidity is the amount of water vapour present in air. An increase in wind speed: With the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding. Evaporation causes cooling The large latent heat of vaporisation of water helps to cool the hot surface. Master Card 1L=1000 mL, 1ml= 1 cm3 0F = 9/5 (0C) + 32 K= 0 C+ 273 , At -400C reading of both the 0 C and 0 F are equal At 40C water has maximum density The critical point ofwater is at a temperature of 374C (705. 2F) and a pressure of218 atmospheres.
Blog: scienceworkplace.blogspot.com
Question and answer 1. Why ice has lower density then water and floats on it? Ans. Ice has lower density than water because it has cage like structure. In this structure a lot of vacant spaces are left., when water molecules are linked in ice.
Blog: scienceworkplace.blogspot.com
2. Which has more energy steam or water and why? Ans. Particles in steam, (at 1000C) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporisation. 3. When ice melts, its temperature remains the same, so where does the heat energy go? Ans. The temperature of the system does not change after the melting point is reached, till all the ice melts. The heat gets used up in changing the state by overcoming the forces of attraction between the particles. 4. Why solid CO2 is called Dry ice? Ans. Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice. 5. Why should we wear cotton clothes in summer? Ans. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation. The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool.
10
Blog: scienceworkplace.blogspot.com
6. Why do we see water droplets on the outer surface of a glass containing ice- cold water? Ans. The water vapour present in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state. 7. Write difference between evaporation and boiling.
8. Write a short note on Plasma and BEC. Ans. Plasma The state consists of super energetic and super excited particles. These particles are in the form of ionised gases. The plasma glows with a special colour depending on the nature of gas. The Sun and the stars glow because of the presence of plasma in them. The plasma is created in stars because of very high temperature.
11
Blog: scienceworkplace.blogspot.com
Bose-Einstein Condensate: The BEC is formed by cooling a gas of extremely low density, about one-hundred-thousandth the density of normal air, to super low temperatures.
12
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Reproduction in Animals Notes For Class 8Document8 pagesReproduction in Animals Notes For Class 8anjupalNo ratings yet
- Control & Coordination - Class XDocument7 pagesControl & Coordination - Class XanjupalNo ratings yet
- Reproduction in Human BeingsDocument6 pagesReproduction in Human BeingsanjupalNo ratings yet
- Sources of Energy - Class XDocument9 pagesSources of Energy - Class XanjupalNo ratings yet
- Periodic Classification of Elements PDFDocument8 pagesPeriodic Classification of Elements PDFanjupal80% (5)
- Improvement in Food ResourcesDocument11 pagesImprovement in Food Resourcesanjupal100% (2)
- Science Main 1 & 2 (ClassGÇôIX) Open Book Exam Sample PDFDocument13 pagesScience Main 1 & 2 (ClassGÇôIX) Open Book Exam Sample PDFanjupalNo ratings yet
- Heredity and EvolutionDocument16 pagesHeredity and EvolutionanjupalNo ratings yet
- Reproduction in PlantsDocument14 pagesReproduction in Plantsanjupal100% (2)
- Improvement in Food REsources - Class IX Mind MappingDocument12 pagesImprovement in Food REsources - Class IX Mind Mappinganjupal83% (6)
- Control and CoordinationDocument17 pagesControl and Coordinationanjupal100% (1)
- Carbon Footprint - Vivek - X-ADocument33 pagesCarbon Footprint - Vivek - X-AanjupalNo ratings yet
- Force and Laws of MotionDocument20 pagesForce and Laws of MotionanjupalNo ratings yet
- Magnetic Effect of Electric CurrentDocument13 pagesMagnetic Effect of Electric Currentanjupal100% (4)
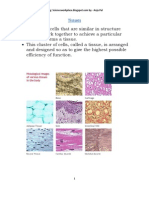
- TissuesDocument16 pagesTissuesanjupalNo ratings yet
- Nutrition in AnimalsDocument19 pagesNutrition in AnimalsanjupalNo ratings yet
- HeatDocument11 pagesHeatanjupalNo ratings yet
- Cell, The Fundamental Unit of LifeDocument12 pagesCell, The Fundamental Unit of LifeanjupalNo ratings yet
- Nutrition in PlantsDocument8 pagesNutrition in PlantsanjupalNo ratings yet
- Life ProcessesDocument28 pagesLife Processesanjupal100% (1)
- L-8 Motion: List of ConceptsDocument11 pagesL-8 Motion: List of ConceptsanjupalNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Springer E-Books To VTU - ConsortiumDocument420 pagesSpringer E-Books To VTU - ConsortiumArie MPNo ratings yet
- CEIC 2002 Exam Preparation Practice Question No. 1 FluxDocument2 pagesCEIC 2002 Exam Preparation Practice Question No. 1 FluxWinnieNo ratings yet
- Hydrotreater Optimization With WpheDocument29 pagesHydrotreater Optimization With Wpheandrei12320003181No ratings yet
- Raymond CBLMDocument35 pagesRaymond CBLManna luna llubit100% (2)
- ME Course Content Guide for AY2011/2012 IntakeDocument4 pagesME Course Content Guide for AY2011/2012 IntakedavidwongbxNo ratings yet
- Engineering Physics BTech NIT CalicutDocument12 pagesEngineering Physics BTech NIT Calicutnitcalicutian100% (1)
- Bhanda Puta Valuka Puta Bhudhara PutaDocument19 pagesBhanda Puta Valuka Puta Bhudhara PutaDr Debasis PanigrahiNo ratings yet
- Freeze-Drying: The Basic Process: Ã 2016 Elsevier Ltd. All Rights ReservedDocument6 pagesFreeze-Drying: The Basic Process: Ã 2016 Elsevier Ltd. All Rights ReservedYashinta PutriNo ratings yet
- Metallurgical Thermo DynamicsDocument567 pagesMetallurgical Thermo Dynamicspahnin100% (4)
- Hidraulica Magazine 3 2013Document87 pagesHidraulica Magazine 3 2013Valentino MiroiuNo ratings yet
- Heat and Temperature ModuleDocument22 pagesHeat and Temperature Modulekiannatherese andradaNo ratings yet
- 2012-Performance Evaluation of A Solar Wind-Ventilated Cabinet DryerDocument7 pages2012-Performance Evaluation of A Solar Wind-Ventilated Cabinet DryerAbhishek SaxenaNo ratings yet
- CompiledDocument114 pagesCompiledRovic Jan Rafael RoaNo ratings yet
- Design and Construction of A Waterproof Sealing MachineDocument46 pagesDesign and Construction of A Waterproof Sealing MachineEkwe PaulNo ratings yet
- MAK 302L Experiment 3 Extended Surface Heat Transfer 1. PurposeDocument5 pagesMAK 302L Experiment 3 Extended Surface Heat Transfer 1. PurposeYEe FaNgNo ratings yet
- MO ch6Document55 pagesMO ch6Mohammad HajeerNo ratings yet
- Lesson 5 Conduction - Fourier Law of Heat ConductionDocument24 pagesLesson 5 Conduction - Fourier Law of Heat Conductionsurya kiranNo ratings yet
- Thermodynamic Exercise EntropyDocument2 pagesThermodynamic Exercise EntropyFarid AimanNo ratings yet
- NSO Solved Paper 2017-18Document9 pagesNSO Solved Paper 2017-18Vatsal NarwalNo ratings yet
- Tropical Architecture - UMNDocument11 pagesTropical Architecture - UMNmNo ratings yet
- Ongc InterviewDocument14 pagesOngc InterviewShubham MishraNo ratings yet
- Heat Transfer: Physical Origins and Rate EquationsDocument29 pagesHeat Transfer: Physical Origins and Rate EquationsAllyth AlqhtaniNo ratings yet
- PureTemp 23 Technical Data SheetDocument1 pagePureTemp 23 Technical Data SheetIkutegbe CharlesNo ratings yet
- Comparison Between Different Heat Sources Types in Thin-Plate Welding Simulation-Hashemzadeh-2014Document8 pagesComparison Between Different Heat Sources Types in Thin-Plate Welding Simulation-Hashemzadeh-2014DonatasNo ratings yet
- G10 CH 6 Heat&Temperature11May07Document17 pagesG10 CH 6 Heat&Temperature11May07Tun Lin AungNo ratings yet
- Grade 12 TH A WorksheetDocument7 pagesGrade 12 TH A Worksheetabdimoh7522No ratings yet
- CH 6 QuizDocument2 pagesCH 6 QuizClaire Elizabeth OlsonNo ratings yet
- 2013 Guie of Student Kroes Aircraft Basic Science 8thDocument154 pages2013 Guie of Student Kroes Aircraft Basic Science 8thJose Tejeda100% (1)
- Rotary Dryer CalculationDocument5 pagesRotary Dryer Calculationsushant_jhawer100% (2)
- Department of Biomedical Engineering (Aait) : Work Sheet #3Document4 pagesDepartment of Biomedical Engineering (Aait) : Work Sheet #3gfsfNo ratings yet