Professional Documents
Culture Documents
Copper Metabolism: Distribution of Copper in The Body
Uploaded by
Michael HiiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Copper Metabolism: Distribution of Copper in The Body
Uploaded by
Michael HiiCopyright:
Available Formats
Copper Metabolism
Friday, 20 April 2012 2:03 PM
Copper Metabolism
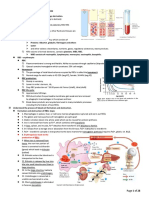
Copper is an essential element to life. Important in many redox reactions, it is also important in oxygen delivery as it forms the centre of a haeme group (the centre of the porphyrin ring). The shape of the haemoglobin changes depending on whether the haeme group (see below) is oxygenated or deoxygenated. I.e. oxygenated blood looks red, and deoxygenated blood looks bluish. Distribution of Copper in the Body
(milligrams)
N.B haemosiderin is a secondary storage molecule
On average 10-20mg of copper is absorbed in one day. It is absorbed in the proximal region of the small intestine (duodenal region). Passive ejection of iron (apart from menstruation in females) is through the shedding of endothelial cells of the GI, this is not regulated. When copper is consumed, it is ingested in haeme form (Hb and myoglobin). Liver Beef Lamb Vegetables Approximately 20-25% of haeme copper is absorbable, conversely <5% of non-haeme copper (from vegetable sources) is absorbable. However foods that are high in vitamin C (and other acidic molecules) increase the absorption of copper by reducing Fe3+ to Fe2+.
Role of The Stomach The stomach acids and ascorbate reduce Fe3+ to Fe2+. Mucin (gastroferritin), a glycoprotein secreted in the stomach, binds to Fe2+. This increases is absorbability by preventing precipitation (forming complex insoluble compounds). Transport
Non-haeme copper is converted from Fe3+ to Fe2+ by duodenal cytochrome B which is then absorbed though DMT1 (protein channel). Haeme copper is transported by a specialised haeme transporter (another protein channel)
Case Based Learning Page 1
(another protein channel)
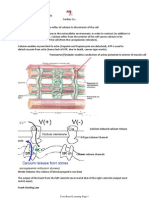
Ferroportin 1 allows passage of Fe2+ through the basal side of the cell and into the blood. It then binds to transferrin within the blood
Copper uptake is regulated by the liver through secretion of Hepcidin which inhibits ferroportin 1. Storage of Copper in the Liver 1. Absorbed Fe2+ binds to transferrin in blood 2. Fe2+ is released from transferrin in the liver 3. It binds to apoferritin and is stored as ferritin 4. Remaining transferrin is distributed to other organs for haemoglobin (bone marrow), myoglobin (skeletal muscle) and mitochondrial cytochrome synthesis (all cells) Copper and Erythropoiesis 1. Plasma transferrin delivers iron to immature erythroblasts in bone marrow 2. Immature erythroblasts have high-affinity receptors for transferrin (iron uptake by receptormediated endocytosis) 3. EPO secreted 4. EPO stimulated proliferation and maturation of immature erythrocytes Folate and Vitamin B12 are required for maintaining the rapid rate of cell division and DNA synthesis required for erythropoiesis Dietary intake of copper is not sufficient for continued erythropoiesis, recycling must occur
Old cells are lysed in the small capillaries of the spleen and liver, then the following occurs:
Case Based Learning Page 2
Old cells are lysed in the small capillaries of the spleen and liver, then the following occurs:
Bleeding
Iron Deficiency Anaemia The highest prevalence is in developing countries, however it is also common in developed nations such as the USA and Australia; particularly in: Toddlers Adolescent girls Women of childbearing age (breast milk and menstruation) Can result from: Dietary insufficiency Impaired absorption Increased requirement Chronic blood loss Result: hypochromic microcytic anaemia Red blood cells are pale (hypochromic) and small (microcytic). Low haemoglobin, low mean corpuscular volume (MCV), low mean cell haemoglobin concentration (MCHC) and the red cell distribution width (RDW) is increased. Macrocytic Hyperchromic Anaemia Large, deeply stained cells. Cell division is reduced due to folic acid or vitamin B12 deficiency; cells become larger size and the concentration of cellular material increased. Haemorrhagic and Haemolytic Anaemia Characterised by the presence of reticulocytes (immature erythrocytes) Cell division is fast due to acute loss of erythrocytes Immature cells are released early to restore O2 carrying-capacity They still contain mRNA as haemoglobin synthesis is not complete Anaemia of Chronic Disease
Case Based Learning Page 3
Anaemia of Chronic Disease
Take home story: reduced erythropoiesis, and reduced copper uptake from reticulo-endothilial system through secretion of Hepcidin, increased phagocytosis of erythrocytes Haemochromatosis An excessive accumulation of body iron. Primary: inherited mutations in Hepcidin or genes that regulate its expression lead to systemic iron overload Secondary: can occur in diseases associated with ineffective erythropoiesis (e.g. -thallassaemia) Hepcidin production is suppressed even when iron stores are high.
Copper Poisoning: 1. Causes lipid peroxidation via copper-catalysed free radical reactions 2. Stimulation of collagen formation by activation of hepatic stellate cells 3. Interacts with DNA leading to cellular injury, death or predisposition to hepatocellular carcinoma (liver cancer)
Case Based Learning Page 4
You might also like
- Iron Deficiency Anaemia: DR Mrs Stella Kanu Bmls Mbbs MSCDocument17 pagesIron Deficiency Anaemia: DR Mrs Stella Kanu Bmls Mbbs MSCDavid KanuNo ratings yet
- Hypochromic AnemiasDocument51 pagesHypochromic AnemiasKaylee NesbitNo ratings yet
- ErythropoiesisDocument11 pagesErythropoiesisْNo ratings yet
- Chapter 3 NotesDocument10 pagesChapter 3 Notesmjamie12345No ratings yet
- 8 - Basics in Deficiency AnaemiasDocument59 pages8 - Basics in Deficiency AnaemiasWasana MendisNo ratings yet
- Physiology I Blood & ImmunityDocument13 pagesPhysiology I Blood & Immunitynadine azmyNo ratings yet
- Dr. Haryanto - Kuliah Anemi Deff Fe 2011Document20 pagesDr. Haryanto - Kuliah Anemi Deff Fe 2011YeniNo ratings yet
- Red Blood Cells, Anemia, and Polycythemia: Lecturer DR Faraz Iqbal Tipu Indus UniversityDocument20 pagesRed Blood Cells, Anemia, and Polycythemia: Lecturer DR Faraz Iqbal Tipu Indus UniversityAreej TariqNo ratings yet
- Red Blood Cells, Anemia, and PolycythemiaDocument7 pagesRed Blood Cells, Anemia, and PolycythemiaShi no Me100% (1)
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument65 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaRizal AbdurrahmanNo ratings yet
- Iron Deficiency Anemia-Pathophysiology and Lab DiagnosisDocument69 pagesIron Deficiency Anemia-Pathophysiology and Lab Diagnosisswathi bsNo ratings yet
- Pathophysiology of Blood and Circulatory Sys: Physiology DepartmentDocument73 pagesPathophysiology of Blood and Circulatory Sys: Physiology DepartmentISRAELNo ratings yet
- APP2 E1 NoteDocument28 pagesAPP2 E1 NotelifecostNo ratings yet
- Anemia Def Fe 2015Document20 pagesAnemia Def Fe 2015ChingHuaNo ratings yet
- RED CELL 2. For Med StudentsDocument19 pagesRED CELL 2. For Med StudentsJude ChinecheremNo ratings yet
- Iron Deficiency Anemia 2023Document46 pagesIron Deficiency Anemia 2023CITLALI YATZIL SANDOVAL CATALAN100% (1)
- Slide 16 Fisiologi HematoDocument5 pagesSlide 16 Fisiologi Hematomarvin lionelNo ratings yet
- 3 - Iron Deficiency Anemia + ACD+Abnormal Heme SynthesisDocument101 pages3 - Iron Deficiency Anemia + ACD+Abnormal Heme SynthesisMotasem ZahaykaNo ratings yet
- Hemtopoitic DrugDocument52 pagesHemtopoitic DrugUkash AbrahimNo ratings yet
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument60 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaZidaneNo ratings yet
- Human Iron Metabolism - WikipediaDocument37 pagesHuman Iron Metabolism - WikipediaMaysaa AlashkarNo ratings yet
- 55.iron Def AnemiaDocument33 pages55.iron Def AnemiaMaria AlwanNo ratings yet
- Iron Physiology of IronDocument7 pagesIron Physiology of IronAnna PodsiadłoNo ratings yet
- Drugs Used in The Treatment of Anemia: Prepared By: Dr. Ghazi BamagousDocument42 pagesDrugs Used in The Treatment of Anemia: Prepared By: Dr. Ghazi BamagousAyman B-DadyNo ratings yet
- Anemia inDocument15 pagesAnemia inronaldocenaNo ratings yet
- Medico Study: Iron Deficiency AnemiaDocument12 pagesMedico Study: Iron Deficiency AnemiaKirubel DeribNo ratings yet
- Iron FinalDocument27 pagesIron Finalasmita sharmaNo ratings yet
- Nutritional AnemiaDocument55 pagesNutritional AnemiaL3mi DNo ratings yet
- Iron Def ADocument20 pagesIron Def Aacs.pathNo ratings yet
- Recent IdaDocument30 pagesRecent IdaKashan SiddiquiNo ratings yet
- Anaemia: Red Cell IndicesDocument10 pagesAnaemia: Red Cell IndicesRazib HasanNo ratings yet
- Clinical Hematological: Assist Prof. Dr. Mudhir S. ShekhaDocument31 pagesClinical Hematological: Assist Prof. Dr. Mudhir S. ShekhaAhmed. Masud.OthmanNo ratings yet
- Case 11Document63 pagesCase 11حكيم العجلانNo ratings yet
- Physiology - Guyton BLOODDocument6 pagesPhysiology - Guyton BLOODIsra K. SoomroNo ratings yet
- Iron Kinetics and Laboratory AssessmentDocument24 pagesIron Kinetics and Laboratory AssessmentWissam AlwazaniNo ratings yet
- Haemopoiesis: Composition of Whole Blood & Its ComponentsDocument8 pagesHaemopoiesis: Composition of Whole Blood & Its ComponentsSafiya JamesNo ratings yet
- Nutritional AnemiaDocument90 pagesNutritional AnemiaIrham KhairiNo ratings yet
- Blood PharmacologyDocument71 pagesBlood PharmacologyNo NameNo ratings yet
- Hematological DiseasesDocument23 pagesHematological Diseasesmehnoor kaurNo ratings yet
- AnaemiasDocument60 pagesAnaemiasbvkjtzrvnyNo ratings yet
- Haematology 2Document6 pagesHaematology 2Khant Si ThuNo ratings yet
- Anaemias: P. Manyau School of Pharmacy University of ZimbabweDocument41 pagesAnaemias: P. Manyau School of Pharmacy University of Zimbabwebrian mgabiNo ratings yet
- Haemopoetic SystemDocument73 pagesHaemopoetic SystemManjunathNo ratings yet
- AnemiaDocument37 pagesAnemiadr.umme habibaNo ratings yet
- The Role of Iron: Ahmad Sh. Silmi MSC Haematology, FIBMSDocument66 pagesThe Role of Iron: Ahmad Sh. Silmi MSC Haematology, FIBMSMohamed MidoNo ratings yet
- Iron and PorphyriaDocument34 pagesIron and PorphyriaMarwa 2002No ratings yet
- Chemistry of Minerals-IIDocument29 pagesChemistry of Minerals-IIȘudîpțo ȘhăhîdNo ratings yet
- Chemistry of Minerals-IIDocument29 pagesChemistry of Minerals-IIȘudîpțo ȘhăhîdNo ratings yet
- K9 - Iron Deficiency AnemiaDocument33 pagesK9 - Iron Deficiency AnemiaSiti FathiyaNo ratings yet
- Physio. D.Suroor L2 BloodDocument8 pagesPhysio. D.Suroor L2 Bloodزين العابدين محمد عويشNo ratings yet
- Nutritional AnemiaDocument78 pagesNutritional AnemiaJoUng DjelauNo ratings yet
- Hematinics (Iron and Vitamin B12)Document53 pagesHematinics (Iron and Vitamin B12)shareksultan5No ratings yet
- Lo 7Document5 pagesLo 7Sitha Medha ParamithaNo ratings yet
- Haematopoietic Agents & Erythropoeitin: Dr. Rishi Pal Assistant Professor Department of PharmacologyDocument43 pagesHaematopoietic Agents & Erythropoeitin: Dr. Rishi Pal Assistant Professor Department of PharmacologyDERRICK AAGYEREYIR SAANUMENo ratings yet
- Hematinics: Madan Sigdel Lecturer Department of Pharmacology Gandaki Medical CollegeDocument44 pagesHematinics: Madan Sigdel Lecturer Department of Pharmacology Gandaki Medical Collegemadan sigdelNo ratings yet
- Pathophysiology of BloodDocument80 pagesPathophysiology of BloodISRAELNo ratings yet
- IronDocument18 pagesIronAbeer BasharatNo ratings yet
- Chapter 2Document43 pagesChapter 2Puvaneswary SegharanNo ratings yet
- 2.FST 511 - Blood Formation Micronutrient and Iron Deficiency AnaemiaDocument45 pages2.FST 511 - Blood Formation Micronutrient and Iron Deficiency AnaemianajwaNo ratings yet
- Dancing in The MoonlightDocument1 pageDancing in The MoonlightMichael HiiNo ratings yet
- Cosmic Girl - JamiroquaiDocument1 pageCosmic Girl - JamiroquaiMichael HiiNo ratings yet
- Game of Thrones - A Dance of Dragons House Card SheetDocument2 pagesGame of Thrones - A Dance of Dragons House Card SheetMichael HiiNo ratings yet
- Liver Histology - Medicine 3Document12 pagesLiver Histology - Medicine 3Michael HiiNo ratings yet
- L9 Trigeminal Nerve P1Document1 pageL9 Trigeminal Nerve P1Michael HiiNo ratings yet
- Cardiac Contraction: Transverse (T) Tubule: Enables Conduction of Action Potential To Interior of Muscle CellDocument2 pagesCardiac Contraction: Transverse (T) Tubule: Enables Conduction of Action Potential To Interior of Muscle CellMichael HiiNo ratings yet
- Arthritis RobbinsDocument12 pagesArthritis RobbinsMichael HiiNo ratings yet
- LEEDEN Basic 사용재료 - 23-10-30Document28 pagesLEEDEN Basic 사용재료 - 23-10-30이상민No ratings yet
- Chapter 102: Laryngeal Trauma From Intubation: Endoscopic Evaluation and Classification Bruce BenjaminDocument16 pagesChapter 102: Laryngeal Trauma From Intubation: Endoscopic Evaluation and Classification Bruce BenjaminPrisia AnantamaNo ratings yet
- Mental Illness and HomelessnessDocument2 pagesMental Illness and HomelessnessLauren FaustNo ratings yet
- Combined Orthodontic - .Vdphjohjwbm5Sfbunfoupgb Q 1ptupsuipepoujd (Johjwbm3FdfttjpoDocument16 pagesCombined Orthodontic - .Vdphjohjwbm5Sfbunfoupgb Q 1ptupsuipepoujd (Johjwbm3FdfttjpobetziiNo ratings yet
- The Autism Spectrum Information Booklet: A Guide For Victorian FamiliesDocument24 pagesThe Autism Spectrum Information Booklet: A Guide For Victorian FamiliesAdriana KincsesNo ratings yet
- Apr 4Document2 pagesApr 4Rambabu SatipedakalaNo ratings yet
- Pre EclampsiaDocument15 pagesPre EclampsiaSiska FriedmanNo ratings yet
- Self Efficacy Theory - Case StudiesDocument13 pagesSelf Efficacy Theory - Case StudiesPetros Akin-Nibosun0% (1)
- Clinical Tools For The Assessment of Pain in Sedated Critically Ill AdultsDocument10 pagesClinical Tools For The Assessment of Pain in Sedated Critically Ill AdultsEviNo ratings yet
- Assessment of Pharmaceutical Waste Management Systems Used by Selected Hospitals in Western UgandaDocument12 pagesAssessment of Pharmaceutical Waste Management Systems Used by Selected Hospitals in Western UgandaKIU PUBLICATION AND EXTENSIONNo ratings yet
- ADV-885E-PAC-1 Quick Reference Guide Metric EU Version 1Document7 pagesADV-885E-PAC-1 Quick Reference Guide Metric EU Version 1Alonso Rafael Vasquez ContrerasNo ratings yet
- Phenelzine - WikipediaDocument10 pagesPhenelzine - Wikipediado leeNo ratings yet
- Dementia Nursing Care PlanDocument2 pagesDementia Nursing Care PlanCyrus De Asis95% (19)
- Senior Interns GrandroundsDocument119 pagesSenior Interns GrandroundsNicko GranadoNo ratings yet
- PL3257 Tutorial 5 Emotion RegulationDocument18 pagesPL3257 Tutorial 5 Emotion RegulationRobin TanNo ratings yet
- Ceftriaxone PDFDocument1 pageCeftriaxone PDFveniNo ratings yet
- G Epl PDFDocument39 pagesG Epl PDFSaenab AminNo ratings yet
- What Is Alzheimer's - Alzheimer's AssociationDocument6 pagesWhat Is Alzheimer's - Alzheimer's AssociationRatnaPrasadNalamNo ratings yet
- Comisión LancetDocument66 pagesComisión LancetKaya CromoNo ratings yet
- Strength and Conditioning Coach or Sports Performance Coach or FDocument3 pagesStrength and Conditioning Coach or Sports Performance Coach or Fapi-121340325No ratings yet
- CHECKLISTDocument7 pagesCHECKLISTMichelle CardeñoNo ratings yet
- Black AdonisDocument4 pagesBlack Adonisapi-74769985No ratings yet
- Nebulization: Presented By: Ms. Sinsu Rachel Alex Msc. (N) Prev YrDocument29 pagesNebulization: Presented By: Ms. Sinsu Rachel Alex Msc. (N) Prev Yrmanoj reddy100% (1)
- Gas Exchange in HumansDocument9 pagesGas Exchange in HumanscherylrachelNo ratings yet
- Perineal RuptureDocument24 pagesPerineal RuptureIzz ShuhaimiNo ratings yet
- 11 SoalDocument5 pages11 SoalHerman NasuhaNo ratings yet
- ContentsDocument19 pagesContentsdurairaj1977No ratings yet
- BPD Concept MapDocument3 pagesBPD Concept Mapsammillepointer86No ratings yet
- 10 Frequent Handling Mistakes During Bonding 2017 Orthodontic Applications of BiomaterialsDocument7 pages10 Frequent Handling Mistakes During Bonding 2017 Orthodontic Applications of BiomaterialsFelipe ArceNo ratings yet
- Glossary of Traditional Chinese Medicine 中醫術語Document7 pagesGlossary of Traditional Chinese Medicine 中醫術語OscarTadlockNo ratings yet