Professional Documents
Culture Documents
Antibiotic Sensitivity Test
Uploaded by
ganesh2gigCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Antibiotic Sensitivity Test
Uploaded by
ganesh2gigCopyright:
Available Formats
ANTIBIOTIC SUSCEPTIBILITY TESTING
Bacteria demonstrate two kinds of resistance to antibiotics, namely intrinsic resistance and acquired resistance. Intrinsic resistance means that the species was resistant to an antibiotic even before its introduction. Acquired resistance means that the species was originally susceptible to an antibiotic, but later became resistant. Bacteria can acquire antibiotic resistance either by mutation or through exchange of genetic material among same or closely related species. The sudden acquisition of resistance to antibiotics poses difficulties in treating infections. Resistance to several different antibiotics at the same time is even more significant problem. It is because of the acquired resistance that bacterial isolates must be subjected to antibiotic susceptibility testing. Bacteria showing reduced susceptibility or resistance to an antibiotic implies that it should not be used on the patient. Various methods of antibiotic susceptibility testing are: 1. Quantitative Methods 2. Qualitative Methods 3. Automated Susceptibility Tests 4. Newer Non-Automated Susceptibility Tests 5. Molecular Techniques Quantitative Methods: In these tests, the minimum amount of antibiotic that inhibits the visible growth of an isolate or minimum inhibitory concentration (MIC) is determined. Bacterial isolate is subjected to various dilutions of antibiotics. The highest dilution of antibiotic that has inhibited the growth of bacteria is considered as MIC. These tests can be performed on broth or agar. 1. Broth dilution methods a. Macrobroth dilution MIC tests b. Microbroth dilution MIC tests 2. Agar dilution methods Macrobroth dilution tests: A serial two-fold dilution of antibiotic are made in test tubes from 0 to maximum concentration that is achieved in vivo without toxic effect on patient. The inoculum density of bacterial isolate to be tested is standardized with 0.5 McFarland turbidity standard. The suspension should have a final inoculum of 5 x 105 cfu/ml. 1ml of bacterial suspension is added to rows of antibiotic solution and incubated at 37oC overnight. The lowest concentration of antibiotic that completely inhibits visual growth of bacteria (no turbidity) is recorded as MIC. Microbroth dilution tests: A polystyrene tray containing 80 wells is filled with small volumes of serial two-fold dilutions of different antibiotics. The inoculum suspension and standardization is done according to McFarland standard. The bacterial inoculum is then inoculated into the wells and incubated at 37oC overnight. MIC is determined as in macrobroth dilution test. Agar dilution method: A serial two-fold dilution of the antibiotic is prepared in Mueller-Hinton agar.The bacterial inoculum is standardized according to McFarland standard. Using calibrated loops a volume of 0.001-0.002 ml is inoculated on the surface of agar and incubated at 37oC overnight. The lowest concentration of antibiotic that inhibits visible growth on surface of agar is taken as MIC. Qualitative Methods: These tests categorize a bacterial isolate as sensitive, intermediate or resistant to a particular antibiotic. Disk diffusion test: In this method the standardized bacterial isolate is spread on an agar plate and then paper disc containing specific concentration of antibiotics are placed and incubated at 37oC overnight. If the isolate is susceptible to the antibiotic,
it does not grow around the disk thus forming a zone of inhibition. Strains resistant to an antibiotic grow up to the margin of disk. The diameter of zone of inhibition must be measured and result read from the Kirby Bauer chart as sensitive, intermediate or resistant.
Automated Susceptibility Methods: Determination of bacterial growth in wells containing antimicrobial agent are performed in short period of time using computer-assisted instruments. Various techniques include: Turbidimetric detection: VITEK (report ready in 4-15 hours) Flourimetric detection: AutoSCAN Walkaway (report ready in 3.5-7 hours) Video Image processing: ALADIN (report ready in 18-24 hours) Newer Non Automated Susceptibility Tests: Alamar system, E-test and Spiral gradient endpoint system Molecular Techniques: Detection of gene coding for resistance to one or several drugs by techniques such as PCR and DNA hybridization. Last edited in June 2006
You might also like
- Analytical MicrobiologyFrom EverandAnalytical MicrobiologyFrederick KavanaghNo ratings yet
- Preparation of Media: Microbiology BIOL 275Document9 pagesPreparation of Media: Microbiology BIOL 275Nur AishaNo ratings yet
- Microbial Growth PhaseDocument2 pagesMicrobial Growth PhaseMahathir Mohmed100% (5)
- Sampling and Isolation of Bacteria From SoilDocument9 pagesSampling and Isolation of Bacteria From SoilKishmala BatoolNo ratings yet
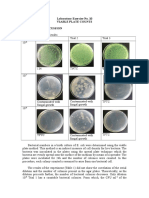
- Laboratory Exercise No. 10 Viable Plate Counts Results and DiscussionDocument3 pagesLaboratory Exercise No. 10 Viable Plate Counts Results and Discussionvanessa olga100% (1)
- Exercise 5Document6 pagesExercise 5triciallorin_190% (1)
- Industrial Importance of MicrobesDocument23 pagesIndustrial Importance of MicrobesDiah AyuningrumNo ratings yet
- Microbiology Methods of Monitoring PopulationsDocument17 pagesMicrobiology Methods of Monitoring PopulationsStephen MooreNo ratings yet
- Bacteria CountingDocument30 pagesBacteria Countingnavidhah 22No ratings yet
- PH Antimicrobial: Ms. Sunisa Thongdee 4518996 Ms - Janjira Sillapee 4518999Document19 pagesPH Antimicrobial: Ms. Sunisa Thongdee 4518996 Ms - Janjira Sillapee 4518999biochemi100% (2)
- Isolation and Identification of Fungi From Fast Food Restaurants in Langa BazarDocument6 pagesIsolation and Identification of Fungi From Fast Food Restaurants in Langa BazarIJEAB JournalNo ratings yet
- Classification and Indentification of BacteriaDocument77 pagesClassification and Indentification of BacteriaAnand MadhavanNo ratings yet
- Microbiology PDFDocument71 pagesMicrobiology PDFDanny Alexander TullumeNo ratings yet
- BIOL 240 Lab Report 1Document11 pagesBIOL 240 Lab Report 1Ben CharlesNo ratings yet
- Mic MBCDocument5 pagesMic MBCOneil CerdenaNo ratings yet
- Exercise 8 Bacterial Motility and FlagellaDocument33 pagesExercise 8 Bacterial Motility and FlagellaJonah Feliza Baradas Mora100% (1)
- Methods To Study Soil Microbial DiversityDocument25 pagesMethods To Study Soil Microbial Diversityrd2165scribdNo ratings yet
- Sterilization and Disinfection MethodsDocument5 pagesSterilization and Disinfection Methodsferdudz schneiderNo ratings yet
- Lecture 6 Microbial ControlDocument63 pagesLecture 6 Microbial ControljowditzNo ratings yet
- Innate Immunity Chapter SummaryDocument11 pagesInnate Immunity Chapter SummaryOrhan AsdfghjklNo ratings yet
- Isolation and Enumeration of Bacteria in Water and FoodDocument30 pagesIsolation and Enumeration of Bacteria in Water and FoodOld Lake100% (1)
- Quorum Sensing in Bacteria and A Glance On Pseudomonas Aeruginosa 2327 5073.1000156Document10 pagesQuorum Sensing in Bacteria and A Glance On Pseudomonas Aeruginosa 2327 5073.1000156Tanjila IslamNo ratings yet
- Unknown Bacterium Identification PDFDocument9 pagesUnknown Bacterium Identification PDFLolNo ratings yet
- BACTERIAL TOXINS: TARGETS AND MECHANISMSDocument20 pagesBACTERIAL TOXINS: TARGETS AND MECHANISMSYogiabrorNo ratings yet
- Inflamatory Process 5.5.2Document7 pagesInflamatory Process 5.5.2Maxwell C Jay KafwaniNo ratings yet
- Enzyme Immobilization Methods and AdvantagesDocument30 pagesEnzyme Immobilization Methods and AdvantagesVidhi ModiNo ratings yet
- Cloning VectorsDocument5 pagesCloning Vectorssashaikh1213No ratings yet
- Kirby-Bauer Disk Diffusion Susceptibility Test ProtocolDocument14 pagesKirby-Bauer Disk Diffusion Susceptibility Test ProtocolmauryaajitNo ratings yet
- NMEICT-MHRD Fermentation Media DesignDocument4 pagesNMEICT-MHRD Fermentation Media DesignriyaNo ratings yet
- Catalytic Antibody ProductionDocument14 pagesCatalytic Antibody ProductionAnisam Abhi100% (1)
- Protein immunoblotting (Western blotting) techniqueDocument19 pagesProtein immunoblotting (Western blotting) techniqueNANDA AHSANINo ratings yet
- Biotechnology Lab ManualDocument34 pagesBiotechnology Lab Manualanon_348923763No ratings yet
- SerialDilutions PDFDocument5 pagesSerialDilutions PDFAmelia_KharismayantiNo ratings yet
- Isolation of Genomic DNADocument16 pagesIsolation of Genomic DNASamra KousarNo ratings yet
- Gene Manipulation Techniques Techniques: - Nucleic Acids Separation and DetectionDocument18 pagesGene Manipulation Techniques Techniques: - Nucleic Acids Separation and DetectionPuainthran NaiduNo ratings yet
- Physiology of BacteriaDocument150 pagesPhysiology of BacteriaМохіт Кумар ЯмпатіNo ratings yet
- Major and Minor Elements.Document5 pagesMajor and Minor Elements.Md Ahsanul HaqueNo ratings yet
- Immunology - SyllabusDocument3 pagesImmunology - SyllabusAlaa' Al-AbdulrazzaqNo ratings yet
- Sterilization and Aseptic TechniqueDocument3 pagesSterilization and Aseptic TechniqueE.coilman100% (3)
- Specific Host Defenses: The Immune ResponseDocument54 pagesSpecific Host Defenses: The Immune Responseadyaly44No ratings yet
- Rheumatoid Factor Latex Test PartolanDocument15 pagesRheumatoid Factor Latex Test Partolanchocoholic potchiNo ratings yet
- Gram Staining Clinical ExerciseDocument10 pagesGram Staining Clinical ExerciseHimani Aggarwal100% (1)
- BT-303 Lab ManualDocument21 pagesBT-303 Lab ManualZakaullah Akhtar50% (2)
- Scope of MicroDocument32 pagesScope of MicroShehram QazafiNo ratings yet
- Gram Stain Prac ReportDocument4 pagesGram Stain Prac ReportToga BrandonNo ratings yet
- Size Exclusion Column ChromatographyDocument8 pagesSize Exclusion Column ChromatographySmeetha Kaur100% (1)
- Isolation and characterization of cellulase producing bacteriaDocument4 pagesIsolation and characterization of cellulase producing bacteriaaj_6No ratings yet
- Biotechnology Lab Manual Offered To Iii Year B.Tech Chemical EngineeringDocument34 pagesBiotechnology Lab Manual Offered To Iii Year B.Tech Chemical Engineeringanon_348923763No ratings yet
- Estimation of Citric Acid From Aspergillus SPDocument4 pagesEstimation of Citric Acid From Aspergillus SPDinithiDahanayake100% (3)
- Downstream ProcessingDocument4 pagesDownstream ProcessingVachaspatiMishraNo ratings yet
- Micro B Lab 3 - Identification of MicroorganismsDocument16 pagesMicro B Lab 3 - Identification of MicroorganismsRachel-Ann SurajNo ratings yet
- Microbial Nutrition and GrowthDocument30 pagesMicrobial Nutrition and GrowthOsaetin AnnNo ratings yet
- Report BacteriaDocument11 pagesReport BacteriaSuzeanni JalilNo ratings yet
- Sutherland 1991Document7 pagesSutherland 1991Isal AbdussalamNo ratings yet
- Bacterial Transformation Lab (6a)Document7 pagesBacterial Transformation Lab (6a)Chris PriceNo ratings yet
- Spectrophotometric Estimation of Escitalopram OxalateDocument3 pagesSpectrophotometric Estimation of Escitalopram Oxalateapi-19918842No ratings yet
- Food MCB II NotesDocument73 pagesFood MCB II NotesRichard Simon KisituNo ratings yet
- Plant Biotechnology (Tissue Culture)Document11 pagesPlant Biotechnology (Tissue Culture)Hewa HusenNo ratings yet
- Membrane Research: Classic Origins and Current ConceptsFrom EverandMembrane Research: Classic Origins and Current ConceptsA. L. Muggleton-HarrisNo ratings yet
- Biosurfactants: Research and DevelopmentFrom EverandBiosurfactants: Research and DevelopmentGloria Soberon-ChavezNo ratings yet
- Cell Counting Neubauer ChamberDocument6 pagesCell Counting Neubauer ChamberHerma ZulaikhaNo ratings yet
- Stability Testing WHO2008Document50 pagesStability Testing WHO2008Mikhail SmirnovNo ratings yet
- 2007Document24 pages2007ganesh2gig100% (1)
- Antioxidant ActivityDocument10 pagesAntioxidant Activityganesh2gigNo ratings yet
- Bacterial Chondronecrosis With OsteomyelitisDocument19 pagesBacterial Chondronecrosis With Osteomyelitisganesh2gigNo ratings yet
- Determination of Chromium in Cocoa ProductsDocument8 pagesDetermination of Chromium in Cocoa Productsganesh2gigNo ratings yet
- Microsoft PowerPoint - Cholesterol Estimation11Document37 pagesMicrosoft PowerPoint - Cholesterol Estimation11ganesh2gigNo ratings yet
- Kim 30 6 10 0608 21Document9 pagesKim 30 6 10 0608 21ganesh2gigNo ratings yet
- Aliyu and BalaDocument8 pagesAliyu and Balaganesh2gigNo ratings yet
- Assessment of Trace Mineral Status of RuminantsDocument10 pagesAssessment of Trace Mineral Status of Ruminantsganesh2gigNo ratings yet
- ReviewDocument1 pageReviewganesh2gigNo ratings yet
- IS 1374:2007 Poultry Feed SpecificationDocument2 pagesIS 1374:2007 Poultry Feed Specificationganesh2gigNo ratings yet
- Microsoft PowerPoint - Cholesterol Estimation11Document37 pagesMicrosoft PowerPoint - Cholesterol Estimation11ganesh2gigNo ratings yet
- Hardness of WaterDocument0 pagesHardness of Waterganesh2gig100% (1)
- The Mineral Supplementation of Poultry Feed in Inorg FarmsDocument8 pagesThe Mineral Supplementation of Poultry Feed in Inorg FarmsVieddaNo ratings yet
- InTech-Soybean Based Surfactants and Their ApplicationsDocument25 pagesInTech-Soybean Based Surfactants and Their Applicationsganesh2gigNo ratings yet
- Microsoft PowerPoint - Cholesterol Estimation11Document37 pagesMicrosoft PowerPoint - Cholesterol Estimation11ganesh2gigNo ratings yet
- Trypan BlueDocument6 pagesTrypan Blueganesh2gigNo ratings yet
- 21030243Document7 pages21030243ganesh2gigNo ratings yet
- Calibration: Constructing A Calibration CurveDocument10 pagesCalibration: Constructing A Calibration Curvedéborah_rosalesNo ratings yet
- Example of Derivative SpectrosDocument6 pagesExample of Derivative Spectrosganesh2gigNo ratings yet
- SOP DigestionDocument6 pagesSOP Digestionganesh2gigNo ratings yet
- Freezin Point To Know Molecular WeightDocument6 pagesFreezin Point To Know Molecular Weightganesh2gigNo ratings yet
- Standard SolutionDocument5 pagesStandard Solutionganesh2gigNo ratings yet
- Manganese Oxide EstimationDocument3 pagesManganese Oxide Estimationganesh2gig60% (5)
- CalciumDocument1 pageCalciumganesh2gigNo ratings yet
- Instruction Manual: FOR DCL-12, 13, 14, 15, 20Document30 pagesInstruction Manual: FOR DCL-12, 13, 14, 15, 20ganesh2gigNo ratings yet
- I. Determining Protein Amino Acid SequenceDocument4 pagesI. Determining Protein Amino Acid Sequenceganesh2gigNo ratings yet
- Instruction Manual: FOR DCL-12, 13, 14, 15, 20Document30 pagesInstruction Manual: FOR DCL-12, 13, 14, 15, 20ganesh2gigNo ratings yet
- Conjunctions and Connectors in Written ExpressionDocument3 pagesConjunctions and Connectors in Written ExpressionVeasna Chann100% (1)
- ICS Course Introduces Incident Management SystemDocument22 pagesICS Course Introduces Incident Management SystemMessias PortoNo ratings yet
- Stress in Teens Causes Mental Health DysfunctionDocument2 pagesStress in Teens Causes Mental Health DysfunctionDelanoAlexanderNo ratings yet
- Perkins 1998Document10 pagesPerkins 1998Emma AzNo ratings yet
- Microbiology Exam QuestionsDocument23 pagesMicrobiology Exam Questionswhitewave25No ratings yet
- Canine Distemper VirusDocument5 pagesCanine Distemper VirusDumapis RichardNo ratings yet
- Extraction of Phoenix Dactylifera (Mariami) Seeds Oil Using Supercritical Carbon Dioxide (SC-CO)Document6 pagesExtraction of Phoenix Dactylifera (Mariami) Seeds Oil Using Supercritical Carbon Dioxide (SC-CO)مرتضى عباسNo ratings yet
- Sodium Bicarbonate SDSDocument8 pagesSodium Bicarbonate SDSMohamed HalemNo ratings yet
- Buteyko - Free EbookDocument37 pagesButeyko - Free EbookDavid Burnet50% (2)
- Grade 7 Cosme - Nail DiseasesDocument22 pagesGrade 7 Cosme - Nail DiseasesLigaya PastorNo ratings yet
- Federal Negarit Gazette: I I I IDocument12 pagesFederal Negarit Gazette: I I I IAlemitu KidaneNo ratings yet
- Senarai Buku Teks Kesihatan AwamDocument5 pagesSenarai Buku Teks Kesihatan AwamMOHAMAD IZAMEER ASHRAF BIN MOHD AMRAN / HPUPMNo ratings yet
- Ted Bundy - Melissa Smith - Autopsy ReportDocument6 pagesTed Bundy - Melissa Smith - Autopsy ReportErin Banks100% (1)
- Grade 6 MAPEH Assessment Tool FINALDocument8 pagesGrade 6 MAPEH Assessment Tool FINALdaniel AguilarNo ratings yet
- Coagulopathy in Cardiac Surgery1Document63 pagesCoagulopathy in Cardiac Surgery1Mohammed Abdul ShafiNo ratings yet
- Revamp of RSBY Phase I - Operational Manual (Released On 11th June 2014) PDFDocument225 pagesRevamp of RSBY Phase I - Operational Manual (Released On 11th June 2014) PDFJeet PrajapatiNo ratings yet
- Q3 - LAS - Health Education 9 - Week 1-4Document7 pagesQ3 - LAS - Health Education 9 - Week 1-4Melody Joy AmoscoNo ratings yet
- Public Relations Key for Hospital SuccessDocument5 pagesPublic Relations Key for Hospital SuccessNia peluNo ratings yet
- Aditya Birla CSR StudyDocument35 pagesAditya Birla CSR StudySONI GUPTA80% (5)
- 10 1111@jocd 13667Document7 pages10 1111@jocd 13667jardel.santos220No ratings yet
- Nduba Landfil1Document6 pagesNduba Landfil1rrutayisire50% (2)
- Reviews in Food and Nutrition Toxicity, Volume 3 (CRC, 2005)Document291 pagesReviews in Food and Nutrition Toxicity, Volume 3 (CRC, 2005)reader1453No ratings yet
- 11 Emergency Preparedness PlanDocument6 pages11 Emergency Preparedness PlanMAHESH SHAWNo ratings yet
- Adjunct Appointments ProcedureDocument6 pagesAdjunct Appointments ProcedureAnglophile123No ratings yet
- Investigatory Project 2014Document27 pagesInvestigatory Project 2014nerkerlNo ratings yet
- (pt2) CHCAGE003 Student Assessment Tool v2.1 04.04.19Document117 pages(pt2) CHCAGE003 Student Assessment Tool v2.1 04.04.19Charina Aubrey90% (10)
- 10-Day Liver & Gallstone CleanseDocument9 pages10-Day Liver & Gallstone Cleanseglyconet100% (9)
- Chapter 15 Nclex Questions ReviewerDocument4 pagesChapter 15 Nclex Questions ReviewerCarolyn Moquerio-serniculaNo ratings yet
- Impact of Covid-19 on Ethiopia's Tourism and Hotel IndustryDocument6 pagesImpact of Covid-19 on Ethiopia's Tourism and Hotel IndustryFuadNo ratings yet
- Medipac (Equivalent)Document3 pagesMedipac (Equivalent)Mohamed GamalNo ratings yet