Professional Documents
Culture Documents
Introduction To Materials and Processes PDF
Uploaded by
jayeshjpillaiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Introduction To Materials and Processes PDF
Uploaded by
jayeshjpillaiCopyright:
Available Formats
FromNDTResources August18,2007nks 2of89
Introduction to Materials and Processes
Introduction
Introduction
GeneralMaterialClassifications
Metals
Ceramics
Polymers
Composites
StructureofMaterials
AtomicBonds
SolidStateStructure
PrimaryMetallicCrystallineStructures
Solidification
AnisotropyandIsotropy
CrystalDefects
Elastic/PlasticDeformation
FatigueCrackInitiation
Diffusion
PropertyModification
CeramicStructures
PolymerStructure
CompositeStructures
PhysicalandChemicalProperties
SectionIntroduction
PhaseTransformationTemperature
Density
SpecificGravity
ThermalConductivity
ThermalExpansion
ElectricalConductivity
MagneticProperties
OxidationandCorrosion
MechanicalProperties
SectionIntroduction
Loading
Stress&Strain
Tensile
Compression,Bearing,&Shear
Hardness
Creep&StressRupture
Toughness
ImpactToughness
NotchToughness
FractureToughness
Fatigue
SNFatigue
FatigueCrackGrowthRate
Introduction to Materials and Processes
FromNDTResources August18,2007nks 3of89
Introduction to Materials
This section will provide a basic introduction to materials and material fabrication
processing. It is important that NDT personnel have some background in material science for
a couple of reasons. First, non-destructive testing almost always involves the interaction of
energy of some type (mechanics, sound, electricity, magnetism or radiation) with a material.
To understand how energy interacts with a material, it is necessary to know a little about the
material. Secondly, NDT often involves detecting manufacturing defects and service induced
damage and, therefore, it is necessary to understand how defects and damage occur.
This section will begin with an introduction to the four common types of engineering
materials. The structure of materials at the atomic level will then be considered, along with
some atomic level features that give materials their characteristic properties. Some of the
properties that are important for the structural performance of a material and methods for
modifying these properties will also be covered.
In the second half of this text, methods used to shape and form materials into useful shapes
will be discussed. Some of the defects that can occur during the manufacturing process, as
well as service induced damage will be highlighted. This section will conclude with a
summary of the role that NDT plays in ensuring the structural integrity of a component.
General Material Classifications
There are thousands of materials available for use in
engineering applications. Most materials fall into one of
three classes that are based on the atomic bonding forces
of a particular material. These three classifications are
metallic, ceramic and polymeric. Additionally, different
materials can be combined to create a composite
material. Within each of these classifications, materials
are often further organized into groups based on their
chemical composition or certain physical or mechanical
properties. Composite materials are often grouped by the
types of materials combined or the way the materials are arranged together. Below is a list of
some of the commonly classification of materials within these four general groups of
materials.
Metals
Ferrous metals and alloys (irons,
carbon steels, alloy steels,
stainless steels, tool and die
steels)
Nonferrous metals and alloys
(aluminum, copper, magnesium,
nickel, titanium, precious
metals, refractory metals,
superalloys)
Polymeric
Thermoplastics plastics
Thermoset plastics
Elastomers
Introduction to Materials and Processes
FromNDTResources August18,2007nks 4of89
Ceramics
Glasses
Glass ceramics
Graphite
Diamond
Composites
Reinforced plastics
Metal-matrix composites
Ceramic-matrix composites
Sandwich structures
Concrete
Each of these general groups will be discussed in more detail in the following pages.
Metals
Metals account for about two thirds of all the elements and about 24% of the mass of the
planet. Metals have useful properties including strength, ductility, high melting points,
thermal and electrical conductivity, and toughness. From the periodic table, it can be seen
that a large number of the elements are classified as being a metal. A few of the common
metals and their typical uses are presented below.
Common Metallic Materials
Iron/Steel - Steel alloys are used for strength critical applications
Aluminum - Aluminum and its alloys are used because they are easy to form, readily
available, inexpensive, and recyclable.
Copper - Copper and copper alloys have a number of properties that make them
useful, including high electrical and thermal conductivity, high ductility, and good
corrosion resistance.
Titanium - Titanium alloys are used for strength in higher temperature (~1000 F)
application, when component weight is a concern, or when good corrosion resistance
is required
Nickel - Nickel alloys are used for still higher temperatures (~1500-2000 F)
applications or when good corrosion resistance is required.
Refractory materials are used for the highest temperature (> 2000 F) applications.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 5of89
The key feature that distinguishes metals from non-metals is their bonding. Metallic
materials have free electrons that are free to move easily from one atom to the next. The
existence of these free electrons has a number of profound consequences for the properties of
metallic materials. For example, metallic materials tend to be good electrical conductors
because the free electrons can move around within the metal so freely. More on the structure
of metals will be discussed later.
Ceramics
A ceramic has traditionally been defined as an inorganic, non-metallic solid that is
prepared from powdered materials, is fabricated into products through the application of heat,
and displays such characteristic properties as hardness, strength, low electrical conductivity,
and brittleness." The word ceramic comes the from Greek word "keramikos", which means
"pottery." They are typically crystalline in nature and are compounds formed between
metallic and non-metallic elements such as aluminum and oxygen (alumina-Al
2
O
3
), calcium
and oxygen (calcia - CaO), and silicon and nitrogen (silicon nitride-Si
3
N
4
).
Depending on their method of formation,
ceramics can be dense or lightweight. Typically, they
will demonstrate excellent strength and hardness
properties; however, they are often brittle in nature.
Ceramics can also be formed to serve as electrically
conductive materials or insulators. Some ceramics, like
superconductors, also display magnetic properties.
They are also more resistant to high temperatures and
harsh environments than metals and polymers. Due to
ceramic materials wide range of properties, they are
used for a multitude of applications.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 6of89
The broad categories or segments that make up the ceramic industry can be classified as:
Structural clay products (brick, sewer pipe, roofing and wall tile, flue linings, etc.)
White wares (dinnerware, floor and wall tile, electrical porcelain, etc.)
Refractories (brick and monolithic products used in metal, glass, cements, ceramics,
energy conversion, petroleum, and chemicals industries)
Glasses (flat glass (windows), container glass (bottles), pressed and blown glass
(dinnerware), glass fibers (home insulation), and advanced/specialty glass (optical
fibers))
Abrasives (natural (garnet, diamond, etc.) and synthetic (silicon carbide, diamond,
fused alumina, etc.) abrasives are used for grinding, cutting, polishing, lapping, or
pressure blasting of materials)
Cements (for roads, bridges, buildings, dams, and etc.)
Advanced ceramics
o Structural (wear parts, bio ceramics, cutting tools, and engine components)
o Electrical (capacitors, insulators, substrates, integrated circuit packages,
piezoelectrics, magnets and superconductors)
o Coatings (engine components, cutting tools, and industrial wear parts)
o Chemical and environmental (filters, membranes, catalysts, and catalyst
supports)
The atoms in ceramic materials are held together by a chemical bond which will be
discussed a bit later. Briefly though, the two most common chemical bonds for ceramic
materials are covalent and ionic. Covalent and ionic bonds are much stronger than in metallic
bonds and, generally speaking, this is why ceramics are brittle and metals are ductile.
Polymers
A polymeric solid can be thought of as a material that contains many chemically
bonded parts or units which themselves are bonded together to form a solid. The word
polymer literally means "many parts." Two industrially important polymeric materials are
plastics and elastomers. Plastics are a large and varied group of synthetic materials which are
processed by forming or moulding into shape. Just as there are many types of metals such as
aluminum and copper, there are many types of
plastics, such as polyethylene and nylon. Elastomers
or rubbers can be elastically deformed a large amount
when a force is applied to them and can return to
their original shape (or almost) when the force is
released.
Polymers have many properties that make them
attractive to use in certain conditions. Many
polymers:
are less dense than metals or ceramics,
resist atmospheric and other forms of
corrosion,
offer good compatibility with human tissue,
or
exhibit excellent resistance to the conduction
of electrical current.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 7of89
The polymer plastics can be divided into two classes, thermoplastics and thermosetting
plastics, depending on how they are structurally and chemically bonded. Thermoplastic
polymers comprise the four most important commodity materials polyethylene,
polypropylene, polystyrene and polyvinyl chloride. There are also a number of specialized
engineering polymers. The term thermoplastic indicates that these materials melt on heating
and may be processed by a variety of moulding and extrusion techniques. Alternately,
thermosetting polymers can not be melted or remelted. Thermosetting polymers include
alkyds, amino and phenolic resins, epoxies, polyurethanes, and unsaturated polyesters.
Rubber is a natural occurring polymer. However, most polymers are created by
engineering the combination of hydrogen and carbon atoms and the arrangement of the
chains they form. The polymer molecule is a long chain of covalent-bonded atoms and
secondary bonds then hold groups of polymer chains together to form the polymeric material.
Polymers are primarily produced from petroleum or natural gas raw products but the use of
organic substances is growing. The super-material known as Kevlar is a man-made polymer.
Kevlar is used in bullet-proof vests, strong/lightweight frames, and underwater cables that are
20 times stronger than steel.
Composites
A composite is commonly defined as a combination of two or more distinct materials,
each of which retains its own distinctive properties, to create a new material with properties
that cannot be achieved by any of the components acting alone. Using this definition, it can
be determined that a wide range of engineering materials fall into this category. For example,
concrete is a composite because it is a mixture of Portland cement and aggregate. Fibreglass
sheet is a composite since it is made of glass fibers imbedded in a polymer.
Composite materials are said to have two phases. The reinforcing phase is the fibers,
sheets, or particles that are embedded in the matrix phase. The reinforcing material and the
matrix material can be metal, ceramic, or polymer. Typically, reinforcing materials are strong
with low densities while the matrix is usually a ductile, or tough, material.
Some of the common classifications of
composites are:
Reinforced plastics
Metal-matrix composites
Ceramic-matrix composites
Sandwich structures
Concrete
Composite materials can take many forms
but they can be separated into three categories
based on the strengthening mechanism. These
categories are dispersion strengthened, particle
reinforced and fiber reinforced. Dispersion
strengthened composites have a fine
distribution of secondary particles in the
matrix of the material.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 8of89
These particles impede the mechanisms that allow a material to deform. (These
mechanisms include dislocation movement and slip, which will be discussed later). Many
metal-matrix composites would fall into the dispersion strengthened composite category.
Particle reinforced composites have a large volume fraction of particle dispersed in the matrix
and the load is shared by the particles and the matrix. Most commercial ceramics and many
filled polymers are particle-reinforced composites. In fiber-reinforced composites, the fiber is
the primary load-bearing component. Fibreglass and carbon fiber composites are examples of
fiber-reinforced composites.
If the composite is designed and fabricated correctly, it combines the strength of the
reinforcement with the toughness of the matrix to achieve a combination of desirable
properties not available in any single conventional material. Some composites also offer the
advantage of being tailorable so that properties, such as strength and stiffness, can easily be
changed by changing amount or orientation of the reinforcement material. The downside is
that such composites are often more expensive than conventional materials.
Structure of Materials
It should be clear that all matter is made of atoms. From the periodic table, it can be
seen that there are only about 100 different kinds of atoms in the entire Universe. These same
100 atoms form thousands of different substances ranging from the air we breathe to the
metal used to support tall buildings. Metals behave differently than ceramics, and ceramics
behave differently than polymers. The properties of matter depend on which atoms are used
and how they are bonded together.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 9of89
The structure of materials can be classified by the general magnitude of various
features being considered. The three most common major classification of structural, listed
generally in increasing size, are:
Atomic structure, which includes features that cannot be seen, such as the types of
bonding between the atoms, and the way the atoms are arranged.
Microstructure which includes features that can be seen using a microscope, but seldom with
the naked eye.
Macrostructure, which includes features that can be seen with the naked eye)
The atomic structure primarily affects the chemical, physical, thermal, electrical,
magnetic, and optical properties. The microstructure and macrostructure can also affect these
properties but they generally have a larger effect on mechanical properties and on the rate of
chemical reaction. The properties of a material offer clues as to the structure of the material.
The strength of metals suggests that these atoms are held together by strong bonds. However,
these bonds must also allow atoms to move since metals are also usually formable. To
understand the structure of a material, the type of atoms present, and how the atoms are
arranged and bonded must be known. Lets first look at atomic bonding.
Atomic Bonding
(Metallic, Ionic, Covalent, and van der Waals Bonds)
From elementary chemistry it is known that the
atomic structure of any element is made up of a
positively charged nucleus surrounded by electrons
revolving around it. An elements atomic number
indicates the number of positively charged protons in
the nucleus. The atomic weight of an atom indicates
how many protons and neutrons in the nucleus. To
determine the number of neutrons in an atom, the
atomic number is simply subtracted from the atomic
weight.
Atoms like to have a balanced electrical charge.
Therefore, they usually have negatively charged
electrons surrounding the nucleus in numbers equal to the number of protons. It is also known
that electrons are present with different energies and it is convenient to consider these
electrons surrounding the nucleus in energy shells. For example, magnesium, with an
atomic number of 12, has two electrons in the inner shell, eight in the second shell and two in
the outer shell.
All chemical bonds involve electrons. Atoms will stay close together if they have a
shared interest in one or more electrons. Atoms are at their most stable when they have no
partially-filled electron shells. If an atom has only a few electrons in a shell, it will tend to
lose them to empty the shell. These elements are metals. When metal atoms bond, a metallic
bond occurs. When an atom has a nearly full electron shell, it will try to find electrons from
another atom so that it can fill its outer shell. These elements are usually described as non-
metals. The bond between two non-metal atoms is usually a covalent bond. Where metal and
non-metal atom comes together an ionic bond occurs. There are also other, less common,
types of bond but the details are beyond the scope of this material. On the next few pages, the
Metallic, Covalent and Ionic bonds will be covered in more detail.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 10of89
Ionic Bonds
Ionic bonding occurs between charged particles. These may be atoms or groups of
atoms, but this discuss will be conducted in terms of single atoms. Ionic bonding occurs
between metal atoms and non-metal atoms. Metals usually have 1, 2, or 3 electrons in their
outermost shell. Non-metals have 5, 6, or 7 electrons in their outer shell. Atoms with outer
shells that are only partially filled are unstable. To become stable, the metal atom wants to get
rid of one or more electrons in its outer shell. Losing electrons will either result in an empty
outer shell or get it closer to having an empty outer shell. It would like to have an empty outer
shell because the next lower energy shell is a stable shell with eight electrons.
Since electrons have a negative charge, the atom that gains electrons becomes a
negatively charged ions (aka anion) because it now has more electrons than protons.
Alternately, an atom that loses electrons becomes a positively charged ion (aka cations). The
particles in an ionic compound are held together because there are oppositely charged
particles that are attracted to one another.
The images above schematically show the process that takes place during the
formation of an ionic bond between sodium and chlorine atoms. Note that sodium has one
valence electron that it would like to give up so that it would become stable with a full outer
shell of eight. Also note that chlorine has seven valence electrons and it would like to gain an
electron in order to have a full shell of eight. The transfer of the electron causes the
previously neutral sodium atom to become a positively charged ion (cation), and the
previously neutral chlorine atom to become a negatively charged ion (anion). The attraction
for the cation and the anion is called the ionic bond.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 11of89
Some Common Features of Materials with Ionic Bonds:
Hard
Good insulators
Transparent
Brittle or cleave rather than deform
Covalent Bonding
Where a compound only contains non-metal atoms, a covalent bond is formed by
atoms sharing two or more electrons. Non-metals have 4 or more electrons in their outer
shells (except boron). With this many electrons in the outer shell, it would require more
energy to remove the electrons than would be gained by making new bonds. Therefore, both
the atoms involved share a pair of electrons. Each atom gives one of its outer electrons to the
electron pair, which then spends some time with each atom. Consequently, both atoms are
held near each other since both atoms have a share in the electrons.
More than one electron pair can be formed with half of the electrons coming from one
atom and the rest from the other atom. An important feature of this bond is that the electrons
are tightly held and equally shared by the participating atoms. The atoms can be of the same
element or different elements. In each molecule, the bonds between the atoms are strong but
the bonds between molecules are usually weak. This makes many solid materials with
covalent bonds brittle. Many ceramic materials have covalent bonds.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 12of89
Compounds with covalent bonds may be solid, liquid or gas at room temperature
depending on the number of atoms in the compound. The more atoms in each molecule, the
higher a compounds melting and boiling temperature will be. Since most covalent
compounds contain only a few atoms and the forces between molecules are weak, most
covalent compounds have low melting and boiling points. However, some, like carbon
compounds, can be very large. An example is the diamond in which carbon atoms each share
four electrons to form giant lattices.
Some Common Features of Materials with Covalent Bonds:
Hard
Good insulators
Transparent
Brittle or cleave rather than deform
Metallic Bonding
A common characteristic of metallic elements is they contain only one to three
electrons in the outer shell. When an element has only one, two or three valence electrons
(i.e. electrons in the outer shell), the bond between these electrons and the nucleus is
relatively weak. So, for example, when aluminum atoms are grouped together in a block of
metal, the outer electrons leave individual atoms to become part of common electron cloud.
In this arrangement, the valence electrons have considerable mobility and are able to conduct
heat and electricity easily. Also, the delocalized nature of the bonds, make it possible for the
atoms to slide past each other when the metal is deformed instead of fracturing like glass or
other brittle material.
Since the aluminum atoms lose two electrons, they end up having a positive charge
and are designated Al
3+
ions (cations). These ions repel each other but are held together in the
block because the negative electrons are attracted to the positively charged ions. A result of
the sharing of electrons is the cations arrange themselves in a regular pattern. This regular
pattern of atoms is the crystalline structure of metals. In the crystal lattice, atoms are packed
closely together to maximize the strength of the bonds. An actual piece of metal consists of
many tiny crystals called grains that touch at grain boundaries.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 13of89
Some Common Features of Materials with Metallic Bonds:
Good electrical and thermal conductors due to their free valence electrons
Opaque
Relatively ductile
Van der Waals Bond
The van der Waal bonds occur to some extent in all materials but are particularly
important in plastics and polymers. These materials are made up of a long string molecules
consisting of carbon atoms covalently bonded with other atoms, such as hydrogen, nitrogen,
oxygen, fluorine. The covalent bonds within the molecules are very strong and rupture only
under extreme conditions. The bonds between the molecules that allow sliding and rupture to
occur are called van der Waal forces.
When ionic and covalent bonds
are present, there is some imbalance in
the electrical charge of the molecule.
Take water as an example. Research has
determined the hydrogen atoms are
bonded to the oxygen atoms at an angle
of 104.5. This angle produces a positive
polarity at the hydrogen-rich end of the
molecule and a negative polarity at the
other end. A result of this charge
imbalance is that water molecules are
attracted to each other. This is the force
that holds the molecules together in a
drop of water.
This same concept can be carried
on to plastics, except that as molecules
become larger, the van der Waal forces between molecules also increases. For example, in
polyethylene the molecules are composed of hydrogen and carbon atoms in the same ratio as
ethylene gas. But there are more of each type of atom in the polyethylene molecules and as
the number of atoms in a molecule increases, the matter passes from a gas to a liquid and
finally to a solid.
Polymers are often classified as being either a thermoplastic or a thermosetting
material. Thermoplastic materials can be easily remelted for forming or recycling and
thermosetting material cannot be easily remelted. In thermoplastic materials consist of long
chainlike molecules. Heat can be used to break the van der Waal forces between the
molecules and change the form of the material from a solid to a liquid. By contrast,
thermosetting materials have a three-dimensional network of covalent bonds. These bonds
cannot be easily broken by heating and, therefore, can not be remelted and formed as easily
as thermoplastics.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 14of89
Solid State Structure
In the previous pages, some of the mechanisms that bond together the multitude of
individual atoms or molecules of a solid material were discussed. These forces may be
primary chemical bonds, as in metals and ionic solids, or they may be secondary van der
Waals forces of solids, such as in ice, paraffin wax and most polymers. In solids, the way the
atoms or molecules arrange themselves contributes to the appearance and the properties of
the materials.
Atoms can be gathered together as an aggregate through a number of different
processes, including condensation, pressurization, chemical reaction, electrode position, and
melting. The process usually determines, at least initially, whether the collection of atoms
will take to form of a gas, liquid or solid. The state usually changes as its temperature or
pressure is changed. Melting is the process most often used to form an aggregate of atoms.
When the temperature of a melt is lowered to a certain point, the liquid will form either a
crystalline solid or and amorphous solid.
Amorphous Solids
A solid substance with its atoms held apart at equilibrium spacing, but with no long-
range periodicity in atom location in its structure is an amorphous solid. Examples of
amorphous solids are glass and some types of plastic. They are sometimes described as
supercooled liquids because their molecules are arranged in a random manner some what as
in the liquid state. For example, glass is commonly made from silicon dioxide or quartz sand,
which has a crystalline structure. When the sand is melted and the liquid is cooled rapidly
enough to avoid crystallization, an amorphous solid called a glass is formed. Amorphous
solids do not show a sharp phase change from solid to liquid at a definite melting point, but
rather soften gradually when they are heated. The physical properties of amorphous solids are
identical in all directions along any axis so they are said to have isotropic properties, which
will be discussed in more detail later
Introduction to Materials and Processes
FromNDTResources August18,2007nks 15of89
.
Crystalline Solids
More than 90% of naturally occurring and artificially prepared solids are crystalline.
Minerals, sand, clay, limestone, metals, carbon (diamond and graphite), salts ( NaCl, KCl
etc.), all have crystalline structures. A crystal is a regular, repeating arrangement of atoms or
molecules. The majority of solids, including all metals, adopt a crystalline arrangement
because the amount of stabilization achieved by anchoring interactions between neighbouring
particles is at its greatest when the particles adopt regular (rather than random) arrangements.
In the crystalline arrangement, the particles pack efficiently together to minimize the total
intermolecular energy.
The regular repeating pattern that the atoms arrange in is called the crystalline lattice.
The scanning tunnelling microscope (STM) makes it possible to image the electron cloud
associated individual atoms at the surface of a material. Below is an STM image of a
platinum surface showing the regular alignment of atoms.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 16of89
Courtesy: IBM Research, Almaden Research Centre.
Crystal Structure
Crystal structures may be conveniently specified by describing the arrangement within the
solid of a small representative group of atoms or molecules, called the unit cell. By
multiplying identical unit cells in three directions, the location of all the particles in the
crystal is determined. In nature, 14 different types of crystal structures or lattices are found.
The simplest crystalline unit cell to picture is the cubic, where the atoms are lined up in a
square, 3D grid. The unit cell is simply a box with an atom at each corner. Simple cubic
crystals are relatively rare, mostly because they tend to easily distort. However, many crystals
form body-centered-cubic (bcc) or face-centered-cubic (fcc) structures, which are cubic with
either an extra atom centered in the cube or centered in each face of the cube. Most metals
form bcc, fcc or Hexagonal Close Packed (hpc) structures; however, the structure can change
depending on temperature. These three structures will be discussed in more detail on the
following page.
Crystalline structure is important because it contributes to the properties of a material.
For example, it is easier for planes of atoms to slide by each other if those planes are closely
packed. Therefore, lattice structures with closely packed planes allow more plastic
deformation than those that are not closely packed. Additionally, cubic lattice structures
allow slippage to occur more easily than non-cubic lattices. This is because their symmetry
provides closely packed planes in several directions. A face-centered cubic crystal structure
will exhibit more ductility (deform more readily under load before breaking) than a body-
centered cubic structure. The bcc lattice, although cubic, is not closely packed and forms
strong metals. Alpha-iron and tungsten have the bcc form. The fcc lattice is both cubic and
closely packed and forms more ductile materials. Gamma-iron, silver, gold, and lead have fcc
structures. Finally, HCP lattices are closely packed, but not cubic. HCP metals like cobalt and
zinc are not as ductile as the fcc metals.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 17of89
Primary Metallic Crystalline Structures
(BCC, FCC, HCP)
As pointed out on the previous page, there are 14 different
types of crystal unit cell structures or lattices are found in nature.
However most metals and many other solids have unit cell structures
described as body center cubic (bcc), face centered cubic (fcc) or
Hexagonal Close Packed (hcp). Since these structures are most
common, they will be discussed in more detail.
Body-Centered Cubic (BCC) Structure
The body-centered cubic unit cell has atoms at each of the eight corners of a cube (like the
cubic unit cell) plus one atom in the center of the cube (left image below). Each of the corner
atoms is the corner of another cube so the corner atoms are shared among eight unit cells. It is
said to have a coordination number of 8. The bcc unit cell consists of a net total of two atoms;
one in the center and eight eighths from corners atoms as shown in the middle image below
(middle image below). The image below highlights a unit cell in a larger section of the lattice.
The bcc arrangement does not allow the atoms to pack together as closely as the fcc or hcp
arrangements. The bcc structure is often the high temperature form of metals that are close-
packed at lower temperatures. The volume of atoms in a cell per the total volume of a cell is
called the packing factor. The bcc unit cell has a packing factor of 0.68.
Some of the materials that have a bcc structure include lithium, sodium, potassium,
chromium, barium, vanadium, alpha-iron and tungsten. Metals which have a bcc structure are
usually harder and less malleable than close-packed metals such as gold. When the metal is
deformed, the planes of atoms must slip over each other, and this is more difficult in the bcc
structure. It should be noted that there are other important mechanisms for hardening
materials, such as introducing impurities or defects which make slipping more difficult.
These hardening mechanisms will be discussed latter.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 18of89
Face Centered Cubic (FCC) Structure
The face centered cubic structure has atoms located at each of the corners and the centers of
all the cubic faces (left image below). Each of the corner atoms is the corner of another cube
so the corner atoms are shared among eight unit cells. Additionally, each of its six face
centered atoms is shared with an adjacent atom. Since 12 of its atoms are shared, it is said to
have a coordination number of 12. The fcc unit cell consists of a net total of four atoms; eight
eighths from corners atoms and six halves of the face atoms as shown in the middle image
above. The image below highlights a unit cell in a larger section of the lattice.
In the fcc structure (and the hcp structure) the atoms can pack closer together than
they can in the bcc structure. The atoms from one layer nest themselves in the empty space
between the atoms of the adjacent layer. To picture packing arrangement, imagine a box
filled with a layer of balls that are aligned in columns and rows. When a few additional balls
are tossed in the box, they will not balance directly on top of the balls in the first layer but
instead will come to rest in the pocket created between four balls of the bottom layer. As
more balls are added they will pack together to fill up all the pockets. The packing factor (the
volume of atoms in a cell per the total volume of a cell) is 0.74 for fcc crystals. Some of the
metals that have the fcc structure include aluminum, copper, gold, iridium, lead, nickel,
platinum and silver.
Hexagonal Close Packed (HPC) Structure
Another common close packed structure is the hexagonal close pack. The hexagonal
structure of alternating layers is shifted so its atoms are aligned to the gaps of the preceding
layer. The atoms from one layer nest themselves in the empty space between the atoms of the
adjacent layer just like in the fcc structure. However, instead of being a cubic structure, the
pattern is hexagonal. (See image below.) The difference between the HPC and FCC structure
is discussed later in this section.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 19of89
The hcp structure has three layers of atoms. In each the top and bottom layer, there are
six atoms that arrange themselves in the shape of a hexagon and a seventh atom that sits in
the middle of the hexagon. The middle layer has three atoms nestle in the triangular
"grooves" of the top and bottom plane. Note that there are six of these "grooves" surrounding
each atom in the hexagonal plane, but only three of them can be filled by atoms.
As shown in the middle image above, there are six atoms in the hcp unit cell. Each of
the 12 atoms in the corners of the top and bottom layers contribute 1/6 atom to the unit cell,
the two atoms in the center of the hexagon of both the top and bottom layers each contribute
atom and each of the three atom in the middle layer contribute 1 atom. The image on the
right above attempts to show several hcp unit cells in a larger lattice.
The coordination number of the atoms in this structure is 12. There are six nearest
neighbours in the same close packed layer, three in the layer above and three in the layer
below. The packing factor is 0.74, which is the same as the fcc unit cell. The hcp structure is
very common for elemental metals and some examples include beryllium, cadmium,
magnesium, titanium, zinc and zirconium.
Similarities and Difference Between the
FCC and HCP Structure
The face centered cubic and hexagonal close packed structures both have a packing
factor of 0.74, consist of closely packed planes of atoms, and have a coordination number of
12. The difference between the fcc and hcp is the stacking sequence. The hcp layers cycle
among the two equivalent shifted positions whereas the fcc layers cycle between three
positions. As can be seen in the image, the hcp structure contains only two types of planes
with an alternating ABAB arrangement. Notice how the atoms of the third plane are in
exactly the same position as the atoms in the first plane. However, the fcc structure contains
three types of planes with a ABCABC arrangement. Notice how the atoms in rows A and C
are no longer aligned. Remember that cubic lattice structures allow slippage to occur more
easily than non-cubic lattices, so hcp metals are not as ductile as the fcc metals.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 20of89
The table below shows the stable room temperature crystal structures for several elemental
metals.
Metal Crystal Structure Atomic Radius (nm)
Aluminum FCC 0.1431
Cadmium HCP 0.1490
Chromium BCC 0.1249
Cobalt HCP 0.1253
Copper FCC 0.1278
Gold FCC 0.1442
Iron (Alpha) BCC 0.1241
Lead FCC 0.1750
Magnesium HCP 0.1599
Molybdenum BCC 0.1363
Nickel FCC 0.1246
Platinum FCC 0.1387
Silver FCC 0.1445
Tantalum BCC 0.1430
Titanium (Alpha) HCP 0.1445
Tungsten BCC 0.1371
Zinc HCP 0.1332
A nanometer (nm) equals 10
-9
meter or 10 Angstrom units.
Solidification
The crystallization of a large amount of material from a single point of nucleation
results in a single crystal. In engineering materials, single crystals are produced only under
carefully controlled conditions. The expense of producing single crystal materials is only
justified for special applications, such as turbine engine blades, solar cells, and piezoelectric
materials. Normally when a material begins to solidify, multiple crystals begin to grow in the
liquid and a polycrystalline (more than one crystal) solid forms.
The moment a crystal begins to grow is know as nucleation and the point where it
occurs is the nucleation point. At the solidification temperature, atoms of a liquid, such as
melted metal, begin to bond together at the nucleation points and start to form crystals. The
Introduction to Materials and Processes
FromNDTResources August18,2007nks 21of89
final sizes of the individual crystals depend on the number of nucleation points. The crystals
increase in size by the progressive addition of atoms and grow until they impinge upon
adjacent growing crystal.
a) Nucleation of crystals, b) crystal growth, c) irregular grains form as crystals grow
together, d) grain boundaries as seen in a microscope.
In engineering materials, a crystal is usually referred to as a grain. A grain is merely a
crystal without smooth faces because its growth was impeded by contact with another grain
or a boundary surface. The interface formed between grains is called a grain boundary. The
atoms between the grains (at the grain boundaries) have no crystalline structure and are said
to be disordered.
Grains are sometimes large enough to be
visible under an ordinary light microscope or even to
the unaided eye. The spangles that are seen on newly
galvanized metals are grains. Rapid cooling
generally results in more nucleation points and
smaller grains (a fine grain structure). Slow cooling
generally results in larger grains which will have
lower strength, hardness and ductility.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 22of89
Dendrites
In metals, the crystals that form in the liquid during freezing generally follow a
pattern consisting of a main branch with many appendages. A crystal with this morphology
slightly resembles a pine tree and is called a dendrite, which means branching. The formation
of dendrites occurs because crystals grow in defined planes due to the crystal lattice they
create. The figure to the right shows how a cubic crystal can grow in a melt in three
dimensions, which correspond to the six faces of the cube. For clarity of illustration, the
adding of unit cells with continued solidification from the six faces is shown simply as lines.
Secondary dendrite arms branch off the primary arm, and tertiary arms off the secondary
arms and etcetera.
During freezing of a polycrystalline material,
many dendritic crystals form and grow until they
eventually become large enough to impinge upon
each other. Eventually, the inter dendritic spaces
between the dendrite arms crystallize to yield a more
regular crystal. The original dendritic pattern may not
be apparent when examining the microstructure of a
material. However, dendrites can often be seen in
solidification voids that sometimes occur in castings
or welds, as shown to the right..
Shrinkage
Most materials contract or shrink during solidification
and cooling. Shrinkage is the result of:
Contraction of the liquid as it cools prior to its
solidification
Contraction during phase change from a liquid to solid
Contraction of the solid as it continues to cool to ambient temperature.
Shrinkage can sometimes cause cracking to occur in component as it solidifies. Since the
coolest area of a volume of liquid is where it contacts a mould or die, solidification usually
begins first at this surface. As the crystals grow inward, the material continues to shrink. If
the solid surface is too rigid and will not deform to accommodate the internal shrinkage, the
stresses can become high enough to exceed the tensile strength of the material and cause a
crack to form. Shrinkage cavitation sometimes occurs because as a material solidifies inward,
shrinkage occurred to such an extent that there is not enough atoms present to fill the
available space and a void is left.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 23of89
Anisotropy and Isotropy
In a single crystal, the physical and mechanical properties often differ with
orientation. It can be seen from looking at our models of crystalline structure that atoms
should be able to slip over one another or distort in relation to one another easier in some
directions than others. When the properties of a material vary with different crystallographic
orientations, the material is said to be anisotropic.
Alternately, when the properties of a material are the same in all directions, the
material is said to be isotropic. For many polycrystalline materials the grain orientations are
random before any working (deformation) of the material is done. Therefore, even if the
individual grains are anisotropic, the property differences tend to average out and, overall, the
material is isotropic. When a material is formed, the grains are usually distorted and
elongated in one or more directions which makes the material anisotropic. Material forming
will be discussed later but lets continue discussing crystalline structure at the atomic level.
Crystal Defects
A perfect crystal, with every atom of the same type in the correct position, does not exist.
All crystals have some defects. Defects contribute to the mechanical properties of metals. In
fact, using the term defect is sort of a misnomer since these features are commonly
intentionally used to manipulate the mechanical properties of a material. Adding alloying
elements to a metal is one way of introducing a crystal defect. Nevertheless, the term defect
will be used, just keep in mind that crystalline defects are not always bad. There are basic
classes of crystal defects:
point defects, which are places where an atom is missing or irregularly placed in the
lattice structure. Point defects include lattice vacancies, self-interstitial atoms,
substitution impurity atoms, and interstitial impurity atoms
linear defects, which are groups of atoms in irregular positions. Linear defects are
commonly called dislocations.
planar defects, which are interfaces between homogeneous regions of the material.
Planar defects include grain boundaries, stacking faults and external surfaces.
It is important to note at this point that plastic deformation in a material occurs due to the
movement of dislocations (linear defects). Millions of dislocations result for plastic forming
operations such as rolling and extruding. It is also important to note that any defect in the
regular lattice structure disrupts the motion of dislocation, which makes slip or plastic
deformation more difficult. These defects not only include the point and planer defects
mentioned above, and also other dislocations. Dislocation movement produces additional
dislocations, and when dislocations run into each other it often impedes movement of the
dislocations. This drives up the force needed to move the dislocation or, in other words,
strengthens the material. Each of the crystal defects will be discussed in more detail in the
following pages.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 24of89
Point Defects
Point defects are where an atom is
missing or is in an irregular place in the lattice
structure. Point defects include self interstitial
atoms, interstitial impurity atoms, substitutional
atoms and vacancies. A self interstitial atom is
an extra atom that has crowded its way into an
interstitial void in the crystal structure. Self
interstitial atoms occur only in low
concentrations in metals because they distort
and highly stress the tightly packed lattice
structure.
A substitutional impurity atom is an
atom of a different type than the bulk atoms,
which has replaced one of the bulk atoms in the
lattice. Substitutional impurity atoms are usually
close in size (within approximately 15%) to the
bulk atom. An example of substitutional
impurity atoms is the zinc atoms in brass. In
brass, zinc atoms with a radius of 0.133 nm have
replaced some of the copper atoms, which have
a radius of 0.128 nm.
Interstitial impurity atoms are much smaller than the atoms in the bulk matrix.
Interstitial impurity atoms fit into the open space between the bulk atoms of the lattice
structure. An example of interstitial impurity atoms is the carbon atoms that are added to iron
to make steel. Carbon atoms, with a radius of 0.071 nm, fit nicely in the open spaces between
the larger (0.124 nm) iron atoms.
Vacancies are empty spaces where an atom should be, but is missing. They are
common, especially at high temperatures when atoms are frequently and randomly change
their positions leaving behind empty lattice sites. In most cases diffusion (mass transport by
atomic motion) can only occur because of vacancies.
Linear Defects - Dislocations
Dislocations are another type of defect in crystals. Dislocations are areas were the
atoms are out of position in the crystal structure. Dislocations are generated and move when a
stress is applied. The motion of dislocations allows slip plastic deformation to occur.
Before the discovery of the dislocation by Taylor, Orowan and Polyani in 1934, no
one could figure out how the plastic deformation properties of a metal could be greatly
changed by solely by forming (without changing the chemical composition). This became
even bigger mystery when in the early 1900s scientists estimated that metals undergo plastic
deformation at forces much smaller than the theoretical strength of the forces that are holding
the metal atoms together. Many metallurgists remained skeptical of the dislocation theory
until the development of the transmission electron microscope in the late 1950s. The TEM
Introduction to Materials and Processes
FromNDTResources August18,2007nks 25of89
allowed experimental evidence to be collected that showed that the strength and ductility of
metals are controlled by dislocations.
There are two basic types of dislocations, the edge dislocation and the screw
dislocation. Actually, edge and screw dislocations are just extreme forms of the possible
dislocation structures that can occur. Most dislocations are probably a hybrid of the edge and
screw forms but this discussion will be limited to these two types.
Edge Dislocations
The edge defect can be easily visualized as an extra half-plane of atoms in a lattice. The
dislocation is called a line defect because the locus of defective points produced in the lattice
by the dislocation lie along a line. This line runs along the top of the extra half-plane. The
inter-atomic bonds are significantly distorted only in the immediate vicinity of the dislocation
line.
Understanding the movement of a dislocation is key to understanding why
dislocations allow deformation to occur at much lower stress than in a perfect crystal.
Dislocation motion is analogous to movement of a caterpillar. The caterpillar would have to
exert a large force to move its entire body at once. Instead it moves the rear portion of its
body forward a small amount and creates a hump. The hump then moves forward and
eventual moves all of the body forward by a small amount.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 26of89
As shown in the set of images above, the dislocation moves similarly moves a small
amount at a time. The dislocation in the top half of the crystal is slipping one plane at a time
as it moves to the right from its position in image (a) to its position in image (b) and finally
image (c). In the process of slipping one plane at a time the dislocation propagates across the
crystal. The movement of the dislocation across the plane eventually causes the top half of
the crystal to move with respect to the bottom half. However, only a small fraction of the
bonds are broken at any given time. Movement in this manner requires a much smaller force
than breaking all the bonds across the middle plane simultaneously.
Screw Dislocations
There is a second basic type of
dislocation, called screw dislocation. The
screw dislocation is slightly more difficult
to visualize. The motion of a screw
dislocation is also a result of shear stress,
but the defect line movement is
perpendicular to direction of the stress and
the atom displacement, rather than parallel.
To visualize a screw dislocation, imagine a
block of metal with a shear stress applied
across one end so that the metal begins to
rip. This is shown in the upper right image.
The lower right image shows the plane of
atoms just above the rip. The atoms
represented by the blue circles have not yet
moved from their original position. The
atoms represented by the red circles have
moved to their new position in the lattice
and have re-established metallic bonds. The
atoms represented by the green circles are in
the process of moving. It can be seen that
only a portion of the bonds are broke at any
given time. As was the case with the edge
dislocation, movement in this manner
requires a much smaller force than breaking
all the bonds across the middle plane
simultaneously.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 27of89
If the shear force is increased, the atoms will continue to slip to the right. A row of the
green atoms will find there way back into a proper spot in the lattice (and become red) and a
row of the blue atoms will slip out of position (and become green). In this way, the screw
dislocation will move upward in the image, which is perpendicular to direction of the stress.
Recall that the edge dislocation moves parallel to the direction of stress. As shown in the
image below, the net plastic deformation of both edge and screw dislocations is the same,
however.
The dislocations move along the densest planes of atoms in a material, because the
stress needed to move the dislocation increases with the spacing between the planes. FCC and
BCC metals have many dense planes, so dislocations move relatively easy and these
materials have high ductility. Metals are strengthened by making it more difficult for
dislocations to move. This may involve the introduction of obstacles, such as interstitial
atoms or grain boundaries, to pin the dislocations. Also, as a material plastically deforms,
more dislocations are produced and they will get into each others way and impede movement.
This is why strain or work hardening occurs.
In ionically bonded materials, the ion must move past an area with a repulsive charge
in order to get to the next location of the same charge. Therefore, slip is difficult and the
materials are brittle. Likewise, the low density packing of covalent materials makes them
generally more brittle than metals.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 28of89
Planar Defects
Stacking Faults and Twin Boundaries
A disruption of the long-range stacking sequence can produce two other common
types of crystal defects: 1) a stacking fault and 2) a twin region. A change in the stacking
sequence over a few atomic spacings produces a stacking fault whereas a change over many
atomic spacings produces a twin region.
A stacking fault is a one or two layer interruption in the stacking sequence of atom
planes. Stacking faults occur in a number of crystal structures, but it is easiest to see how they
occur in close packed structures. For example, it is know from a previous discussion that face
centered cubic (fcc) structures differ from hexagonal close packed (hcp) structures only in
their stacking order. For hcp and fcc structures, the first two layers arrange themselves
identically, and are said to have an AB arrangement. If the third layer is placed so that its
atoms are directly above those of the first (A) layer, the stacking will be ABA. This is the hcp
structure, and it continues ABABABAB. However it is possible for the third layer atoms to
arrange themselves so that they are in line with the first layer to produce an ABC
arrangement which is that of the fcc structure. So, if the hcp structure is going along as
ABABAB and suddenly switches to ABABABCABAB, there is a stacking fault present.
Alternately, in the fcc arrangement the pattern is ABCABCABC. A stacking fault in
an fcc structure would appear as one of the C planes missing. In other words the pattern
would become ABCABCAB_ABCABC.
If a stacking fault does not corrects itself immediately but continues over some
number of atomic spacings, it will produce a second stacking fault that is the twin of the first
one. For example if the stacking pattern is ABABABAB but switches to ABCABCABC for a
period of time before switching back to ABABABAB, a pair of twin stacking faults is
produced. The red region in the stacking sequence that goes ABCABCACBACBABCABC is
the twin plane and the twin boundaries are the A planes on each end of the highlighted
region.
Grain Boundaries in Polycrystals
Another type of planer defect is the grain boundary. Up to this point, the discussion
has focused on defects of single crystals. However, solids generally consist of a number of
crystallites or grains. Grains can range in size from nanometers to millimeters across and
their orientations are usually rotated with respect to neighbouring grains. Where one grain
stops and another begins is know as a grain boundary. Grain boundaries limit the lengths and
motions of dislocations. Therefore, having smaller grains (more grain boundary surface area)
strengthens a material. The size of the grains can be controlled by the cooling rate when the
material cast or heat treated. Generally, rapid cooling produces smaller grains whereas slow
cooling result in larger grains. For more information, refer to the discussion on solidification.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 29of89
Bulk Defects
Bulk defects occur on a much bigger
scale than the rest of the crystal defects
discussed in this section. However, for the
sake of completeness and since they do affect
the movement of dislocations, a few of the
more common bulk defects will be
mentioned. Voids are regions where there are
a large number of atoms missing from the
lattice. The image to the right is a void in a
piece of metal The image was acquired using
a Scanning Electron Microscope (SEM).
Voids can occur for a number of reasons.
When voids occur due to air bubbles becoming trapped when a material solidifies, it is
commonly called porosity. When a void occurs due to the shrinkage of a material as it
solidifies, it is called cavitation.
Another type of bulk defect occurs when impurity atoms cluster together to form
small regions of a different phase. The term phase refers to that region of space occupied by
a physically homogeneous material. These regions are often called precipitates. Phases and
precipitates will be discussed in more detail latter.
Elastic/Plastic Deformation
When a sufficient load is applied to a metal or other structural material, it will cause
the material to change shape. This change in shape is called deformation. A temporary shape
change that is self-reversing after the force is removed, so that the object returns to its
original shape, is called elastic
deformation. In other words, elastic
deformation is a change in shape of a
material at low stress that is
recoverable after the stress is removed.
This type of deformation involves
stretching of the bonds, but the atoms
do not slip past each other.
When the stress is sufficient to
permanently deform the metal, it is
called plastic deformation. As
discussed in the section on crystal
defects, plastic deformation involves
the breaking of a limited number of
atomic bonds by the movement of
dislocations. Recall that the force
needed to break the bonds of all the
atoms in a crystal plane all at once is
very great. However, the movement of
dislocations allows atoms in crystal
planes to slip past one another at a
Introduction to Materials and Processes
FromNDTResources August18,2007nks 30of89
much lower stress levels. Since the energy required to move is lowest along the densest
planes of atoms, dislocations have a preferred direction of travel within a grain of the
material. This results in slip that occurs along parallel planes within the grain. These parallel
slip planes group together to form slip bands, which can be seen with an optical microscope.
A slip band appears as a single line under the microscope, but it is in fact made up of closely
spaced parallel slip planes as shown in the image.
Fatigue Crack Initiation
While on the subject of dislocations, it is
appropriate to briefly discuss fatigue. Fatigue is one of the
primary reasons for the failure of structural components.
The life of a fatigue crack has two parts, initiation and
propagation. Dislocations play a major role in the fatigue
crack initiation phase. It has been observed in laboratory
testing that after a large number of loading cycles
dislocations pile up and form structures called persistent
slip bands (PSB). An example of a PSB is shown in the
micrograph image to the right.
PSBs are areas that rise above (extrusion) or fall below
(intrusion) the surface of the component due to movement
of material along slip planes. This leaves tiny steps in the
surface that serve as stress risers where fatigue cracks can
initiate. A crack at the edge of a PSB is shown in the
image below taken with a scanning electron microscope
(SEM).
Introduction to Materials and Processes
FromNDTResources August18,2007nks 31of89
Diffusion
Diffusion is the migration of atoms from a region of high
concentration to a region of low concentration. In a homogeneous
material, atoms are routinely moving around but the movement is
random (i.e. there is always an equal number of atoms moving in all
directions). In an inhomogeneous material, all the atoms are moving
near randomly, but there is a migration of atoms to areas where their
concentrations are lower. In other words, there is a net diffusion.
Atom diffusion can occur by the motion of host or
substitutional atoms to vacancies (vacancy diffusion), or interstitial
impurities atoms to different interstitial positions (interstitial
diffusion). In order to move, an atom must overcome the bond
energy due to nearby atoms. This is more easily achieved at high
temperatures when the atoms are vibrating strongly. Carburizing,
which will be discussed later, is an example of diffusion is used.
Property Modification
Many structural metals undergo some special treatment to modify their properties so
that they will perform better for their intended use. This treatment can include mechanical
working, such as rolling or forging, alloying and/or thermal treatments. Consider aluminum
as an example. Commercially pure aluminum (1100) has a tensile strength of around 13,000
psi, which limits its usefulness in structural applications. However, by cold-working
aluminum, its strength can be approximately doubled. Also, strength increases are obtained
by adding alloying metals such as manganese, silicon, copper, magnesium and zinc. Further,
many aluminum alloys are strengthened by heat treatment. Some heat-treatable aluminum
alloys obtain tensile strengths that can exceed 100,000 psi.
Strengthening/Hardening Mechanisms
As discussed in the previous section, the ability of a crystalline material to plastically
deform largely depends on the ability for dislocation to move within a material. Therefore,
impeding the movement of dislocations will result in the strengthening of the material. There
are a number of ways to impede dislocation movement, which include:
controlling the grain size (reducing continuity of atomic planes)
strain hardening (creating and tangling dislocations)
alloying (introducing point defects and more grains to pin dislocation)
Introduction to Materials and Processes
FromNDTResources August18,2007nks 32of89
Control of Grain Size
The size of the grains within a material also has
an effect on the strength of the material. The boundary
between grains acts as a barrier to dislocation
movement and the resulting slip because adjacent
grains have different orientations. Since the atom
alignment is different and slip planes are discontinuous
between grains. The smaller the grains, the shorter the
distance atoms can move along a particular slip plane. Therefore, smaller grains improve the
strength of a material. The size and number of grains within a material is controlled by the
rate of solidification from the liquid phase.
Strain Hardening
Strain hardening (also called work-hardening or cold-working) is the process of
making a metal harder and stronger through plastic deformation. When a metal is plastically
deformed, dislocations move and additional dislocations are generated. The more dislocations
within a material, the more they will interact and become pinned or tangled. This will result
in a decrease in the mobility of the dislocations and a strengthening of the material. This type
of strengthening is commonly called cold-working. It is called cold-working because the
plastic deformation must occurs at a temperature low enough that atoms cannot rearrange
themselves. When a metal is worked at higher temperatures (hot-working) the dislocations
can rearrange and little strengthening is achieved.
Strain hardening can be easily demonstrated with piece of wire or a paper clip. Bend a
straight section back and forth several times. Notice that it is more difficult to bend the metal
at the same place. In the strain hardened area dislocations have formed and become tangled,
increasing the strength of the material. Continued bending will eventually cause the wire to
break at the bend due to fatigue cracking. (After a large number of bending cycles,
dislocations form structures called Persistent Slip Bands (PSB). PSBs are basically tiny areas
where the dislocations have piled up and moved the material surface out leave steps in the
surface that act as stress risers or crack initiation
points.)
It should be understood, however, that increasing the
strength by cold-working will also result in a reduction
in ductility. The graph to the right shows the yield
strength and the percent elongation as a function of
percent cold-work for a few example materials. Notice
that for each material, a small amount of cold-working
results in a significant reduction in ductility.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 33of89
Effects of Elevated Temperature on Strain Hardened Materials
When strain hardened materials are exposed to elevated temperatures, the
strengthening that resulted from the plastic deformation can be lost. This can be a bad thing if
the strengthening is needed to support a load. However, strengthening due to strain hardening
is not always desirable, especially if the material is being heavily formed since ductility will
be lowered.
Heat treatment can be used to remove the effects of strain hardening. Three things can
occur during heat treatment:
1. Recovery
2. Recrystallization
3. Grain growth
Recovery
When a stain hardened
material is held at an elevated
temperature an increase in atomic
diffusion occurs that relieves some of
the internal strain energy. Remember
that atoms are not fixed in position
but can move around when they have
enough energy to break their bonds.
Diffusion increases rapidly with
rising temperature and this allows
atoms in severely strained regions to
move to unstrained positions. In other
words, atoms are freer to move
around and recover a normal position in the lattice structure. This is known as the recovery
phase and it results in an adjustment of strain on a microscopic scale. Internal residual
stresses are lowered due to a reduction in the dislocation density and a movement of
dislocation to lower-energy positions. The tangles of dislocations condense into sharp two-
dimensional boundaries and the dislocation density within these areas decrease. These areas
are called subgrains. There is no appreciable reduction in the strength and hardness of the
material but corrosion resistance often improves.
Recrystallization
At a higher temperature, new, strain-free grains nucleate and grow inside the old
distorted grains and at the grain boundaries. These new grains grow to replace the deformed
grains produced by the strain hardening. With recrystallization, the mechanical properties
return to their original weaker and more ductile states. Recrystallization depends on the
temperature, the amount of time at this temperature and also the amount of strain hardening
that the material experienced. The more strain hardening, the lower the temperature will be at
which recrystallization occurs. Also, a minimum amount (typically 2-20%) of cold work is
necessary for any amount of recrystallization to occur. The size the new grains is also
partially dependant on the amount of strain hardening. The greater the stain hardening, the
more nuclei for the new grains, and the resulting grain size will be smaller (at least initially).
Introduction to Materials and Processes
FromNDTResources August18,2007nks 34of89
Grain Growth
If a specimen is left at the high temperature beyond the time needed for complete
recrystallization, the grains begin to grow in size. This occurs because diffusion occurs across
the grain boundaries and larger grains have less grain boundary surface area per unit of
volume. Therefore, the larger grains lose fewer atoms and grow at the expense of the smaller
grains. Larger grains will reduce the strength and toughness of the material.
Alloying
Only a few elements are widely used commercially in their pure form. Generally, other
elements are present to produce greater strength, to improve corrosion resistance, or simply
as impurities left over from the refining process. The addition of other elements into a metal
is called alloying and the resulting metal is called an alloy. Even if the added elements are
non-metals, alloys may still have metallic properties.
Copper alloys were produced very early in our history. Bronze, an alloy of copper and tin,
was the first alloy known. It was easy to produce by simply adding tin to molten copper.
Tools and weapons made of this alloy were stronger than pure copper ones. The typical
alloying elements in some common metals are presented in the table below.
Alloy Composition
Brass Copper, Zinc
Bronze Copper, Zinc, Tin
Pewter Tin, Copper, Bismuth, Antimony
Cast Iron Iron, Carbon, Manganese, Silicon
Steel Iron, Carbon (plus small amounts of other elements)
Stainless Steel Iron, Chromium, Nickel
The properties of alloys can be manipulated by varying composition. For example steel
formed from iron and carbon can vary substantially in hardness depending on the amount of
carbon added and the way in which it was processed.
When a second element is added, two basically different structural changes are possible:
1. Solid solution strengthening occurs when the atoms of the new element form a solid
solution with the original element, but there is still only one phase. Recall that the
term phase refers to that region of space occupied by a physically homogeneous
material.
2. The atoms of the new elements form a new second phase. The entire microstructure
may change to this new phase or two phases may be present.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 35of89
Solid Solution Strengthening
Solid solution strengthening involves the addition of other metallic elements that will
dissolve in the parent lattice and cause distortions because of the difference in atom size
between the parent metal and the solute metal. Recall from the section on crystal point
defects that it is possible to have substitutional impurity atoms, and interstitial impurity
atoms. A substitutional impurity atom is an atom of a different type than the bulk atoms,
which has replaced one of the bulk atoms in the lattice. Substitutional impurity atoms are
usually close in size (within approximately 15%) to the bulk atom. Interstitial impurity atoms
are much smaller than the atoms in the bulk matrix. Interstitial impurity atoms fit into the
open space between the bulk atoms of the lattice structure.
Since the impurity atoms are smaller or larger than the surrounding atoms they
introduce tensile or compressive lattice strains. They disrupt the regular arrangement of ions
and make it more difficult for the layers to slide over each other. This makes the alloy
stronger and less ductile than the pure metal. For example, an alloy of 30% nickel raises the
cast tensile strength of copper from 25,000 PSI to 55,000 PSI.
Multiphase Metals
Still another method of strengthening the metal is adding elements that have no or
partial solubility in the parent metal. This will result in the appearance of a second phase
distributed throughout the crystal or between crystals. These secondary phases can raise or
reduce the strength of an alloy. For example, the addition of tin, zinc, or aluminum to copper
will result in an alloy with increased strength, but alloying with lead or bismuth with result in
a lower strength alloy. The properties of a polyphase (two of more phase) material depend on
the nature, amount, size, shape, distribution, and orientation of the phases. Greek letters are
commonly used to distinguish the different solid phases in a given alloy.
Phases can be seen on a microscopic scale with an optical microscope after the surface has
been properly polished and etched. Below is a micrograph take at 125x of lead-tin alloy
composed of two phases. The light colored regions are a tin-rich phase and the dark colored
regions are a lead-rich phase.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 36of89
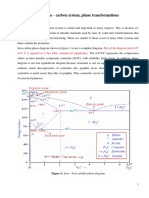
Phase Diagrams
As previously stated, the phase diagram is simply a map showing the structure of
phases present as the temperature and overall composition of the alloy are varied. It is a very
useful tool for understanding and controlling the structures of polyphase materials. A binary
phase diagram shows the phases formed in differing mixtures of two elements over a range of
temperatures. When an alloy exhibits more than two phases, a different type of phase diagram
must be used, such as a ternary diagram for three phase alloys. This discussion will focus on
the binary phase diagram.
On the binary phase diagram, compositions
run from 100% Element A on the left,
through all possible mixtures, to 100%
Element B on the right. The composition of
an alloy is given in the form A - x%B. For
example, Cu - 20%Al is 80% copper and
20% aluminum. Weight percentages are
often used to specify the proportions of the
alloying elements, but atomic percent are
sometimes used. Weight percentages will be
used throughout this text.
Alloys generally do not have a single melting
point, but instead melt (or alternately
solidify) over a range of temperatures. At
each end of the phase diagram only one of
the elements is present (100% A or 100% B)
so a specific melting point does exists.
Additionally, there is sometimes a mixture of
the constituent elements which produces
melting at a single temperature like a pure
element. This is called the eutectic point.
At compositions other than at the pure A,
pure B and the eutectic points, when the
alloy is cooled from a high temperature it
will begin to solidify at a certain temperature
but will remain in a mushy (liquid plus solid)
condition over a range of temperatures. If
experiments are conducted over a range of
compositions to determine the temperature at
which the alloys start to solidify, this data
can be potted on the phase diagram to
produce a curve. This start of solidification
curve will join the three single solidification
points and is called the liquidus line.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 37of89
Up to a few percent of composition, it
is possible for one element to remain
dissolve in another while both are in the solid
state. This is called solid solubility and the
solubility limit normally changes with
temperature. The extent of the solid
solubility region can be plotted onto the
phase diagram. In this example, the alpha
phase is the region of solid solution where
some of B atoms have dissolved in a matrix
of A atoms. The beta phase is the region
where a small percentage of A atoms have dissolved in a matrix of B atoms. It is important to
note that some elements have zero solid solubility in other elements. An example is
aluminum/silicon alloys, where aluminum has zero solid solubility in silicon.
If an alloy's composition does not
place it within the alpha or beta solid
solution regions, the alloy will become fully
solid at the eutectic temperature. The
eutectic line on the phase diagram indicates
where this transformation will occur over the
range of compositions. At alloy compositions
and temperatures between the liquidus
temperature and the eutectic temperature, a
mushy mix of either alpha or beta phase will
exist as solid masses within a liquid mixture
of A and B. These are the alpha plus liquid and the beta plus liquid areas on the phase
diagram. The region below the eutectic line, and outside the solid solution region, will be a
solid mixture of alpha and beta.
Tie and Lever Rules
Simply by looking at a phase diagram it is possible to tell what phase or phases an
alloy will have at a given temperature. But, it is also possible to get quantitative information
from the diagram. Consider the alloy at the temperature shown on the phase diagram. It is
easy to see that at this temperature, it is a
mixture of alpha and liquid phases. Using a
tie line it is also possible to determine the
composition of the phases at this
temperature. A tie line is an isothermal
(constant temperature) line drawn through
the alloy's position on the phase diagram
when it is in a two phase field. The points
where the ends of the tie line intersect the
two adjacent solubility curves indicate the
compositions of the two phases that exist in
equilibrium at this temperature.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 38of89
In this example, the tie line shows that the alpha phase is 5.2%B and the liquid phase
is 34.5%B at this temperature. It is important to keep in mind that the tie rule addresses the
determination of the compositions of the constituent phases within the sample and it does not
address the overall chemical composition of the sample, which remains unchanged.
It is also possible to determine how much of each phase exists at the given
temperature using the lever rule. It is important to know the amounts of each phase present
because the properties of the alloy depend on the amount of each phase present. The lever
rule uses the tie line and the basic scientific principle of the conservation of mass to
determine the ratio of the two phases present. The tie-line gives the chemical compositions of
each of the two phases, and the combined amounts of these two compositions must add up to
the alloy's overall composition (C
o
), which is known. In other words, C
o
must be composed
of the appropriate amount of at composition C
and of liquid at C
liq
. So basically, the
proportions of the phases present are given by the relative lengths of the two sections of the
tie line.
The fraction of alpha phase present is the given by the ratio of the C
o
to C
liq
portion of
the tie line and the total length of the tie line (C
liq
to C
). Mathematically the relationships can
be written as f
<< (C
liq
C
o
)/(C
liq
- C
). The fraction of liquid phase present is given by the
ratio of the C
o
to C
portion of the tie line and the total length of the tie line (C
liq
to C
).
Mathematically this relationships can be written as f
liq
<< (C
o
- C
)/(C
liq
- C
). Of course, the
two values must total to equal one.
Note that the right side of the tie line gives the proportion of the phase on the left (
phase in this example) and left side of the tie line gives the proportion of the phase to the
right (liquid phase in this example). It is easy to keep this relationship straight by simply
considering what the ratio would be near one of the tie line intersect points. For example, if
C
o
were near the liquidus line the ratio of the liquid section of the line to the total length of
the line will be nearly one.
Composition, Microstructure, and the Phase Diagram
Lets finish this discussion on phase diagrams by briefly looking at three different
compositions of elements A and B, and how their microstructures will differ because of their
positions on the phase diagram. First a eutectic alloy, which is an alloy with composition
right at the eutectic point, will be considered. Then compositions on both sides of the eutectic
point will be discussed. An alloy with a composition that lies to the left of the eutectic point
on the phase diagram is called a hypoeutectic alloy, and an alloy with a composition that lies
to the right of the eutectic point is called hypereutectic alloy. At this point, only the condition
of slow cooling, which will allow the alloy to solidify into it equilibrium condition, will be
considered. The microstructure can be controlled by manipulating the speed of cooling the
alloy, but this will be covered in the section on heat treatments.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 39of89
Eutectic Alloys
First, consider the eutectic alloy of elements
A and B as it is cooled from a temperature at
location 1 to location 4 on the phase diagram.
At location 1, the alloy is at a high enough
temperature to make the mixture fully liquid.
The circles below show a representation of
the alloy's microstructure at each of the
locations numbered on the phase diagram.
At location 1, there is nothing of interest as
the alloy is completely liquid. As the alloy is
slow cooled, it remains liquid until it reaches the eutectic temperature (location 2) where it
starts to solidify at any favorable nucleation sites. From the microstructure image 2, it can be
see that as the alloy solidifies it forms into alternate layers of alpha and beta phase. This
layered microstructure is known as lamellar microstructure and the layers are often only of
the order of 1 micron across. The reason that a eutectic alloy forms in this way has to do with
the diffusion times required to form the solid.
The grains grow by adding alpha to alpha and beta to beta until they encounter
another grain (location 3). Further nucleation sites will also continue to form within the liquid
parts of the mixture. This solidification happens very rapidly as any given volume of liquid in
the melt reaches the eutectic temperature. Remember that a eutectic composition solidifies at
a single temperature like a pure element and not over a temperature range.
As the now sold alloy cools to location 4, the composition of the layers of alpha and
beta continue to change as it cools. Atoms of A and B will diffuse between the two phases to
produce the equilibrium compositions of alpha and beta phase at a given temperature. By
drawing tie lines at various temperatures the eutectic point on the phase diagram, it can be
seen that the solubility of A in the beta phase and B in the alpha phase decreases as the
temperature decreases. Since this phase composition change is due to diffusion, which is a
relatively a slow process), it is important that eutectic alloys be allowed to cool slowly to
produce the correct microstructure.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 40of89
Hypoeutectic Alloys
Next, consider an alloy of A and B
that has an overall composition that places it
to the left of the eutectic point. When an
alloy falls to the left of the eutectic point it is
called a hypoeutectic alloy. At location 1, the
alloy is at a temperature that is high enough
to put it in a fully liquid phase.
When the alloy is cooled, it remains
in the liquid state until it reaches the
temperature where it crosses the liquidus line
(location 2). At this temperature, the alpha phase starts to solidify at any favorable nucleation
sites. The alpha solidifies as dendrites which grow to become grains of alpha. The first solid
phase to form is called the primary phase so, in this case, primary alpha is formed.
As the alloy continues to cool (location 3) the existing nucleation sites will grow as
dendrites and further nucleation sites will form within the liquid part of the mixture. The melt
will have that mushy consistency of chunks in liquid while it is in the alpha + liquid region
of the phase diagram. Since the alpha phase is mostly element A (with a small amount of B
atoms in solid solution), the remaining liquid becomes slightly richer in B as the liquid cools,
which is indicated by the liquidus line. The composition of the solid alpha phase also
becomes slightly richer in B atoms as the solid solution line shows.
This primary alpha phase growth and the accompanying phase composition shifts
continue until enough A atoms have been removed so that the remaining liquid is of eutectic
composition. This composition is achieved at the point where the temperature crosses the
eutectic line (location 4). At this point the primary alpha phase stops forming. The remaining
liquid starts to solidify into the lamellar (alternating layers of alpha and beta phases) structure
of a eutectic composition. The eutectic structure will grow; adding alpha to the layers of
alpha and beta to the layers of beta in the eutectic regions, and new solidification sites will
continue to form. Remember that solidification occurs rapidly and without the need for a
further decrease in temperature once the liquid reaches the eutectic line. At this point, the
entire alloy has solidified into a mixture comprised of grains of alpha and grains of eutectic
mixture (alpha and beta). The microstructure from this point at the eutectic line down to
ambient temperature will look something like that shown in micro 5.
Diffusion occurs as the alloy cools since the amount of element B in the alpha phase
changes with temperature. This occurs exactly like it did for the eutectic alloy. Diffusion
must also occur in the grains of pure alpha, as the composition of alpha phase also changes
with temperature.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 41of89
Hypereutectic
Finally, consider an alloy of A and B
that has an overall composition that places it
to the right of the eutectic point. When an
alloy falls to the right of the eutectic point it
is called a hypereutectic alloy. This alloy will
solidify like the hypoeutectic alloy did except
it will pass through the beta + liquid region
of the phase diagram rather than the alpha +
liquid region. This will result in a
microstructure comprised of grains of beta
and grains of eutectic mixture (alpha and
beta) rather than grains of alpha and grains of eutectic mixture (alpha and beta) as the
hypoeutectic alloy had.
At location 1, the alloy is at a temperature that is high enough to put it in a fully liquid
phase. When the alloy is cooled, it remains in the liquid state until it reaches the temperature
where it crosses the liquidus line (location 2). At this temperature, the beta phase starts to
solidify at any favorable nucleation sites. The beta solidifies as dendrites which grow to
become grains of beta. The first solid phase to form is called the primary phase so, in this
case, primary beta is formed.
As the alloy continues to cool (location 3) the existing nucleation sites will grow as
dendrites and further nucleation sites will form within the liquid part of the mixture. Since the
beta phase is mostly element B (with a small amount of A atoms in solid solution), the
remaining liquid becomes richer in A as the liquid cools, which is indicated by the liquidus
line. The composition of the solid beta phase also becomes slightly richer in A atoms as the
solid solution line shows.
This primary beta phase growth and the accompanying phase composition shifts
continue until enough B atoms have been removed so that the remaining liquid is of eutectic
composition. This composition is achieved at the point where the temperature crosses the
eutectic line (location 4). At this point the primary beta phase stops forming. The remaining
liquid starts to solidify into the lamellar (alternating layers of alpha and beta phases) structure
of a eutectic composition. The eutectic structure will grow; adding alpha to the layers of
alpha and beta to the layers of beta in the eutectic regions, and new solidification sites will
continue to form. At this point, the entire alloy quickly solidifies into a mixture of beta grains
and eutectic mixture (alpha and beta) grains. The microstructure from this point at the
eutectic line down to ambient temperature will look something like that shown in micro 5.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 42of89
Diffusion occurs as the alloy cools since the amount of element B in the alpha phase
changes with temperature. This occurs exactly like it did for the eutectic alloy. Diffusion
must also occur in the grains of pure alpha, as the composition of alpha phase also changes
with temperature.
Thermal Treatments (Heat-Treating)
In the previous pages on the subjects of alloying and the binary phase diagram, the
microstructures of alloys that were allowed to solidify by slow cooling were considered. It
should also be known, however, that it is possible to modify the microstructure of an alloy by
subjecting it to various thermal treatments. Heat-treating is a term used to describe all of the
controlled heating and cooling operations performed on a material in the solid state for the
purpose of altering its microstructure and/or properties. The focus of this discussion will be
on metals but is should be noted that heat-treatment is also used on ceramics and composites
to modify their properties.
The major objectives of the different kinds of thermal treatments are:
1. Soften the material for improved workability.
2. Increase the strength or hardness of the material.
3. Increase the toughness or resistance to fracture of the material.
4. Stabilize mechanical or physical properties against changes that might occur during
exposure to service environments.
5. Insure part dimensional stability.
6. Relieve undesirable residual stresses induced during part fabrication.
Different metals respond to treatment at different temperatures. Each metal has a specific
chemical composition, so changes in physical and structural properties take place at different,
critical temperatures. Even small percentages of elements in the metal composition, such as
carbon, will greatly determine the temperature, time, method and rate of cooling that needs to
be used in the heat treating process. Depending on the thermal treatment used, the atomic
structure and/or microstructure of a material may change due to movement of dislocations, an
increase or decrease in solubility of atoms, an increase in grain size, the formation of new
grains of the same or different phase, a change in the crystal structure, and others
mechanisms.
Since there are so many ways in which metals are heat treated, it is not practical to discuss
them all. But, as an example, lets look at how heat treatment is used to strengthen a copper
aluminum alloy.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 43of89
Precipitation Hardening
In designing alloys for strength, an approach often taken is to develop an alloy with a
structure that consists of particles (which impede dislocation movement) dispersed in a
ductile matrix. Such a dispersion can be obtained by choosing an alloy that is a single phase
at elevated temperature but on cooling will precipitate another phase in the matrix. A thermal
process is then developed to produce the desired distribution of precipitate in the matrix.
When the alloy is strengthened by this thermal treatment, it is called precipitation
strengthening or hardening.
Precipitation hardening consists of three main steps: solution treatment, quenching,
and aging. Solution treatment involves heating the alloy to a temperature that allows the
alloying atoms (called the solute) to dissolve into the solution. This results in a homogeneous
solid solution of one phase. Quenching rapidly cools the solution and freezes the atoms in
solution. In more technical terms, the quenching cools the material so fast that the atoms of
the alloying elements do not have time to diffuse out of the solution. In the as-quenched
condition, the solute is supersaturated meaning that the lattice is overly stressed by the
alloying atoms. Aging is the process where the solute particles diffuse out of solution and into
clusters that distort and strengthen the material.
The precipitation hardening process for a copper-aluminum alloy is shown graphically
in the image below. On the right is phase diagram, which is a very useful tool for
understanding and controlling polyphase structures. The phase diagram is simply a map
showing the structure of phases present as the temperature and overall composition of the
alloy are varied. The images on the right in the image show the resulting microstructure at
each step in the process.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 44of89
Common Heat Treating Processes
A few of the more common terms used in heat treating are introduced below. It should
be noted that not all of the term are applicable to all alloys.
Age Hardening is a relatively low-temperature heat treatment process that strengthens a
material by causing the precipitation of components or phases of alloy from a super-saturated
solid solution condition.
Annealing is a softening process in which metals are heated and then allowed to cool slowly.
The purpose of annealing is to soften the material for improve machinability, formability, and
sometimes to control magnetic properties.
Normallizing is much like annealing, but the cooling process is much faster. This results in
increased strength but less ductility in the metal. Its purpose is to refine grain structure,
produce more uniform mechanical properties, and sometimes to relieve internal and surface
stresses.
Precipitation Heat Treatment is the three step process of solution treating, quenching, and
age hardening to increase the strength or hardness of an alloy.
Solution Heat Treatment involves heating the material to a temperature that puts all the
elements in solid solution and then cooling very rapidly to freeze the atoms in place.
Stress Relieving is a low temperature heat treat process that is used to reduce the level of
residual stresses in a material.
Tempering involves gently heating a hardened metal and allowing it to cool slowly will
produce a metal that is still hard but also less brittle. This process is known as tempering.
Quenching is the rapid cooling of a hot material. The medium used to quench the material
can vary from forced air, oil, water and others. Many steels are hardened by heating and
quenching. Quenching results in a metal that is very hard but also brittle.
More information on heat treatment can be found in the material (ie aluminum, steel,
titanium,etc.)sections
Introduction to Materials and Processes
FromNDTResources August18,2007nks 45of89
Ceramic Structures
As discussed in the introduction, ceramics and related materials cover a wide range of
objects. Ceramics are a little more complex than metallic structures, which is why metals
were covered first. A ceramic has traditionally been defined as an inorganic, non-metallic
solid that is prepared from powdered materials and is fabricated into products through the
application of heat. Most ceramics are made up of two or more elements. This is called a
compound. For example, alumina (Al2O3) is a compound made up of aluminum atoms and
oxygen atoms.
The two most common chemical bonds for ceramic materials are covalent and ionic.
The bonding of atoms together is much stronger in covalent and ionic bonding than in
metallic. This is why ceramics generally have the following properties: high hardness, high
compressive strength, and chemical inertness. This strong bonding also accounts for the less
attractive properties of ceramics, such as low ductility and low tensile strength. The absence
of free electrons is responsible for making most ceramics poor conductors of electricity and
heat.
However, it should be noted that the crystal structures of ceramics are many and
varied and this results in a very wide range of properties. For example, while ceramics are
perceived as electrical and thermal insulators, ceramic oxide (initially based on Y-Ba-Cu-O)
is the basis for high temperature superconductivity. Diamond and silicon carbide have a
higher thermal conductivity than aluminum or copper. Control of the microstructure can
overcome inherent stiffness to allow the production of ceramic springs, and ceramic
composites which have been produced with a fracture toughness about half that of steel. Also,
the atomic structures are often of low symmetry that gives some ceramics interesting
electromechanical properties like piezoelectricity, which is used in sensors and transducers.
The structure of most ceramics varies from relatively simple to very complex. The
microstructure can be entirely glassy (glasses only); entirely crystalline; or a combination of
crystalline and glassy. In the latter case, the glassy phase usually surrounds small crystals,
bonding them together. The main compositional classes of engineering ceramics are the
oxides, nitrides and carbides.
Ceramic Glass
Ceramics with an entirely glassy structure have certain properties that are quite
different from those of metals. Recall that when metal in the liquid state is cooled, a
crystalline solid precipitates when the melting freezing point is reached. However, with a
glassy material, as the liquid is cooled it becomes more and more viscous. There is no sharp
melting or freezing point. It goes from liquid to a soft plastic solid and finally becomes hard
and brittle. Because of this unique property, it can be blown into shapes, in addition to being
cast, rolled, drawn and otherwise processed like a metal.
Glassy behaviour is related to the atomic structure of the material. If pure silica (SiO
2
)
is fused together, a glass called vitreous silica is formed on cooling. The basic unit structure
of this glass is the silica tetrahedron, which is composed of a single silicon atom surrounded
by four equidistant oxygen atoms. The silicon atoms occupy the openings (interstitials)
between the oxygen atoms and share four valence electrons with the oxygen atoms through
covalent bonding. The silica atom has four valence electrons and each of the oxygen atoms
Introduction to Materials and Processes
FromNDTResources August18,2007nks 46of89
has two valence electrons so the silica tetrahedron has four extra valence electrons to share
with adjacent tetrahedral. The silicate structures can link together by sharing the atoms in two
corners of the SiO
2
tetrahedrons, forming chain or ring structures. A network of silica
tetrahedral chains form, and at high temperatures these chains easily slide past each other. As
the melt cools, thermal vibrational energy decreases and the chains can not move as easily so
the structure becomes more rigid. Silica is the most important constituent of glass, but other
oxides are added to change certain physical characteristics or to lower the melting point.
Ceramic Crystalline or Partially Crystalline Material
Most ceramics usually contain both metallic and non-metallic elements with ionic or
covalent bonds. Therefore, the structure the metallic atoms, the structure of the non-metallic
atoms, and the balance of charges produced by the valence electrons must be considered. As
with metals, the unit cell is used in describing the atomic structure of ceramics. The cubic and
the hexagonal cells are most common. Additionally, the difference in radii between the
metallic and non-metallic ions plays an important role in the arrangement of the unit cell.
In metals, the regular arrangement of atoms into densely packed planes led to the
occurrence of slip under stress, which gives metal their characteristic ductility. In ceramics,
brittle fracture rather than slip is common because both the arrangement of the atoms and the
type of bonding is different. The fracture or cleavage planes of ceramics are the result of
planes of regularly arranged atoms.
The building criteria for the crystal structure are:
maintain neutrality
charge balance dictates chemical formula
achieve closest packing
A few of the different types of ceramic materials outside of the glass family are described
below.
Silicate Ceramics
As mentioned previously, the silica structure is the
basic structure for many ceramics, as well as glass. It has an
internal arrangement consisting of pyramid (tetrahedral or four-
sided) units. Four large oxygen (0) atoms surround each
smaller silicon (Si) atom. When silica tetrahedrons share three
corner atoms, they produce layered silicates (talc, kaolinite
clay, mica). Clay is the basic raw material for many building
products such as brick and tile. When silica tetrahedrons share
four comer atoms, they produce framework silicates (quartz,
tridymite). Quartz is formed when the tetrahedra in this
material are arranged in a regular, orderly fashion. If silica in
the molten state is cooled very slowly it crystallizes at the
freezing point. But if molten silica is cooled more rapidly, the
resulting solid is a disorderly arrangement which is glass.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 47of89
Cement
Cement (Portland cement) is one of the main ingredients of concrete. There are a
number of different grades of cement but a typical Portland cement will contain 19 to 25%
SiO
2
, 5 to 9% Al
2
O
3
, 60 to 64% CaO and 2 to 4% FeO. Cements are prepared by grinding
the clays and limestone in proper proportion, firing in a kiln, and regrinding. When water is
added, the minerals either decompose or combine with water, and a new phase grows
throughout the mass. The reaction is solution, recrystallization, and precipitation of a silicate
structure. It is usually important to control the amount of water to prevent an excess that
would not be part of the structure and would weaken it. The heat of hydration (heat of
reaction in the adsorption of water) in setting of the cement can be large and can cause
damage in large structures.
Nitride Ceramics
Nitrides combine the superior hardness of
ceramics with high thermal and mechanical stability,
making them suitable for applications as cutting tools,
wear-resistant parts and structural components at high
temperatures. TiN has a cubic structure which is perhaps
the simplest and best known of structure types. Cations
and anions both lie at the nodes of separate fcc lattices.
The structure is unchanged if the Ti and N atoms
(lattices) are interchanged.
Ferroelectric Ceramics
Depending on the crystal structure, in some
crystal lattices, the centers of the positive and negative
charges do not coincide even without the application of
external electric field. In this case, it is said that there
exists spontaneous polarization in the crystal. When the
polarization of the dielectric can be altered by an electric
field, it is called ferroelectric. A typical ceramic
ferroelectric is barium titanate, BaTiO
3
. Ferroelectric
materials, especially polycrystalline ceramics, are very
promising for varieties of application fields such as
piezoelectric/electrostrictive transducers, and
electrooptic.
Phase Diagram
The phase diagram is important in understanding the formation and control of the
microstructure of the microstructure of polyphase ceramics, just as it is with polyphase
metallic materials. Also, nonequilibrium structures are even more prevalent in ceramics
because the more complex crystal structures are more difficult to nucleate and to grow from
the melt.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 48of89
Imperfections in Ceramics
Imperfections in ceramic crystals
include point defects and impurities like in
metals. However, in ceramics defect
formation is strongly affected by the
condition of charge neutrality because the
creation of areas of unbalanced charges
requires an expenditure of a large amount of
energy. In ionic crystals, charge neutrality
often results in defects that come as pairs of
ions with opposite charge or several nearby
point defects in which the sum of all charges
is zero. Charge neutral defects include the
Frenkel and Schottky defects. A Frenkel-
defect occurs when a host atom moves into a
nearby interstitial position to create a vacancy-interstitial pair of cations. A Schottky-defect is
a pair of nearby cation and anion vacancies. Schottky defect occurs when a host atom leaves
its position and moves to the surface creating a vacancy-vacancy pair.
Sometimes, the composition may alter slightly to arrive at a more balanced atomic
charge. Solids such as SiO
2
, which have a well-defined chemical formula, are called
stoichiometric compounds. When the composition of a solid deviates from the standard
chemical formula, the resulting solid is said to be nonstoichiometric. Nonstoichiometry and
the existence of point defects in a solid are often closely related. Anion vacancies are the
source of the nonstoichiometry in SiO
2-x
,
Introduction of impurity atoms in the lattice is likely in conditions where the charge is
maintained. This is the case of electronegative impurities that substitute a lattice anion or
electropositive substitutional impurities. This is more likely for similar ionic radii since this
minimizes the energy required for lattice distortion. Defects will appear if the charge of the
impurities is not balanced.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 49of89
Polymer Structure
Engineering polymers include natural materials such as rubber and synthetic materials
such as plastics and elastomers. Polymers are very useful materials because their structures
can be altered and tailored to produce materials 1) with a range of mechanical properties 2) in
a wide spectrum of colors and 3) with different transparent properties.
Polymers
A polymer is composed of many
simple molecules that are repeating
structural units called monomers. A single
polymer molecule may consist of
hundreds to a million monomers and may
have a linear, branched, or network
structure. Covalent bonds hold the atoms in the polymer molecules together and secondary
bonds then hold groups of polymer chains together to form the polymeric material.
Copolymers are polymers composed of two or more different types of monomers.
Polymer Chains (Thermoplastics and Thermosets)
A polymer is an organic material and the backbone of every organic material is a
chain of carbon atoms. The carbon atom has four electrons in the outer shell. Each of these
valence electrons can form a covalent bond to another carbon atom or to a foreign atom. The
key to the polymer structure is that two carbon atoms can have up to three common bonds
and still bond with other atoms. The elements found most frequently in polymers and their
valence numbers are: H, F, Cl, Bf, and I with 1 valence electron; O and S with 2 valence
electrons; n with 3 valence electrons and C and Si with 4 valence electrons.
The ability for molecules to form
long chains is a vital to producing polymers.
Consider the material polyethylene, which
is made from ethane gas, C
2
H
6
. Ethane gas
has a two carbon atoms in the chain and
each of the two carbon atoms share two
valence electrons with the other. If two
molecules of ethane are brought together,
one of the carbon bonds in each molecule
can be broken and the two molecules can be
joined with a carbon to carbon bond. After
the two mers are joined, there are still two
free valence electrons at each end of the
chain for joining other mers or polymer chains. The process can continue liking more mers
and polymers together until it is stopped by the addition of anther chemical (a terminator),
that fills the available bond at each end of the molecule. This is called a linear polymer and is
building block for thermoplastic polymers.
The polymer chain is often shown in two dimensions, but it should be noted that they
have a three dimensional structure. Each bond is at 109 to the next and, therefore, the carbon
backbone extends through space like a twisted chain of TinkerToys. When stress is applied,
Mer The repeating unit in a polymer chain
Monomer A single mer unit (n=1)
Polymer Many mer-units along a chain (n=10
3
or
more)
Degree of Polymerization The average number of
mer-units in a chain.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 50of89
these chains stretch and the elongation of polymers can be thousands of times greater than it
is in crystalline structures.
The length of the polymer chain is very important. As the number of carbon atoms in
the chain is increased to beyond several hundred, the material will pass through the liquid
state and become a waxy solid. When the number of carbon atoms in the chain is over 1,000,
the solid material polyethylene, with its characteristics of strength, flexibility and toughness,
is obtained. The change in state occurs because as the length of the molecules increases, the
total binding forces between molecules also increases.
It should also be noted that the molecules are not generally straight but are a tangled
mass. Thermoplastic materials, such as polyethylene, can be pictured as a mass of intertwined
worms randomly thrown into a pail. The binding forces are the result of van der Waals forces
between molecules and mechanical entanglement between the chains. When thermoplastics
are heated, there is more molecular movement and the bonds between molecules can be
easily broken. This is why thermoplastic materials can be remelted.
There is another group of polymers in which
a single large network, instead of many molecules is
formed during polymerization. Since polymerization
is initially accomplished by heating the raw materials
and brining them together, this group is called
thermosetting polymers or plastics. For this type of
network structure to form, the mers must have more than two places for boning to occur;
otherwise, only a linear structure is possible. These chains form jointed structures and rings,
and may fold back and forth to take on a partially crystalline structure.
Since these materials are essentially comprised of one giant molecule, there is no
movement between molecules once the mass has set. Thermosetting polymers are more rigid
and generally have higher strength than thermoplastic polymers. Also, since there is no
opportunity for motion between molecules in a thermosetting polymer, they will not become
plastic when heated.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 51of89
Types of polymers
o Commodity plastics
PE =Polyethylene
PS =Polystyrene
PP =Polypropylene
PVC =Poly(vinyl chloride)
PET =Poly(ethylene terephthalate)
o Specialty or Engineering Plastics
Teflon (PTFE) =
Poly(tetrafluoroethylene)
PC =Polycarbonate (Lexan)
Polyesters and Polyamides (Nylon)
Composite Structures
Components of Composite Materials
Matrix phase: bulk materials such as:
Metals Ceramics Polymers
Reinforcement: fibers and particulates such as:
Glass Carbon Kevlar
Silicon Carbide Boron Ceramic
Ceramic Metallic Aggregate
Interface: area of mechanical
A composite material is basically a combination of two or more materials, each of
which retains it own distinctive properties. Multiphase metals are composite materials
on a micro scale, but generally the term composite is applied to materials that are
created by mechanically bonding two or more different materials together. The
resulting material has characteristics that are not characteristic of the components in
isolation. The concept of composite materials is ancient. An example is adding straw
to mud for building stronger mud walls. Most commonly, composite materials have a
bulk phase, which is continuous, called the matrix; and a dispersed, non-continuous,
phase called the reinforcement. Some other examples of basic composites include
concrete (cement mixed with sand and aggregate), reinforced concrete (steel rebar in
concrete), and fiberglass (glass strands in a resin matrix).
Introduction to Materials and Processes
FromNDTResources August18,2007nks 52of89
In about the mid 1960s, a new
group of composite materials,
called advanced engineered
composite materials (aka
advanced composites), began
to emerge. Advanced
composites utilize a
combination of resins and
fibers, customarily
carbon/graphite, kevlar, or
fiberglass with an epoxy resin.
The fibers provide the high stiffness, while the surrounding polymer resin matrix
holds the structure together. The fundamental design concept of composites is that the
bulk phase accepts the load over a large surface area, and transfers it to the
reinforcement material, which can carry a greater load. The significance here lies in
that there are numerous matrix materials and as many fiber types, which can be
combined in countless ways to produce just the desired properties. These materials
were first developed for use in the aerospace industry because for certain application
they have a higher stiffness to weight or strength-to-weight ratio than metals. This
means metal parts can be replaced with lighter weight parts manufactured from
advanced composites. Generally, carbon-epoxy composites are two thirds the weight
of aluminum, and two and a half times as stiff. Composites are resistant to fatigue
damage and harsh environments, and are repairable.
Composites meeting the criteria of having mechanical bonding can also be produced
on a micro scale. For example, when tungsten carbide powder is mixed with cobalt
powder, and then pressed and sintered together, the tungsten carbide retains its
identity. The resulting material has a soft cobalt matrix with tough tungsten carbide
particles inside. This material is used to produce carbide drill bits and is called a
metal-matrix composite. A metal matrix composite is a type of metal that is reinforced
with another material to improve strength, wear or some other characteristics.
Classification of Composite Materials
Since the reinforcement material is of primary importance in the strengthening
mechanism of a composite, it is convenient to classify composites according to the
characteristics of the reinforcement. The following three categories are commonly used.
1. Fiber Reinforced In this group of composites, the fiber is the primary load-bearing
component.
2. Dispersion Strengthened In this group, the matrix is the major load-bearing
component.
3. Particle Reinforced In this group, the load is shared by the matrix and the particles.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 53of89
Fiber Reinforced Composites
Fiberglass is likely the best know fiber reinforced composite but carbon-epoxy and
other advanced composites all fall into this category. The fibers can be in the form of long
continuous fibers, or they can be discontinuous fibers, particles, whiskers and even weaved
sheets. Fibers are usually combined with ductile matrix materials, such as metals and
polymers, to make them stiffer, while fibers are added to brittle matrix materials like
ceramics to increase toughness. The length-to diameter ratio of the fiber, the strength of the
bond between the fiber and the matrix, and the amount of fiber are variables that affect the
mechanical properties. It is important to have a high length-to-diameter aspect ratio so that
the applied load is effectively transferred form the matrix to the fiber.
Fiber materials include:
Glass glass is the most common and inexpensive fiber and is usually use for the
reinforcement of polymer matrices. Glass has a high tensile strength and fairly low density
(2.5 g/cc).
Carbon-graphite - in advance composites, carbon fibers are the material of choice. Carbon
is a very light element, with a density of about 2.3 g/cc and its stiffness is considerable higher
than glass. Carbon fibers can have up to 3 times the stiffness of steel and up to 15 times the
strength of construction steel. The graphitic structure is preferred over the diamond-like
crystalline forms for making carbon fiber because the graphitic structure is made of densely
packed hexagonal layers, stacked in a lamellar style. This structure results in mechanical and
thermal properties are highly anisotropic and this gives component designers the ability to
control the strength and stiffness of components by varying the orientation of the fiber.
Polymer the strong covalent bonds of polymers can lead to impressive properties when
aligned along the fiber axis of high molecular weight chains. Kevlar is an aramid (aromatic
polyamide) composed of oriented aromatic chains, which makes them rigid rod-like
polymers. Its stiffness can be as high as 125 GPa and although very strong in tension, it has
very poor compression properties. Kevlar fibers are mostly used to increase toughness in
otherwise brittle matrices.
Ceramic fibers made from materials such as Alumina and SiC (Silicon carbide) are
advantageous in very high temperature applications, and also where environmental attack is
an issue. Ceramics have poor properties in tension and shear, so most applications as
reinforcement are in the particulate form.
Metallic - some metallic fibers have high strengths but since there density is very high they
are of little use in weight critical applications. Drawing very thin metallic fibers (less than
100 micron) is also very expensive.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 54of89
Dispersion Strengthen Composites
In dispersion strengthened composites, small particles on the order of 10-5 mm to 2.5
x 10-4 mm in diameter are added to the matrix material. These particles act to help the matrix
resist deformation. This makes the material harder and stronger. Consider a metal matrix
composite with a fine distribution of very hard and small secondary particles. The matrix
material is carrying most of the load and deformation is accomplished by slip and dislocation
movement. The secondary particles impede slip and dislocation and, thereby, strengthen the
material. The mechanism is that same as precipitation hardening but effect is not quite as
strong. However, particles like oxides do not react with the matrix or go into solution at high
temperatures so the strengthening action is retained at elevated temperatures.
Particle Reinforced Composites
The particles in these composite are larger than in dispersion strengthened
composites. The particle diameter is typically on the order of a few microns. In this case, the
particles carry a major portion of the load. The particles are used to increase the modulus and
decrease the ductility of the matrix. An example of particle reinforced composites is an
automobile tire which has carbon black particles in a matrix of polyisobutylene elastomeric
polymer. Particle reinforced composites are much easier and less costly than making fiber
reinforced composites. With polymeric matrices, the particles are simply added to the
polymer melt in an extruder or injection molder during polymer processing. Similarly,
reinforcing particles are added to a molten metal before it is cast.
Interface
1. The interface is a bounding surface or zone where a discontinuity occurs, whether
physical, mechanical, chemical etc.
2. The matrix material must "wet" the fiber. Coupling agents are frequently used to
improve wettability. Well "wetted" fibers increase the interface surface area.
3. To obtain desirable properties in a composite, the applied load should be effectively
transferred from the matrix to the fibers via the interface. This means that the
interface must be large and exhibit strong adhesion between fibers and matrix. Failure
at the interface (called debonding) may or may not be desirable. This will be
explained later in fracture propagation modes.
4. Bonding with the matrix can be either weak van der Walls forces or strong covalent
bonds.
5. The internal surface area of the interface can go as high as 3000 cm2/cm3.
6. Interfacial strength is measured by simple tests that induce adhesive failure between
the fibers and the matrix. The most common is the Three-point bend test or ILSS
(interlaminar shear stress test)
We will consider the results of incorporating fibers in a matrix. The matrix, besides
holding the fibers together, has the important function of transferring the applied load to the
fibers. It is of great importance to be able to predict the properties of a composite, given the
component properties and their geometric arrangement.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 55of89
Isotropy and Anisotropy in Composites
1. Fiber reinforced composite materials typically exhibit anisotropy. That is, some
properties vary depending upon which geometric axis or plane they are measured
along.
2. For a composite to be isotropic in a specific property, such as CTE or Youngs
modulus, all reinforcing elements, whether fibers or particles, have to be randomly
oriented. This is not easily achieved for discontinuous fibers, since most processing
methods tend to impart a certain orientation to the fibers.
3. Continuous fibers in the form of sheets are usually used to deliberately make the
composite anisotropic in a particular direction that is known to be the principally
loaded axis or plane.
Physical and Chemical Properties
Physical properties are those that can be observed without changing the identity of the
substance. The general properties of matter such as color, density, hardness, are examples of
physical properties. Properties that describe how a substance changes into a completely
different substance are called chemical properties. Flammability and corrosion/oxidation
resistance are examples of chemical properties.
The difference between a physical and chemical property is straightforward until the
phase of the material is considered. When a material changes from a solid to a liquid to a
vapor it seems like them become a difference substance. However, when a material melts,
solidifies, vaporizes, condenses or sublimes, only the state of the substance changes. Consider
ice, liquid water, and water vapor, they are all simply H
2
O. Phase is a physical property of
matter and matter can exist in four phases solid, liquid, gas and plasma.
Some of the more important physical and chemical properties from an engineering
material standpoint will be discussed in the following sections.
Phase Transformation Temperatures
Density
Specific Gravity
Thermal Conductivity
Linear Coefficient of Thermal Expansion
Electrical Conductivity and Resistivity
Magnetic Permeability
Corrosion Resistance
Introduction to Materials and Processes
FromNDTResources August18,2007nks 56of89
Phase Transformation Temperatures
When temperature rises and pressure
is held constant, a typical substance
changes from solid to liquid and then
to vapor. Transitions from solid to
liquid, from liquid to vapor, from
vapor to solid and visa versa are
called phase transformations or
transitions. Since some substances
have several crystal forms,
technically there can also be solid to
another solid form phase
transformation.
Phase transitions from solid to liquid,
and from liquid to vapor absorb heat.
The phase transition temperature
where a solid changes to a liquid is called the melting point. The temperature at which
the vapor pressure of a liquid equals 1 atm (101.3 kPa) is called the boiling point.
Some materials, such as many polymers, do not go simply from a solid to a liquid
with increasing temperature. Instead, at some temperature below the melting point,
they start to lose their crystalline structure but the molecules remain linked in chains,
which results in a soft and pliable material. The temperature at which a solid, glassy
material begins to soften and flow is called the glass transition temperature.
Density
Mass can be thinly distributed as in a pillow, or tightly packed as in a block of lead.
The space the mass occupies is its volume, and the mass per unit of volume is its
density.
Mass (m) is a fundamental measure of the amount of matter. Weight (w) is a measure
of the force exerted by a mass and this force is force is produced by the acceleration
of gravity. Therefore, on the surface of the earth, the mass of an object is determined
by dividing the weight of an object by 9.8 m/s
2
(the acceleration of gravity on the
surface of the earth). Since we are typically comparing things on the surface of the
earth, the weight of an object is commonly used rather than calculating its mass.
The density (r) of a material depends on the phase it is in and the temperature. (The
density of liquids and gases is very temperature dependent.) Water in the liquid state
has a density of 1 g/cm
3
= 1000g/m
3
at 4
o
C. Ice has a density of 0.917 g/cm
3
at 0
o
c,
and it should be noted that this decrease in density for the solid phase is unusual. For
almost all other substances, the density of the solid phase is greater than that of the
liquid phase. Water vapor (vapor saturated air) has a density of 0.051 g/cm
3
.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 57of89
Some common units used for expressing density are grams/cubic centimeter,
kilograms/cubic meter, grams/milliliter, grams/liter, pounds for cubic inch and pounds
per cubic foot; but it should be obvious that any unit of mass per any unit of volume
can be used.
Substance Density (g/cm
3
)
Air 0.0013
Gasoline 0.7
Wood 0.85
Water (ice) 0.92
Water (liquid) 1.0
Aluminum 2.7
Steel 7.8
Silver 10.5
Lead 11.3
Mercury 13.5
Gold 19.3
Specific Gravity
Specific gravity is the ratio of density of a substance compared to the density of fresh
water at 4C (39 F). At this temperature the density of water is at its greatest value and equal
1 g/mL. Since specific gravity is a ratio, so it has no units. An object will float in water if its
density is less than the density of water and sink if its density is greater that that of water.
Similarly, an object with specific gravity less than 1 will float and those with a specific
gravity greater than one will sink. Specific gravity values for a few common substances are:
Au, 19.3; mercury, 13.6; alcohol, 0.7893; benzene, 0.8786. Note that since water has a
density of 1 g/cm3, the specific gravity is the same as the density of the material measured in
g/cm3.
The Discovery of Specific Gravity
The discovery of specific gravity makes for an interesting story. Sometime around
250 B.C., the Greek mathematician Archimedes was given the task of determining whether a
craftsman had defrauded King Heiro II of Syracuse. The king had provided a metal smith
with gold to make a crown. The king suspected that the metal smith had added less valuable
silver to crown and kept some of the gold for himself. The crown weighed the same as other
crowns but due to its intricate designs it was impossible to measure the exact volume of the
crown so its density could be determined. The king challenged Archimedes to determine if
the crown was pure gold. Archimedes had no immediate answer and pondered this question
for sometime.
One day while entering a bath, he noticed that water spilled over the sides of the pool, and
realized that the amount of water that spilled out was equal in volume to the space that his
body occupied. He realized that a given mass of silver would occupy more space than an
equivalent mass of gold. Archimedes first weighed the crown and weighed out an equal mass
of pure gold. Then he placed the crown in a full container of water and the pure gold in a
container of water. He found that more water spilled over the sides of the tub when the
craftsmans crown was submerged. It turned out that the craftsman had been defrauding the
King! Legend has it that Archimedes was so excited about his discovery that he ran naked
through the streets of Sicily shouting Eureka! Eureka! (Which is Greek for I have found
it!).
Introduction to Materials and Processes
FromNDTResources August18,2007nks 58of89
Thermal Conductivity
Thermal conductivity () is the intrinsic property of a material which relates its ability
to conduct heat. Heat transfer by conduction involves transfer of energy within a material
without any motion of the material as a whole. Conduction takes place when a temperature
gradient exists in a solid (or stationary fluid) medium. Conductive heat flow occurs in the
direction of decreasing temperature because higher temperature equates to higher molecular
energy or more molecular movement. Energy is transferred from the more energetic to the
less energetic molecules when neighboring molecules collide.
Thermal conductivity is defined as the quantity of heat (Q) transmitted through a unit
thickness (L) in a direction normal to a surface of unit area (A) due to a unit temperature
gradient (T) under steady state conditions and when the heat transfer is dependent only on
the temperature gradient. In equation form this becomes the following:
Thermal Conductivity = heat distance / (area temperature gradient)
= Q L / (A T)
Approximate values of thermal conductivity for some common materials are presented in the
table below.
Material
Thermal Conducti vi ty
W/m,
o
K
Thermal Conducti vity
(cal/sec)/(cm
2
,
o
C/cm)
Air at 0 C 0.024 0.000057
Aluminum 205.0 0.50
Brass 109.0 -
Concrete 0.8 0.002
Copper 385.0 0.99
Glass, ordinary 0.8 0.0025
Gold 310 -
Ice 1.6 0.005
Iron - 0.163
Lead 34.7 0.083
Polyethylene HD 0.5 -
Polystyrene expanded 0.03 -
Silver 406.0 1.01
Styrofoam 0.01 -
Steel 50.2 -
Water at 20 C - 0.0014
Wood 0.12-0.04 0.0001
Introduction to Materials and Processes
FromNDTResources August18,2007nks 59of89
Linear Coefficient of Thermal Expansion
When heat is added to most materials, the average amplitude of the atoms' vibrating
within the material increases. This, in turn, increases the separation between the atoms
causing the material to expand. If the material does not go through a phase change, the
expansion can be easily related to the temperature change. The linear coefficient of thermal
expansion ( a) describes the relative change in length of a material per degree temperature
change. As shown in the following equation, a is the ratio of change in length ( Dl) to the
total starting length (l
i
) and change in temperature ( DT).
By rearranging this equation, it can be seen that if the linear coefficient of thermal
expansion is known, the change in components length can be calculated for each degree of
temperature change. This effect also works in reverse. That is to say, if energy is removed
from a material then the object's temperature will decrease causing the object to contract.
Thermal expansion (and contraction) must be taken into account when designing
products with close tolerance fits as these tolerances will change as temperature changes if
the materials used in the design have different coefficients of thermal expansion. It should
also be understood that thermal expansion can cause significant stress in a component if the
design does not allow for expansion and contraction of components. The phenomena of
thermal expansion can be challenging when designing bridges, buildings, aircraft and
spacecraft, but it can be put to beneficial uses. For example, thermostats and other heat-
sensitive sensors make use of the property of linear expansion.
Linear Coefficient of Thermal Expansion for a Few Common Materials
Material
a
(m/m/
o
K)
a
(mm/m/
o
K)
Aluminum 23.8 x 10
-6
0.0238
Concrete 12.0 x 10
-6
0.011
Copper 17.6 x 10 -6 0.0176
Brass 18.5 x 10
-6
0.0185
Steel 12.0 x 10
-6
0.0115
Timber 40.0 x 10
-6
0.04
Quartz Glass 0.5 x 10
-6
0.0005
Polymeric
Materials
40-200 x 10
-6
0.040-
0.200
Acrylic 75.0 x 10
-6
0.075
Introduction to Materials and Processes
FromNDTResources August18,2007nks 60of89
Electrical Conductivity and Resistivity
It is well known that one of the subatomic particles of an atom is the electron. The
electrons carry a negative electrostatic charge and under certain conditions can move from
atom to atom. The direction of movement between atoms is random unless a force causes the
electrons to move in one direction. This directional movement of electrons due to an
electromotive force is what is known as electricity.
Electrical Conductivity
Electrical conductivity is a measure of how well a material accommodates the
movement of an electric charge. It is the ratio of the current density to the electric field
strength. Its SI derived unit is the Siemens per meter, but conductivity values are often
reported as percent IACS. IACS is an acronym for International Annealed Copper Standard
or the material that was used to make traditional copper-wire . The conductivity of the
annealed copper (5.8108 x 10
7
S/m) is defined to be 100% IACS at 20C. All other
conductivity values are related back to this conductivity of annealed copper. Therefore, iron
with a conductivity value of 1.044 x 10
7
S/m, has a conductivity of approximately 18% of
that of annealed copper and this is reported as 18% IACS. An interesting side note is that
commercially pure copper products now often have IACS conductivity values greater than
100% because processing techniques have improved since the adoption of the standard in
1913 and more impurities can now be removed from the metal.
Conductivity values in Siemens/meter can be converted to % IACS by multiplying the
conductivity value by 1.7241 x10
-6
. When conductivity values are reported in
microSiemens/centimeter, the conductivity value is multiplied by 172.41 to convert to the %
IACS value.
Electrical conductivity is a very useful property since values are affected by such
things as a substances chemical composition and the stress state of crystalline structures.
Therefore, electrical conductivity information can be used for measuring the purity of water,
sorting materials, checking for proper heat treatment of metals, and inspecting for heat
damage in some materials.
Electrical Resistivity
Electrical resistivity is the reciprocal of conductivity. It is the is the opposition of a
body or substance to the flow of electrical current through it, resulting in a change of
electrical energy into heat, light, or other forms of energy. The amount of resistance depends
on the type of material. Materials with low resistivity are good conductors of electricity and
materials with high resistivity are good insulators.
The SI unit for electrical resistivity is the ohm meter. Resistivity values are more
commonly reported in micro ohm centimeters units. As mentioned above resistivity values
are simply the reciprocal of conductivity so conversion between the two is straightforward.
For example, a material with two micro ohm centimeter of resistivity will have
microSiemens/centimeter of conductivity. Resistivity values in microhm centimeters units
can be converted to % IACS conductivity values with the following formula:
172.41 / resistivity = % IACS
Introduction to Materials and Processes
FromNDTResources August18,2007nks 61of89
Temperature Coefficient of Resistivity
As noted above, electrical conductivity values (and resistivity values) are typically
reported at 20
o
C. This is done because the conductivity and resistivity of material is
temperature dependant. The conductivity of most materials decreases as temperature
increases. Alternately, the resistivity of most material increases with increasing temperature.
The amount of change is material dependant but has been established for many elements and
engineering materials.
The reason that resistivity increases with increasing temperature is that the number of
imperfection in the atomic lattice structure increases with temperature and this hampers
electron movement. These imperfections include dislocations, vacancies, interstitial defects
and impurity atoms. Additionally, above absolute zero, even the lattice atoms participate in
the interference of directional electron movement as they are not always found at their ideal
lattice sites. Thermal energy causes the atoms to vibrate about their equilibrium positions. At
any moment in time many individual lattice atoms will be away from their perfect lattice sites
and this interferes with electron movement.
When the temperature coefficient is known, an adjusted resistivity value can be
computed using the following formula:
R
1
= R
2
* [1 + a * (T
1
T
2
)]
Where: R
1
= resistivity value adjusted to T
1
R
2
= resistivity value known or measured at temperature T
2
a = Temperature Coefficient
T
1
= Temperature at which resistivity value needs to be known
T
2
= Temperature at which known or measured value was obtained
For example, suppose that resistivity measurements were being made on a hot piece
of aluminum. Normally when measuring resistivity or conductivity, the instrument is
calibrated using standards that are at the same temperature as the material being measured,
and then no correction for temperature will be required. However, if the calibration standard
and the test material are at different temperatures, a correction to the measured value must be
made. Presume that the instrument was calibrated at 20
o
C (68
o
F) but the measurement was
made at 25
o
C (77
o
F) and the resistivity value obtained was 2.706 x 10
-8
ohm meters.
Using the above equation and the following temperature coefficient value, the
resistivity value corrected for temperature can be calculated.
R
1
= R
2
* [1 + a * (T
1
T
2
)]
Where: R
1
= ?
R
2
= 2.706 x 10
-8
ohm meters (measured resistivity at 25
o
C)
a = 0.0043/
o
C
T
1
= 20
o
C
T
2
= 25
o
C
R
1
= 2.706 x 10
-8
ohm meters * [1 + 0.0043/
o
C * (20
o
C 25
o
C)]
R
1
= 2.648 x 10
-8
ohm meters
Introduction to Materials and Processes
FromNDTResources August18,2007nks 62of89
Note that the resistivity value was adjusted downward since this example involved
calculating the resistivity for a lower temperature.
Since conductivity is simply the inverse of resistivity, the temperature coefficient is
the same for conductivity and the equation requires only slight modification. The equation
becomes:
s
1
= s
2
/ [1 + a * (T
1
T
2
)]
Where: s
1
= conductivity value adjusted to T
1
s
2
= conductivity value known or measured at temperature T
2
a = Temperature Coefficient
T
1
= Temperature at which conductivity value needs to be known
T
2
= Temperature at which known or measured value was obtained
In this example lets consider the same aluminum alloy with a temperature coefficient
of 0.0043 per degree centigrade and a conductivity of 63.6% IACS at 25
o
C. What will the
conductivity be when adjusted to 20
o
C?
s
1
= 63.6% IACS / [1 + 0.0043 * (20
o
C 25
o
C)]
s
1
= 65.0% IASC
The temperature coefficient for a few metallic elements is shown below.
Material
Temperature Coefficient
(/
o
C)
Nickel 0.0059
Iron 0.0060
Molybdenum 0.0046
Tungsten 0.0044
Aluminum 0.0043
Copper 0.0040
Silver 0.0038
Platinum 0.0038
Gold 0.0037
Zinc 0.0038
Introduction to Materials and Processes
FromNDTResources August18,2007nks 63of89
Magnetic Permeability
Magnetic permeability or simply permeability is the ease with which a material can be
magnetized. It is a constant of proportionality that exists between magnetic induction and
magnetic field intensity. This constant is equal to approximately 1.257 x 10
-6
Henry per meter
(H/m) in free space (a vacuum). In other materials it can be much different, often
substantially greater than the free-space value, which is symbolized
0
.
Materials that cause the lines of flux to move farther apart, resulting in a decrease in
magnetic flux density compared with a vacuum, are called diamagnetic. Materials that
concentrate magnetic flux by a factor of more than one but less than or equal to ten are called
paramagnetic; materials that concentrate the flux by a factor of more than ten are called
ferromagnetic. The permeability factors of some substances change with rising or falling
temperature, or with the intensity of the applied magnetic field.
In engineering applications, permeability is often expressed in relative, rather than in
absolute, terms. If o represents the permeability of free space (that is, 4p X10
-7
H/m or
1.257 x 10
-6
H/m) and represents the permeability of the substance in question (also
specified in henrys per meter), then the relative permeability,
r
, is given by:
r
= /
0
For non-ferrous metals such as copper, brass, aluminum etc., the permeability is the
same as that of "free space", i.e. the relative permeability is one. For ferrous metals however
the value of r may be several hundred. Certain ferromagnetic materials, especially
powdered or laminated iron, steel, or nickel alloys, have
r
that can range up to about
1,000,000. Diamagnetic materials have
r
less than one, but no known substance has relative
permeability much less than one. In addition, permeability can vary greatly within a metal
part due to localized stresses, heating effects, etc.
When a paramagnetic or ferromagnetic core is inserted into a coil, the inductance is
multiplied by
r
compared with the inductance of the same coil with an air core. This effect is
useful in the design of transformers and eddy current probes.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 64of89
Corrosion
Corrosion involves the deterioration of a material as it reacts with its environment.
Corrosion is the primary means by which metals deteriorate. Corrosion literally consumes the
material reducing load carrying capability and causing stress concentrations. Corrosion is
often a major part of maintenance cost and corrosion prevention is vital in many designs.
Corrosion is not expressed in terms of a design property value like other properties but rather
in more qualitative terms such as a material is immune, resistant, susceptible or very
susceptible to corrosion.
The corrosion process is usually electrochemical in nature, having the essential
features of a battery. Corrosion is a natural process that commonly occurs because unstable
materials, such as refined metals want to return to a more stable compound. For example,
some metals, such as gold and silver, can be found in the earth in their natural, metallic state
and they have little tendency to corrode. Iron is a moderately
active metal and corrodes readily in the presence of water. The
natural state of iron is iron oxide and the most common iron ore
is Hematite with a chemical composition of Fe
2
0
3
. Rust, the
most common corrosion product of iron, also has a chemical
composition of Fe
2
O
3
.
The difficulty in terms of energy required to extract
metals from their ores is directly related to the ensuing
tendency to corrode and release this energy. The electromotive
force series (See table) is a ranking of metals with respect to
their inherent reactivity. The most noble metal is at the top and
has the highest positive electrochemical potential. The most
active metal is at the bottom and has the most negative
electrochemical potential.
Corrosion involve two chemical processesoxidation and reduction. Oxidation is the process of
stripping electrons from an atom and reduction occurs when an electron is added to an atom. The
oxidation process takes place at an area known as the anode. At the anode, positively charged
atoms leave the solid surface and enter into an electrolyte as ions. The ions leave their
corresponding negative charge in the form of electrons in the metal which travel to the location of
the cathode through a conductive path. At the cathode, the corresponding reduction reaction takes
place and consumes the free electrons. The electrical balance of the circuit is restored at the
cathode when the electrons react with neutralizing positive ions, such as hydrogen ions, in the
electrolyte. From this description, it can be seen that there are four essential components that are
needed for a corrosion reaction to proceed. These components are an anode, a cathode, an
electrolyte with oxidizing species, and some direct electrical connection between the anode and
cathode. Although atmospheric air is the most common environmental electrolyte, natural
waters, such as seawater rain, as well as man-made solutions, are the environments most
frequently associated with corrosion problems.
Partial Electromotive Force Series
Standard Potential
Electrode Reaction
(at 25
o
C), V-SHE
Au
3+
+ 3e
-
-> Au 1.498
Pd
2+
+ 2e
-
-> Pd 0.987
Hg
2+
+ 2e
-
-> Hg 0.854
Ag
+
+ e
-
-> Au 0.799
Cu
+
+ e
-
-> Cu 0.521
Cu
2+
+ 2e
-
-> Cu 0.337
2H
+
+ 2e
-
-> H2 0.000 (Ref.)
Pb
2+
+ 2e
-
-> Pb -0.126
Sn
2+
+ 2e
-
-> Sn -0.136
Ni
2+
+ 2e
-
-> Ni -0.250
Co
2+
+ 2e
-
-> Co -0.277
Cd
2+
+ 2e
-
-> Cd -0.403
Fe
2+
+ 2e
-
-> Fe -0.440
Cr
3+
+ 3e
-
-> Cr -0.744
Cr
2+
+ 2e
-
-> Cr -0.910
Zn
2+
+ 2e
-
-> Zn -0.763
Mn
2+
+ 2e
-
-> Mn -1.180
Ti
2+
+ 2e
-
-> Ti -1.630
Al
3+
+ 3e
-
-> Al -1.662
Be
2+
+ 2e
-
-> Be -1.850
Mg
2+
+ 2e
-
-> Mg -2.363
Li
+
+ e
-
-> Li -3.050
Note that aluminum, as indicated by its position in the series, is a
relatively reactive metal; among structural metals, only beryllium and
magnesium are more reactive. Aluminum owes its excellent corrosion
resistance to the barrier oxide film that is bonded strongly to the surface
and if damaged reforms immediately in most environments. On a surface
freshly abraded and exposed to air, the protective film is only 10
Angstroms thick but highly effective at protecting the metal from
corrosion.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 65of89
A typical situation might involve a
piece of metal that has anodic and cathodic
regions on the same surface. If the surface
becomes wet, corrosion may take place
through ionic exchange in the surface water
layer between the anode and cathode.
Electron exchange will take place through
the bulk metal. Corrosion will proceed at the
anodic site according to a reaction such as
M M
++
+ 2e
-
where M is a metal atom. The resulting
metal cations (M
++
) are available at the metal
surface to become corrosion products such as
oxides, hydroxides, etc. The liberated
electrons travel through the bulk metal (or
another low resistance electrical connection) to the cathode, where they are consumed by
cathodic reactions such as
2H
+
+ 2e
-
H 2
The basic principles of corrosion that were just covered, generally apply to all
corrosion situation except certain types of high temperature corrosion. However, the process
of corrosion can be very straightforward but is often very complex due to variety of variable
that can contribute to the process. A few of these variable are the composition of the material
acting in the corrosion cell, the heat treatment and stress state of the materials, the
composition of the electrolyte, the distance between the anode and the cathode, temperature,
protective oxides and coating, etc.
Types of Corrosion
Corrosion is commonly classified based on the appearance of the corroded material.
The classifications used vary slightly from reference to reference but there is generally
considered to be eight different forms of corrosion. There forms are:
Uniform or general corrosion that is distributed more or less uniformly over a surface.
Localized corrosion that is confined to small area. Localized corrosion often occurs due to
a concentrated cell. A concentrated cell is an electrolytic cell in which the electromotive force
is caused by a concentration of some components in the electrolyte. This difference leads to
the formation of distinct anode and cathode regions.
Pitting corrosion that is confined to small areas and take the form of cavities on a
surface.
Crevice corrosion occurring at locations where easy access to the bulk environment
is prevented, such as the mating surfaces of two components.
Filiform Corrosion that occurs under some coatings in the form of randomly
distributed threadlike filaments.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 66of89
Intergranular preferential corrosion at or along the grain boundaries of a metal.
Exfoliation a specific form of
corrosion that travels along
grain boundaries parallel to the
surface of the part causing
lifting and flaking at the surface.
The corrosion products expand
between the uncorroded layers
of metal to produce a look that
resembles pages of a book.
Exfoliation corrosion is
associated with sheet, plate and
extruded products and usually
initiates at unpainted or unsealed edges or holes of susceptible metals.
Galvanic corrosion associated primarily with the electrical coupling of materials with
significantly different electrochemical potentials.
Environmental Cracking brittle fracture of a normally ductile material that occurs
partially due to the corrosive effect of an environment.
Corrosion fatigue fatigue cracking that is characterized by uncharacteristically short
initiation time and/or growth rate due to the damage of corrosion or buildup of
corrosion products.
High temperature hydrogen attack the loss of strength and ductility of steel due to a
high temperature reaction of absorbed hydrogen with carbides. The result of the
reaction is decarburization and internal fissuring.
Hydrogen Embrittlement the loss of ductility of a metal resulting from absorption of
hydrogen.
Liquid metal cracking cracking caused by contact with a liquid metal.
Stress corrosion cracking of a metal due to the combined action of corrosion and a
residual or applied tensile stress.
Erosion corrosion a corrosion reaction accelerated by the relative movement of a
corrosive fluid and a metal surface.
Fretting corrosion damage at the interface of two contacting surfaces under load
but capable of some relative motion. The damage is accelerated by movement at the interface
that mechanically abraded the surface and exposes fresh material to corrosive attack.
Dealloying the selective corrosion of one or more components of a solid solution alloy.
Dezincification corrosion resulting in the selective removal of zinc from copper-
zinc alloys.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 67of89
Mechanical Properties
The mechanical properties of a material are those properties that involve a reaction to an
applied load. The mechanical properties of metals determine the range of usefulness of a
material and establish the service life that can be expected. Mechanical properties are also
used to help classify and identify material. The most common properties considered are
strength, ductility, hardness, impact resistance, and fracture toughness.
Most structural materials are anisotropic, which means that their material properties vary with
orientation. The variation in properties can be due to directionality in the microstructure
(texture) from forming or cold working operation, the controlled alignment of fiber
reinforcement and a variety of other causes. Mechanical properties are generally specific to
product form such as sheet, plate, extrusion, casting, forging, and etc. Additionally, it is
common to see mechanical property listed by the directional grain structure of the material. In
products such as sheet and plate, the rolling direction is called the longitudinal direction, the
width of the product is called the transverse direction, and the thickness is called the short
transverse direction. The grain orientations in standard wrought forms of metallic products are
shown the image.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 68of89
The mechanical properties of a material are not constants and often change as a function of
temperature, rate of loading, and other conditions. For example, temperatures below room
temperature generally cause an increase in strength properties of metallic alloys; while ductility,
fracture toughness, and elongation usually decrease. Temperatures above room temperature usually
cause a decrease in the strength properties of metallic alloys. Ductility may increase or decrease with
increasing temperature depending on the same variables
It should also be noted that there is often significant variability in the values obtained when
measuring mechanical properties. Seemingly identical test specimen from the same lot of material will
often produce considerable different results. Therefore, multiple tests are commonly conducted to
determine mechanical properties and values reported can be an average value or calculated
statistical minimum value. Also, a range of values are sometimes reported in order to show variability.
Loading
The application of a force to an object is known as loading. Materials can be subjected to many
different loading scenarios and a materials performance is dependant on the loading conditions.
There are five fundamental loading conditions; tension, compression, bending, shear, and torsion.
Tension is the type of loading in which the two sections of material on either side of a plane tend to be
pulled apart or elongated. Compression is the reverse of tensile loading and involves pressing the
material together. Loading by bending involves applying a load in a manner that causes a material to
curve and results in compressing the material on one side and stretching it on the other. Shear
involves applying a load parallel to a plane which caused the material on one side of the plane to want
to slide across the material on the other side of the plane. Torsion is the application of a force that
causes twisting in a material.
If a material is subjected to a constant force, it is called static loading. If the loading of the
material is not constant but instead fluctuates, it is called dynamic or cyclic loading. The way a
material is loaded greatly affects its mechanical properties and largely determines how, or if, a
component will fail; and whether it will show warning signs before failure actually occurs.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 69of89
Stress and Strain
Stress
The term stress (s) is used to express the loading in terms of force applied to a certain cross-
sectional area of an object. From the perspective of loading, stress is the applied force or system of
forces that tends to deform a body. From the perspective of what is happening within a material,
stress is the internal distribution of forces within a body that balance and react to the loads applied to
it. The stress distribution may or may not be uniform, depending on the nature of the loading
condition. For example, a bar loaded in pure tension will essentially have a uniform tensile stress
distribution. However, a bar loaded in bending will have a stress distribution that changes with
distance perpendicular to the normal axis.
Simplifying assumptions are often used to represent stress as a vector quantity for many
engineering calculations and for material property determination. The word " vector" typically refers to
a quantity that has a "magnitude" and a "direction". For example, the stress in an axially loaded bar is
simply equal to the applied force divided by the bar's cross-sectional area.
Some common measurements of stress are:
Psi = lbs/in
2
(pounds per square inch)
ksi or kpsi = kilopounds/in
2
(one thousand or 10
3
pounds per square inch)
Pa = N/m 2 (Pascals or Newtons per square meter)
kPa = Kilopascals (one thousand or 10
3
Newtons per square meter)
GPa = Gigapascals (one million or 10
6
Newtons per square meter)
*Any metric prefix can be added in front of psi or Pa to indicate the multiplication factor
It must be noted that the stresses in most 2-D or 3-D solids are actually more complex and
need be defined more methodically. The internal force acting on a small area of a plane can be
resolved into three components: one normal to the plane and two parallel to the plane. The normal
force component divided by the area
gives the normal stress (s), and parallel
force components divided by the area
give the shear stress (t). These
stresses are average stresses as the
area is finite, but when the area is
allowed to approach zero, the stresses
become stresses at a point. Since
stresses are defined in relation to the
plane that passes through the point
under consideration, and the number of
such planes is infinite, there appear an
infinite set of stresses at a point.
Fortunately, it can be proven that the
stresses on any plane can be
computed from the stresses on three
orthogonal planes passing through the
point. As each plane has three
stresses, the stress tensor has nine
stress components, which completely
describe the state of stress at a point.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 70of89
Strain
Strain is the response of a system to an applied stress. When a material is loaded with
a force, it produces a stress, which then causes a material to deform. Engineering strain is
defined as the amount of deformation in the direction of the applied force divided by the
initial length of the material. This results in a unitless number, although it is often left in the
unsimplified form, such as inches per inch or meters per meter. For example, the strain in a
bar that is being stretched in tension is the amount of elongation or change in length divided
by its original length. As in the case of stress, the strain distribution may or may not be
uniform in a complex structural element, depending on the nature of the loading condition.
If the stress is small, the material may only strain a small amount and the material will
return to its original size after the stress is released. This is called elastic deformation,
because like elastic it returns to its unstressed state. Elastic deformation only occurs in a
material when stresses are lower than a critical stress called the yield strength. If a material is
loaded beyond it elastic limit, the material will remain in a deformed condition after the load
is removed. This is called plastic deformation.
Engineering and True Stress and Strain
The discussion above focused on engineering stress and strain, which use the fixed,
undeformed cross-sectional area in the calculations. True stress and strain measures account
for changes in cross-sectional area by using the instantaneous values for the area. The
engineering stress-strain curve does not give a true indication of the deformation
characteristics of a metal because it is based entirely on the original dimensions of the
specimen, and these dimensions change continuously during the testing used to generate the
data.
Engineering stress and strain data is commonly used because it is easier to generate
the data and the tensile properties are adequate for engineering calculations. When
considering the stress-strain curves in the next section, however, it should be understood that
metals and other materials continues to strain-harden until they fracture and the stress
required to produce further deformation also increase.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 71of89
Stress Concentration
When an axial load is applied to a piece of
material with a uniform cross-section, the norm al stress
will be uniformly distributed over the cross-section.
However, if a hole is drilled in the material, the stress
distribution will no longer be uniform. Since the material
that has been removed from the hole is no longer
available to carry any load, the load must be
redistributed over the remaining material. It is not
redistributed evenly over the entire remaining cross-
sectional area but instead will be redistributed in an
uneven pattern that is highest at the edges of the hole
as shown in the image. This phenomenon is known as
stress concentration.
Tensile Properties
Tensile properties indicate how the material will
react to forces being applied in tension. A tensile test is
a fundamental mechanical test where a carefully
prepared specimen is loaded in a very controlled
manner while measuring the applied load and the
elongation of the specimen over some distance. Tensile tests are used to determine the modulus of
elasticity, elastic limit, elongation, proportional limit, reduction in area, tensile strength, yield point,
yield strength and other tensile properties.
The main product of a tensile test is a load versus elongation curve which is then converted
into a stress versus strain curve. Since both the engineering stress and the engineering strain are
obtained by dividing the load and elongation by constant values (specimen geometry information), the
load-elongation curve will have the same shape as the engineering stress-strain curve. The stress-
strain curve relates the applied stress to the resulting strain and each material has its own unique
stress-strain curve. A typical engineering stress-strain curve is shown below. If the true stress, based
on the actual cross-sectional area of the specimen, is used, it is found that the stress-strain curve
increases continuously up to fracture.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 72of89
Linear-Elastic Region and Elastic Constants
As can be seen in the figure, the stress and strain initially increase with a linear relationship.
This is the linear-elastic portion of the curve and it indicates that no plastic deformation has occurred.
In this region of the curve, when the stress is reduced, the material will return to its original shape. In
this linear region, the line obeys the relationship defined as Hooke's Law where the ratio of stress to
strain is a constant.
The slope of the line in this region where stress is proportional to strain and is called the
modulus of elasticity or Young's modulus. The modulus of elasticity (E) defines the properties of a
material as it undergoes stress, deforms, and then returns to its original shape after the stress is
removed. It is a measure of the stiffness of a given material. To compute the modulus of elastic ,
simply divide the stress by the strain in the material. Since strain is unitless, the modulus will have the
same units as the stress, such as kpi or MPa. The modulus of elasticity applies specifically to the
situation of a component being stretched with a tensile force. This modulus is of interest when it is
necessary to compute how much a rod or wire stretches under a tensile load.
There are several different kinds of moduli depending on the way the material is being
stretched, bent, or otherwise distorted. When a component is subjected to pure shear, for instance, a
cylindrical bar under torsion, the shear modulus describes the linear-elastic stress-strain relationship.
Axial strain is always accompanied by lateral strains of opposite sign in the two directions
mutually perpendicular to the axial strain. Strains that result from an increase in length are
designated as positive (+) and those that result in a decrease in length are designated as negative (-
). Poisson's ratio is defined as the negative of the ratio of the lateral strain to the axial strain for a
uniaxial stress state.
Poisson's ratio is sometimes also defined as the ratio of the absolute values of lateral and
axial strain. This ratio, like strain, is unitless since both strains are unitless. For stresses within the
elastic range, this ratio is approximately constant. For a perfectly isotropic elastic material, Poisson's
Ratio is 0.25, but for most materials the value lies in the range of 0.28 to 0.33. Generally for steels,
Poissons ratio will have a value of approximately 0.3. This means that if there is one inch per inch of
deformation in the direction that stress is applied, there will be 0.3 inches per inch of deformation
perpendicular to the direction that force is applied.
Only two of the elastic constants are independent so if two constants are known, the third can be
calculated using the following formula:
E =2 (1 +n) G.
Where: E = modulus of elasticity (Young's modulus)
n = Poisson's ratio
G = modulus of rigidity (shear modulus).
A couple of additional elastic constants that may be encountered include the bulk modulus
(K), and Lame's constants (m and l). The bulk modulus is used describe the situation where a piece of
material is subjected to a pressure increase on all sides. The relationship between the change in
pressure and the resulting strain produced is the bulk modulus. Lame's constants are derived from
modulus of elasticity and Poisson's ratio.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 73of89
Yield Point
In ductile materials, at some point, the stress-strain curve deviates from the straight-
line relationship and Law no longer applies as the strain increases faster than the stress. From
this point on in the tensile test, some permanent deformation occurs in the specimen and the
material is said to react plastically to any further increase in load or stress. The material will
not return to its original, unstressed condition when the load is removed. In brittle materials,
little or no plastic deformation occurs and the material fractures near the end of the linear-
elastic portion of the curve.
With most materials there is a gradual transition from elastic to plastic behavior, and
the exact point at which plastic deformation begins to occur is hard to determine. Therefore,
various criteria for the initiation of yielding are used depending on the sensitivity of the strain
measurements and the intended use of the data. (See Table) For most engineering design and
specification applications, the yield strength is used. The yield strength is defined as the stress
required to produce a small, amount of plastic deformation. The offset yield strength is the
stress corresponding to the intersection of the stress-strain curve and a line parallel to the
elastic part of the curve offset by a specified strain (in the US the offset is typically 0.2% for
metals and 2% for plastics).
To determine the yield strength using this offset, the point is found on the strain axis
(x-axis) of 0.002, and then a line parallel to the stress-strain line is drawn. This line will
intersect the stress-strain line slightly after it begins to curve,
and that intersection is defined as the yield strength with a 0.2%
offset. A good way of looking at offset yield strength is that
after a specimen has been loaded to its 0.2 percent offset yield
strength and then unloaded it will be 0.2 percent longer than
before the test. Even though the yield strength is meant to
represent the exact point at which the material becomes permanently deformed, 0.2%
elongation is considered to be a tolerable amount of sacrifice for the ease it creates in
defining the yield strength.
Some materials such as gray cast iron or soft copper exhibit essentially no linear-elastic
behaviour. For these materials the usual practice is to define the yield strength as the stress
required to produce some total amount of strain.
True elastic limit is a very low value and is related to the motion of a few hundred
dislocations. Micro strain measurements are required to detect strain on order of 2 x 10 -6
in/in.
Proportional limit is the highest stress at which stress is directly proportional to strain. It is
obtained by observing the deviation from the straight-line portion of the stress-strain curve.
Elastic limit is the greatest stress the material can withstand without any measurable
permanent strain remaining on the complete release of load. It is determined using a tedious
incremental loading-unloading test procedure. With the sensitivity of strain measurements
usually employed in engineering studies (10 -4in/in), the elastic limit is greater than the
proportional limit. With increasing sensitivity of strain measurement, the value of the elastic
limit decreases until it eventually equals the true elastic limit determined from micro strain
measurements.
Yield strength is the stress required to produce a small-specified amount of plastic
deformation. The yield strength obtained by an offset method is commonly used for
engineering purposes because it avoids the practical difficulties of measuring the elastic limit
or proportional limit.
In Great Britain, the yield
strength is often referred to
as the proof stress. The
offset value is either 0.1% or
0.5%
Introduction to Materials and Processes
FromNDTResources August18,2007nks 74of89
Ultimate Tensile Strength
The ultimate tensile strength (UTS) or, more simply, the tensile strength, is the
maximum engineering stress level reached in a tension test. The strength of a material is its
ability to withstand external forces without breaking. In brittle materials, the UTS will at the
end of the linear-elastic portion of the stress-strain curve or close to the elastic limit. In
ductile materials, the UTS will be well outside of the elastic portion into the plastic portion of
the stress-strain curve.
On the stress-strain curve above, the UTS is the highest point where the line is
momentarily flat. Since the UTS is based on the engineering stress, it is often not the same as
the breaking strength. In ductile materials strain hardening occurs and the stress will continue
to increase until fracture occurs, but the engineering stress-strain curve may show a decline in
the stress level before fracture occurs. This is the result of engineering stress being based on
the original cross-section area and not accounting for the necking that commonly occurs in
the test specimen. The UTS may not be completely representative of the highest level of
stress that a material can support, but the value is not typically used in the design of
components anyway. For ductile metals the current design practice is to use the yield strength
for sizing static components. However, since the UTS is easy to determine and quite
reproducible, it is useful for the purposes of specifying a material and for quality control
purposes. On the other hand, for brittle materials the design of a component may be based on
the tensile strength of the material.
Measures of Ductility (Elongation and Reduction of Area)
The ductility of a material is a measure of the extent to which a material will deform
before fracture. The amount of ductility is an important factor when considering forming
operations such as rolling and extrusion. It also provides an indication of how visible
overload damage to a component might become before the component fractures. Ductility is
also used a quality control measure to assess the level of impurities and proper processing of
a material.
The conventional measures of
ductility are the engineering strain at fracture
(usually called the elongation ) and the
reduction of area at fracture. Both of these
properties are obtained by fitting the
specimen back together after fracture and
measuring the change in length and cross-
sectional area. Elongation is the change in
axial length divided by the original length of
the specimen or portion of the specimen. It is
expressed as a percentage. Because an
appreciable fraction of the plastic
deformation will be concentrated in the
necked region of the tensile specimen, the
value of elongation will depend on the gage length over which the measurement is taken. The
smaller the gage length the greater the large localized strain in the necked region will factor
into the calculation. Therefore, when reporting values of elongation , the gage length should
be given.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 75of89
One way to avoid the complication from necking is to base the elongation
measurement on the uniform strain out to the point at which necking begins. This works well
at times but some engineering stress-strain curve are often quite flat in the vicinity of
maximum loading and it is difficult to precisely establish the strain when necking starts to
occur.
Reduction of area is the change in cross-sectional area divided by the original cross-
sectional area. This change is measured in the necked down region of the specimen. Like
elongation, it is usually expressed as a percentage.
As previously discussed, tension is just one of the way that a material can be loaded.
Other ways of loading a material include compression, bending, shear and torsion, and there
are a number of standard tests that have been established to characterize how a material
performs under these other loading conditions. A very cursory introduction to some of these
other material properties will be provided on the next page.
Compressive, Bearing, & Shear Properties
Compressive Properties
In theory, the compression test is simply the opposite of the
tension test with respect to the direction of loading. In compression
testing the sample is squeezed while the load and the displacement are
recorded. Compression tests result in mechanical properties that
include the compressive yield stress, compressive ultimate stress, and
compressive modulus of elasticity.
Compressive yield stress is measured in a manner identical to
that done for tensile yield strength. When testing metals, it is defined
as the stress corresponding to 0.002 in./in. plastic strain. For plastics,
the compressive yield stress is measured at the point of permanent
yield on the stress-strain curve. Moduli are generally greater in
compression for most of the commonly used structural materials.
Ultimate compressive strength is the stress required to rupture a specimen. This value
is much harder to determine for a compression test than it is for a tensile test since many
material do not exhibit rapid fracture in compression. Materials such as most plastics that do
not rupture can have their results reported as the compressive strength at a specific
deformation such as 1%, 5%, or 10% of the sample's original height.
For some materials, such as concrete, the compressive strength is the most important material
property that engineers use when designing and building a structure. Compressive strength is
also commonly used to determine whether a concrete mixture meets the requirements of the
job specifications.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 76of89
Bearing Properties
Bearing properties are used when designing mechanically fastened joints. The
purpose of a bearing test is to determine the the deformation of a hole as a function of the
applied bearing stress. The test specimen is basically a piece of sheet or plate with a carefully
prepared hole some standard distance from the edge. Edge-to-hole diameter ratios of 1.5 and
2.0 are common. A hardened pin is inserted through the hole and an axial load applied to the
specimen and the pin. The bearing stress is computed by dividing the load applied to the pin,
which bears against the edge of the hole, by the bearing area (the product of the pin diameter
and the sheet or plate thickness). Bearing yield and ultimate stresses are obtained from
bearing tests. BYS is computed from a bearing stress deformation curve by drawing a line
parallel to the initial slope at an offset of 0.02 times the pin diameter. BUS is the maximum
stress withstood by a bearing specimen.
Shear Properties
A shearing stress acts parallel to the stress plane, whereas a tensile or compressive
stress acts normal to the stress plane. Shear properties are primarily used in the design of
mechanically fastened components, webs, and torsion members, and other components
subject to parallel, opposing loads. Shear properties are dependant on the type of shear test
and their is a variety of different standard shear tests that can be performed including the
single-shear test, double-shear test, blanking-shear test, torsion-shear test and others. The
shear modulus of elasticity is considered a basic shear property. Other properties, such as the
proportional limit stress and shear ultimate stress, cannot be treated as basic shear properties
because of form factor effects.
Hardness
Hardness is the resistance of a material to localized deformation. The term can apply to
deformation from indentation, scratching, cutting or bending. In metals, ceramics and most polymers,
the deformation considered is plastic deformation of the surface. For elastomers and some polymers,
hardness is defined at the resistance to elastic deformation of the surface. The lack of a fundamental
definition indicates that hardness is not be a basic property of a material, but rather a composite one
with contributions from the yield strength, work hardening, true tensile strength, modulus, and others
factors. Hardness measurements are widely used for the quality control of materials because they are
quick and considered to be nondestructive tests when the marks or indentations produced by the test
are in low stress areas.
There are a large variety of methods used for determining the hardness of a substance. A few of the
more common methods are introduced below.
Mohs Hardness Test
One of the oldest ways of measuring hardness was devised by the German mineralogist
Friedrich Mohs in 1812. The Mohs hardness test involves observing whether a materials surface is
scratched by a substance of known or defined hardness. To give numerical values to this physical
property, minerals are ranked along the Mohs scale, which is composed of 10 minerals that have
been given arbitrary hardness values. Mohs hardness test, while greatly facilitating the identification
of minerals in the field, is not suitable for accurately gauging the hardness of industrial materials such
as steel or ceramics. For engineering materials, a variety of instruments have been developed over
the years to provide a precise measure of hardness. Many apply a load and measure the depth or
size of the resulting indentation. Hardness can be measured on the macro-, micro- or nano- scale.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 77of89
Brinell Hardness Test
The oldest of the hardness test methods in common use on engineering materials today is the
Brinell hardness test. Dr. J . A. Brinell invented the Brinell test in Sweden in 1900. The Brinell test uses
a desktop machine to applying a specified load to a hardened sphere of a specified diameter. The
Brinell hardness number, or simply the Brinell number, is obtained by dividing the load used, in
kilograms, by the measured surface area of the indentation, in square millimeters, left on the test
surface. The Brinell test is frequently used to determine the hardness metal forgings and castings that
have a large grain structures. The Brinell test provides a measurement over a fairly large area that is
less affected by the course grain structure of these materials than are Rockwell or Vickers tests.
A wide range of materials can be tested using a Brinell test simply by varying the test load and
indenter ball size. In the USA, Brinell testing is typically done on iron and steel castings using a
3000Kg test force and a 10mm diameter ball. A 1500 kilogram load is usually used for aluminum
castings. Copper, brass and thin stock are frequently tested using a 500Kg test force and a 10 or
5mm ball. In Europe Brinell testing is done using a much wider range of forces and ball sizes and it is
common to perform Brinell tests on small parts using a 1mm carbide ball and a test force as low as
1kg. These low load tests are commonly referred to as baby Brinell tests. The test conditions should
be reported along with the Brinell hardness number. A value reported as "60 HB 10/1500/30" means
that a Brinell Hardness of 60 was obtained using a 10mm diameter ball with a 1500 kilogram load
applied for 30 seconds.
Rockwell Hardness Test
The Rockwell Hardness test also uses a machine to apply a specific load and then measure
the depth of the resulting impression. The indenter may either be a steel ball of some specified
diameter or a spherical diamond-tipped cone of 120 angle and 0.2 mm tip radius, called a brale. A
minor load of 10 kg is first applied, which causes a small initial penetration to seat the indenter and
remove the effects of any surface irregularities. Then, the dial is set to zero and the major load is
applied. Upon removal of the major load, the depth reading is taken while the minor load is still on.
The hardness number may then be read directly from the scale. The indenter and the test load used
determine the hardness scale that is used (A, B, C, etc).
For soft materials such as copper alloys, soft steel, and aluminum alloys a 1/16" diameter
steel ball is used with a 100-kilogram load and the hardness is read on the "B" scale. In testing harder
materials, hard cast iron and many steel alloys, a 120 degrees diamond cone is used with up to a 150
kilogram load and the hardness is read on the "C" scale. There are several Rockwell scales other
than the "B" & "C" scales, (which are called the common scales). A properly reported Rockwell value
will have the hardness number followed by "HR" (Hardness Rockwell) and the scale letter. For
example, 50 HRB indicates that the material has a hardness reading of 50 on the B scale.
A -Cemented carbides, thin steel and shallow case hardened steel
B -Copper alloys, soft steels, aluminum alloys, malleable iron, etc.
C -Steel, hard cast irons, pearlitic malleable iron, titanium, deep case hardened
steel and other materials harder than B 100
D -Thin steel and medium case hardened steel and pearlitic malleable iron
E -Cast iron, aluminum and magnesium alloys, bearing metals
F -Annealed copper alloys, thin soft sheet metals
G -Phosphor bronze, beryllium copper, malleable irons
H -Aluminum, zinc, lead
K, L, M, P, R, S, V -Bearing metals and other very soft or thin materials,
including plastics.
Rockwell Superficial Hardness Test
The Rockwell Superficial Hardness Tester is used to test thin materials, lightly carburized
steel surfaces, or parts that might bend or crush under the conditions of the regular test. This tester
uses the same indenters as the standard Rockwell tester but the loads are reduced. A minor load of 3
kilograms is used and the major load is either 15 or 45 kilograms depending on the indenter used.
Using the 1/16" diameter, steel ball indenter, a "T" is added (meaning thin sheet testing) to the
superficial hardness designation. An example of a superficial Rockwell hardness is 23 HR15T, which
indicates the superficial hardness as 23, with a load of 15 kilograms using the steel ball.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 78of89
Vickers and Knoop Microhardness Tests
The Vickers and Knoop Hardness Tests are a modification of the Brinell test and are used to
measure the hardness of thin film coatings or the surface hardness of case-hardened parts. With
these tests, a small diamond pyramid is pressed into the sample under loads that are much less than
those used in the Brinell test. The difference between the Vickers and the Knoop Tests is simply the
shape of the diamond pyramid indenter. The Vickers test uses a square pyramidal indenter which is
prone to crack brittle materials. Consequently, the Knoop test using a rhombic-based (diagonal ratio
7.114:1) pyramidal indenter was developed which produces longer but shallower indentations. For the
same load, Knoop indentations are about 2.8 times longer than Vickers indentations.
An applied load ranging from 10g to 1,000g is used. This low amount of load creates a small
indent that must be measured under a microscope. The measurements for hard coatings like TiN
must be taken at very high magnification (i.e. 1000X), because the indents are so small. The surface
usually needs to be polished. The diagonals of the impression are measured, and these values are
used to obtain a hardness number (VHN), usually from a lookup table or chart. The Vickers test can
be used to characterize very hard materials but the hardness is measured over a very small region.
The values are expressed like 2500 HK25 (or HV25) meaning 2500 Hardness Knoop at 25
gram force load. The Knoop and Vickers hardness values differ slightly, but for hard coatings, the
values are close enough to be within the measurement error and can be used interchangeably.
Scleroscope and Rebound Hardness Tests
The Scleroscope test is a very old test that involves dropping a diamond tipped hammer,
which falls inside a glass tube under the force of its own weight from a fixed height, onto the test
specimen. The height of the rebound travel of the hammer is measured on a graduated scale. The
scale of the rebound is arbitrarily chosen and consists on Shore units, divided into 100 parts, which
represent the average rebound from pure hardened high-carbon steel. The scale is continued higher
than 100 to include metals having greater hardness. The Shore Scleroscope measures hardness in
terms of the elasticity of the material and the hardness number depends on the height to which the
hammer rebounds, the harder the material, the higher the rebound.
The Rebound Hardness Test Method is a recent advancement that builds on the Scleroscope. There
are a variety of electronic instruments on the market that measure the loss of energy of the impact
body. These instruments typically use a spring to accelerate a spherical, tungsten carbide tipped
mass towards the surface of the test object. When the mass contacts the surface it has a specific
kinetic energy and the impact produces an indentation (plastic deformation) on the surface which
takes some of this energy from the impact body. The impact body will lose more energy and it
rebound velocity will be less when a larger indentation is produced on softer material. The velocities
of the impact body before and after impact are measured and the loss of velocity is related to Brinell,
Rockwell, or other common hardness value.
Durometer Hardness Test
A Durometer is an instrument that is commonly used for measuring the indentation hardness
of rubbers/elastomers and soft plastics such as polyolefin, fluoropolymer, and vinyl. A Durometer
simply uses a calibrated spring to apply a specific pressure to an indenter foot. The indenter foot can
be either cone or sphere shaped. An indicating device measures the depth of indentation. Durometers
are available in a variety of models and the most popular testers are the Model A used for measuring
softer materials and the Model D for harder materials.
Barcol Hardness Test
The Barcol hardness test obtains a hardness value by measuring the penetration of a sharp
steel point under a spring load. The specimen is placed under the indenter of the Barcol hardness
tester and a uniform pressure is applied until the dial indication reaches a maximum. The Barcol
hardness test method is used to determine the hardness of both reinforced and non-reinforced rigid
plastics and to determine the degree of cure of resins and plastics.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 79of89
Creep and Stress Rupture Properties
Creep Properties
Creep is a time-dependent
deformation of a material while under an
applied load that is below its yield
strength. It is most often occurs at elevated
temperature, but some materials creep at
room temperature. Creep terminates in
rupture if steps are not taken to bring to a
halt.
Creep data for general design use
are usually obtained under conditions of
constant uniaxial loading and constant
temperature. Results of tests are usually plotted as strain versus time up to rupture. As
indicated in the image, creep often takes place in three stages. In the initial stage, strain
occurs at a relatively rapid rate but the rate gradually decreases until it becomes
approximately constant during the second stage. This constant creep rate is called the
minimum creep rate or steady-state creep rate since it is the slowest creep rate during the test.
In the third stage, the strain rate increases until failure occurs.
Creep in service is usually affected by changing conditions of loading and
temperature and the number of possible stress-temperature-time combinations is infinite.
While most materials are subject to creep, the creep mechanisms is often different between
metals, plastics, rubber, concrete.
Stress Rupture Properties
Stress rupture testing is similar to creep testing except that the stresses are higher than
those used in a creep testing. Stress rupture tests are used to determine the time necessary to
produce failure so stress rupture testing is always done until failure. Data is plotted log-log
as in the chart above. A straight line or best fit curve is usually obtained at each temperature
of interest. This information can then be used to extrapolate time to failure for longer times.
A typical set of stress rupture curves is shown below.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 80of89
Toughness
The ability of a metal to deform plastically and to absorb energy in the process before fracture
is termed toughness. The emphasis of this definition should be placed on the ability to absorb energy
before fracture. Recall that ductility is a measure of how much something deforms plastically before
fracture, but just because a material is ductile does not make it tough. The key to toughness is a good
combination of strength and ductility. A material with high strength and high ductility will have more
toughness than a material with low strength and high ductility. Therefore, one way to measure
toughness is by calculating the area under the stress strain curve from a tensile test. This value is
simply called material toughness and it has units of energy per volume. Material toughness equates
to a slow absorption of energy by the material.
There are several variables that have a profound influence on the toughness of a material. These
variables are:
Strain rate (rate of loading)
Temperature
Notch effect
A metal may possess satisfactory toughness under static loads but may fail under dynamic loads or
impact. As a rule ductility and, therefore, toughness decrease as the rate of loading increases.
Temperature is the second variable to have a major influence on its toughness. As temperature is
lowered, the ductility and toughness also decrease. The third variable is termed notch effect, has to
due with the distribution of stress. A material might display good toughness when the applied stress is
uniaxial; but when a multiaxial stress state is produced due to the presence of a notch, the material
might not withstand the simultaneous elastic and plastic deformation in the various directions.
There are several standard types of toughness test that generate data for specific loading conditions
and/or component design approaches. Three of the toughness properties that will be discussed in
more detail are 1) impact toughness, 2) notch toughness and 3) fracture toughness.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 81of89
Impact Toughness
The impact toughness (AKA Impact strength) of a material can be determined with a
Charpy or Izod test. These tests are named after their inventors and were developed in the
early 1900s before fracture mechanics theory was available. Impact properties are not
directly used in fracture mechanics calculations, but the economical impact tests continue to
be used as a quality control method to assess notch sensitivity and for comparing the relative
toughness of engineering materials.
The two tests use different specimens
and methods of holding the specimens, but
both tests make use of a pendulum-testing
machine. For both tests, the specimen is
broken by a single overload event due to the
impact of the pendulum. A stop pointer is used
to record how far the pendulum swings back
up after fracturing the specimen. The impact
toughness of a metal is determined by
measuring the energy absorbed in the fracture
of the specimen. This is simply obtained by
noting the height at which the pendulum is
released and the height to which the pendulum
swings after it has struck the specimen . The
height of the pendulum times the weight of the
pendulum produces the potential energy and
the difference in potential energy of the pendulum at the start and the end of the test is equal
to the absorbed energy.
Since toughness is greatly affected by
temperature, a Charpy or Izod test is often repeated
numerous times with each specimen tested at a
different temperature. This produces a graph of impact
toughness for the material as a function of
temperature. An impact toughness versus temperature
graph for a steel is shown in the image. It can be seen
that at low temperatures the material is more brittle
and impact toughness is low. At high temperatures the
material is more ductile and impact toughness is
higher. The transition temperature is the boundary
between brittle and ductile behavior and this
temperature is often an extremely important
consideration in the selection of a material.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 82of89
Notch-Toughness
Notch toughness is the ability that a material possesses to absorb energy in the
presence of a flaw. As mentioned previously, in the presence of a flaw, such as a notch or
crack, a material will likely exhibit a lower level of toughness. When a flaw is present in a
material, loading induces a triaxial tension stress state adjacent to the flaw. The material
develops plastic strains as the yield stress is exceeded in the region near the crack tip.
However, the amount of plastic deformation is restricted by the surrounding material, which
remains elastic. When a material is prevented from deforming plastically, it fails in a brittle
manner.
Notch-toughness is measured with a variety of specimens such as the Charpy V-notch
impact specimen or the dynamic tear test specimen. As with regular impact testing the tests
are often repeated numerous times with specimens tested at a different temperature. With
these specimens and by varying the loading speed and the temperature, it is possible to
generate curves such as those shown in the graph. Typically only static and impact testing is
conducted but it should be recognized that many components in service see intermediate
loading rates in the range of the dashed red line.
Fracture Toughness
Fracture toughness is an indication of the amount of stress required to propagate a
preexisting flaw. It is a very important material property since the occurrence of flaws is not
completely avoidable in the processing, fabrication, or service of a material/component.
Flaws may appear as cracks, voids, metallurgical inclusions, weld defects, design
discontinuities, or some combination thereof. Since engineers can never be totally sure that a
material is flaw free, it is common practice to assume that a flaw of some chosen size will be
present in some number of components and use the linear elastic fracture mechanics (LEFM)
approach to design critical components. This approach uses the flaw size and features,
component geometry, loading conditions and the material property called fracture toughness
to evaluate the ability of a component containing a flaw to resist fracture.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 83of89
A parameter called the stress-intensity factor (K) is used to
determine the fracture toughness of most materials. A Roman
numeral subscript indicates the mode of fracture and the three modes
of fracture are illustrated in the image to the right. Mode I fracture is
the condition in which the crack plane is normal to the direction of
largest tensile loading. This is the most commonly encountered mode
and, therefore, for the remainder of the material we will consider K
I
The stress intensity factor is a function of loading, crack size,
and structural geometry. The stress intensity factor may be
represented by the following equation:
Where: K
I
is the fracture toughness in
s is the applied stress in MPa or psi
a is the crack length in meters or inches
B
is a crack length and component geometry factor that is different for
each specimen and is dimensionless.
Role of Material Thickness
Specimens having standard
proportions but different absolute
size produce different values for
K
I
. This results because the stress
states adjacent to the flaw changes
with the specimen thickness (B)
until the thickness exceeds some
critical dimension. Once the
thickness exceeds the critical
dimension, the value of K
I
becomes relatively constant and
this value, K
IC
, is a true material
property which is called the plane-
strain fracture toughness. The
relationship between stress
intensity, K
I
, and fracture
toughness, K
IC
, is similar to the relationship between stress and tensile stress. The stress
intensity, K
I
, represents the level of stress at the tip of the crack and the fracture toughness,
K
IC
, is the highest value of stress intensity that a material under very specific (plane-strain)
conditions that a material can withstand without fracture. As the stress intensity factor
reaches the K
IC
value, unstable fracture occurs. As with a materials other mechanical
properties, K
IC
is commonly reported in reference books and other sources.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 84of89
Plane-Strain and Plane-Stress
When a material with a crack is loaded in tension, the
materials develop plastic strains as the yield stress is exceeded in the
region near the crack tip. Material within the crack tip stress field,
situated close to a free surface, can deform laterally (in the z-direction
of the image) because there can be no stresses normal to the free
surface. The state of stress tends to biaxial and the material fractures
in a characteristic ductile manner, with a 45
o
shear lip being formed at
each free surface. This condition is called plane-stress" and it occurs
in relatively thin bodies where the stress through the thickness cannot
vary appreciably due to the thin section.
However, material away from the free surfaces of a relatively
thick component is not free to deform laterally as it is constrained by
the surrounding material. The stress state under these conditions tends to triaxial and there is zero
strain perpendicular to both the stress axis and the direction of crack propagation when a material is
loaded in tension. This condition is called plane-strain and is found in thick plates. Under plane-
strain conditions, materials behave essentially elastic until the fracture stress is reached and then
rapid fracture occurs. Since little or no plastic deformation is noted, this mode fracture is termed brittle
fracture.
Plane-Strain Fracture Toughness Testing
When performing a fracture toughness test, the most common test specimen configurations
are the single edge notch bend (SENB or three-point bend), and the compact tension (CT)
specimens. From the above discussion, it is clear that an accurate determination of the plane-strain
fracture toughness requires a specimen whose thickness exceeds some critical thickness (B). Testing
has shown that plane-strain conditions generally prevail when:
Plane Strain - a condition of a
body in which the displacements
of all points in the body are
parallel to a given plane, and the
values of theses displacements
do not depend on the distance
perpendicular to the plane
Plane Stress a condition of a
body in which the state of stress
is such that two of the principal
stresses are always parallel to a
given plane and are constant in
the normal direction.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 85of89
Where: B is the minimum thickness that produces a condition where
plastic strain energy at the crack tip in minimal
K
IC
is the fracture toughness of the material
s
y
is the yield stress of material
When a material of unknown fracture toughness is tested, a specimen of full material section
thickness is tested or the specimen is sized based on a prediction of the fracture toughness. If the
fracture toughness value resulting from the test does not satisfy the requirement of the above
equation, the test must be repeated using a thicker specimen. In addition to this thickness calculation,
test specifications have several other requirements that must be met (such as the size of the shear
lips) before a test can be said to have resulted in a K
IC
value.
When a test fails to meet the thickness and other test requirement that are in place to insure
plane-strain condition, the fracture toughness values produced is given the designation K
C
.
Sometimes it is not possible to produce a specimen that meets the thickness requirement. For
example when a relatively thin plate product with high toughness is being tested, it might not be
possible to produce a thicker specimen with plain-strain conditions at the crack tip.
Plane-Stress and Transitional-Stress States
For cases where the plastic energy at the crack tip is not negligible, other fracture mechanics
parameters, such as the J integral or R-curve, can be used to characterize a material. The toughness
data produced by these other tests will be dependant on the thickness of the product tested and will
not be a true material property. However, plane-strain conditions do not exist in all structural
configurations and using K
IC
values in the design of relatively thin areas may result in excess
conservatism and a weight or cost penalty. In cases where the actual stress state is plane-stress or,
more generally, some intermediate- or transitional-stress state, it is more appropriate to use J integral
or R-curve data, which account for slow, stable fracture (ductile tearing) rather than rapid (brittle)
fracture.
Uses of Plane-Strain Fracture Toughness
K
IC
values are used to determine the critical crack length when a given stress is applied to a
component.
Where: s
c
is the critical applied stress that will cause failure
K
IC
is the plane-strain fracture toughness
Y is a constant related to the sample's geometry
a
is the crack length for edge cracks
or one half crack length for internal crack
K
IC
values are used also used to calculate the critical stress value when a crack of a given length is
found in a component.
Where: a
is the crack length for edge cracks
or one half crack length for internal crack
s is the stress applied to the material
K
IC
is the plane-strain fracture toughness
Y is a constant related to the sample's geometry
Introduction to Materials and Processes
FromNDTResources August18,2007nks 86of89
Orientation
The fracture toughness of a material commonly varies with grain direction. Therefore,
it is customary to specify specimen and crack orientations by an ordered pair of grain
direction symbols. The first letter designates the grain direction normal to the crack plane.
The second letter designates the grain direction parallel to the fracture plane. For flat sections
of various products, e.g., plate, extrusions, forgings, etc., in which the three grain directions
are designated (L) longitudinal, (T) transverse, and (S) short transverse, the six principal
fracture path directions are: L-T, L-S, T-L, T-S, S-L and S-T.
Fatigue Properties
Fatigue cracking is one of the primary damage mechanisms of structural components.
Fatigue cracking results from cyclic stresses that are below the ultimate tensile stress, or even
the yield stress of the material. The name fatigue is based on the concept that a material
becomes tired and fails at a stress level below the nominal strength of the material. The
facts that the original bulk design strengths are not exceeded and the only warning sign of an
impending fracture is an often hard to see crack, makes fatigue damage especially dangerous.
The fatigue life of a component can be expressed as the number of loading cycles
required to initiate a fatigue crack and to propagate the crack to critical size. Therefore, it can
be said that fatigue failure occurs in three stages crack initiation; slow, stable crack growth;
and rapid fracture.
As discussed previously, dislocations play a major role in the
fatigue crack initiation phase. In the first stage, dislocations
accumulate near surface stress concentrations and form
structures called persistent slip bands (PSB) after a large
number of loading cycles. PSBs are areas that rise above
(extrusion) or fall below (intrusion) the surface of the
component due to movement of material along slip planes. This
leaves tiny steps in the surface that serve as stress risers where
tiny cracks can initiate. These tiny crack (called microcracks)
nucleate along planes of high shear stress which is often 45
o
to
the loading direction.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 87of89
In the second stage of fatigue, some of the tiny microcracks join together and begin to propagate
through the material in a direction that is perpendicular to the maximum tensile stress. Eventually, the
growth of one or a few crack of the larger cracks will dominate over the rest of the cracks. With
continued cyclic loading, the growth of the dominate crack or cracks will continue until the remaining
uncracked section of the component can no longer support the load. At this point, the fracture
toughness is exceeded and the remaining cross-section of the material experiences rapid fracture.
This rapid overload fracture is the third stage of fatigue failure.
Factors Affecting Fatigue Life
In order for fatigue cracks to initiate, three basic factors are necessary. First, the loading
pattern must contain minimum and maximum peak values with large enough variation or fluctuation.
The peak values may be in tension or compression and may change over time but the reverse loading
cycle must be sufficiently great for fatigue crack initiation. Secondly, the peak stress levels must be of
sufficiently high value. If the peak stresses are too low, no crack initiation will occur. Thirdly, the
material must experience a sufficiently large number of cycles of the applied stress. The number of
cycles required to initiate and grow a crack is largely dependant on the first to factors.
In addition to these three basic factors, there are a host of other variables, such as stress
concentration, corrosion, temperature, overload, metallurgical structure, and residual stresses which
can affect the propensity for fatigue. Since fatigue cracks generally initiate at a surface, the surface
condition of the component being loaded will have an effect on its fatigue life. Surface roughness is
important because it is directly related to the level and number of stress concentrations on the
surface. The higher the stress concentration the more likely a crack is to nucleate. Smooth surfaces
increase the time to nucleation. Notches, scratches, and other stress risers decrease fatigue life.
Surface residual stress will also have a significant effect on fatigue life. Compressive residual stresses
from machining, cold working, heat treating will oppose a tensile load and thus lower the amplitude of
cyclic loading
Introduction to Materials and Processes
FromNDTResources August18,2007nks 88of89
The figure shows several types of loading that could initiate a fatigue crack. The
upper left figure shows sinusoidal loading going from a tensile stress to a compressive stress.
For this type of stress cycle the maximum and minimum stresses are equal. Tensile stress is
considered positive, and compressive stress is negative. The figure in the upper right shows
sinusoidal loading with the minimum and maximum stresses both in the tensile realm. Cyclic
compression loading can also cause fatigue. The lower figure shows variable-amplitude
loading, which might be experienced by a bridge or airplane wing or any other component
that experiences changing loading patterns. In variable-amplitude loading, only those cycles
exceeding some peak threshold will contribute to fatigue cracking.
S-N Fatigue Properties
There are two general types of fatigue
tests conducted. One test focuses on the
nominal stress required to cause a fatigue
failure in some number of cycles. This test
results in data presented as a plot of stress (S)
against the number of cycles to failure (N),
which is known as an S-N curve. A log scale
is almost always used for N.
The data is obtained by cycling
smooth or notched specimens until failure.
The usual procedure is to test the first
specimen at a high peak stress where failure is expected in a fairly short number of cycles.
The test stress is decreased for each succeeding specimen until one or two specimens do not
fail in the specified numbers of cycles, which is usually at least 10
7
cycles. The highest stress
at which a runout (non-failure) occurs is taken as the fatigue threshold. Not all materials have
a fatigue threshold (most nonferrous metallic alloys do not) and for these materials the test is
usually terminated after about 10
8
or 5x10
8
cycles.
Since the amplitude of the cyclic loading has a major effect on the fatigue
performance, the S-N relationship is determined for one specific loading amplitude. The
amplitude is express as the R ratio value, which is the minimum peak stress divided by the
maximum peak stress. (R=
min
/
max
). It is most common to test at an R ratio of 0.1 but
families of curves, with each curve at a different R ratio, are often developed.
A variation to the cyclic stress controlled fatigue test is the cyclic strain controlled
test. In this test, the strain amplitude is held constant during cycling. Strain controlled cyclic
loading is more representative of the loading found in thermal cycling, where a component
expands and contracts in response to fluctuations in the operating temperature.
It should be noted that there are several short comings of S-N fatigue data. First, the
conditions of the test specimens do not always represent actual service conditions. For
example, components with surface conditions, such as pitting from corrosion, which differs
from the condition of the test specimens will have significantly different fatigue performance.
Furthermore, there is often a considerable amount of scatter in fatigue data even when
carefully machined standard specimens out of the same lot of material are used. Since there is
considerable scatter in the data, a reduction factor is often applied to the S-N curves to
provide conservative values for the design of components.
Introduction to Materials and Processes
FromNDTResources August18,2007nks 89of89
Fatigue Crack Growth Rate Properties
For some components the crack propagation life is neglected in design because stress levels
are high, and/or the critical flaw size small. For other components the crack growth life might be a
substantial portion of the total life of the assembly. Moreover, preexisting flaws or sharp design
features may significantly reduce or nearly eliminate the crack initiation portion of the fatigue life of a
component. The useful life of these components may be governed by the rate of subcritical crack
propagation.
Aircraft fuselage structure is a good example of structure that is based largely on a slow crack
growth rate design. Many years ago, the USAF reviewed a great number of malfunction reports from
a variety of aircraft. The reports showed that the preponderance of structural failures occurred from 1)
built-in preload stresses, 2) material flaws and 3) flaw caused by in-service usage. These facts led to
a design approach that required the damage tolerance analysis to assume a material flaw exists in
the worst orientation and at the most undesirable location. The analysis helps to ensure that
structures are designed that will support slow stable crack growth until the crack reaches a length
where it can reliable be detected using NDT methods.
The rate of fatigue crack propagation is determined by subjecting fatigue-cracked specimens,
like the compact specimen used in fracture toughness testing, to constant-amplitude cyclic loading.
The incremental increase in crack length is recorded along with the corresponding number of elapsed
load cycles acquire stress intensity (K), crack length (a), and cycle count (N) data during the test. The
data is presented in an a versus N curve as shown in the image to the right. Various a versus N
curves can be generated by varying the magnitude of the cyclic loading and/or the size of the initial
crack.
The data can be reduced to a single curve by presenting the data in terms of crack growth rate per
cycle of loading (Da/ DN or da/dN) versus the fluctuation of the stress-intensity factor at the tip of the
crack (DK
I
). DK
I
is representative of the mechanical driving force, and it incorporates the effect of
changing crack length and the magnitude of the cyclic loading. (See the page on fracture toughness
for more information on the stress-intensity factor.) The most common form of presenting fatigue
crack growth data is a log-log plot of da/dN versus DK
I
.
The fatigue crack propagation behavior of many
materials can be divided into three regions as shown in
the image. Region I is the fatigue threshold region where
the Dk is too low to propagate a crack. Region II
encompasses data where the rate of crack growth
changes roughly linearly with a change in stress intensity
fluctuation. In region III, small increases in the stress
intensity amplitude, produce relatively large increases in
crack growth rate since the material is nearing the point
of unstable fracture.
You might also like
- Heat Treatment by Quenching - DiagramsDocument20 pagesHeat Treatment by Quenching - Diagramssunilmathew4477No ratings yet
- Deformation & StrengthDocument35 pagesDeformation & StrengthcolorofstoneNo ratings yet
- Engineering MetallurgyDocument46 pagesEngineering Metallurgyहेमंत कुमार मीणाNo ratings yet
- Engineering Materials Classification and Properties GuideDocument53 pagesEngineering Materials Classification and Properties GuideGeno Martinez100% (1)
- Metal CastingDocument28 pagesMetal CastingAngel ChanteyNo ratings yet
- Alloys and their PropertiesDocument12 pagesAlloys and their PropertiesNikhil ShelarNo ratings yet
- Engineering MaterialsDocument110 pagesEngineering MaterialsAthith D100% (1)
- Plastic Deformation of Single Crystals ExplainedDocument42 pagesPlastic Deformation of Single Crystals ExplainedNaresh DeshpandeNo ratings yet
- Module 1 FundamentalsDocument90 pagesModule 1 Fundamentalshari0118No ratings yet
- Structural Analysis of Multi-Plate ClutchDocument5 pagesStructural Analysis of Multi-Plate ClutchseventhsensegroupNo ratings yet
- Chapter2 Bonding and PropertiesDocument71 pagesChapter2 Bonding and PropertiesShahd AlhamaydaNo ratings yet
- ME 303 Study Set PDFDocument44 pagesME 303 Study Set PDFFajar RumantoNo ratings yet
- Beginners Guide To CorrosionDocument10 pagesBeginners Guide To Corrosionshamu081No ratings yet
- Non-Traditional Machining: Electro Chemical Machining (ECM)Document14 pagesNon-Traditional Machining: Electro Chemical Machining (ECM)NimoNo ratings yet
- Basic of Mechanical Engineering PDFDocument143 pagesBasic of Mechanical Engineering PDFpsaayoNo ratings yet
- 5 Whys Training Oct 12 2011Document23 pages5 Whys Training Oct 12 2011jayeshjpillaiNo ratings yet
- c1 Mechanical PropertiesDocument46 pagesc1 Mechanical PropertiesHusnal TaufiqNo ratings yet
- 1.0 TitleDocument10 pages1.0 TitlezackziffiNo ratings yet
- Engineering Materials: Learning ObjectivesDocument11 pagesEngineering Materials: Learning Objectives38Zeeshan ZameerNo ratings yet
- Principles of Heat Treating of SteelsDocument30 pagesPrinciples of Heat Treating of Steelssatish_trivediNo ratings yet
- Ferrous Metals and AlloysDocument44 pagesFerrous Metals and AlloysLeonardDacaymatNo ratings yet
- Introduction to Materials Science for NDTDocument96 pagesIntroduction to Materials Science for NDTMircea Dubenco100% (1)
- Non-Ferrous Metals and AlloysDocument58 pagesNon-Ferrous Metals and AlloysPradeep Kumar BagadiNo ratings yet
- Material Science: Structure and PropertiesDocument127 pagesMaterial Science: Structure and PropertiesViraj Babar100% (1)
- Material Science & MetallurgyDocument2 pagesMaterial Science & Metallurgysameer_m_daniNo ratings yet
- Elements of Machine DesignDocument18 pagesElements of Machine DesignDexter Frank Virtucio CalderonNo ratings yet
- Pachakkari KrishiDocument58 pagesPachakkari Krishijayeshjpillai100% (2)
- Materials Science CHAPTER 1Document5 pagesMaterials Science CHAPTER 1KTINE08No ratings yet
- MetalDocument57 pagesMetalPrashant PuriNo ratings yet
- Materials Science Lec 04 Phase & Iron-Carbon DiagramDocument53 pagesMaterials Science Lec 04 Phase & Iron-Carbon DiagramKrishna SarkarNo ratings yet
- Metal FormingDocument80 pagesMetal Formingashok PradhanNo ratings yet
- Mechanical Properties of MetalsDocument258 pagesMechanical Properties of MetalsIsza Marie N. SocorinNo ratings yet
- Nonferrous Alloys Al-6069Document16 pagesNonferrous Alloys Al-6069FMMServicesNo ratings yet
- Measuring Your Process Capability: PreambleDocument13 pagesMeasuring Your Process Capability: PreamblejayeshjpillaiNo ratings yet
- Standardized Work and Problem SolvingDocument34 pagesStandardized Work and Problem Solvingjayeshjpillai100% (3)
- Thermomechanical Processing and Constitutive Strength of Hot Rolled Mild SteelDocument104 pagesThermomechanical Processing and Constitutive Strength of Hot Rolled Mild SteelvishwanathanskNo ratings yet
- Mechanical Properties of Metals ChapterDocument25 pagesMechanical Properties of Metals ChapterJesse McClure100% (9)
- Calculating Critical Temperatures in Steels Using Empirical FormulasDocument6 pagesCalculating Critical Temperatures in Steels Using Empirical FormulasAndress SsalomonnNo ratings yet
- Bonga University: Engineering Material (Meng2091)Document19 pagesBonga University: Engineering Material (Meng2091)Mul'isaa JireenyaaNo ratings yet
- Classifying Materials by Structure, Properties & ApplicationsDocument109 pagesClassifying Materials by Structure, Properties & ApplicationsM.41Mohd AnasNo ratings yet
- Raw Materials and Classifications of Metallic and Non-Metallic MaterialsDocument91 pagesRaw Materials and Classifications of Metallic and Non-Metallic Materialshemarubini96No ratings yet
- 11 Introduction To Engineering MaterialsDocument20 pages11 Introduction To Engineering MaterialsomkardashetwarNo ratings yet
- 12 - Fatigue of MetalsDocument55 pages12 - Fatigue of Metalsvoldemorts100% (1)
- Lecture 3 Plastic DeformationDocument31 pagesLecture 3 Plastic DeformationAmmar SafwtNo ratings yet
- Engineering MaterialsDocument53 pagesEngineering MaterialsRAGINI PASUPULETINo ratings yet
- All Functional Materials For Material EngineeringDocument27 pagesAll Functional Materials For Material Engineeringeddula ganeshNo ratings yet
- Lecture1460085501 2Document526 pagesLecture1460085501 2Smruti Ranjan Pattanayak100% (1)
- Chapter 9d FractureDocument70 pagesChapter 9d FracturenaveenaNo ratings yet
- The effect of annealing on aluminum clad steel sheet propertiesDocument6 pagesThe effect of annealing on aluminum clad steel sheet propertiesRina OktapianiNo ratings yet
- Engineering MaterialsDocument25 pagesEngineering MaterialsNichan CanilloNo ratings yet
- Magnetic PropertiesDocument21 pagesMagnetic PropertiesHosamHasanNo ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- Chapter2 - AJMDocument13 pagesChapter2 - AJMravish kumarNo ratings yet
- Material Science NotesDocument11 pagesMaterial Science NotesRyan Ryan RyanNo ratings yet
- Shape Memory AlloyDocument22 pagesShape Memory AlloyCSETUBENo ratings yet
- Aluminium Titanate: Chemical FormulaDocument6 pagesAluminium Titanate: Chemical FormulaaadhanNo ratings yet
- Abrasion-Resistant Cast Irons1 PDFDocument1 pageAbrasion-Resistant Cast Irons1 PDFjlplazaolaNo ratings yet
- 04 - Plastic Deformation of Single CrystalDocument42 pages04 - Plastic Deformation of Single Crystalshanthakumar100% (1)
- Surface Hardening of SteelDocument50 pagesSurface Hardening of SteelTeptep GonzalesNo ratings yet
- Plastic Deformation of Metals and Related PropertiesDocument23 pagesPlastic Deformation of Metals and Related PropertiesVinayak Bhustalimath50% (2)
- Production Gas Carburising: The Pergamon Materials Engineering Practice SeriesFrom EverandProduction Gas Carburising: The Pergamon Materials Engineering Practice SeriesNo ratings yet
- The Concept of Construction Materiales: Ingles TecnicoDocument12 pagesThe Concept of Construction Materiales: Ingles TecnicoMir DJairoNo ratings yet
- Handout On Civil EngineeringDocument10 pagesHandout On Civil EngineeringKevinEdwuardBerriosFloresNo ratings yet
- Material Science PrinciplesDocument5 pagesMaterial Science PrinciplesIsaiah LuleNo ratings yet
- Unit-II EME AerospaceDocument51 pagesUnit-II EME AerospacePranil Vijay SawalakheNo ratings yet
- Railway TimeDocument1 pageRailway TimejayeshjpillaiNo ratings yet
- Kerala Govt FundingDocument56 pagesKerala Govt FundingjayeshjpillaiNo ratings yet
- VAT TaxDocument8 pagesVAT Taxsankar_rao333No ratings yet
- KVATDocument147 pagesKVATjayeshjpillaiNo ratings yet
- PoP Adhoc OrganicDocument209 pagesPoP Adhoc OrganicummuzzzNo ratings yet
- MSC Application Booklet For NUSDocument52 pagesMSC Application Booklet For NUSjayeshjpillaiNo ratings yet
- PF Proposed Hand-Over MeetingDocument22 pagesPF Proposed Hand-Over MeetingjayeshjpillaiNo ratings yet
- Low Cost Green Houses For Vegetable ProductionDocument19 pagesLow Cost Green Houses For Vegetable Productionqfarms100% (2)
- Malayalam Translation of World Famous Totto ChanDocument108 pagesMalayalam Translation of World Famous Totto Chanjayeshjpillai50% (6)
- How To Measure Flatness With Optical FlatsDocument3 pagesHow To Measure Flatness With Optical FlatsmanchiprasadNo ratings yet
- Adujeevitham by BenyaminDocument103 pagesAdujeevitham by BenyaminjayeshjpillaiNo ratings yet
- TEM Characterization of Dislocation ImageDocument11 pagesTEM Characterization of Dislocation ImagerahmatsaptonoNo ratings yet
- Defect SolidDocument21 pagesDefect SolidSafialMojnabin100% (1)
- BTech Metallurgical CurriculumDocument32 pagesBTech Metallurgical CurriculumiyomasaNo ratings yet
- Ch04 Ppts Callister7e Imperfection Is SolidsDocument32 pagesCh04 Ppts Callister7e Imperfection Is SolidsOdarie HunterNo ratings yet
- MSM R19 - Unit-1Document62 pagesMSM R19 - Unit-1Madheswaran DharmapuriNo ratings yet
- GX12Document10 pagesGX12Adriano OliveiraNo ratings yet
- International Journal of Plasticity: Angela Y. Ku, Akhtar S. Khan, Thomas GN Aupel-HeroldDocument32 pagesInternational Journal of Plasticity: Angela Y. Ku, Akhtar S. Khan, Thomas GN Aupel-HeroldpurnashisNo ratings yet
- A Course On Damage Mechanics-Springer-Verlag Berlin Heidelberg (1996) Professor Jean Lemaitre (Auth.)Document243 pagesA Course On Damage Mechanics-Springer-Verlag Berlin Heidelberg (1996) Professor Jean Lemaitre (Auth.)Nguyễn Văn ThườngNo ratings yet
- High-Temperature Alloys For Industrial EngineeringDocument67 pagesHigh-Temperature Alloys For Industrial EngineeringScott LongmireNo ratings yet
- Test Materials Engineering and ProcessingDocument142 pagesTest Materials Engineering and Processinglarla SinghalNo ratings yet
- B.Tech Engineering Physics SyllabusDocument17 pagesB.Tech Engineering Physics SyllabuskrishcvrNo ratings yet
- Crystalline ImperfectionsDocument17 pagesCrystalline Imperfectionsps4haris.ch3534No ratings yet
- Chapter 3 Nucleation and GrowthDocument30 pagesChapter 3 Nucleation and Growthserdelix100% (1)
- Top-Down Synthesis of Nanostructured Materials: Mechanical and Thermal Processing MethodsDocument9 pagesTop-Down Synthesis of Nanostructured Materials: Mechanical and Thermal Processing MethodsGloria HendersonNo ratings yet
- Chapt 04Document22 pagesChapt 04Jesse McClureNo ratings yet
- Metallurgy Fundamentals & Structure Property RelationDocument280 pagesMetallurgy Fundamentals & Structure Property RelationAbhishek ChavanNo ratings yet
- Correlation Between Process Parameters Grain Size and Hardness of FrictionDocument12 pagesCorrelation Between Process Parameters Grain Size and Hardness of FrictionAhmadNo ratings yet
- Structural and Mechanical Behaviour of Severe Plastically Deformed High Purity Aluminium Sheets Processed by Constrained Groove Pressing TechniqueDocument7 pagesStructural and Mechanical Behaviour of Severe Plastically Deformed High Purity Aluminium Sheets Processed by Constrained Groove Pressing TechniqueMoin ANo ratings yet
- Computational Materials Science: Yunan Prawoto, Roslinda Idris, Nazri Kamsah, Nasir TaminDocument9 pagesComputational Materials Science: Yunan Prawoto, Roslinda Idris, Nazri Kamsah, Nasir TaminpakdeYPNo ratings yet
- Material Science (Unit 1)Document18 pagesMaterial Science (Unit 1)Gaurav AgarwalNo ratings yet
- Eshelby 1951Document6 pagesEshelby 1951ayan849No ratings yet
- Caracterização de Materiais Por UltrassomDocument15 pagesCaracterização de Materiais Por UltrassomCarlos MartinsNo ratings yet
- Chapter2. DiffusionDocument61 pagesChapter2. DiffusionCường Nguyễn ĐứcNo ratings yet
- 5.3.2 Generation of Dislocations: Dislocations in The First Place!Document2 pages5.3.2 Generation of Dislocations: Dislocations in The First Place!Shakira ParveenNo ratings yet
- EGR 2206-Mechanical PropertiesDocument25 pagesEGR 2206-Mechanical PropertiesSolomonNo ratings yet
- MIT Solution Key of Reactions & Kinetics ExaminationDocument6 pagesMIT Solution Key of Reactions & Kinetics ExaminationAstrialdelinaNo ratings yet
- New Design of Process For Cold Forging To Improve Multi-Stage Gas FittingDocument12 pagesNew Design of Process For Cold Forging To Improve Multi-Stage Gas FittingDegaga KebedeNo ratings yet