Professional Documents
Culture Documents
Carlson Embryology Notes
Uploaded by
Hira Tufail MalikOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carlson Embryology Notes
Uploaded by
Hira Tufail MalikCopyright:
Available Formats
Carlson Embryology Notes
Aaron Geller May 18, 2008
Gametogenesis
1. 4 phases of gametogenesis (a) extraembryonic origin of germ cells & migration into gonads germ cells arise in yolk sac around day 24 they are large and high in ALP they move into the hindgut & arrive at primordia of gonads if they get lost they can develop into teratomas (tumors with multiple germ layers) in oral region, mediastinum, sacrococcygeal region
(b) mitotic proliferation of germ cells in females, oogonia go through 1-time proliferation period at 5th month of gestation after this a lot of oogonia degenerate (atresia) number of active oogonia diminishes continually until menopause in males, spermatogonia continue to proliferate in seminiferous tubules for whole lifespan (c) ploidy reduction by meiosis meiosis vs. mitosis i. mitosis: cells start 2n and 4c: 2 copies of each chromosome, with chromosomes replicated 4 total chromosomes at anaphase, each replicated chromosome is split at the centromere, so you get 2 genetically identical 2n, 2c daughter cells 1
ii. meiosis meiosis also starts with 2n, 4c cells the chromosomes line up as homologous pairs of replicated chromosomes, so you get tetrads with crossing over at anaphase I the replicated homologs are pulled apart, so after 1st division you have non-identical n, 2c daughter cells after meiosis II you have n, c daugter cells meiosis in females i. as mentioned, before entering meiosis, oogonia cells proliferate by mitosis ii. oogonia descend from germ cells, but they are surrounded by at epithelial cells, called follicular cells, which arent iii. before or at birth, all non-atretic oogonia enter meiosis I, becoming 1 oocytes iv. they stop at the diplotene (lacy chromosomes after initial crossing over, but before metaphase) phase of meiosis I many species (esp. lower vertebrates) intensively synthesize RNA & proteins here in preparation for ovulation arrested state maintained by oocyte maturation inhibitor (OMI) the oocytes stay this way indenitely, until some of them are allowed to mature after onset of puberty in males as in females, you have germ cells spermatogonia surrounded by supporting cells sustentacular or Sertoli cells no synchronized transition to meiosis like in females, many spermatogonia stay mitotic for whole life type A spermatogonia replicate several times, nally becoming type B spermatogonia at last pre-meiotic division each type B spermatogonium gives 2 1 spermatocytes meiosis II of a 2 spermatocyte produces 2 spermatids prophase I takes several weeks, meiosis II hours cytokinesis is incomplete so the dierent generations are joined by cytoplasmic bridges (S Fig 2.22) 2
(d) maturation of gametes into eggs & spermatozoa females: i. primordial follicle: a 1 oocyte + its follicular cells ii. at puberty, some primordial follicles transition to 1 (or pre-antral) follicles at follicular cells become cuboidal, becoming granulosa cells basement membrane separates granulosa from outside cells, which are called theca folliculi; stimulated by GDF-9 zona pellucida: layer of glycoproteins secreted by oocyte & granulosa cells as oocyte ECM (important for transport into oocyte) iii. as uid accumulates between granulosa cells, a cavity is formed between oocyte called the antrum, at which point the follicle becomes an 2 (or antral) follicle iv. follicular cells surrounding the oocyte in the follicle are the cumulus oophorus v. during ovulation A. surge in luteinizing hormone (LH) causes one 2 follicle to complete meiosis I B. one of the daughter cells receives most of the cytoplasm, becoming the 2 oocyte; the other becomes the rst polar body C. 2 oocyte enters meiosis II but stops at metaphse 3h before ovulation D. meiosis II is only completed if fertilization occurs (e) males: as in females, stimulated by LH i. bound by Leydig cells which make testosterone ii. testosterone bound by Sertoli cells, stimulates spermatogenesis FSH also important because it triggers upregulation of androgen receptor genes nal maturation: spermiogenesis i. acrosome ii. condensation (picnosis) of nucleus 3
iii. neck, middle piece & tail iv. jettisoning of cytoplasm sperm become motile in the epididymis
Molecular Basis
1. two categories of molecules guide development: (a) transcription factors (b) signalling molecules (cytokines) category includes growth factors bind to membrane receptors & trigger signal transduction cascade Reznick lecture 1: distinguish between paracrine & juxtacrine 2. transcription factors two kinds: (a) helix-loop-helix (b) Zn nger homeodomain proteins (helix-loop-helix family) contain 60 AA stretch which provides helix-loop-helix, called the homeodomain the 180 nucleotides which code for the homeodomain are called the homeobox general family called Hox Hox genes are expressed in sequential order along the chromosome, from 3 to 5, and determine the cranial-caudal traits of the tissue expressing them (68). loss-of-function (LOF) of a givne Hox gene neighboring anterior segment will be stretched into segment with LOF mutation; vice-versa for GOF subfamilies: Pax, Sox (complexes with other TFs; see g. 410), Lim, T-box 3. paracrine signalling molecules (a) overview 4
(b)
(c)
(d)
(e) (f)
growth factors most important families: i. transforming growth factor (TGF- ) ii. broblast growth factor other GFs: nerve, epidermal, insulin-like hedgehog proteins typical interaction: regulators compete to exert positive or negative eects: chordin induces neurulation by inhibiting action of BMP, which is inhibitory (74) hedgehog pathway i. pre-hedgehog protein produced in ER with non-signalling C end intracellular signal at N end ii. (intracellular) signal cleaved o of N end iii. C end cleaves itself o iv. remaining segment covalently bonds to cholesterol and is secreted from cell v. shh protein (with cholesterol) binds to Ptc (patched) receptor on target cell vi. Ptc stops inhibiting smo (smoothened) membrane protein, which (via intermediate steps) activates a transcription factor membrane receptor proteins can either have intrinsic kinase activity (e.g., FGF and TGF- receptors) or use second-messenger systems which activate kinases downstream signalling cascade: G-protein sequence can activate transcription factors retinol (vitamin A) can interfere with development if missing or present in excess retinol is taken up by special membrane proteins in the cell it isdoubly-oxidized to retinoic acid in the nucleus it complexes with proteins to make a transcription factor RARE (retinoic acid response element) RARE acts as an enhancer extra limbs on tadpole when grown in vitamin A solution
4. juxtacrine (close contact) signalling; 3 modes (a) protein on cell A interacts with receptor on cell B e.g. Notch receptor provides lateral inhibition for cell dierentiation 5
i. one cell decides its own fate and is dominant ii. it signals neighboring cells (via Delta or Jagged proteins) not to dierentiate into the same kind of cell iii. Notch receptor on submissive cells transduces the signal to choose a dierent path: iv. when it binds its ligand, a protease cleaves o the intracellular domain v. the intracellular domain complexes with other proteins which enhance transcription of a transcription factor which supresses expression of the dominant phenotype (b) secreted ligands in ECM interact with receptors on neighboring cells: bronectin, laminin interact with integrins, aecting actin cytoskeleton (c) gap junctions in epithelia 5. cancer can arise in 2 ways: (a) GOF in a proto-oncogene (b) (double) LOF in a tumor-suppressor gene (ex: patched inhibition of smoothened) 6. histogenesis depends on CAMs (cell adhesion molecules; R 2) (a) 1 N L (b) 2 Ng Cell CAM 105 (hepatocytes) I (epithelia)
First Week of Development
1. ovulation (S 31) hypothalamus cyclically secretes gonadotrophin-releasing hormone (GnRH) this stimulates the pituitary to secrete LH & FSH 6
(a) FSH rescues 15-20 of the current pool of 1 follicles (preventing atresia) stimulates maturation of granulosa cells granulosa & theca cells produce estrogens which cause i. endometrium to enter proliferative phase 2 layers: basal, spongy ii. secrete thinner cervical mucus (help sperm move) iii. stimulate pituitary to secrete more LH (positive feedback) (b) LH spike results (36h before ovulation), causing secretion of maturation promoting factor (MPF) to promote meiosis oocytes to complete meiosis I follicular stromal cells to secrete progesterone (and not estrogen) follicular rupture i. follicle bulges, with avascular area, the stigma ii. LH increases collagenase activity, breaking down ovarian wall iii. prostaglandins trigger muscular contractions, which eject oocyte together with some cumulus oophorus cells (which become the corona radiata) 2. corpus luteum (S 32) remainder of follicle stuck in ovary becomes vascularized under inuence of LH, they become yellow & develop into corpus luteum corpus luteum secretes progesterone progesterone + estrogens trigger uterine progestational (secretory) phase 3 layers: basal, spongy (intermediate), compact (supercial) 3. motion of mbriae and contractions of uterine tubes suck the oocyte into the tube; takes 2-3 days to reach lumen (S 32-3) 4. if no fertilization:
corpus luteum degenerates & turns into scar tissue: corpus albicans progesterone levels fall o, triggering menstruation 5. if yes fertilization: embryos syncitiotrophoblast secretes human chorionic gonadotrophin (hCG), which keeps corpus luteum from degenerating corpus lutem becomes corpus luteum of pregnancy gets bigger keeps making progesterone until 4th month after that, placenta takes over progesterone secretion instead of menstrual phase, uterus is in gravid phase (S 42) 6. fertilization without ovulation, sperm lose motility in the isthmus of the fallopian tube with ovulation, they are able to use chemoattraction to swim to ampulla, where the action happens prerequisites for fertilization (a) capacitation: removal of glycoproteins & plasma proteins from membrane over acrosome allows penetration of the corona radiata (b) acrosome reaction: release of enzymes for penetration of the zona pellucida (acrosin, trypsin-like substances) triggered by zona phases (a) penetration of corona radiata (b) penetration of zona pellucida: as soon as rst sperm penetrates, the zona reaction causes a change in permeability which prevents further penetrations i. sperm penetration raises intracellular Ca2+ ii. in response, exocytosis of cortical granules occurs (c) fusion of sperm & oocyte cell membranes 8
similar to zona reaction, penetration by sperm causes oocyte membrane to become impenetrable meiosis II is completed, creating a second polar body and the denitive oocyte the resulting chromosomes of the denitive oocyte arrange themselves in a the female pronucleus sperm loses its tail & leaves the male pronucleus both karyotypes lose their nuclear envelopes & replicate chromosomes fertilization is now complete; mitosis takes over from here 7. blastomeres mitotic divisions increase number of cells (called blastomeres) & decreases their volume after 3rd division (8 cells), compaction occurs cell outlines become indistinct; cells are connected via tight junctions, maximizing cell-cell contact inner cells (connected via gap junctions) dierentiate from outer cells next divsion (day 3) produces morula (16 cells) (a) inner cells embryo (b) outer cells trophoblast 8. blastocyst uid penetrates intercellular space as cells proliferate space coalesces into a single cavity: blastocoele zona disappears, allowing implantation 9. implantation (a) initial capture similar mechanism to capture of WBCs from bloodstream onto endothelium trophoblast cells express L-selectin, uterine wall a carbohydrate receptor (b) anchoring & penetration through endometrium 9
further attachment uses integrins, laminin, bronectin matrix metalloproteases (MMPs) for ECM breakdown in stroma
Formation of Germ Layers (Gastrulation)
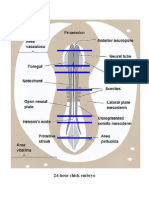
1. early in 2nd week: cells of inner cell mass are arranged in two layers: (a) epiblast (on top=dorsal) (b) hypoblast (underneath=ventral) 2. epiblast develops amniotic cavity, and hypoblast cells spread around cytotrophoblast to make primitive yolk sac (cf. Sadler p. 43) 3. some cells of yolk sac dierentiate extraembryonic mesoderm, and proliferate around inner wall of cytotrophoblast, squeezing between epiblast and cytotrophoblast. 4. primitive streak (visual proof of craniocaudal dierentiation) forms and becomes primitive groove 5. epiblast cells migrate through streak (changing shape and character from epithelial to mesenchymal/broblastic) and become (a) embryonic endoderm (b) embryonic mesoderm (c) extraembryonic mesoderm (d) prechordal plate (induced formation of forebrain) (e) notochord The cells need hyaluronic acid and bronectin to migrate successfully. at cranial end, theres a region without mesoderm (endo meets ectoderm) called buccopharyngeal membrane (aka oropharyngeal) induction of mesoderm (p. 91) experiment: isolate animal pole (epiblast) of blastula on its own, remains ectoderm (makes keratin) when stuck directly on vegetal pole (endoderm), becomes mesoderm (makes actin) 10
on its own + added activin, becomes mesoder (makes actin) 6. notochord development: (a) rst, its a tube (closed at the cranial end, open at the caudal end) extending cranially from primitive pit (anterior end of primitive groove) (b) bottom of caudal end tube and the endoderm it sits on erode, so amniotic cavity communicates with the yolk sac (connection is the neurenteric canal (c) the tube lls in anterior to the canal so it is a solid cylinder of cells sacrococcygeal teratomas are thought to result from retention of part of the primitive streak, which has germ cells for all layers pp. 88-89 notochord laid out by regression of primitive streak ? 7. induction of nervous system experiment: notochord mesoderm required for induction of neverous system; shown in salamanders where removal of notochordanalogue prevents foormation of nervous system, and transplantation of an extra notochord-analogue results in extra nervous sytem (89-90). signalling molecules: noggin, follistatin and chordin trigger induction by blocking BMP-4 g 5-14: regressing primitive streak coincides with development of neural plate 8. box: ciliary currents may be mechanism for establishing left-right axis (95-96) nodal expressed selectively on left side of disc lefty is expressed on left side of primitive streak and may keep lateralization molecules from diusing to right side 9. Cell Adhesion Molecules (CAMs) before gastrulation, both epiblast and hypblast exress N-CAM and L-CAM (=E-cadherin)
11
in the process of migrating though primitive streak, cells stop expressing both CAMs; when they arrive at their destination, CAMs are re-expressed in neural plate, only N-CAM is expressed (also N-cadherin) in non-neural ectoderm, only L-CAM is expressed
Establishment of Basic Body Plan
1. steps in neurulation (a) thickening of neural plate (b) narrowing & lengthening (c) folding up along neural groove (d) apposition and fusion of folds & separation of neural crest cells 2. segmentation (a) anterior region segments into 3 vesicles of brain i. forebrain (prosenchephalon) ii. midbrain (mesencephalon) iii. hindbrian (rhombencephalon) (b) forebrain and hindbrain subdivide into neuromeres and rhombomeres (c) anterior visceral endoderm (near buccopharyngeal membrane) and prechordal plate induce the dierentiation of forebrain/midbrain from hindbrain/spinal cord (establishing the rhombenchephalic isthmus; Otx is transcription factor for forebrain/midbrain, Gbx2 for hindbrain/spinal cord (106). (d) transcription factors trigger individual rhombomeres (107) (e) segmentation of SC is induced by and organized by paraxial mesoderm, not neural tissue (107-8) 3. ectodermal placodes develop concurrently with neurulation and will team up with neural crest cells to become peripheral ganglia and cranial nerves 4. g 6-6 (a) as neural tube is closing, paraxial mesoderm segments into somites 12
(b) at the lateral edge of the mesoderm (where embryonic mesoderm meets extraembryonic mesoderm), coelomic vesicles fuse causing bifurcated of mesoderm into somatic mesoderm and splanchnic mesoderm (c) the bifurcation creates intraembryonic coelom (continuous with extraembryonic coelom) (d) at this point there are paired aortas (e) somites start to form (around day 20; after somitomeres) (f) lateral ectoderm and somatic mesoderm droops down (2 layers together = somatopleure) (g) when neural tube is closed, part of endoderm closes into canal, rises dorsally & is surrounded by splanchnic mesoderm (2 layers together = splanchnopleure) gut 5. g 6-7 (a) regressing primitive streak stimulates development of somitomeres in mesoderm as it moves (b) after 7 cranial and 13 caudal pairs have formed, somites develop out of the rst caudal pair and (c) development into somites proceeds caudally, as does somitomere development 6. somite development (a) complex induction mechanism causes some mesodermic mesenchyme to become epithelial sphere (enclosing somitocoel) Notch, WNT retinoic acid in rostrocaudal gradient FGF8 in caudorostral gradient (S 75) (b) hedgehog signal from notochord & neural tube causes: i. mitosis ii. loss of adhesion molecules iii. reversion to mesenchymal shape, becoming the sclerotome spinal column & rib cage (bones & cartilage) (c) remaining dorsal half of somite becomes dermatome dermis & subcutaneous skin
13
(d) dorsomedial somite cells migrate ventrally, underneath dermatome, to form myotome (S 76) epaxial (back) muscles (e) dermatome + myotome = dermomyotome (f) Each myotome and dermatome retains its innervation from its segment of origin, no matter where the cells migrate. Hence, each somite forms its own sclerotome (the tendon cartilage & bone compartment), its own myotome (providing the segmental muscle compartment), and its own dermatome, which forms the dermis of the back. (S 76) (g) signalling for somite development (S g 6.11) structure notochord, neural tube oor sclerotome dorsal neural tube dorsal somite dorsal neural tube DML dermomyotome lateral plate mesoderm epidermis VLL dermomyotome signal target Shh, ventromedial somite noggin PAX1 sclerotome WNT dorsal somite PAX3 WNT dermomyotome DML MYF5, MYOD BMP4 dermomyotome VLL WNT MYF5, MYOD result sclerotome cartilage, bone dierentiation dermomyotome epaxial muscle limb, body wall muscle
DML = dorsomedial lip, VLL = ventrolateral lip Shh, WNT, BMP are diusable paracrine factors aka growth and dierentiation factors (GDFs; S 8) PAX1, MYF5, MYOD are transcription factors (S 143) (h) g 6-10 i. cells migrate from sclerotome to notochord to make spinal column ii. anterior (rostral) cells from sclerotome n + 1 coalesce with posterior (caudal) cells from sclerotome n to make a vertebra iii. since sclerotomes (not vertebrae) make spinal muscles, you get spinal muscles which span each sequential pair of vertebrae 14
7. early development of circulatory sytem (a) epiblast cells which migrate through caudal part of primitive streak become cadiogenic mesoderm & arrange in -shaped pattern N-cadherin positive cardiac myocytes N-cadherin negative endocardial cells (b) cardiogenic plate rst recognizable precardiac mesoderm special area anterior to buccopharyngeal membrane adjacent is forerunner of pericardial cavity (c) pericardiac splanchnic mesoderm thickens, becoming myocardial primordium (d) nearby mesoderm vescicles start fusing to make up endocardial primordia (future interior of heart) (e) myocardium is formed by fusion of its primordia from the sides towards the center (f) epicardium is formed from its primodium (??) (g) endocardium is fused by fused of its primordia, surrounded by cardiac jelly (ECM) (h) by day 22 heart starts beating 8. blood & blood vessels blood & blood vessels start growing in extraembryonic splanchnopleuric mesoderm of yolk sac (induced by yolk sac endoderm), as blood islands which contain hemangioblasts centrally located hemangioblasts hemocytoblasts peripherally located hemangioblasts endothelial lining cells vessels fuse & grow towards embryo, where they eventually dump blood into developing heart & embryonic vessels 9. endoderm development (a) together with lateral folding which brings splanchnopleure down around endoderm, ventral folding pushes mesoderm in around yolk sac. (b) ventral folding (driven caudally by bulging of head) separates the cavity of the gut from the yolk sac by working from the ends: 15
foregut formed from rostral to caudal hindgut from caudal to rostral midgut where gut ows straight into yolk sac (connection eventually narrows to become the vitelline duct) (c) at rostral & caudal ends, gut meets ectoderm directly and bilayer will break down buccopharyngeal membrane rostrally proctodeal membrane (caudal to allantois) caudally; aka cloacal plate 10. at week 4, 3 circulatory arcs (a) intraembryonic: heart ventral aorta pharyngeal arches dorsal aorta rest of body (b) vitelline: goes to yolk sac (c) allantoic/umbilical
Placenta & Extraembryonic Membranes
1. overview fetal/maternal interface (placenta & chorion) derived from trophoblast mebranes derived from inner cell mass: (a) (b) (c) (d) 2. amnion 2 periods of uid production, corresponding to keratinization of fetal skin (becomes keratinized at week 21) initially transudate of maternal blood plasma, increasing contributions from fetal urine, ltration from fetal vessels (120) fetus drinks it late in pregnancy amniotic uid problems 16 amnion (from epiblast/ectoderm) yolk sac (from endoderm) allantois (from endoderm) extraembryonic mesoderm
hydramnios (excessive uid): often results from fetal inability to swallow, like in esophageal atresia or anencephaly oligohydramnios (not enough uid): bilateral renal agenesis (fetus not urinating) or rupture of amniotic membrane amniocentesis: examine cells (chromosomes) & proteins in uid -fetoprotein indicative of neural tube defect monitor Rh-factor responses monitor lung maturity with lecithin/sphingomyelin ratio 3. yolk sac progressive narrowing and eventual closure of connection with gut small percentage have vestige of gut/yolk sac connection: Meckels diverticulum 4. allantois vestigial excretory route embedded in umbilical cord turns into urachus: ligament from bladder to umbilicus 5. chorion & placenta (a) chorionic plate: outermost extraembryonic mesoderm and trophoblast wrapping; villi emerge from this (b) 1 villus: cytotrophoblast aggregates sticking into syncitiotrophoblast (end of 2nd week); do not touch maternal decidua (c) 2 villus: mesenchyme pushes into 1 villus (d) 3 villus: embryonic vessens penetrate 2 villus (end of 3rd week) (e) at tips of villi, just (cyto) trophoblastic cells: cytotrophoblastic cell column (f) cell columns grow and reach through syncitiotrophoblast to maternal decidua; villi that reach maternal cells are anchoring villi (g) proliferating distal cytotrophoblast cells from neighboring villi meet and form the cytotrophoblastic shell 6. uteroplacental circulation invasive cytotrophoblastic cells which extend from anchoring villi perforate maternal spiral arteries 17
by secreting special ECM they cause structural changes, including widening to reduce local BP layers of interface: syncitiotrophoblast its basal lamina early on: cytotrophoblast (disappears) capillary basal lamina (sometime 2 laminae are consolidated) capillary epithelium
7. gross relations decidua: stroma cells swollen from inux of glycogen & lipid (days after implantation) decidua capsularis: decidua covering chorionic vesicle (the inner bump of a) decidua basalis: decidua underlying chorionic vesicle (the downstroke of a) decidua parietalis: decidua not touching chorionic vesicle (top curve of a) increasing asymmetry in villi development more develop where body stalk connects embryo (embryonic pole) chorion frondosum less at abembryonic pole chorion laeve chorion frondosum + neighboring decidua = placenta eventually, decidua capsularis disappears/fuses with parietalis and chorionoic laeve is covered by parietalis 8. villus structure inside: mesensenchyme + Hofbauer cells (macrophages) outside: syncitiotrophoblast covered with many microvilli to increase surface area & facilitate diusion stress causes increase in microvilli arranged into specialized territories (? 140) (maternal ?) placental surface lacks histocompatibility antigens, keeps mom from rejecting baby 18
9. materials transported (a) gases: O2 , CO2 , anesthetics (b) H2 O, electrolytes (c) fetal wastes: urea, creatinine, bilirubin (d) glucose but not fructose (e) AAs, fatty acids, vitamins (f) steroid hormones but generally not peptide hormones (g) IgG passive immunity (h) Fe via transferrin carrier 10. placental hormone synthesis (142) syncitiotrophoblast peptide hormones (a) Human Chorionic Gonadotropin (HCG) secretion begins before implantation use in pregnancy test tells corpus luteum to keep making progesterone & estrogen production peaks at 8th week; by end of 1st trimester progesterone placenta is progesterone-independent and can make estrogen with adrenal gland & liver (b) chorionic somatomammotropin (aka human placental lactogen) promotes growth, lactation, lipid & carbohydrate metabolism (c) human placental growth hormone closely resembles normal GH helps regulate maternal glucose levels cytotrophoblast peptide hormone: homologue of Gonadotropinreleasing hormone (GnRH); stimulates release of HCG from syncitiotrophoblast 11. placental immunology: why the fetus is not rejected as foreign is an open question; partial answers/theories: lack of antigens from fetus inactivation of maternal immune system Reznick: 19
(a) immunusuppressive cytokines secreted by syncitiotrophoblast (b) HLA-G (human leukocyte antigen G) class IB histocompatibility complex protein interference with immunity signal or response at the decidua inactivation of antibodies by fetus 12. multiple pregnancies (a) if implanted far from each other (as in dizygotic or early-split monozygotic), separate chorions & placentas (b) normal monozygotic: partially fused chorions & placentas (c) late-split monozygotic: shared chorion & placenta, separate amnions vascular systems can be separate or fused if fused, one twin can siphon from the other, causing potentially fatal stunting of growth (d) conjoined twins or (rarely) dizygotic, can share chorion, placenta & amnion (why not monozygotic?) 13. placenta problems placenta previa: placenta covers cervical opening (os of uterus) & obstructs fetus exit; can cause fatal bleeding velamentous insertion of umbilical cord: cord stuck in thin chorionic membrane, not in placenta hyatidiform moles: grape-like deformation of villi associated with non-viable fetus; results from double paternal dose of DNA with nothing maternal
Skeletal System
1. skull/skeletal defects (S 129) (a) cranioschisis: cranial vault fails to close (b) craniosynostosis: premature closure of a suture sagittal sacphocephaly (long skull) coronal acrocephaly (tower skull) coronal & lambdoid brachycephaly (short skull) 20
(c) achondroplasia: autosomal dominant (d) possible molecular mechanism: mutations in FGFRs (see S table 9.1) they are tyrosine kinase receptors FGFR1, FGFR2 (Resnick: TGF also) are expressed in prebone & cartilage regions (important for proliferation & dierentiation, respectively) & are implicated in craniosynostoses FGFR3 is expressed in long bones & is implicated in achondroplasia 2. limb growth at week 4 (Reshef: week 5), limb buds emerge from ventrolateral body wall; 2 kinds of tissue: (a) ectoderm: at distal border, apical ectodermal ridge forms it induces adjacent tissue to proliferate and not to differentiate as growth happens & AER moves out, tissue behind it dierentiates (b) mesenchyme from somatic layer of lateral plate mesoderm; 2 components: i. progress zone ii. the rest (c) somite mesoderm develops into limb muscles nished by week 8 3. limbs develop along 3 axes: (a) proximodistal (hand goes at end of arm) (b) dorsoventral (hair goes on back of hand) (c) anteroposterior (thumb goes on lateral side of hand; for AP orientation, think of pectoral ns of sh) 4. hand development hyaline cartilage models are precursors for bones, including digits separation between digits occurs by apoptosis between the condensing cartilage 5. chicken wing is homologous to y wing! 21
same embryonic tissues same signalling pathways 6. proximodistal development: AER & progress zone the longer the cell stays in the progress zone, the more distal it will travel experiments: (a) removal of AER at increasingly late stages in development leaves longer (less shortened) limbs (b) removal of AER and replacement with a bead soaked in FGF produces a normal limb (wing) (c) removal of AER and replacement with 1 FGF bead restores part of growth removal of AER and replacement with 2 successive FGF beads restores more growth (d) extra AER produces double wing replace wing mesoderm with leg mesoderm produces leg in place of wing replace wing mesoderm with non-limb mesoderm causes regression of AER & stopping of limb development (e) FGF-soaked beads can induce ectopic limbs signalling FGF-8 signals initial budding of limb radical fringe is expressed dorsally serrate 2 (Ser-2) at the apical ridge, which induces the AER ventrally, engrailed-1 inhibits expression of radical fringe, keeping AER region restricted to ridge absence of radical fringe ventrally and high concentration dorsally both suppress Ser-2 in those regions FGF2, FGF4 also involved also controlled by nested expression of Hoxa genes, e.g.: only Hoxa-9 proximally Hoxa-9 through Hoxa-11 in between Hoxa-9 through Hoxa-13 distally Hoxa-13 mutations implicated in hand-foot-genital syndrome 22
7. anteroposterior development: ZPA & Shh ZPA is located posteriorly; it secretes a morphogene which has a concentration gradient: high concentration posteriorly, low concentration anteriorly experiments: (a) add an additional ZPA anteriorly and you get bilaterally symmetrical limb-ends (hand/foot/wing) (b) FISH identied Shh in ZPA (c) Shh expressing cells can cause the same abnormal symmetry as an extra ZPA (retinoic acid can also do this) cases of polydactyly may result from insucient Shh gradient also controlled by Hoxd genes Hoxd-9 most anteriorly Hoxd-9 through Hoxd-11 in between Hoxd-9 through Hoxd-13 posteriorly Hoxd-13 mutations implicated in polysyndactyly 8. dorsoventral development: WNTs WNT-7a expressed in dorsal ectoderm LMX1 expressed in dorsal mesoderm engrailed expressed in ventral ectoderm 9. signalling for limb bud initiation some signal from intermediate mesoderm (S 135: FGF10, TBX4/5) induces ectoderm to start to bulge & secrete FGF8 FGF8 causes proliferation of lateral-plate mesoderm induction of ZPA & Shh secretion Shh induces secretion of FGF4 in ectoderm, which (together with FGF8) signal ZPA to keep making Shh positive feedback FGF4, FGF8 maintain (lateral plate) mesoderm proliferation
23
10
Muscular System
origin paraxial mesoderm (somites) splanchnic mesoderm near heart tube splanchnic mesoderm near gut
type of muscle skeletal 1. cardiac smooth 2. skeletal muscle 2 origins:
(a) ventrolateral lip (VLL) of dermomyotome hypomeric aka hypaxial muscles (limbs & body wall) (b) dorsomedial lip (DML) of dermomyotome epimeric aka epaxial muscles (back) see signalling for somite development Patterns of muscle formation are controlled by connective tissue into which myoblasts migrate (S 143) head: neural crest cells neck: somite mesoderm body wall/limbs: somatic mesoderm limb musculature at rst, the limbs are innevated by a dorsal branch and a ventral branch from each segment the dorsal branches from each segment merge and the ventral branches from each segment merge to give large dorsal & ventral nerves ex: radial nerve (innervates extensor) is collection of dorsal branches, ulnar & median (innervate exor) are collections of ventral branches
11
Nervous System
(a) once neural tube is formed, cells organized into an epithelium (234-5) (b) DNA synthesis occurs in lateral neural tube cells external limiting membrane (=marginal zone) 24
1. proliferation within neural tube
(c) cells migrate towards neural tube lumen (d) if cell splits vertically (metaphase plate perpendicular to NT inner surface): both daughter cells migrate back to marginal zone & repeat cycle (e) if cell splits horizontally: lateral daughter cell i. migrates laterally ii. has high dose of Notch receptor iii. stops dividing & is a postmitotic neuroblast becomes neuron medial daughter cell i. slowly migrates away ii. continues proliferating 2. cell lineages (236-7) (a) multipotential stem cells (mitotic) bipotential stem cells (b) bipotential stem (=bipolar progenitor) cells (express nestin) i. neuronal progenitor cells (express NFs) ii. glial progenitor cells (express glial brillary acidic protein) (c) neuronal progenitor cell (non-mitotic) unipolar neuroblast multipolar neuroblast (d) glial progenitor cell (mitotic) 3 lines, including radial glial cells (e) radial glial cells act as guide wires for migrating neurons; neurons prevent proliferation and nal dierentiation of radial glial cells into nal form 3. neural tube cross-sectional organaziation (a) most medial: marginal zone mitotic cells; becomes ependyma (b) intermediate zone cell bodies and radially developing processes; becomes grey matter of SC sulcus limitans separates IZ into basal plate (ventral) and alar plate (dorsal) basal plate motor neurons & interneurons dierentiation controlled by shh gradient netrin attracts axons from dorsal region which descend & decussate (commisural axons) 25
repels axons of trochlear nerve alar plate sensory interneurons & autonomic system (IML columns) oor plate: main receiver for notochord signals; important for organizing exiting axons into ventral and dorsal roots (experiments with removed notochord and extra notochord) roof plate (c) marginal zoneonly neuronal processes (axons); becomes white matter of SC 4. patterns in rhombomere development (240-2) 5. isthmic organizer induces dorsal midbrain and cerebellum (243) 6. forebrain neuromereric organization (numbered caudal to rotral) (a) p1p3: diencephalon (b) p4p6: telencephalon + optic vesicles ventral forebrain induced by shh, secreted by midline axial structures; insuent shh fusion of optic vesicles & holoprosencephaly 7. pns unmylinated neurons are still wrapped by Schwann cells, just not with spiral axons communicate with Schwann cells via neuregulins, which tell the Schwann cells not to apoptose and whether to myelinate. growth cone overview (245-7) development of neuromuscular junction (247-8) presence of a target (e.g. a limb) increases # of axons heading in its direction; apoptosis of neurons if they dont get trophic factors (249) 8. ans overview sympathetic & para (249-250) some neural crest cells need to migrate a long way to destinations in abdomen (where they become postganglionic neurons); gut facilitates by inducing mitosis of the cells that do make it (251) 26
aganglionic megacolon (Hirschsprungs disease): failure of parasympathetic neural crest cells to colonize colon (252) neural maturation (a) start o able to become either symp or para, then commit (b) by default, they are both noradrenergic, then choose which transmitter (get signal from target tissue; see g 11-21) 9. cns structural development (a) overview migration cells start near ventricle postmitotic cells migrate out along radial glial cell guide wires grey matter is on outside of brain, unlike SC for most of brain, accumulation is inside-out: 6th layer (innermost) arrives rst, then 5th pushes through that & on top of it, etc. exceptions: i. migration parallel to surface (in cerebellum) ii. outside-in development (3 layers of hippocampus & superior colliculi) cortex as a whole may be organized by columnar unit (b) spinal cord: during gestation column grows more than SC, so caudal nerve roots need to elongate inside of column; in adult SC terminates at L2 (253-4) (c) rhombencephalon i. myelencephalon medulla transitional between SC and brain has general layout of SC (g 11-6), but roof plate stretches out laterally & becomes very thin, overlying an enlarged central canal ( 4th ventricle) 3 pairs of ventral (motor) and dorsal (sensory) nuclei cells from alar plate migrate ventrally and become ventral olivary nuclei (g 11-25) ii. metencephalpon cerebellum & pons like medulla, has expanded & thinner roof plate over big central canal (= 4th ventricle) 27
like medulla, has 3 pairs of motor & sensory nuclei cerebellum develops from anterior rhombic lips (dorsolateral bulges); posterior rhombic lips migrate ventrally & become pontine nuclei (cf. olivary nuclei) cells migrate anterolaterally to form a temporary layer called external granular layer, then migrate back in & form the (inner) granular layer; in 2nd phase purkinje cells migrate in opposite direction (d) mesencephalon i. basal plate tegmentum eerent nuclei for cranial nerves III, IV (eye) ii. alar plate tectum corpora quadrigemina = superior & inferior colliculi iii. cerebral peduncles (e) diencephalon from here anteriorly, hard to see basal/alar plate organization; most structures probably come from ala central canal 3rd ventricle thalamus develops surrounding 3rd ventricle & impinges on it from both sides, eventually meeting in middle (interthalamic adhesion aka massa intermedia) ventral to thalamus: hypothalamus caudal to thalamus: epiphysis (=pineal gland); secretes melatonin for circadian rhythm hypophysis (=pituitary gland) (259) made from 2 parts: infundibular process & Rathkes pouch i. infundibular process: ventral growth from oor of diencephalon, posterior to hypothalamus neural lobe of hypophysis ii. in stomodeum, anterior to buccopharyngeal membrane, Rathkes pouch invaginates dorsally & wraps around infundibulum (g 11-31) iii. outer wall of pouch thickens & becomes glandular pars distalis of hypophysis iv. inner wall pars intermedia, separated from from anterior part by residual lumen v. connection of internal pouch with stomodeum degenerates and canal regresses 28
optic cups (f) telencephalon base of each telencephalic vesiscle becomes basal ganglia (=striatum + glubus pallidus): lentiform nucleus (ball-shaped): composed of globus pallidus medially & putamen laterally, separated by internal capsule; sits anterolateral to thalamus caudate nucleus (C-shaped elongation) see Netter p. 110 general developmental pattern: i. migration of cell bodies ii. outgrowth of axons to appropriate targets lamina terminalis: thick anterior wall of 3rd ventricle (g 11-32) site of development of important interhemispheric connections: i. anterior commisure ii. hippocampal commisure (=fornix) iii. corpus callosum rhinencephalon (=archicortex) small layer immediately covering lateral ventricles, primarily for olfaction (receives inputs from olfactory bulbs) 10. CSF circulation (264-5) Arnold-Chiari malformation: herniation of cerebellum into foramen magnum, blocking CSF ow and causing hydrocephalus 11. cranial nerves 3 categories (a) extensions of brain tracts: i. olfactory (I) ii. optic (II) (b) pure motor nerves: i. oculomotor (III) ii. trochlear (IV) iii. abducens (VI) iv. hypoglossal (XII) 29
(c) mixed nerves mapped to specic pharyngeal arches i. trigeminal (V, arch I) ii. facial (VII, arch II) iii. glossopharyngeal (IX, arch III) iv. vagus (X, arch IV) (d) others (?) i. auditory (VIII) ii. accessory (XI) sensory part of nerves from category c + VIII dervied both from neural crest and placode ectoderm cells 12. development of function (a) generally, reexes appear in craniocaudal order (267-272); rst step: neural dierention to put components of reex arc in place (b) reex arc goes online (c) then, vertical (& supplementary horizontal) connections are made; these arent completed till early adulthood (d) during postnatal development the intersegmental connections are rened and myelinated (e) myelination begins in SC at week 11 and continues craniocaudally, motor neurons rst (f) in brain, sensory neurons myelinated rst 13. defects (268-71) (a) cranioschisis: failure of neural tube closure at the brain (b) rachischisis: failure of neural tube closure at the SC (c) spina bida occulta: failure of spinal column to close around SC at 1 or more vertebrae (d) meningocele: protrusion of arachnoid out through defect in spinal column or skull; only minor neurological problems (gs 11-42, 44) (e) myelomeningocele: protrusion of SC through spinal column defect; severe neurological problems (f) meningoencephalocele: protrusion of brain through skull defect (g) meningohydroencephalocele: protrusion of brain + part of ventricle through skull defect (h) lissencephaly: smooth cortices due to failure of cell migration 30
12
Neural Crest
lateral edges of neural plate they need to transition from epithelial to mesenchymal structure; they lose CAMs they migrate, and migration is controlled by ECM of basal laminas of neural tube and surface ectoderm and, of somites they selectively penetrate anterior parts of somites, since posterior part is rich in repellent chondroitin sulfate earliest-migrating cells have most possibilities for dierentiation dierent environmental cues trigger dierent phenotypes (279-80)
1. origins of neural crest cells:
2. trunk neural crest: 3 migratory paths (a) dorsolateral: between ectoderm & somites pigment cells (b) ventral: via space between anterior halves of somites & neural tube, ventral to aorta sympathoadrenal cells (c) ventrolateral: to space between anterior halves of somites & neural tube sensory ganglia 3. circumpharyngeal neural crest (282-4) cells migrate behind 6th pharyngeal arch then turn rostrally, with 2 turn-os: (a) cardiac crest parts of aorta, heart, thyroid, parathyroid, thymus; DiGeorge syndrome (due to chromosomal deletion) occurs when this ow of cells is decient (b) vagal crest parasympathetic innervation of gut 4. cranial neural crest may be morphological substrate for evolution of vertebrate head outow of rhomobomeres 2, 4 and 6 ow into (and constitue bulk of) pharyngeal arches 1, 2 and 3, respectively mesenchyme at r3 and r5 repulses crest cells and causes them to join ow from the neighboring rhomobomeres (g 12-8) g 12-9: pattern of Hoxb expression in pharyngeal arches reects rhombomere expression 31
5. defects (a) DiGeorge syndrome: see above (b) CHARGE association i. ii. iii. iv. v. vi. coloboma heart disease atresia of nsal choanae retardation of development genital hypoplasia ear anomalies
(c) Waardenburgs syndrome mutation in transcription factor Pax-3 (Hox relative; 69, g 4-9) pigmentation defects, deafness, cleft palate, (in Type I) hypoplasia of limb muscles (d) neurobromatosis: tumors of neural crest descendents (e) frontonasal dysplasia: facial abnormalities 6. dierences between cranial and trunk crest cells (a) cranial cells can form skeletal, muscle & connective components (b) more morphogenetic information encoded in cranial cells (e.g., craniocaudal level)
14
Face & Neck
(a) neurocranium; 2 parts: i. membranous part, made of at bones, which form vault of brain derived from neural crest cells & paraxial mesoderm made by membranous ossication (no cartilage intermediate) growth happens by adding bone on outside & resorption on inside at bones connected at exible sutures, which intersect at fontanelles allow molding of head during delivery 32
1. 2 parts of skull (S 125)
ii. chondrocranium, cartilaginous base of skull; 2 parts (S g 9.5) A. prechordal chondrocranium anterior to end of notochord (level of sella turcica) derived from neural crest B. chordal chondrocranium posterior to end of notochord derived from paraxial mesoderm (b) viscerocranium 2. as evolution progresses, 1st pharyngeal arch becomes (a) upper & lower jaw (b) bones of inner ear 3. anterior face vs. viscerocranium viscerocranium is segmented and controlled by Hox anterior face is from NC & begins (anatomically) at anterior end of notochord 4. in addition to NC cells, paraxial and prechordal mesoderm migrate into head/neck region, contributing to (a) connective tissue (b) muscle (c) skeleton: anterior/posterior distinction (S g 16.1) occipital bones of skull from mesoderm (parietal, occipital, petrous temporal) anterior bones of skull from neural crest cells (frontal, sphenoid, squamous temporal, hyoid) laryngeal from endoderm 5. pharynx structure overview (g 14-4) (a) pharyngeal pouches: 4 pairs of pouches o of pharyngeal foregut (b) pharyngeal arches: ectoderm bulges over each pair of pouches, appear in weeks 4 and 5 33
components (S g 16.6): i. artery (medial) ii. cartilage (intermediate) iii. nervous (lateral) iv. muscle (c) pharyngeal grooves: grooves between arches (d) each arch fed by aortic arches owing dorsoventrally; weird numbering: 6th arch follows 4th (?) 6. primordial face frontonasal prominence medially nasomedial & nasolateral processes make U-shaped primordial nose maxillary processes grow toward midline from both sides of stomodeum nasolacrimal groove runs between maxillary process & nasal prominence, from nasolateral processes to each eye near eye, develops into lacrimal sac becomes nasolacrimal duct, drains tears into nasal cavity as face develops, frontonasal prominence recedes (g 14-5) 7. development of frontonasal prominence depends on retinoic acid/shh signalling between forebrain & overlying ectoderm (323) 8. similar Hox expression pattern for limb growth & facial development (324) 9. phylogenetic history of TMJ (325-6) 10. palate separates nasal and oral cavities forms between weeks 6 and 10 Y-shaped intersection of 3 primordial shelves: (a) medial palatine process anteriorly (b) paired lateral palatine processes shelves rst grow down, then rotate up so they meet in middle 34
incisive foramen of palate marks the Y intersection 11. pharyngeal arches (gs 14-21, 22) (a) 1st includes maxillary process dorsally & mandibular prominence/process ventrally (S 259) contributes to face & ear; its cartilage strut (Meckels cartilage) is primordial jawbone, but ultimately resorbed i. maxillary process quadrate cartilage ii. mandibular process Meckels cartilage quadrate Meckel dorsal zygomatic, part of temporal malleus, incus premaxilla, maxilla mandible ventral trigeminal nerve & the muscles it innervates (mastication) no Hox genes expressed (b) 2nd (hyoid arch) muscles of facial expression Richert cartilage, becomes: stylohyoid ligament styloid process of temporal bone stapes part of hyoid bone Hoxa expressed; knockout results in mirror image of arch 1 (c) 3rd rest of hyoid bone (d) 4th cartilages of larynx 12. pharyngeal grooves (clefts) 1st groove becomes external acoustic meatus 2nd & 3rd grooves covered by growth of 2nd arch & become the cervical sinus (340) 2nd arch meets forward projection of 4th arch, obliterating cervical sinus (hence neck is smooth) 13. pharyngeal pouches (340-2) (a) 1st: tubotympanic recess becomes eustachian tube, connecting pharynx & middle ear 35
(b) 2nd shrinks, becomes supratonsillar fossae (c) 3rd disappears, sends the thymic & parathyroid tissue which develops there caudally (g 23), becoming thymus and inferior parathyroid glands. (d) 4th: similar to 3rd, with thymic (but not in humans) & parathyroid components; becomes superior parathyroid glands 14. thyroid (342) starts as thickening just caudal to tongue bud, between 1st and 2nd pouches migrates caudally, growing as it goes in the thyroid diverticulum arrives at front of esophagus connection to its origin, the thyroglossal duct regresses by 7th week, but part near thyroid can persist as pyramidal lobe of thyroid point of origin persists as foramen cecum depression 15. tongue formation (g 14-25, S 16.17) (a) all 4 arches contribute, but component from 3rd arch overgrows the component from the 2nd part (b) from inferior 1st arch, get paired lateral lingual swellings and tuberculum impar in middle (c) lateral swellings grow anteriorly & push together, with tuberculum impar growing behind, in middle (d) anterior 2/3, aka body (component from 1st arch) ends at terminal sulcus which runs through foramen cecum (e) posterior 1/3 aka root (from 3rd & 4th arches) epiglottis innervation (S 269-270) general sensory special sensory body lingual branch of chorda tympani branch of (a) mandibular division of trigeminal facial nerve root glossopharyngeal & vagus glossopharyngeal (b) motor: hypoglossal 16. defects 36
(a) facial clefts i. cleft lip: lack of fusion of nasomedial & maxillary processes ii. cleft palate: lack of fusion of palatal shelves iii. oblique facial cleft: lack of fusion of nasolateral & maxillary processes iv. macrostomia: lack of fusion of maxillary & mandibular processes v. medial cleft lip: lack of fusion of nasomedial processes (b) holoprosencephaly failure to develop midline structures, in extreme cases resulting in cyclopia factors: i. heredity (esp. shh mutation, e.g. Meckels syndrome), ii. shh malfunction from retinoic acid iii. maternal alcoholism, or diabetes (c) frontonasal dysplasia: too much tissue in nose region: broad nasal bridge, hypertelorism can include lack of fusion of nasomedial processes separate external nares, median cleft lip (d) rst arch related (jaw hypoplasias) i. Pierre Robin ii. Treacher Collins (autosomal dominant gene) iii. agnathia - no jaw (e) lateral cysts, sinuses, stulas i. from cervical sinus appear in front of sternocleidomastoid all 3 possible (?) (f) from ridges on 1st 2 arches: preauricular sinuses & stulas true cervicoaural stulas come from 1st pharyngeal groove (g) ectopic thyroid tissue anywhere along migratory route
16
16.1
Urogenital
Urinary
1. overview: recurring themes 37
(a) interconnectedness of urinary & genital systems (b) recapitulation in kidney ontogeny of phylogenetic kidney history (c) epithelial-mesenchymal interactions (d) sexualization of structures 2. pronephros homologue of lowest vertebrate kidney starts developing around day 21, disappear by day 28 only in cervical region (S g 15.1, 15.2) nephrotomes: mediolateral tubules; in pronephric system they remain segmented (discrete) 3. mesonephros homologue of sh/amphibian kidney (no need for water retention) nephrotomes connect with longitudinal primary nephric ducts, which grow caudally towards cloaca as PNDs grow they stimulate growth of new mesonephric tubules (dierent from nephrotomes??) mesonephric unit: (a) (b) (c) (d) glomerulus glomerular capsule surrounding glomerulus glomerular capsule feeds into mesonephric tubule mesonephric tubules dump into 1 nephric duct (becomes mesonephric [Wolan] duct)
also, there are external glomeruli where blood ltrate dumps into embryonic coelom (S g 15.1) by end of week 4 mesonephric ducts attach to cloaca near attachment to cloaca duct outgrowth occurs ureteric bud ( metanephros, future kidney) as metanephros matures, mesonephric duct regresses, but in males, testis attaches to it and it becomes vas deferens (sperm conduit to urethra; g 16-2) 4. metanephros
38
metanephrogenic blastema: mesenchyme cells in vicinity of bud from which metanephros arises 2 parts of kidney development (396) (a) branching of ureteric bud signalled by mesenchyme via GDNF (glial-derived neurotrophic factor), HGF (hepatocyte growth factor) GDNF and HGF genes turned on by WT1 transcription factor signal transduced in bud epithelium by c-RET and MET (b) condensation of new tubules from metanephrogenic blastema proliferation signalled (in response to part a) by epithelium via FGF-2, BMP-7, LIF (leukemia inhibitory factor) WT1 enables mesenchyme response Pax-2, Wnt-4 signal epithelialization of mesenchyme & cause dierentiation into excretory tubules Action is reciprocal; without blastema bud doesnt branch, without bud blastema doesnt make new tubules. 3 kinds of mesoderm in a nephron (a) ureteric bud epithelium ( excretory/renal tubule) (b) metanephrogenic blastema ( collecting tubule) (c) vascular endothelial cells mesenchyme development (a) metanephric duct branches (happens 15 successive times) (b) 1st condensation of mesenchyme makes renal vesicle (S g 15.6) (c) renal vesicle renal tubule: distal part (becomes proximal, from glomerulus) with epithelium (podocytes) (d) renal tubules & collecting tubules establish continuity (S g 15.6C); renal cell carcinomas can occur at the interface (e) slit forms beneath future podocyte cells (f) precursors of vascular endothelial cells grow into the slit (g) glomerulus forms from interface of blood vessels (glomerular endothelium) and podocyte epithelium; ltration done by special basement membrane between the 2 5. epithelium polarization (g 16-6) 39
(a) cells adhere to laminin in basement membrane, but not each other (b) cells adhere to each other via E-cadherin (c) cells adhere to each oterh via tight junction & develop brush border 6. mature kidney calices: penultimate conuences of collecting tubules pelvis: conuence of calices, last stop before ureter kidneys move up from pelvis and rotate 90 degrees (from facing front to facing inwards) & land on suprarenal glands 7. bladder (399) 8. defects (a) renal agenesis if unilateral, kidney grows to compensate fatal if bilateral bilateral renal agenesis symptoms: i. oligohydramnios (less amniotic uid) ii. attened Potter face from mechanical pressure (due to oligohydramnios) iii. tapered, fat ngers (b) renal hypoplasia: small kidney (c) polycystic disease of kidney (d) urachal cyst/sinus/stula (e) exstrophy of bladder frequently with epispadias
16.2
Genital
indierent stage: until 7th week, only way to determine sex is by looking for Barr bodies (no sex phenotype) sexualization starts in gonads & spreads from there genetic gender can be overridden by environment 40
1. overview (401)
example: maleness depends on virilizing action of testes; if male sex hormones not secreted or receptors not active, female phenotype occurs despite genotype 2. migration of germ cells (a) primordial germ cells start as epiblast cells which migrate through primitive streak (b) they wind up as endoderm, lining hindgut near yolk sac (c) they migrate from there to genital ridges (see next) (d) if they land in the wrong place, they become oogonia, regardless of genetic sex; they normally degenerate when this happens but if they dont they can become teratomas 3. origin of gonads: elongated region of steroidogenic mesoderm along border of mesonephros (a) rostral end adrenocortical primordial (b) caudal end genital ridges i. coelomic epithelium (medial part of roof of embryonic coelom) ii. mesonephric ridge (lateral part) 4. testes (a) development i. Sry triggers testes by inhibiting Dax-1, which prevents testes ii. 5th week: coelomic epithelium thickens, PGCs arrive iii. 6th week: columns of cells grow from epitheliun inward primitive sex cords iv. myoid cells (become interstitial cells) migrate from mesonephros v. sex cords thicken (precursors of Sertoli cells) distal part of sex cords seminiferous tubules proximal part rete testis rete ow into eerent tubules vi. tunica albuginea: connective tissue separating testis from epithelium, develops (b) Sertoli secretions i. cause migration of mesonephric mesenchyme into testis 41
ii. iii. iv. v.
prevent male germ cells from entering meiosis induce Leydig cells m ullerian inhibiting substance (see ducts) androgen-binding factor
(c) Leydig cells appear during 8th week secrete androgens i. testosterone ii. androstenedione 5. ovaries in contrast to testes, need PGCs in order to develop like testes, have coelomic & mesonephric cell descendants PGCs become oogonia in medulla (??) oogonia divide mitotically but some enter meiosis oocytes in cortex only mitosis 6. duct system (a) indierent i. mesonephric (wolan) ducts ii. paramesonephric (m ullerian) ducts ventral to mesonephric ducts appear between 44 & 48 days fate depends on gender (b) male because of m ullerian inhibiting substance (secreted by Sertoli cells), m ullerian ducts degenerate under inuence of testosterone, mesonephric ducts develop into ductus (vas) deferens mesonephric tubules can persist near testis as paradidymis development of accessory sex glands (414) Hoxa & Hoxd specify layout (414) (c) female 42
with no testosterone (with ovaries or without), mesonephric tubules regress, paramesonephric tubules develop as in male, depends on Hoxa and Hoxd genes directed by Wnt-7a (g 16-30) cranial parts uterine tubes caudal parts go to midline & meet region of fusion becomes vagina 7. descent of testes begins at 7th month 3 phases (a) release/degradation of cranial suspensory ligament (b) transabdominal descent: testis arrives at inguinal ring (c) transinguinal descent: traversal of inguinal canal
17
Cardiovascular
drive ow of blood through fetus & to placenta take over job of oxygenation postnatally (pulmonary circulation) spare load on underdeveloped fetal lungs by shunting
1. introfunctions of the heart
2. hematopoiesis starts in yolk sac, but by 28th day starts inside embryo in paraaortic clusters in aorta/genital ridge/mesonephros (AGM) region then main site moves to liver secretion of cortisol causes switch from liver to bone marrow cell lines: puripotent stem cells become (a) myeloid SCs (b) lymphoid SCs 3. 2 mechanisms of vascular development (S 78, 180) (a) vasculogenesis vessels arise by coalescence of (hem)angioblasts 43
only major vessels develop this way pathway: (S g 6.13) receptor transformation factor FGF2 FGFR mesoderm hemangioblast VEGF VEGF-R1 hemangioblast epithelial cell VEGF VEGF-R2 epithelial cell tube (b) angiogenesis: vessels arise from pre-existing ones all other vessels 4. early heart devcelopment (a) cardiogenic eld: epiblast cells which migrate through the primitive streak, land anterior to buccopharyngeal membrane (b) development begins around day 18-19, when coelomic vesicles of lateral mesoderm (see g 6-6B) are fusing, causing separation of somatic and splanchninc mesoderm (c) heart originates underneath space which develops but doesnt fuse with the extraembryonic coelom (like the space formed at the same level along the sides of the embryo; g 6-6C, 6-14A) the space is (as mentioned) anterior to buccopharyngeal membrane the space becomes the pericardial cavity the splanchnic mesoderm underneath it becomes the myocardial priomordium the growth of the brain pushes the mesoderm with cardiac primordia ventrally then caudally, rotating it 180 (d) the lateral curling of the embryo pushes shape in in the middle i. caudal ends become venous inow ii. top part broadens, grows arterial outow (e) initially, heart tube is suspended from dorsal roof of pericardial cavity by mesoderm called mesocardium, but this deteriorates so heart is oating in the pericardial space (f) septum transversum: semicircular horizontal shelf which separates heart from liver (382) (g) proepicardium starts forming on septum transversum and spread over the hear to form epicardium (S 161) aka visceral pericardium 44
5. formation of loop (446, S 162-3) outow tract arises from: cephalic paraxial & lateral mesoderm (endothelium) cranial neural crest (wall) atrial portion twists rostrally, dorsally and to left so that it resides in pericardium; driven by HAND-1, 2 bulbus cordis: connection of ventricle to aorta (446) middle part: conus cordis distal part: truncus arteriosus proximal aorta & pulmonary artery narrowings: (a) atrioventricular junction atrioventricular canal (b) truncus arteriosus (c) 1 interventricular foramen, between ventricle and bulbus; marked on outside by bulboventricular sulcus trabeculation (S 163) proximal bulbus cordis becomes trabeculated primitive RV whole ventricle becomes trabeculated primitive LV 6. aortic arches (436-8) (a) g 17-7: phylogenetic history: instead of pulmonary circuit, blood used to ow from common ventricle ventral aorta branchial arches for oxygenation paired dorsal aortas (b) in humans, never observe all arches at once, instead see cradiocaudal progression arch 13 4 (c) 5 6 right left carotid as right subclavian arch of aorta vestigial right pulmonary pulmonary trunk, left pulmonary, ductus arteriosus cf. table 17-3 45
(d) asymmetrical development of 6th arch derivatives reected in trajectories of R & L recurrent laryngeal nerves (438) i. right: curves under 4th arch subclavian ii. left: stays under ductus arteriosus ligamentum arteriosum 7. skipping 438444 since not on syllabus 8. 444-6 lymphatic system 9. valves (446-7, S 171) (a) atrioventricular cushions: dorsal & ventral thickenings formed at AV junction (thickening also happens between ventricle & outow) (b) at the cushions, cardiac jelly (mesenchyme) protrudes into canal (c) induced by myocardium, some endocardial cells transform from epithelium to mesenchyme & migrate into jelly (of AV cushion-?) (d) blood ow hollows out the mesenchyme & (as mesenchyme replaced by connective tissue) valves form (S 171) (e) denitive valves come from epicardium i. right AV canal: tricuspid ii. left AV canal: bicuspid aka mitral iii. they are connected to venetricular trabeculae called papillary muscles by the chordae tendinae 10. partitioning (448-451, g 17-20) (a) atria i. interatrial septum primum grows down from ceiling of heart to split common atrium into right & left ii. before it fuses with the AV cushion the atria are connected by the interatrial foramen (ostium) primum, which shunts blood away from pumonary circuit to systemic iii. dont want to shunt all blood away from R ventricle, though, because it would be hypoplastic; it is allowed to pump at 70-80% capacity, & its output shunted away from lungs by ductus arteriosus (451) iv. as interatrial septum primum fuses with AV cushion, perforations appear at top (cephalic) end, and coalesce to become interatrial foramen secundum, to keep shunting going 46
v. septum secundum grows out from dorsal atrial wall, right to the right of the septum primum vi. the two septa with the foramen make the foramen ovale, which acts as a valve, preventing backow from L to R atrium (b) ventricles: originally (day 30), blood ows through AV canal primitive LV 1 interventricular foramen primitive RV bulbus cordis I.e., ow is sequential from L to R, and is deected L by bulboventricular ange (S g 12.8) by day 35, AV canal enlarged to the right & ange has retracted enough so blood ows into both RV and LV simultaneously (S g 12.17) muscular interventricular septum grows up from (caudal) oor of common ventricle towards AV cushion membranous part of septum contributed by AV cushion; when it meets the conus septum (see next), the interventricular foramen is completely closed (S g 12.22) (c) outow i. truncoconal ridges appear & grow spirally distally, superior & inferior truncus swellings (S 173-5) proximally, dorsal & ventral conus swellings ii. they fuse to make a spiral partition, the aorticopulmonary septum iii. RV reaches around ventrally to access its outow, LV dorsally, the R & L outow tubes slide past each other (g 17-22) iv. depends on NCC migration, potential defect source (d) outow (semilunar) valves: from cranial NCC & cardiac mesoderm protect backow of blood into ventricles i. aortic valve ii. pulmonary valve 11. sinus venosus starts in middle, but with twisting of heart, winds up dumping exclusively into RA (5th week); its left horn becomes coronary sinus (also dumps into RA) 47
confusing terminology: aps around right input each called valves, right and left venous valves (S 167) as the oricie shrinks, wall develops along line of fusion: septum spurium left valve & septum spurium fuse with developing atrial septum right valve: superior part disappears, inferior part valve of IVC & valve of coronary sinus 12. pulmonary vein (S 170) starts as outgrowth of left atrial wall & meets up with developing veins of lung buds atrial attachment grows & becomes smooth-walled part of atrium; displaces original trabeculated part (which remains as appendage) 13. innervation (452) sympathetic bers grow from trunk neural crest (adrenergic, excitatory) parasympathetic bers grow from crania neural crest (cholinergic, inhibitory) cardiac ganglia: migrate from cardiac neural crest; synapse with parasympathetic sensory bers: from nodose ganglion parasympathetic & sensory carried by vagus nerve 14. electrical conduction system as sinus venosus becomes RA, SA node develops in RA. AV node develops in interatrial septum AV node communicates with ventricles via the AV bundle AV bundle extend Purkinje bers into ventricles cellular aspects: conducting bundles are specialized myocytes, with high glycogen content coronary arteries induce conducting bundles by secreting endothelin1 responding cells express engrailed-2 48
15. initiation of function ductus venosus allows blood from placenta to skip liver & proceed directly to IVC there may be a sphincter which enables/disables ductus venosus shunt, to prevent backup of bypassed blood from liver in RA: (a) high pressure blood from IVC (oxygenated) goes through foramen ovale to LA (b) low pressure blood from SVC (deoxygenated) goes through tricuspid valve to RV 16. defects: heart defects are most common birth defect (1% of live births, S 172-3) cardiovascular teratogens (?): rubella virus, thalidomide risk factors: maternal diabetes, hypertension genetic problems holt-oram syndrome heart-hand syndrome tetralogy of Fallot endocardial cushion formation: since neural crest implicated associated with craniofacial defects (S 168) (a) AV septal defects (b) transposition of great vessels (c) tetralogy of Fallot issues at atrial septum (a) atrial septal defect (ASD): patent foramen ovale (b) absence of atrial septum: cor triloculare biventriculare (c) premature closure of foramen ovale: hypoplasia of left side of heart (d) issues at AV canal i. persistent AV canal ii. ostium primum defect?? iii. tricuspid atresia A. patent foramen ovale 49
B. ventricular septal defect (see next) C. hypertrophy of LV & hypoplasia of RV ventricular septal defect (VSD): most common usually restricted to membranous part of septum causes more blood to pulmonary circuit than systemic issues from conotruncal septum (a) tetralogy of Fallot: unequal division of conus; conotruncal septum displaced anteriorly so aorta gets more volume; not fatal i. pulmonary infundibular stenosis ii. defective interventricular septum iii. aorta originating right on top of septal defect iv. RV hypertrophy, since higher pressure (b) persistent truncus arteriosus: conotruncal rides dont fuse always defective interventricular septum (c) transposition of great vessels: septum doesnt spiral usually patent ductus arteriosus
18
Intro:Reznick lecture 1
1. paracrine
19
Birth Defects (Sadler 8, Reznick lecture 2)
ectrodactyly: missing digits amelia: missing limbs meromelia: partial absence of limb phocomeila: ipper limb (phoco=seal)
1. denitions
2. frequency of congenital malformations 50
2% of all births 1% are minor .5% are lethal .5% are serious (only partially treatable) but nonlethal 3. components genetic: 5% mutations, 5% chromosomal defects 10% purely environmental 80% combined environment & genetics 4. see tables in lecture notes 5. environmental factors: especially powerful during weeks 38 (organogenesis); see S table 8.1 (a) infection: bacteria, viruses, parasites, protozoa rubella cytomegalovirus: mother is often asymptomatic herpes simplex HIV varicella- maternal infection has 25% defect rate thalidomide valium alcohol: most common cause of mental retardation (S 115) aminopterin (chemotherapy)
(b) chemicals
(c) hormones progestins administered to prevent abortion cause masculinization of female genitalia diethystilbestrol (synthetic estrogen) given for same reason carcinogenic for female fetuses later in life (d) malnutrition (e) hypoxia CNS damage (f) ionizing radiation: kills rapidly proliferating cells 6. preventing congenital malformations 51
(a) genetic counseling (b) environmental factors: avoid mutagens & infections, especially during critical period good nutrition 7. prenatal diagnosis method trimester 1 CVS 2 ultrasound 2 fetoscopy 2 amniocentesis 2 chordocentesis 2 -fetoprotein 2 nuchal translucency 8. syndrome vs. association (111) syndrome: group of co-occurring symptoms whose cause is known association: group of co-occurring symptoms whose cause is unknown 1-3 % risk to fetus
.5% risk fetal blood sample test for trisomy 21
52
You might also like
- Embryo NotesDocument52 pagesEmbryo NotesSharona Avgush100% (1)
- Anbt - 608: Submitted ToDocument50 pagesAnbt - 608: Submitted ToMayuriGulhaneNo ratings yet
- Lecture: Male Reproductive PhysiologyDocument8 pagesLecture: Male Reproductive Physiologyvalerie obehiNo ratings yet
- General Embryology: Gametogenesis: Formation of Male & Female GametesDocument19 pagesGeneral Embryology: Gametogenesis: Formation of Male & Female Gametes이희승No ratings yet
- Gametogenesis, Gamete Transport & FertilizationDocument56 pagesGametogenesis, Gamete Transport & FertilizationvictorNo ratings yet
- OogenesisDocument23 pagesOogenesisFeri ArdiansyahNo ratings yet
- USMLE NotesDocument165 pagesUSMLE NotesHerliani HalimNo ratings yet
- LZT 304 M.sc. III RD Seema Rai 22.12.14Document28 pagesLZT 304 M.sc. III RD Seema Rai 22.12.14naveen kumarNo ratings yet
- Nzary El 3maliDocument13 pagesNzary El 3maliGana KhaledNo ratings yet
- Seminar 1Document8 pagesSeminar 1gatorssoccer1313No ratings yet
- WWW - Studyguide.pk: Aspects of Human Reproduction Key ObjectivesDocument9 pagesWWW - Studyguide.pk: Aspects of Human Reproduction Key ObjectivesP.P.KarthikeyanNo ratings yet
- Embryo PDFDocument80 pagesEmbryo PDFMOHSEN PMNo ratings yet
- Understanding the Process of OogenesisDocument52 pagesUnderstanding the Process of OogenesisBharat ThapaNo ratings yet
- Molecular Biology of Reproductive ProcessesDocument53 pagesMolecular Biology of Reproductive ProcessesBhaskar GangulyNo ratings yet
- SPERMATOGENESISDocument58 pagesSPERMATOGENESISkathyNo ratings yet
- 2 GametogenesisDocument56 pages2 GametogenesisIRENE SEBASTIANNo ratings yet
- Gametogenesis: The Formation of Eggs and SpermDocument52 pagesGametogenesis: The Formation of Eggs and SpermMUDIN ABDELLANo ratings yet
- Germ cell migration and differentiation in amphibians, birds and mammalsDocument26 pagesGerm cell migration and differentiation in amphibians, birds and mammalsSales Him IT ServicesNo ratings yet
- Female Reproductive HormonesDocument64 pagesFemale Reproductive HormonesJoryll AtienzaNo ratings yet
- GametogenesisDocument8 pagesGametogenesisSOPHIA ALESNANo ratings yet
- Applications of Biology 9700 - Nos - As - 5Document9 pagesApplications of Biology 9700 - Nos - As - 5Nana_Banana_94No ratings yet
- DR Anangwe-GametogenesisDocument23 pagesDR Anangwe-GametogenesisStanley OdiraNo ratings yet
- Embryolo GY: Presented By: Ms. Sweta K. Gaude M.Sc. (N) 1 Year SDM InsDocument31 pagesEmbryolo GY: Presented By: Ms. Sweta K. Gaude M.Sc. (N) 1 Year SDM InsSavita HanamsagarNo ratings yet
- 3rd Lecture Morphological Changes During Maturation o Te GameteaDocument25 pages3rd Lecture Morphological Changes During Maturation o Te GameteaHussein Al Saedi100% (1)
- 1 GametogenesisDocument41 pages1 Gametogenesishaladaqqa1No ratings yet
- Cell CycleDocument21 pagesCell CycleKiran HassanNo ratings yet
- 2 Spermatogenesis DR - GosaiDocument31 pages2 Spermatogenesis DR - GosaiDr.B.B.GosaiNo ratings yet
- Lecture 8 - 30.12.2022Document17 pagesLecture 8 - 30.12.2022Adnan Mohammad Adnan HailatNo ratings yet
- 03 OogenesistxtDocument23 pages03 OogenesistxtLina AnielaNo ratings yet
- Chapter 3 - Tissue Renewal, Regeneration, and RepairDocument12 pagesChapter 3 - Tissue Renewal, Regeneration, and RepairAgnieszka WisniewskaNo ratings yet
- Unit4review CellcommunicationcellcycleDocument2 pagesUnit4review Cellcommunicationcellcycleapi-286357921No ratings yet
- EmbryologyDocument101 pagesEmbryologytawandarukwavamadisonNo ratings yet
- Female Reproductive SystemDocument42 pagesFemale Reproductive SystemMaulidyaAlistaNo ratings yet
- in Vitro GrowthDocument23 pagesin Vitro GrowthYến NguyễnNo ratings yet
- Embryology Study GuideDocument255 pagesEmbryology Study GuideSiddharth KatyalNo ratings yet
- Gametogenesis and SpermatogenesisDocument46 pagesGametogenesis and SpermatogenesisDenis KaguiNo ratings yet
- Embryology01-FertilizationToGastrulationbackupDocument42 pagesEmbryology01-FertilizationToGastrulationbackupArini Sabila FaizaNo ratings yet
- Q & A Notebook Chap 03Document13 pagesQ & A Notebook Chap 03umamahfarooq75No ratings yet
- Econtent PDF EmbryologyGametogenesisDocument9 pagesEcontent PDF EmbryologyGametogenesisVipin BhartiNo ratings yet
- CELL CYCLE-WPS OfficeDocument40 pagesCELL CYCLE-WPS OfficeShubhendu ChattopadhyayNo ratings yet
- Genetics (Introduction)Document10 pagesGenetics (Introduction)Mielah RuthNo ratings yet
- CC-13 Unit 1Document10 pagesCC-13 Unit 1Subhradeep SinghaNo ratings yet
- Proliferasi SelDocument50 pagesProliferasi SelIkan SeluangNo ratings yet
- Reproduction 2024 PDFDocument1 pageReproduction 2024 PDFKhan MuhammadNo ratings yet
- SAQ Unit 2Document9 pagesSAQ Unit 2Shilpa DuttaNo ratings yet
- Fetal Development from Embryonic to Fetal Period Week by WeekDocument71 pagesFetal Development from Embryonic to Fetal Period Week by WeeklisafelixNo ratings yet
- Lec - 5Document14 pagesLec - 5abhikumar22113344No ratings yet
- Female Reproductive SystemDocument42 pagesFemale Reproductive SystemDoki NatsuNo ratings yet
- Gonad & GametogenesisDocument40 pagesGonad & GametogenesisAnggita DaniellaNo ratings yet
- Presentation GametogenesisDocument33 pagesPresentation GametogenesisAndy ZempaNo ratings yet
- Male ReproductionDocument3 pagesMale Reproductionmelly nazwa juwitaNo ratings yet
- 02 Protein Transport Within A Cell MAT 2021Document18 pages02 Protein Transport Within A Cell MAT 2021Mishaal IrfanNo ratings yet
- STEM Cell Cycle and Cell DivisionDocument5 pagesSTEM Cell Cycle and Cell DivisionErolin PobleteNo ratings yet
- Module 3 - Gen Bio 1 - MidtermDocument7 pagesModule 3 - Gen Bio 1 - MidtermAngel Cuacko GacmatanNo ratings yet
- Embryology 1 GametogenesisDocument64 pagesEmbryology 1 Gametogenesiselphas walelaNo ratings yet
- General Embryology 2022Document130 pagesGeneral Embryology 2022KAMAL ALSOFIANYNo ratings yet
- 1 - EmbryologyDocument19 pages1 - EmbryologynohacksNo ratings yet
- Gametogenesis BiotechDocument16 pagesGametogenesis Biotechnica onicaNo ratings yet
- Camp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.From EverandCamp's Zoology by the Numbers: A comprehensive study guide in outline form for advanced biology courses, including AP, IB, DE, and college courses.No ratings yet
- Exercise 8 Pig Embryo (Posterior Sections)Document16 pagesExercise 8 Pig Embryo (Posterior Sections)Gail AmuraoNo ratings yet
- Developmental Biology Exam #1Document5 pagesDevelopmental Biology Exam #1Pearl PascuaNo ratings yet
- Embryology GastrulationDocument9 pagesEmbryology GastrulationRivera Gómez América LucíaNo ratings yet
- Human Anatomy 7th Edition Marieb Test BankDocument20 pagesHuman Anatomy 7th Edition Marieb Test BankKerriAdamsdwroi100% (14)
- Germ Layer Establishment in Embryonic DevelopmentDocument52 pagesGerm Layer Establishment in Embryonic DevelopmentAngela Nicole ManasNo ratings yet
- Development of Frog EmbryoDocument13 pagesDevelopment of Frog EmbryoNexieNo ratings yet
- Langman Medical Embryology Made Easy PDFDocument19 pagesLangman Medical Embryology Made Easy PDFAsma SaleemNo ratings yet
- Claire Bolalin - Quiz (Neurulation)Document1 pageClaire Bolalin - Quiz (Neurulation)Claire BolalinNo ratings yet
- 18 Hour Chick EmbryoDocument7 pages18 Hour Chick Embryoaa628100% (2)
- Embryology of Musculoskeletal SystemDocument14 pagesEmbryology of Musculoskeletal SystemdiahNo ratings yet
- Muscle TissueDocument3 pagesMuscle Tissuekugmo89No ratings yet
- Presentation Fascial ManipulationDocument8 pagesPresentation Fascial ManipulationricardoNo ratings yet
- MSC ZoologyDocument183 pagesMSC ZoologyShruti VermaNo ratings yet
- PHYSIOLOGY OF HUMAN DEVELOPMENT FROM FERTILIZATION TO IMPLANTATIONDocument68 pagesPHYSIOLOGY OF HUMAN DEVELOPMENT FROM FERTILIZATION TO IMPLANTATIONانس ابوهيبةNo ratings yet
- 4 MM FrogDocument5 pages4 MM FrogMarina of The SeaNo ratings yet
- Embryology MCQsDocument7 pagesEmbryology MCQsSafiya James100% (1)
- Sclerotome Dermatome MyotomeDocument5 pagesSclerotome Dermatome Myotomeerick evanderNo ratings yet
- Chicken Embryo Lab ReportDocument10 pagesChicken Embryo Lab Reportgmk5031No ratings yet
- 4th To 8th WeekDocument48 pages4th To 8th WeekjefferyNo ratings yet
- Skeletal Muscle Development and Regeneration: Concise ReviewDocument12 pagesSkeletal Muscle Development and Regeneration: Concise Reviewtesfaye mekonnenNo ratings yet
- Dermatome Myotome SclerotomeDocument4 pagesDermatome Myotome SclerotomeAdhya TiaraNo ratings yet
- NEO, April 01, 2021 Volume 22, Issue 4Document70 pagesNEO, April 01, 2021 Volume 22, Issue 4Majo EscobarNo ratings yet
- 4mm Frog EmbryoDocument3 pages4mm Frog EmbryoIvy CruzNo ratings yet
- 1Document223 pages1Isaac Tan100% (2)
- Irisan Seri Berudu KatakDocument10 pagesIrisan Seri Berudu KatakfarahNo ratings yet
- Chick Embryo (Embryology Lab)Document9 pagesChick Embryo (Embryology Lab)humanupgrade100% (1)
- Exercise 16 Whole Mount of A 33 Hour Chick Embryo PDFDocument3 pagesExercise 16 Whole Mount of A 33 Hour Chick Embryo PDFMichaela FayeNo ratings yet
- แกะเทป Developmental of Musculoskeletal SystemDocument50 pagesแกะเทป Developmental of Musculoskeletal Systemjulesarojinee100% (1)
- Quail Birds (Salwa)Document5 pagesQuail Birds (Salwa)mamusa113No ratings yet
- Embryology Practice Questions With Answers Questions and Study Guide - Quizlet Flashcards by Ypapo-1Document118 pagesEmbryology Practice Questions With Answers Questions and Study Guide - Quizlet Flashcards by Ypapo-1LoisNo ratings yet