Professional Documents
Culture Documents
F321 Module 1 Practice 4
Uploaded by
coughsyrup123Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
F321 Module 1 Practice 4
Uploaded by
coughsyrup123Copyright:
Available Formats
F321 module 1 Practice 4:
1.
Calcium oxide neutralises acids such as nitric acid. A student neutralised 1.50 g of CaO
with 2.50 mol dm3 nitric acid, HNO3. The equation for this reaction is shown below.
CaO(s) + 2HNO3(aq) Ca(NO3)2(aq) + H2O(l)
(i)
How many moles of CaO were reacted?
mol
[2]
(ii)
Calculate the volume of 2.50 mol dm3 HNO3 needed to exactly neutralise
1.50 g of CaO.
volume = cm3
[2]
[Total 4 marks]
2.
The nitrate ion, NO3, in Ca(NO3)2 contains both covalent and dative covalent bonds.
(i)
What is the difference between a covalent bond and a dative covalent bond?
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[1]
(ii)
Calcium nitrate decomposes on heating to form calcium oxide, oxygen and
nitrogen(IV) oxide, NO2.
Construct a balanced equation for this reaction.
.........................................................................................................................
[1]
[Total 2 marks]
The King's CE School
3.
Aqueous silver nitrate can be used as a test for halide ions. A student decided to carry
out this test on a solution of magnesium chloride. The bottle of magnesium chloride
that the student used showed the formula MgCl2.6H2O.
The student dissolved a small amount of MgCl2.6H2O in water and added aqueous
silver nitrate to the aqueous solution.
(i)
What is the molar mass of MgCl2.6H2O?
molar mass = g mol1
[1]
(ii)
What would the student see after adding the aqueous silver nitrate, AgNO3(aq)?
.........................................................................................................................
[1]
(iii)
Write an ionic equation for this reaction. Include state symbols.
.........................................................................................................................
[2]
(iv)
Using aqueous silver nitrate, it is sometimes difficult to distinguish between
chloride, bromide and iodide ions.
How can aqueous ammonia be used to distinguish between these three ions?
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[3]
[Total 7 marks]
The King's CE School
4.
A small amount of solid magnesium oxide, MgO, was reacted with excess dilute
hydrochloric acid.
(i)
Define an acid.
.........................................................................................................................
[1]
(ii)
Write a balanced equation for this reaction.
.........................................................................................................................
[1]
[Total 2 marks]
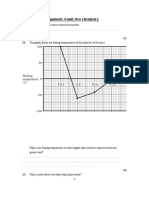
5.
Carbon is in the p-block of the Periodic Table. Naturally occurring carbon contains a
mixture of two isotopes, 12C and 13C.
Complete the table below for the atomic structure of the isotopes 12C and 13C.
isotope
protons
neutrons
electrons
12
13
C
[Total 2 marks]
6.
A sample of carbon was found to contain 95% of 12C and 5% of 13C.
(i)
How could this information be obtained experimentally?
.........................................................................................................................
[1]
The King's CE School
(ii)
The 13C isotope has a relative isotopic mass of 13.00.
Define the term relative isotopic mass.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
(iii)
Calculate the relative atomic mass of this sample of carbon to three significant
figures.
Ar = ............................................
[2]
[Total 5 marks]
7.
In 2000, the mass of CO2 emitted in the UK was equivalent to 1 kg per person in every
hour.
(i)
Calculate the volume of 1 kg of carbon dioxide. Assume that 1 mole of CO2
occupies 24 dm3.
volume = .......................... dm3
[2]
(ii)
The UK has set a target to cut CO2 emissions by 60% of the 2000 value by 2050.
Calculate the reduction needed in the volume of CO2 emissions each hour per
person if the target is to be met.
answer: ........................... dm3
[1]
[Total 3 marks]
The King's CE School
8.
A student prepared an aqueous solution of calcium chloride by reacting calcium with
+
hydrochloric acid. Calcium chloride contains Ca2 and Cl ions.
(a)
Complete and balance the following equation for this reaction.
Ca(s) + HCl(aq) . CaCl2(aq) +
[2]
(b)
This is a redox reaction.
Use oxidation states to show that calcium has been oxidised.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[2]
[Total 4 marks]
9.
To prepare the aqueous calcium chloride, the student added the exact amount of
calcium so that all the hydrochloric acid had reacted. She used 50 cm3 of
2.0 mol dm3 HCl.
(i)
How many moles of HCl had she used?
[1]
(ii)
Calculate the mass of calcium that she used.
[2]
The King's CE School
(iii)
The student added some more calcium and she was surprised that a reaction still
took place.
Explain this observation.
Write a balanced equation for this reaction.
.........................................................................................................................
.........................................................................................................................
.........................................................................................................................
[3]
[Total 6 marks]
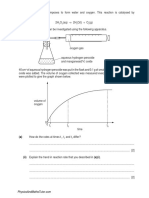
10.
The element titanium, Ti, atomic number 22, is a metal that is used in the aerospace
industry for both airframes and engines.
A sample of titanium for aircraft construction was analysed using a mass spectrometer
and was found to contain three isotopes, 46Ti, 47Ti and 48Ti. The results of the analysis
are shown in the table below.
46
isotope
Ti
47
Ti
48
Ti
relative isotopic mass
46.00
47.00
48.00
percentage composition
8.9
9.8
81.3
(a)
(i)
Explain the term isotopes.
................................................................................................................
................................................................................................................
[1]
(ii)
Complete the table below for atoms of two of the titanium isotopes.
isotope
46
Ti
47
Ti
protons
neutrons
electrons
[2]
The King's CE School
(b)
Using the information in the first table, calculate the relative atomic mass of this
sample of titanium.
Give your answer to three significant figures.
[2]
[Total 5 marks]
The King's CE School
You might also like
- Periodic Table, Group 2 and The Halogens 2 QPDocument14 pagesPeriodic Table, Group 2 and The Halogens 2 QPmalakNo ratings yet
- Ligand Coupling Reactions with Heteroatomic CompoundsFrom EverandLigand Coupling Reactions with Heteroatomic CompoundsRating: 4 out of 5 stars4/5 (1)
- Energy Changes, Rate, Reversible, NH3, H2SO4Document5 pagesEnergy Changes, Rate, Reversible, NH3, H2SO4HudaNo ratings yet
- As Level Chemistry: Topic 2 - Amount of Substance TestDocument10 pagesAs Level Chemistry: Topic 2 - Amount of Substance Testkarima akterNo ratings yet
- GP 7 AsDocument129 pagesGP 7 Ashussainjunaid210No ratings yet
- Mixed Topic Revision 1 DiffusionDocument23 pagesMixed Topic Revision 1 DiffusionYaakkwNo ratings yet
- Chemical Energetics 2 QPDocument11 pagesChemical Energetics 2 QPWilliam TsuiNo ratings yet
- General Properties of Transition Metals 1 QPDocument10 pagesGeneral Properties of Transition Metals 1 QPaanammanaNo ratings yet
- A-Level Chemistry: Paper 3 Practice Paper 3Document20 pagesA-Level Chemistry: Paper 3 Practice Paper 3Jesus ChristNo ratings yet
- 1.1.3 AcidsDocument8 pages1.1.3 AcidsKel1209No ratings yet
- Assignment 4 U2 Chem RedoxDocument34 pagesAssignment 4 U2 Chem RedoxMoshiurNo ratings yet
- The Particulate Nature of Matter 3 QPDocument10 pagesThe Particulate Nature of Matter 3 QPSajia EhsaniNo ratings yet
- Isomerism 2 QPDocument9 pagesIsomerism 2 QPPragna AnanthNo ratings yet
- Question Database For Amount of SubstancesDocument23 pagesQuestion Database For Amount of SubstancesKamrul Alam MasumNo ratings yet
- 2.04 - 2.06 Redox Reactions, Halogens and Alkali Earth MetalsDocument37 pages2.04 - 2.06 Redox Reactions, Halogens and Alkali Earth Metalsgobod52280No ratings yet
- Mock 1Document15 pagesMock 1G M Ali KawsarNo ratings yet
- Metal p4 Ws 4Document10 pagesMetal p4 Ws 4NonnoonoNo ratings yet
- The Particulate Nature of Matter 3 QPDocument10 pagesThe Particulate Nature of Matter 3 QPBara' HammadehNo ratings yet
- P7 Oxidation Reduction and RedoxDocument42 pagesP7 Oxidation Reduction and Redox/ “Nu” /No ratings yet
- Periodicity Past QuestionsDocument17 pagesPeriodicity Past QuestionsMohammad KhanNo ratings yet
- As-Level Paper 1 pp9Document15 pagesAs-Level Paper 1 pp9ConorNo ratings yet
- My TestDocument20 pagesMy TestHidayah TeacherNo ratings yet
- Extra Analysis Qs - 3Document6 pagesExtra Analysis Qs - 3sureshthevanNo ratings yet
- Topic 4 - Group 7Document9 pagesTopic 4 - Group 7Abirame SivakaranNo ratings yet
- t2 Chem Revision Ex 22Document19 pagest2 Chem Revision Ex 22Nicholas OwNo ratings yet
- Quiz - 1 - Matter & StoichiometryDocument4 pagesQuiz - 1 - Matter & StoichiometryDaniel Ngenokesho WandyaNo ratings yet
- 1.3 Question DatabaseDocument42 pages1.3 Question DatabasedaminNo ratings yet
- Balanced Equations & Associated Calc's 10 QPDocument8 pagesBalanced Equations & Associated Calc's 10 QPjade.davis0019No ratings yet
- Born-Haber Cycles 1 QP PDFDocument9 pagesBorn-Haber Cycles 1 QP PDFTalha KhanNo ratings yet
- Equilibria A2Document48 pagesEquilibria A2javedkaleemNo ratings yet
- 9701 - s14 - QP - 22 (Kairos)Document11 pages9701 - s14 - QP - 22 (Kairos)MCHNo ratings yet
- Redox 2 QPDocument7 pagesRedox 2 QPPramitaNo ratings yet
- Redox Questions Igcse ChemDocument7 pagesRedox Questions Igcse ChemCaylinNo ratings yet
- t2 Chem Revision Ex 22 - Answer SchemeDocument20 pagest2 Chem Revision Ex 22 - Answer SchemeNicholas Ow50% (2)
- Revision ExerciseDocument7 pagesRevision ExerciseNana BNo ratings yet
- Balanced Equations & Associated Calc's 05 QPDocument8 pagesBalanced Equations & Associated Calc's 05 QPlmao lmaoNo ratings yet
- t2 Chem Revision Ex 17Document16 pagest2 Chem Revision Ex 17Nicholas OwNo ratings yet
- Jason 291123Document12 pagesJason 291123msyahrulramdan050199No ratings yet
- Shapes of Simple Molecules - Ions 5 QPDocument8 pagesShapes of Simple Molecules - Ions 5 QPSiddhant DuggalNo ratings yet
- Acid-Base Exam Questions 3Document18 pagesAcid-Base Exam Questions 3Jake RobinsonNo ratings yet
- All AS F331 QuestionsDocument116 pagesAll AS F331 QuestionsSalvador__DaliNo ratings yet
- Organic Chemisty - Reaction MechanismsDocument67 pagesOrganic Chemisty - Reaction MechanismsKamrul Alam MasumNo ratings yet
- Halogenoalkanes Alcohol and Spectra Unit 2Document8 pagesHalogenoalkanes Alcohol and Spectra Unit 2Barminga KamurenNo ratings yet
- Mock PaperDocument9 pagesMock PaperjunetaskinNo ratings yet
- I6 Reactions of Ions in Aqueous SolutionDocument16 pagesI6 Reactions of Ions in Aqueous Solution/ “Nu” /No ratings yet
- BondingDocument14 pagesBondingMuizzudin AzaliNo ratings yet
- Extra Moles QuestionsDocument6 pagesExtra Moles QuestionschenzNo ratings yet
- Rate of Reaction 2 QP (Tomek)Document9 pagesRate of Reaction 2 QP (Tomek)Tomasz OstrowskiNo ratings yet
- IB Acids and BasesDocument45 pagesIB Acids and BasesAhmad Hajj AliNo ratings yet
- 9701 s17 QP 42 RemovedDocument18 pages9701 s17 QP 42 RemovedSherise EeNo ratings yet
- Periodic Table, Group 2 and The Halogens 1 QPDocument13 pagesPeriodic Table, Group 2 and The Halogens 1 QPmalakNo ratings yet
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocument9 pages1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereNo ratings yet
- Chemi 401 QDocument13 pagesChemi 401 QPenang Home TuitionNo ratings yet
- 2.4, 2.5, 2.6 TestDocument7 pages2.4, 2.5, 2.6 Testzafarchem_iqbalNo ratings yet
- ChemistryDocument4 pagesChemistryMartin LayneNo ratings yet
- Drip Too HardDocument176 pagesDrip Too HardNedaal AnwarNo ratings yet
- Exampro GCSE Chemistry: C3 Chapter 1 HigherDocument25 pagesExampro GCSE Chemistry: C3 Chapter 1 HigherSamuel KalemboNo ratings yet
- Group 2 - The Alkaline Earth Metals 1 QPDocument8 pagesGroup 2 - The Alkaline Earth Metals 1 QPWilliam TsuiNo ratings yet
- Chemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructureDocument25 pagesChemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructuresechosNo ratings yet
- Edexcel C3 Summary NotesDocument5 pagesEdexcel C3 Summary Notesbloodyinspired50% (2)
- Unit Level Raw Mark and Ums Grade Boundaries June 2014Document39 pagesUnit Level Raw Mark and Ums Grade Boundaries June 2014coughsyrup123No ratings yet
- Topic 1-Lifestyle, Health & Risk: WaterDocument11 pagesTopic 1-Lifestyle, Health & Risk: Waternimz1992No ratings yet
- Further Mechanics Exam Pack MSDocument45 pagesFurther Mechanics Exam Pack MScoughsyrup123No ratings yet
- C1 Gold 3Document15 pagesC1 Gold 3coughsyrup123No ratings yet
- UKMT Senior Challenge 2012Document2 pagesUKMT Senior Challenge 2012Daniel CarpenterNo ratings yet
- F321 Module 3 Practice 5 AnswersDocument4 pagesF321 Module 3 Practice 5 Answerscoughsyrup123No ratings yet
- RES9 ChemflashcardsDocument4 pagesRES9 Chemflashcardspleb123No ratings yet
- Mark Scheme June 2007 6683 Statistics S1Document8 pagesMark Scheme June 2007 6683 Statistics S1Ting Phin YuanNo ratings yet
- F321 Module 3 Practice 5Document4 pagesF321 Module 3 Practice 5coughsyrup123No ratings yet
- AS Edexcel Biology Topic 1 Lifestyle, Health and Risk 1.2 The Heart and HealthDocument17 pagesAS Edexcel Biology Topic 1 Lifestyle, Health and Risk 1.2 The Heart and HealthSammy MacuraNo ratings yet
- F321 Module 2 Practice 4Document6 pagesF321 Module 2 Practice 4coughsyrup123No ratings yet
- RES5 Bio Revision PlannerDocument3 pagesRES5 Bio Revision PlannerKawthar SmithNo ratings yet
- F321 Module 3 Practice 4Document7 pagesF321 Module 3 Practice 4coughsyrup123No ratings yet
- F321 Module 2 Practice 6Document2 pagesF321 Module 2 Practice 6coughsyrup123No ratings yet
- F321 Module 3 Practice 3Document10 pagesF321 Module 3 Practice 3coughsyrup123No ratings yet
- F321 Module 3 Practice 4 AnswersDocument4 pagesF321 Module 3 Practice 4 Answerscoughsyrup123No ratings yet
- F321 Module 3 Practice 1Document10 pagesF321 Module 3 Practice 1coughsyrup123No ratings yet
- F321 Module 3 Practice 2 AnswersDocument4 pagesF321 Module 3 Practice 2 Answerscoughsyrup123No ratings yet
- F321 Module 3 Practice 2Document7 pagesF321 Module 3 Practice 2coughsyrup123No ratings yet
- F321 Module 2 Practice 6 AnswersDocument2 pagesF321 Module 2 Practice 6 Answerscoughsyrup123No ratings yet
- F321 Module 2 Practice 5Document5 pagesF321 Module 2 Practice 5coughsyrup123No ratings yet
- F321 Module 2 Practice 5 AnswersDocument3 pagesF321 Module 2 Practice 5 Answerscoughsyrup123No ratings yet
- F321 Module 3 Practice 3 AnswersDocument4 pagesF321 Module 3 Practice 3 Answerscoughsyrup123No ratings yet
- F321 Module 3 Practice 1 AnswersDocument6 pagesF321 Module 3 Practice 1 Answerscoughsyrup123No ratings yet
- F321 Module 2 Practice 3Document7 pagesF321 Module 2 Practice 3coughsyrup123No ratings yet
- F321 Module 2 Practice 4 AnswersDocument4 pagesF321 Module 2 Practice 4 Answerscoughsyrup123No ratings yet
- F321 Module 2 Practice 2 AnswersDocument5 pagesF321 Module 2 Practice 2 Answerscoughsyrup123No ratings yet
- F321 Module 2 Practice 3 AnswersDocument4 pagesF321 Module 2 Practice 3 Answerscoughsyrup123No ratings yet
- F321 Module 2 Practice 2Document8 pagesF321 Module 2 Practice 2coughsyrup123No ratings yet
- Assessment - UK Forestry Data ICT THEORY For CAT1Document13 pagesAssessment - UK Forestry Data ICT THEORY For CAT1Joanna AchemaNo ratings yet
- Reinforced Concrete Design PDFDocument1 pageReinforced Concrete Design PDFhallelNo ratings yet
- UpdateJul2007 3julDocument10 pagesUpdateJul2007 3julAnshul SinghNo ratings yet
- 한국항만 (영문)Document38 pages한국항만 (영문)hiyeonNo ratings yet
- Ec 0301Document25 pagesEc 0301Silvio RomanNo ratings yet
- Installation of Submarine PE PipesDocument84 pagesInstallation of Submarine PE Pipeswaseemiqbal133100% (2)
- Economics - Economics - Cheat - SheetDocument1 pageEconomics - Economics - Cheat - SheetranaurNo ratings yet
- Embedded Software Development ProcessDocument34 pagesEmbedded Software Development ProcessAmmar YounasNo ratings yet
- Pipe Freezing StudyDocument8 pagesPipe Freezing StudymirekwaznyNo ratings yet
- World of Self, Family and Friends UNIT 4 - Lunchtime Speaking 37 Wednesday Friendship LanguageDocument11 pagesWorld of Self, Family and Friends UNIT 4 - Lunchtime Speaking 37 Wednesday Friendship LanguageAin NawwarNo ratings yet
- Chapter 1 INTRODUCTION TO LITERATUREDocument4 pagesChapter 1 INTRODUCTION TO LITERATUREDominique TurlaNo ratings yet
- A-1660 11TH Trimester From Mcdowell To Vodafone Interpretation of Tax Law in Cases. OriginalDocument18 pagesA-1660 11TH Trimester From Mcdowell To Vodafone Interpretation of Tax Law in Cases. OriginalPrasun TiwariNo ratings yet
- Mossbauer SpectrosDocument7 pagesMossbauer SpectroscyrimathewNo ratings yet
- Nails Care: Word Search: Name: - DateDocument1 pageNails Care: Word Search: Name: - DateDeverly Hernandez Balba-AmplayoNo ratings yet
- Sustainable Strategic Management BarbosaDocument11 pagesSustainable Strategic Management BarbosapurwawardhaniNo ratings yet
- Banin Cawu 1: Panitia Ujian Perguruan Islam Mathali'Ul FalahDocument4 pagesBanin Cawu 1: Panitia Ujian Perguruan Islam Mathali'Ul FalahKajen PatiNo ratings yet
- Chapter 5 IppDocument24 pagesChapter 5 IppRoseann EnriquezNo ratings yet
- Technical Rockwell Automation FactoryTalk HistorianDocument6 pagesTechnical Rockwell Automation FactoryTalk HistorianAmit MishraNo ratings yet
- Full Download University Physics With Modern Physics 14th Edition Young Test Bank PDF Full ChapterDocument13 pagesFull Download University Physics With Modern Physics 14th Edition Young Test Bank PDF Full Chapterpoetrycloudyzjm12q100% (19)
- Materials Management - 1 - Dr. VP - 2017-18Document33 pagesMaterials Management - 1 - Dr. VP - 2017-18Vrushabh ShelkarNo ratings yet
- Shoshana Bulka PragmaticaDocument17 pagesShoshana Bulka PragmaticaJessica JonesNo ratings yet
- Pot-Roasted Beef BrisketDocument4 pagesPot-Roasted Beef Brisketmarcelo nubileNo ratings yet
- WD Support Warranty Services Business Return Material Authorization RMA Pre Mailer For ResellerDocument3 pagesWD Support Warranty Services Business Return Material Authorization RMA Pre Mailer For ResellerZowl SaidinNo ratings yet
- 835 (Health Care Claim PaymentAdvice) - HIPAA TR3 GuideDocument306 pages835 (Health Care Claim PaymentAdvice) - HIPAA TR3 Guideअरूण शर्माNo ratings yet
- Maskote WB Zinc Stop-OffDocument7 pagesMaskote WB Zinc Stop-OffbondsivamaniNo ratings yet
- Lesson Plan 1Document3 pagesLesson Plan 1api-311983208No ratings yet
- Gandhi and The Non-Cooperation MovementDocument6 pagesGandhi and The Non-Cooperation MovementAliya KhanNo ratings yet
- Class 1 KeyDocument3 pagesClass 1 Keyshivamsingh.fscNo ratings yet
- Nascsa - Sponsor Solicitation List: January 06, 2021Document35 pagesNascsa - Sponsor Solicitation List: January 06, 2021Prasoon SimsonNo ratings yet
- PID Marcado Operación Del Paquete Del Compresor de Hidrogeno PHP-K-002 PDFDocument7 pagesPID Marcado Operación Del Paquete Del Compresor de Hidrogeno PHP-K-002 PDFDenisNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Tribology: Friction and Wear of Engineering MaterialsFrom EverandTribology: Friction and Wear of Engineering MaterialsRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet