Professional Documents
Culture Documents
Composition of The Human Body
Uploaded by
raschensOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Composition of The Human Body
Uploaded by
raschensCopyright:
Available Formats
Composition of the human body
Composition of the human body
The composition of the human body can be looked at from the point of view of either mass composition, or atomic composition. To illustrate both views, the human body is ~70% water, and water is ~11% hydrogen by mass but ~67% hydrogen by atomic percent. Thus, most of the mass of the human body is oxygen, but most of the atoms in the human body are hydrogen atoms. Both mass-composition and atomic composition figures are given below. Almost 99% of the mass of the human body is made up of the six elements oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. Only about 0.85% is composed of another five elements: potassium, sulfur, sodium, chlorine, and magnesium. All are necessary to life. The remaining elements are trace elements, of which more than a dozen are thought to be necessary for life, or play an active role in health (e.g., fluorine, which hardens dental enamel but seems to have no other function). Not all elements which are found in the human body in trace quantities play a role in life. Some of these elements are thought to be simple bystander contaminants without function (examples: caesium, titanium), while many others are thought to be active toxins, depending on amount (cadmium, mercury, radioactives). The possible utility and toxicity of a few elements at levels normally found in the body (aluminum) is debated. Functions have been proposed for trace amounts of cadmium and lead, but these are almost certainly toxic in amounts normally found in the body. There is evidence that one element normally considered a toxin (arsenic) is essential in ultratrace quantities, even in mammals. Some elements that are clearly used in lower organisms and plants (arsenic, silicon, boron, nickel, vanadium) are probably needed by mammals also, but in far smaller doses. Two halogens used abundantly by lower organisms (fluorine and bromine) are presently known to be used by mammals only opportunistically. However, a general rule is that elements found in active biochemical use in lower organisms are often eventually found to be used in some way, by higher organisms.[citation needed]
Elemental composition
The average 70kg adult human body contains approximately 6.7 x 1027 atoms and contains at least detectable traces of 60 chemical elements. About 24 or 25 of these elements are thought to play an active positive role in life and health in humans.[1] The relative amounts of each element vary by individual, with the largest contributor due to fat/muscle/bone body composition ratio differences from person to person. The numbers in the table are averages of different numbers reported by different references. The human body is ~65% water, and water is ~11% hydrogen by mass but ~67% hydrogen by atomic percent.
Atomic number 8 Element Mass Oxygen Percent of [2][3][4][5][6][7] 65 Mass [8] (kg) 43 Atomic percent 24 [9] Positive health role in mammals Group
Yes (water, electron acceptor) /No (Reactive Oxygen Species) Yes (organic compounds are hydrocarbon derivatives) Yes (e.g. water) Yes (e.g. DNA and amino acids) Yes (e.g. Calmodulin and Hydroxylapatite in bones) Yes (e.g. DNA and phosphorylation) Yes (e.g. Na+/K+-ATPase)
16
Carbon
18
16
12
14
1 7 20
Hydrogen Nitrogen Calcium
10 3 1.4
7 1.8 1.0
63 0.58 0.24
1 15 2
15 19
Phosphorus Potassium
1.1 0.25
0.78 0.14
0.14 0.033
15 1
Composition of the human body
2
0.25 0.15 0.15 0.05 0.006 0.0037 0.14 0.10 0.095 0.019 0.0042 0.0026 0.038 0.037 0.024 0.0070 0.00067 0.0012 Yes (e.g.Cysteine, Methionine, Biotin, Thiamine) Yes (e.g. Na+/K+-ATPase) Yes (e.g. Cl-transporting ATPase) Yes (e.g. binding to ATP and other nucleotides) Yes (e.g. Hemoglobin, Cytochromes) Yes/No (topically hardens teeth; toxic in higher amounts) Yes (e.g. Zinc finger proteins) Yes (probable) No (?) No (?) No (?) No (?) (toxic in higher amounts) Yes (e.g. copper proteins) No(?) (toxic?) No(?) (toxic in higher amounts) No 0.0000012 6.0e-7 7.5e-7 No? (toxic) No(?) Yes (e.g. thyroxine, triiodothyronine) No 0.0000030 4.5e-8 0.0000015 8.9e-8 0.0000015 8.9e-8 0.0000015 Yes (probable) Yes (toxic in higher amounts) Yes (e.g. urease) Yes (not confirmed) Yes (e.g. Mn-SOD) Yes (not confirmed). Toxic in higher amounts Yes (not confirmed). Toxic in high amounts. Useful medically (mood stabilizer). No (toxic) No Yes (e.g. the molybdenum oxotransferases, Xanthine oxidase and Sulfite oxidase) No (?) 3.0e-7 Yes (e.g. vitamin B12) No (toxic) No (toxic) No 3.0e-7 No No 2 14 17 4 13 16 10 6 7 15 1 16 1 17 2 8 17
16 11 17 12 26 9
Sulfur Sodium Chlorine Magnesium Iron* Fluorine
30 14 37 38 35 82 29 13 48 58 56 50 53 22 5 34 28 24 25 33 3
Zinc Silicon Rubidium Strontium Bromine Lead Copper Aluminium Cadmium Cerium Barium Tin Iodine Titanium Boron Selenium Nickel Chromium Manganese Arsenic Lithium
0.0032 0.002 0.00046 0.00046 0.00029 0.00017 0.0001 0.000087 0.000072 0.000057 0.000031 0.000024 0.000016 0.000013 0.000069 0.000019 0.000014 0.0000024 0.000017 0.000026 0.0000031
0.0023 0.0010 0.00068 0.00032 0.00026 0.00012 0.000072 0.000060 0.000050 0.000040 0.000022 0.000020 0.000020 0.000020 0.000018 0.000015 0.000015 0.000014 0.000012 0.000007 0.000007
0.00031 0.0058 0.000033 0.000033 0.000030 0.0000045 0.0000104 0.000015 0.0000045
12 14 1 2 17 14 11 13 12
80 55 42
Mercury Caesium Molybdenum
0.000019 0.0000021 0.000013
0.000006 0.000006 0.000005
8.9e-8 1.0e-7 4.5e-8
12 1 6
32 27 51 47 41 40 57
Germanium Cobalt Antimony Silver Niobium Zirconium Lanthanum 0.0000021 0.000011 0.000001 0.00016 0.0006 0.000137
0.000005 0.000003 0.000002 0.000002 0.0000015 0.000001 8e-7
14 9 15 11 5 4
Composition of the human body
3
0.000012 7e-7 7e-7 6e-7 5e-7 5e-7 4e-7 0.000014 2e-7 2e-7 2e-7 0.000026 1.1e-7 1e-7 1.3e-7 1e-7 5.0e-8 2.0e-8 5e-9 1e-17 3.6e-8 3e-14 4.5e-8 1e-17% 3.0e-9 1.2e-8 3.0e-7 No No No No No (toxic) No No No No Yes (not confirmed) No (toxic) No (toxic) No No No (toxic) No (toxic) 6 2 2 16 13 3 15 13 13 11 3 5 5
52 31 39 83 81 49 79 21 73 23 90 92 62 74 4 88
Tellurium Gallium Yttrium Bismuth Thallium Indium Gold Scandium Tantalum Vanadium Thorium Uranium Samarium Tungsten Beryllium Radium
*Iron = ~3 g in men, ~2.3 g in women The elements needed for life are relatively common in the Earth's crust, and conversely most of the common elements are necessary for life. An exception is aluminium, which is the third most common element in the Earth's crust (after oxygen and silicon), but seems to serve no function in living cells. Rather, it is harmful in large amounts.[citation needed] Transferrins can bind aluminium.[10] Periodic table highlighting dietary elements[1]
H Li Be B Al Ti V Cr Mn Fe Ru Os Hs Co Ni Cu Zn Ga In Tl C Si Ge Sn Pb Fl Tm N P As Sb Bi O S Se Te Po F Cl Br I At He Ne Ar Kr Xe Rn
Na Mg K Ca Sc Y *
Rb Sr
Zr Nb Mo Tc Hf Ta W Re Bh
Rh Pd Ag Cd Ir Pt Au Hg
Cs Ba La
Fr Ra Ac ** Rf Db Sg *
Mt Ds Rg Cn Uut Gd Tb Dy Ho Er
Uup Lv Uus Uuo Yb No Lu Lr
Ce Pr Nd Pm Sm Eu U Np
** Th Pa
Pu Am Cm Bk Cf Es Fm Md
The four organic basic elements
Quantity elements
Essential trace elements
Function suggested from active handling in mammals, but no specific identified biochemical function
Composition of the human body
Composition by molecule type
The composition can also be expressed in terms of chemicals, such as: Water Proteins including those of hair, connective tissue, etc. Fats (or lipids) Apatite in bones Carbohydrates such as glycogen and glucose DNA Dissolved inorganic ions such as sodium, potassium, chloride, bicarbonate, phosphate Gases such as oxygen, carbon dioxide, nitrogen oxide, hydrogen, carbon monoxide, methanethiol. These may be dissolved or present in the gases in the lungs or intestines. Ethane and pentane are produced by oxygen free radicals.[11] Many other small molecules, such as amino acids, fatty acids, nucleobases, nucleosides, nucleotides, vitamins, cofactors. Free radicals such as superoxide, hydroxyl, and hydroperoxyl. The composition of the human body can be viewed on an atomic and molecular scale as shown in this article. The estimated gross molecular contents of a typical 20-micrometre human cell is as follows:[]
Molecule Water Other Inorganics Lipids Other Organics Protein RNA DNA Percent of Mass Mol.Weight (daltons) Molecules Percent of Molecules 65* 1.5 12 0.4 20 1.0 0.1 18* N/A N/A N/A N/A N/A 1e11 1.74e14* 1.31e12 8.4e11 7.7e10 1.9e10 5e7 46* 98.73* 0.74 0.475 0.044 0.011 3e-5 3e-11
Water: Obviously the amount of water is highly dependent on the level of hydration. DNA: A human cell also contains mitochondrial DNA. Sperm cells contain less mitochondrial DNA than other cells. A mammalian red blood cell contains no nucleus and thus no DNA.
Materials and tissues
Body composition can also be expressed in terms of various types of material, such as: Muscle Fat Bone and teeth Brain and nerves Connective tissue Blood 7% of body weight. Lymph Contents of digestive tract, including intestinal gas Urine Air in lungs
Composition of the human body
Composition by cell type
There are many species of bacteria and other microorganisms that live on or inside the healthy human body. In fact, 90% of the cells in (or on) a human body are microbes, by number[12][13] (much less by mass or volume). Some of these symbionts are necessary for our health. Those that neither help nor harm us are called commensal organisms.
References
[1] Ultratrace minerals. Authors: Nielsen, Forrest H. USDA, ARS Source: Modern nutrition in health and disease / editors, Maurice E. Shils ... et al.. Baltimore : Williams & Wilkins, c1999., p. 283-303. Issue Date: 1999 URI: (http:/ / hdl. handle. net/ 10113/ 46493) [2] Thomas J. Glover, comp., Pocket Ref, 3rd ed. (Littleton: Sequoia, 2003), p. 324 (), which in [3] turn cites Geigy Scientific Tables, Ciba-Geigy Limited, Basel, Switzerland, 1984. [5] Distribution of elements in the human body (by weight) (http:/ / www. daviddarling. info/ encyclopedia/ E/ elbio. html) Retrieved on 2007-12-06 [7] ) [8] J. Emsley, The Element, 3rd ed., Oxford: Clarendon Press, 1998. [9] Neilsen, cited (http:/ / hdl. handle. net/ 10113/ 46493) [11] Douglas Fox, "The speed of life" (http:/ / www. newscientist. com/ article/ mg18024195. 500-the-speed-of-life. html), New Scientist, No 2419, 1 November 2003.
Article Sources and Contributors
Article Sources and Contributors
Composition of the human body Source: http://en.wikipedia.org/w/index.php?oldid=543997864 Contributors: 0paco0, 1exec1, AznBurger, Betacommand, Carstensen, Chem8700, Cmglee, Cutelyaware, Double sharp, Download, Dratman, Epbr123, Eric Kvaalen, Eric Norby, Essent, Evagrim, Fishal, Frietjes, G716, Glacialfox, Hamiltondaniel, Iain.meek83, Icairns, Jmh649, Kzyn, Littlealien182, MeekMark, Memstone, Michael Hardy, Narayansg, Narrul, Nergaal, One-ply, Paul A, Philosophia X Known, Regstuff, Sbharris, Sp, SpaceFlight89, SpecMode, Spinningspark, Szczureq, TYelliot, Timwi, Wikipelli, Woohookitty, Wtshymanski, Zemyla, , 81 anonymous edits
License
Creative Commons Attribution-Share Alike 3.0 Unported //creativecommons.org/licenses/by-sa/3.0/
You might also like
- Nutrition BiochemDocument45 pagesNutrition BiochemKesha Marie TalloNo ratings yet
- Minerals and Their Significance To A Human BodyDocument3 pagesMinerals and Their Significance To A Human BodyengghomNo ratings yet
- Reproductive System Study GuideDocument6 pagesReproductive System Study GuideJames DaurayNo ratings yet
- Last Minute RevisionDocument229 pagesLast Minute RevisionSelvaArockiamNo ratings yet
- Cations, Anions, and the Human BodyDocument1 pageCations, Anions, and the Human BodyKathryn Dominique PachecoNo ratings yet
- Easy Tips for a Healthy DietDocument11 pagesEasy Tips for a Healthy Dietdiop1959No ratings yet
- Homeostasis Booklet: Done By: Sonali AmbasanaDocument4 pagesHomeostasis Booklet: Done By: Sonali AmbasanaambasanaNo ratings yet
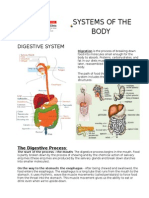
- Systems of The Body (Digestive)Document3 pagesSystems of The Body (Digestive)KryztalGhail LlanoraNo ratings yet
- Nutrition PaperDocument4 pagesNutrition Paperapi-433705780No ratings yet
- Development of The Body Cavities: EmbryologyDocument9 pagesDevelopment of The Body Cavities: EmbryologyRania FadillaNo ratings yet
- Food Energy: Energy Usage in The Human BodyDocument11 pagesFood Energy: Energy Usage in The Human Bodyzharell1019No ratings yet
- Binomial NomenclatureDocument20 pagesBinomial NomenclatureSumairaAlviNo ratings yet
- Human Body Systems VocabularyDocument4 pagesHuman Body Systems Vocabularyapi-231946379No ratings yet
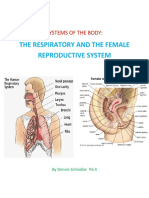
- Systems of The BodyDocument10 pagesSystems of The BodyKatrina SchindlarNo ratings yet
- Enzymes and Their Importance in Plants and AnimalsDocument4 pagesEnzymes and Their Importance in Plants and Animalsanili50% (2)
- Basal Metabolic RateDocument23 pagesBasal Metabolic RateMRITUNJAY KUMAR SHARMANo ratings yet
- Medical Terminology SyllabusDocument3 pagesMedical Terminology SyllabusAzza RezkNo ratings yet
- Asexual Vs Sexual RepoductionDocument11 pagesAsexual Vs Sexual Repoductiontssandoval100% (1)
- Telomere PDFDocument2 pagesTelomere PDFsoumita100% (1)
- Body System ChecklistDocument6 pagesBody System Checklistapi-422967453No ratings yet
- Shifting Paradigms: Matt Wallden, MSC Ost Med, Do, ND, Associate EditorDocument10 pagesShifting Paradigms: Matt Wallden, MSC Ost Med, Do, ND, Associate EditorStephen HonesNo ratings yet
- EYE ASSESSMENT AND DISORDERSDocument374 pagesEYE ASSESSMENT AND DISORDERSFirifan DiribaNo ratings yet
- Endocrine System PhysiologyDocument2 pagesEndocrine System PhysiologyLuisGabitoNo ratings yet
- Autoimmune EncephalitisDocument39 pagesAutoimmune EncephalitisnurulmaulidyahidayatNo ratings yet
- Phytoecdysteroids RkleinDocument11 pagesPhytoecdysteroids RkleintrungkunmingNo ratings yet
- Chapter 12 Coordination and ControlDocument22 pagesChapter 12 Coordination and ControlXaundrae McDonaldNo ratings yet
- Chapter 1: An Introduction To The Human BodyDocument7 pagesChapter 1: An Introduction To The Human BodyShyra EntradaNo ratings yet
- Endocrine SystemDocument21 pagesEndocrine SystemMark DimarucutNo ratings yet
- Sources Hormone FunctionDocument2 pagesSources Hormone FunctionKatherine Joy MaderajeNo ratings yet
- Human Health and DiseasesDocument24 pagesHuman Health and DiseasesAnujith AnuNo ratings yet
- Natural Hybridization in Butterflies and PlantsDocument8 pagesNatural Hybridization in Butterflies and PlantsJohn MewburnNo ratings yet
- 3.6 - The Respiratory SystemDocument4 pages3.6 - The Respiratory SystemDjej100% (1)
- Body CavitiesDocument22 pagesBody Cavitiesapi-421876553No ratings yet
- Elements in Our Body and in TechnologyDocument46 pagesElements in Our Body and in TechnologySherilyn Closa BunagNo ratings yet
- Elements Found in Blood and BonesDocument2 pagesElements Found in Blood and BonesMARIEL MUTUCNo ratings yet
- Human BodyDocument2 pagesHuman BodyTinaTin PareulidzeNo ratings yet
- FMD of A Human BodyDocument2 pagesFMD of A Human Bodyvangiecapili09271995No ratings yet
- Chemistry of Life Elements and CompoundsDocument31 pagesChemistry of Life Elements and CompoundsHapsah MuhammadNo ratings yet
- What Elements Are Essential To The Human Body? Give Its ImportanceDocument3 pagesWhat Elements Are Essential To The Human Body? Give Its ImportanceDynie Anne PoliquitNo ratings yet
- GENERAL BIOCHEMISTRY COURSE NOTES 2019-2020.pdf - ProtectedDocument47 pagesGENERAL BIOCHEMISTRY COURSE NOTES 2019-2020.pdf - ProtectedChia Oliver100% (2)
- Toxic Chemicals in The EnvironmentDocument18 pagesToxic Chemicals in The Environmenthariette kimby gonzalesNo ratings yet
- The Main Elements Found in the Human BodyDocument1 pageThe Main Elements Found in the Human BodyJose Miguel Delacruz MalanaNo ratings yet
- Bio ElementsDocument28 pagesBio ElementsBioinformatics Biotechnology100% (1)
- Invertebrates 1 @chetanbhagatDocument269 pagesInvertebrates 1 @chetanbhagatEka Dyah ANo ratings yet
- Anatomy and Physiology AssignmentDocument2 pagesAnatomy and Physiology AssignmentAj MirandaNo ratings yet
- Урок 43Document3 pagesУрок 43Ольга ХОМИШАКNo ratings yet
- Bioelements & BiomoleculesDocument13 pagesBioelements & Biomoleculesjennifer nogueraNo ratings yet
- Molecules of LifeDocument55 pagesMolecules of LifeAnabelle Gonzales MontevirgenNo ratings yet
- Concept: Nutrients and NutritionDocument3 pagesConcept: Nutrients and NutritionLe DioNo ratings yet
- Biomolecules and Water Ch-1 Biochemistry of ProteinsDocument14 pagesBiomolecules and Water Ch-1 Biochemistry of ProteinsAditi SharmaNo ratings yet
- 3.1 - Chemical Elements and WaterDocument5 pages3.1 - Chemical Elements and WaterAlvin JovanNo ratings yet
- BiochemistryDocument43 pagesBiochemistryIza StudennaNo ratings yet
- Types and Functions of Organic and Inorganic CompoundsDocument2 pagesTypes and Functions of Organic and Inorganic CompoundsSofia Coleen Naluaran TemplonuevoNo ratings yet
- Module 2: The Chemical Level of Organization: Topic: Basic Chemistry & Biochemistry Learning TargetsDocument15 pagesModule 2: The Chemical Level of Organization: Topic: Basic Chemistry & Biochemistry Learning Targetsalmira garciaNo ratings yet
- An Introductory Course in Organic: Dosen: Dr. Ir. Sukirno M.Eng Asisten ArivaDocument84 pagesAn Introductory Course in Organic: Dosen: Dr. Ir. Sukirno M.Eng Asisten ArivaMiranda Hasanah ArrasyidNo ratings yet
- From Dust To Man Scientific ProofDocument3 pagesFrom Dust To Man Scientific ProofandreuxandrearosasNo ratings yet
- Cristy AlfonsoDocument17 pagesCristy AlfonsoAnonymous yiqdcRgYdJNo ratings yet
- Basic K. 7Document3 pagesBasic K. 7Demisachew TenaNo ratings yet
- Unit Five Kingdom Classification: StructureDocument23 pagesUnit Five Kingdom Classification: Structurekaladhar reddyNo ratings yet
- The Didactical ChallengeDocument315 pagesThe Didactical ChallengeraschensNo ratings yet
- Division of A StringDocument26 pagesDivision of A StringraschensNo ratings yet
- Mesmerism and Its OpponentsDocument403 pagesMesmerism and Its OpponentsraschensNo ratings yet
- A Rudimentary Treatise On Clock and Watc PDFDocument307 pagesA Rudimentary Treatise On Clock and Watc PDFraschensNo ratings yet
- COMPLETE Letters of Petrus PeregrinusDocument76 pagesCOMPLETE Letters of Petrus PeregrinusDan BeesonNo ratings yet
- Rationale of MesmerisimDocument254 pagesRationale of Mesmerisimraschens100% (1)
- The Sacred CircleDocument611 pagesThe Sacred CircleraschensNo ratings yet
- Mechanical Philosophy Horology and Astro PDFDocument597 pagesMechanical Philosophy Horology and Astro PDFraschensNo ratings yet
- The Principles of MR Harrison S Timekeep PDFDocument63 pagesThe Principles of MR Harrison S Timekeep PDFraschensNo ratings yet
- Turning and Mechanical Manipulation PDFDocument499 pagesTurning and Mechanical Manipulation PDFraschensNo ratings yet
- The Circle of The Mechanical Arts PDFDocument711 pagesThe Circle of The Mechanical Arts PDFraschensNo ratings yet
- A System of Mechanical Philosophy PDFDocument737 pagesA System of Mechanical Philosophy PDFraschensNo ratings yet
- Home etching circuit boards with toner transfer in under 40 stepsDocument5 pagesHome etching circuit boards with toner transfer in under 40 stepsraschensNo ratings yet
- Dinural Variation of Terrestrial MagnetismDocument42 pagesDinural Variation of Terrestrial MagnetismraschensNo ratings yet
- 18 Man Is A Compound Electrical MagnetDocument192 pages18 Man Is A Compound Electrical MagnetKeith WarlickNo ratings yet
- Kepler's Laws of Planetary MotionDocument11 pagesKepler's Laws of Planetary MotionraschensNo ratings yet
- The Power Source: The 25 Amp & 60 Amp RectifierDocument0 pagesThe Power Source: The 25 Amp & 60 Amp RectifierraschensNo ratings yet
- CNCDocument26 pagesCNCraschensNo ratings yet
- Embellishing Your Woodturning With Inlay TechniquesDocument5 pagesEmbellishing Your Woodturning With Inlay TechniquesraschensNo ratings yet
- Euclid ElementsDocument571 pagesEuclid ElementsraschensNo ratings yet
- The Art of ChannelingDocument14 pagesThe Art of ChannelingEarthcat100% (6)
- Euclid ElementsDocument449 pagesEuclid ElementsraschensNo ratings yet
- Kepler's Laws of Planetary MotionDocument11 pagesKepler's Laws of Planetary MotionraschensNo ratings yet
- Chinese ChronologyDocument290 pagesChinese ChronologyraschensNo ratings yet
- The Word Made Manifest Through Sacred GeometryDocument384 pagesThe Word Made Manifest Through Sacred GeometryDerek Gerlach100% (15)
- COMPLETE Letters of Petrus PeregrinusDocument76 pagesCOMPLETE Letters of Petrus PeregrinusDan BeesonNo ratings yet
- Cosmic Law - Patterns in The Universe by Dean BrownDocument67 pagesCosmic Law - Patterns in The Universe by Dean BrownTaur100% (9)
- Dinural Variation of Terrestrial MagnetismDocument42 pagesDinural Variation of Terrestrial MagnetismraschensNo ratings yet
- Knowledge: ManualDocument36 pagesKnowledge: ManualraschensNo ratings yet
- Composition of The Human BodyDocument6 pagesComposition of The Human BodyraschensNo ratings yet
- Common Sense Renewed R. C. ChristianDocument276 pagesCommon Sense Renewed R. C. Christianwarhed76100% (3)
- AmadeusDocument3 pagesAmadeusCleofe Mae Piñero AseñasNo ratings yet
- Auto IntroductionDocument90 pagesAuto IntroductionShivanand ArwatNo ratings yet
- Hoa ReviewerDocument3 pagesHoa ReviewerRachel Mae BahoyNo ratings yet
- Never Fear, Never Quit - A Story of Courage and Perseverance PDFDocument68 pagesNever Fear, Never Quit - A Story of Courage and Perseverance PDFnaveen100% (2)
- CP I-O Modules PDFDocument91 pagesCP I-O Modules PDFVlad ChioreanNo ratings yet
- Intelligence, Reasoning, Creativity, and WisdomDocument3 pagesIntelligence, Reasoning, Creativity, and WisdomSammy DeeNo ratings yet
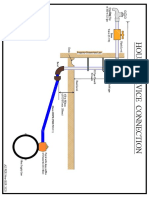
- House Service Connection NEW BSR 2020-1Document1 pageHouse Service Connection NEW BSR 2020-1Deshraj BairwaNo ratings yet
- TATA Gluco PDFDocument5 pagesTATA Gluco PDFsidharth dasNo ratings yet
- Adept Conveyor Technologies Product ManualDocument32 pagesAdept Conveyor Technologies Product ManualBagus Eko BudiyudhantoNo ratings yet
- FMDocument12 pagesFMGajera HarshadNo ratings yet
- CBSE Class 11 English Sample Paper Set 5Document8 pagesCBSE Class 11 English Sample Paper Set 5Shantam BasuNo ratings yet
- Application of Operations Research in Banking FinanceDocument13 pagesApplication of Operations Research in Banking FinanceInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Countable UncountableDocument4 pagesCountable UncountablePaoLo Mena la TorreNo ratings yet
- Mibk - TDS PDFDocument3 pagesMibk - TDS PDFMardianus U. RihiNo ratings yet
- 123 Rule For TRS MMD DG ShippingDocument2 pages123 Rule For TRS MMD DG ShippingGurjit SinghNo ratings yet
- Baobawon Island Seafood Capital Bless Amare Sunrise Resort Sam Niza Beach Resort Oklahoma Island Rafis Resort Inday-Inday Beach ResortDocument42 pagesBaobawon Island Seafood Capital Bless Amare Sunrise Resort Sam Niza Beach Resort Oklahoma Island Rafis Resort Inday-Inday Beach ResortAmber WilsonNo ratings yet
- TTBR 10 January 2024 LDocument22 pagesTTBR 10 January 2024 Lfossil.tractor0sNo ratings yet
- NSBI 2022-2023 FormsDocument16 pagesNSBI 2022-2023 FormsLove MaribaoNo ratings yet
- 2G - 4G Network Module - Data Sheet - EnglishDocument8 pages2G - 4G Network Module - Data Sheet - EnglishbbwroNo ratings yet
- DVRP Newsletter Resilience Vol. 2, Issue 2Document6 pagesDVRP Newsletter Resilience Vol. 2, Issue 2Lucius Doxerie Sr.No ratings yet
- Simple LED Flasher CircuitsDocument5 pagesSimple LED Flasher CircuitsVivek BNo ratings yet
- Sir ClanDocument109 pagesSir ClanJames AbendanNo ratings yet
- Premchand Deliverance Download in PDFDocument4 pagesPremchand Deliverance Download in PDFRiya W100% (3)
- Portland Traffic Crash Report 2021Document11 pagesPortland Traffic Crash Report 2021KGW NewsNo ratings yet
- IMG - 0092 PSME Code 2012 90Document1 pageIMG - 0092 PSME Code 2012 90Bugoy2023No ratings yet
- University of Toronto Astronomy 101 Midterm Test QuestionsDocument6 pagesUniversity of Toronto Astronomy 101 Midterm Test QuestionsTrash RowzanNo ratings yet
- Chips Unlimited Blend LibraryDocument20 pagesChips Unlimited Blend Librarymizan sallehNo ratings yet
- Diversification in Flavoured Milk: A ReviewDocument6 pagesDiversification in Flavoured Milk: A ReviewInternational Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- Improving MV Underground Cable Performance - Experience of TNB MalaysiaDocument4 pagesImproving MV Underground Cable Performance - Experience of TNB Malaysialbk50No ratings yet