Professional Documents
Culture Documents
Introduction
Uploaded by
Saleha ShamsudinOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Introduction
Uploaded by
Saleha ShamsudinCopyright:
Available Formats
Recently, the bioconversion of renewable lignocellulosic biomass into fuels and chemicals as an alternative to petroleum is gaining importance due
to the realization of diminishing natural oil and gas resources. Lignocellulose into biofuels and chemicals is the most feasible conversion route strategy in terms of sustainability. In general, lignocellulosic biomass is composed of cellulose, hemicellulose and lignin. Approximately, 70% of dry mass of lignocellulosic materials are present as cellulose and hemicellulose. They are polysaccharides of the required fermentable sugars for almost fermentation process by microorganism into fuels and chemicals.
Currently more than 46 900 km2 of oil palm are cultivated in Malaysia, the worlds largest exporter of palm oil [1]. As one of the biggest exporters of palm oil and palm oil products, palm oil industries in Malaysia generate huge quantities of biomass in the form of oil palm empty fruit bunch (EFB), oil palm shells (OPS) and oil palm fibers (OPF). The potential of these are yet to be exploited. Out of these biomass, the EFB generated during processing of palm oil, can be considered as primary feedstock for the production of sugars which can be further used as carbon source for biological conversion through fermentation.
Efficient pretreatment method can significantly enhance the hydrolysis of biomass into fermentable sugars. Therefore, a detailed knowledge of pretreatment is required in order to investigate on the potential utilization of EFB as a substrate for sugars production. The structure of EFB needs to be examined to facilitate optimal utilization of this bioresource for biofuels and chemicals production.
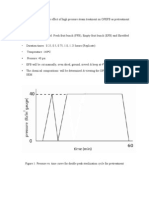
Steam has the potential to degrade (thereby pretreat) the complex structure of lignocellulosic biomass. In steam pretreatment, the biomass is simultaneously treated at high
pressures and high temperatures steam of 140 C to 260 C, for few to several minutes. Steam pretreatment has been reported to be efficient in partially hydrolyzing hemicelluloses, modifying the lignin, increasing access to surface area, decreasing the crystallinity of cellulose and its degree of polymerization [5]. Steam pretreatment on EFB for sugars production is an economic option that can be implemented in the palm oil mill. During palm oil processing, steam is continuously being generated in the mill for electricity and sterilizing the fruits. The boilers produce superheated steam which is used to generate electricity through turbine generator. The low-pressure steam (140 C, 0.28 MPa) is used for heating purposes in the factory. Every year, palm oil mills produce 50 Gkg of OPS and OPF; but only 60 % are used as solid fuel for steam boilers [10]. This amount is sufficient to support the application of steam for the pretreatment of EFB in the palm oil mill. Overall, this pretreatment is attractive to be practiced in the palm oil mill as it include renewable resources (water, OPS and OPF), as all these could be considered as inexpensive resources and is readily available in the mill.
In this study, a pretreatment step is intended to open up the biomass (EFB) for enzymatic attack into sugars from the hemicellulose and cellulose breakdown components. Saturated steam pretreatment or commonly recognized as autohydrolysis is the process of converting lignocellulose into sugars by an exposure to the high temperature and pressure of steam with no addition of external catalysts. Lignin and other components not converted can be burned to provide the heat and electricity that needed to run the overall process involved (Wyman 2007) or to be use in the palm oil mill production. On the other hand, lignin could also be potentially used as a substrate for highly value added product of lignophenol production. Therefore, the pretreatment used is a cost-effective pretreatment method strategy which employed an excess steam from the mill to pretreat (autohydrolyse) the EFB as feasibility
study on EFB. Chemical composition, sugars concentration and structural changes were determined in order to assess the effect of steam pretreatment on the different types of EFB biomass.
A preliminary study on the different substrates and enzymes concentration together with varied temperature and pH is evaluated using common commercial cellulase enzyme, Cellulclast by Novozymes for enhancement of sugars yield. Three pretreatment conditions were chose purposely to study the effects of different parameters tested in saccharification. Later experiments were done to increase the temperatures and pressures of pretreatment for enhancement of saccharification. In this study, varied of pretreatment time is used in order to investigate the best saturated pretreatment severity factor on sugars enhancement with minor loses of polysaccharides components. The best steam pretreated EFB was then be evaluated using the scanning electron micrography, fiber analysis, FTIR spectroscopy, X-ray diffractogram, BET and thermal analysis. While the steam condensate was determined by GCMS, HPLC and colorimetric method in estimating the value of degradation products. A proposed energy balances based on mass balances calculation for the direct separation xylose and glucose from saturated steam pretreatment and saccharification is described in detail.
Since some researchers have claimed that biomass pretreatment as the second most expensive unit cost [11].
Cellulose, a principle component of all plant materials, is considered one of the most abundant renewable resources in the world. Cellulose is made of linked glucose molecules connected by 1, 4 glycosidic bonds. Hemicellulose is a complex, heterogeneous mixture of sugars and sugar derivatives that form a highly branched network. The monomers that comprise hemicelluloses are hexoses (glucose, galactose and mannose) and pentoses (arabinose and xylose). Hemicellulose can be classified into three groups, namely xylans, mannans and galactans based on the polymer backbone that is very often homopolymeric with 1, 4 linkage. Cellulosic materials also contain lignin, a three dimensional phenylpropane polymer with phenylpropane units held together by ether and carbon-carbon bonds (Sun, Y 2002).
The structures of the lignocellulosic biomass, especially cellulose crystallinity, the sheathing of hemicelluloses, and the lignin barrier, make it more recalcitrant enzymatic hydrolysis compared to corn starch. Mechanical or chemical pretreatment is used to break down the hemicelluloses and lignin structures in order to improve the substrate digestibility (Sun, Y 2002).
Cellulose is a polymer of glucose, a 6-carbon sugar. Hemicellulose is consists of a mixture of 5-carbon and 6-carbon sugar such as xylose, mannose, glucose, arabinose, galactose and uronic acids. Lignin is a phenolic polymer that makes up the bulk of the remaining dry mass and cannot be utilized by ethanol fermenting microorganisms (Greer 2005).
The varied raw materials used in manufacture of ethanol via fermentation are classified into three main types of raw materials. There are sugars (from sugarcane, sugar beets, molasses and fruits), starches (from corn, cassava, potatoes and root crops) and lignocellulosic biomass (from wood, agricultural residues and paper mills). Starches and cellulose must first be hydrolyzed to fermentable sugars by the action of enzymes or mineral acids (Lin & Tanaka 2006).
2.2
LIGNOCELLULOSIC BIOMASS
Lignocellulosic biomass is principally composed of the compounds cellulose, hemicelluloses and lignin.
The African oil palm, Elaeis guineensis is used as commercial agriculture in the production of palm oil in Malaysia (Ibrahim 2006). At present, Malaysia is the largest producer and exporter of oil palm produced about 51% of all oil palm obtained in the world (Rodrguez et al 2007). However, palm residues are largely wasted. Palm oil production generates 8.8 106 t y-1 of empty fruit bunches, 5.4 106 t y-1 of fibre and 2.3 106 t y-1 of shell (Husain et al 2002). Oil palm empty fruit bunch (OPEFB) is the major component of all solid wastes at the mill. According to Kittikun (2002), in one ton of fresh fruit bunches (FFB) composed of 230-250 kg of empty fruit bunch. Recently, it was reported that, 15 million tons
of OPEFB waste is generated annually in Malaysia by oil palm mills. In the past, OPEFB often be used as fuel to generate steam at the mills which creates environmental pollution problems in nearby localities (Rahman et al 2007). However, burning is now prohibited by regulations to prevent air pollution. Therefore nowadays, OPEFB can be used as an alternative raw material to produce bioethanol due to it contains of cellulose and hemicellulose that can be degraded into fermentable sugars through enzymatic hydrolysis. The OPEFB has a fibrous structure and the fibers stick together to form vascular bundles. The chemical composition of OPEFB is shown in Table 1 (% dry weight, w/w). The conversion of lignocellulosic biomass to ethanol is more challenging than regular food biomass due to the complex structure of the plant cell wall (Rebecca et al 2007). In order to obtain high fermentable sugars, the structure of OPEFB has to be altered or removed using suitable pretreatment method. The pretreatment method is used to facilitate the enzymatic hydrolysis of lignocellulosic materials (hgren et al 2007). It has been noted that heating lignocellulosic materials to high temperature changes its physical and chemical properties. Heat treatment using moist heat or steam on lignocellulosic materials has been applied by many researchers as a pretreatment in lignocellulosic bioethanol production (hgren et al 2007, Linde et al 2007, Viola et al 2007, Negro et al 2003, Kaar et al 1998).
In the palm oil mill, sterilization process on fresh fruit bunch (FFB) has been used to inactivate the natural enzymes, loosen the fruits off the bunch and soften the mesocarp, resulting in easier extraction of oil. Typical steam temperature used in a horizontal direct contact steam sterilizer at the mill is 140oC for 50 minutes (Prasertsan & Prasertsan 1996). In this study, the effect of heat treatment using high pressure steam generated from the boiler in the mill on OPEFB as a pretreatment will be investigated.
In this study, steaming and mechanical treatment might be combined to effectively disrupt the cellulosic structure and make it more susceptible to enzymatic hydrolysis. The conversion of hemicelluloses and cellulose through enzymatic hydrolysis result in the production of pentoses and hexoses sugars. Enzymatic hydrolysis of cellulose to glucose consists of the cellulase adsorption onto the surface of the cellulose, the biodegradation of cellulose to fermentable sugars and the desorption of the cellulose. Enzymatic degradation of cellulose to glucose is generally accomplished by at least three major classes of enzymes: endo-glucanases, exo-glucanases and -glucosidase. These enzymes are usually called together cellulose or cellulolytic enzymes (Wyman 1996). Whereas since hemicellulose contains different sugar units, the hemicellulytic enzymes are more complex and involve at least endo-1,4--D-xylanases, exo-1,4--D-xylosidases, endo-1,4--D-mannanases, -
mannosidases, acetyl xylan esterases, -glucuronidase, -L-arabinofuranosidases and galactosidases (Taherzadeh & Karimi 2007).
Current industrial fermentation organisms are design to only convert glucose to ethanol and other products. In order to utilize lignocellulosic biomass which contains 5- and 6- carbon sugars, the microorganism that can ferment efficiently 5- and 6- carbon sugars must be used and developed. The co-fermentation using single or consotia of yeast is oriented to complete assimilation of all the sugars previously released during the hydrolysis of lignocellulosic biomass. Hence, in this study, the utilizing of local yeast isolates that capable of assimilating both hexoses and pentoses in an optimal way could allow high conversion and ethanol yield.
2.4
BIOMASS PRETREATMENT
The purpose of preatment of lignocellulosic materials is to remove lignin and hemicelluloses, reduce cellulose crystallinity and increase the porosity of the materials. Pretreatment must meet the following requirements: (1) improve the formation of sugars or the ability to subsequently form sugars by enzymatic hydrolysis; (2) avoid the degradation or loss of carbohydrate; (3) avoid the formation of byproducts inhibitory to the subsequent hydrolysis and fermentation processes; be cost-effective. For the pretreatment of lignocellulosics, several physical, physical-chemical, chemical and biological processes have been developed (Snchez & Cardona 2007). The main pretreatment methods reported in literature are shown in Table 2. Methods Procedures Examples of pretreated materials / Remarks References
Table 2: Pretreatment methods of lignocellulosic biomass for fuel ethanol production 2.5 ENZYME HYDROLYSIS
Cellulose can be hydrolysed by the action of cellulases into glucose units. The classical cellulase system includes endoglucanase, exoglucanase and cellobiase (-glucosidase). Endoglucanase attacks more or less randomly at sites within (1-4)--D-glucan chains in amorphous regions of cellulose or at the surface of microfibrils. Exoglucanase releases cellobiose from non-reducing ends of -D-glucan chains. Cellobiase hydrolyzes cellobiose and water-soluble cellodextrins to glucose (Lee, J 1997).
2.6
SACCHARIFICATION
OR
FERMENTATION
OF
BIOMASS
HYDROLYZATES
Once the cellulose and hemicelulose have been broken down to simple sugars, fermentation can then take place (Murphy & McCarthy 2005). The classical configuration employed for fermenting biomass hydrolyzates involves a sequential process where the hydrolysis of cellulose and fermentation are carried out in different units. This configuration is known as separate hydrolysis and fermentation (SHF). In the alternative variant, the simultaneous saccharification and fermentation (SSF), the hydrolysis and fermentation are performed in a single unit (Snchez & Cardona 2007).
Balusu, R., Paduru, R., Kuravi, S.K., Seenayya, G. & Reddy, G. 2005. Optimization of critical medium components using response surface methodology for ethanol production from cellulosic biomass by Clostridium thermocellum SS19. Process Biochemistry 40:3025-3030. Cheung, S.W. & Anderson, B.C. 1996. Laboratory investigation of ethanol production from municipal primary wastewater solids. Bioresource Technology 59:81-96. Delgenes, J.P., Laplace, J.M., Moletta, R. & Navarro, J.M. 1996. Comparative study of separated fermentations and cofermentation processes to produce ethanol from hardwood derived hydrolysates. Biomass and Bioenergy 11(4):353-360. Greer, D. 2005. Creating cellulosic ethanol: spinning straw into fuel. (online) http://www.harvestcleanenergy.org (22nd August 2007) Levin, D.B., Islam, R., Cicek, N.Sparling, R. 2006. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Hydrogen Energy 31:14961503. Mes-Hartree, M. & Saddler, J.N. 1982. Butanol production of Clostridium acetobutylicum grown on sugars found in hemicelluloses hydrolysates. Biotechnology Letters 4:247252. Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426. hgren, K., Vehmannper, J., Siika-Aho, M., Galbe, M., Viikari, L. & Zacchi, G. 2006. High temperature enzymatic prehydrolysis prior to simultaneous saccharification and fermentation of steam pretreated corn stover for ethanol production. Enzyme and Microbial Technology 40:607-613.
Rahman, S.H.A., Choudhury, J.P. & Ahmad, A.L. 2007. Production of xylose from oil palm empty fruit bunch fiber using sulfuric acid. Biochemical Engineering Journal 30:97103. Rani, K.S, Swamy, M.V. & Seenayya, G. 1998. Production of ethanol from various pure and natural cellulosic biomass by Clostridium thermocellum strains SS21 and SS22. Process Biochemistry 33(4):435-440. Srinivas, D., Rao, K.J., Thdore, K. & Panda, T. 1995. Direct conversion of cellulosic material to ethanol by the intergenetic fusant Trichoderma reesei QM 9414/Saccharomyces cerevisiae NCIM 3288. Enzyme and Micobial Technology 17:418-423. Wyman, C.E. 2007. What is (and is not) vital to advancing cellulosic ethanol. Trends in Biotechnology 25(4):153-157. Yu, Z. & Zhang, H. 2003. Ethanol fermentation of acid-hydrolyzed cellulosic pyrolysate with Saccharomyces cerevisiae. Bioresource Technology 90:95-100.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Fed-Batch CultivationDocument14 pagesFed-Batch CultivationSaleha ShamsudinNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Mammalian Cell Bioreactor SystemDocument41 pagesMammalian Cell Bioreactor SystemSaleha ShamsudinNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- PID Tuning FormulaDocument1 pagePID Tuning FormulaSaleha ShamsudinNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- Group 3Document15 pagesGroup 3Saleha ShamsudinNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Comparison Between Simultaneous Saccharification and Fermentation and Separate Hydrolysis and Fermentation Using Steam-Pretreated Corn StoverDocument2 pagesA Comparison Between Simultaneous Saccharification and Fermentation and Separate Hydrolysis and Fermentation Using Steam-Pretreated Corn StoverSaleha ShamsudinNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Organic Chemistry: Carbonyl Compounds IIDocument73 pagesOrganic Chemistry: Carbonyl Compounds IISaleha ShamsudinNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- PID Tuning FormulaDocument1 pagePID Tuning FormulaSaleha ShamsudinNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- PID ControllerDocument11 pagesPID ControllerSaleha ShamsudinNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Quiz 1: A) With The Aid of The Diagram, Described The Characteristics of The FollowingDocument1 pageQuiz 1: A) With The Aid of The Diagram, Described The Characteristics of The FollowingSaleha ShamsudinNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Group 7Document12 pagesGroup 7Saleha ShamsudinNo ratings yet
- Quiz 1: A) With The Aid of The Diagram, Described The Characteristics of The FollowingDocument1 pageQuiz 1: A) With The Aid of The Diagram, Described The Characteristics of The FollowingSaleha ShamsudinNo ratings yet
- Aldehyde Ketone Guide ReactionsDocument12 pagesAldehyde Ketone Guide ReactionsSaleha ShamsudinNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- How Cells Grow in Continuous CultureDocument6 pagesHow Cells Grow in Continuous CultureSaleha ShamsudinNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Continous Fermentation1Document38 pagesContinous Fermentation1Saleha ShamsudinNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Fig 1: Schematic Overview of Biomass To Ethanol Conversion ProcessDocument2 pagesFig 1: Schematic Overview of Biomass To Ethanol Conversion ProcessSaleha ShamsudinNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Sterilise R 1Document1 pageSterilise R 1Saleha ShamsudinNo ratings yet
- Experiment 1: To Study The Effect of High Pressure Steam Treatment On OPEFB As PretreatmentDocument5 pagesExperiment 1: To Study The Effect of High Pressure Steam Treatment On OPEFB As PretreatmentSaleha ShamsudinNo ratings yet
- House For SaleDocument1 pageHouse For SaleSaleha ShamsudinNo ratings yet
- Jurnal Saleha 1923-1928Document6 pagesJurnal Saleha 1923-1928Saleha ShamsudinNo ratings yet
- Sealed EfbDocument1 pageSealed EfbSaleha ShamsudinNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Horiba A GUIDEBOOK To Particle Size AnalysisDocument32 pagesHoriba A GUIDEBOOK To Particle Size AnalysislouispriceNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Mini Project 2008Document1 pageMini Project 2008Saleha ShamsudinNo ratings yet
- Unit 10R - CarbohydratesDocument20 pagesUnit 10R - CarbohydratesGovind ManglaniNo ratings yet
- 24 Axens Julie Maillot PDFDocument17 pages24 Axens Julie Maillot PDFErikoNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Non-Alcoholic Beverages: Sustainability Accounting StandardDocument32 pagesNon-Alcoholic Beverages: Sustainability Accounting StandardLeslie TerronesNo ratings yet
- Monosaccharides DisaccharidesDocument10 pagesMonosaccharides Disaccharidesanujaa19100% (1)
- 270 274Document5 pages270 274Vicha FatanahNo ratings yet
- Notes CarbohydratesDocument21 pagesNotes CarbohydratesChris_Barber09100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Biomass and Bioenergy Unit 1Document6 pagesBiomass and Bioenergy Unit 1Rafia Rayana btbcNo ratings yet
- Isolation and Characterization of CarbohydratesDocument7 pagesIsolation and Characterization of CarbohydratesKyle Dillan LansangNo ratings yet
- TOLLENSDocument5 pagesTOLLENSDDS (Dingdong Dantes Supporter)No ratings yet
- For 80 Cents More Ielts Reading AnswerDocument2 pagesFor 80 Cents More Ielts Reading AnswerAnóòp Paudel0% (2)
- Renewable and Sustainable Energy Reviews: Tatsuji KoizumiDocument8 pagesRenewable and Sustainable Energy Reviews: Tatsuji KoizumiRASH nurNo ratings yet
- Science Reviewer - FinaDocument3 pagesScience Reviewer - FinaAmamore Lorenzana PlazaNo ratings yet
- Biochemistry Lab Report on Benedict's Test for Reducing SugarsDocument5 pagesBiochemistry Lab Report on Benedict's Test for Reducing SugarsVARO Da GamerNo ratings yet
- Application of Clean Fuels in Combustion EnginesDocument251 pagesApplication of Clean Fuels in Combustion EnginesFernando CardenasNo ratings yet
- Starch Properties and ReactionsDocument28 pagesStarch Properties and ReactionsHappie DilaoNo ratings yet
- Carbohydrates Practical ReportDocument9 pagesCarbohydrates Practical ReportshubaNo ratings yet
- Exercise 4:: Organic Components: CarbohydratesDocument20 pagesExercise 4:: Organic Components: CarbohydratespikachuzingungaNo ratings yet
- Introduction CarbohydratesDocument32 pagesIntroduction CarbohydratesvershaparchaNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Sbt1102 - Biochemistry Unit 1 CarbohydratesDocument22 pagesSbt1102 - Biochemistry Unit 1 CarbohydratesJeremy CorrenNo ratings yet
- Cellulosic Ethanol From CornDocument21 pagesCellulosic Ethanol From CornSujala BhattaraiNo ratings yet
- Biofuel Chemistry ProjectDocument18 pagesBiofuel Chemistry ProjectThiviNo ratings yet
- Sweetening Agents in Confectionery ProductsDocument14 pagesSweetening Agents in Confectionery ProductsSandhya DeviNo ratings yet
- Biodiesel From JatrophaDocument15 pagesBiodiesel From JatrophaSergio A Mtz BhaNo ratings yet
- Details of Sugar Industries in Tamil NaduDocument3 pagesDetails of Sugar Industries in Tamil NadugouthamNo ratings yet
- Carbohydrates Lecture 1Document52 pagesCarbohydrates Lecture 1hafsaharshadNo ratings yet
- Laboratory Activity On Carbohydrates Part IIDocument5 pagesLaboratory Activity On Carbohydrates Part IIZAIRA N. CNo ratings yet
- Formal Report (Tests For Carbohydrates)Document15 pagesFormal Report (Tests For Carbohydrates)Angelo TolentinoNo ratings yet
- Day13 ICISPanAmericanGlycerineStructuralShiftOctober2018FinalDocument42 pagesDay13 ICISPanAmericanGlycerineStructuralShiftOctober2018FinalKUKUNo ratings yet
- Meoma SpecDocument1 pageMeoma SpecRio FransenNo ratings yet
- Test Result Discussion/Explanation: CarbohydratesDocument11 pagesTest Result Discussion/Explanation: CarbohydratesAnnapril TasicNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)