Professional Documents
Culture Documents
Sexual Determination DX: LH Elevated E Elevated
Uploaded by
samantha_schnei9354Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sexual Determination DX: LH Elevated E Elevated
Uploaded by
samantha_schnei9354Copyright:
Available Formats
REPRO DISEASES Disease Name Sexual Determination Dx Gonadal Agensis if no germ cell => no gonad bowing of the can
look femur and tibia phenotypically (long bones); female anomalies of ribs from Sox9 - on an autosome; if you have an XY female with bowing of the long bones - think Sox 9 mutation Clinical presentation Associated Signs/ Symptoms Genetics Labs / Imaging Gross LM Tx Notes
Campomelic Dysplasia
phenotypic female 46, XY; with small vagina, Gonads = Androgen no uterus, testes but Insensitivity (aka intraabdominal receptors are Testicular testes; scant pubic bad => can't Feminization) hair and large recognize the breasts T or DHT phenotypic female (or ambiguous 46,XY; genitalia) with gonads are small vagina, no testes but 5-alpha reductase uterus, can't convert deficiency ("penis undescended amenorrhea T to DHT at 13") testes; AT => can't PUBERTY: virilize "override" external androgen receptor genitalia and start to phenotypic female 46,XX but Mullerian with small vagina, Mullerian Agenesis (Mayer- no uterus, normal amenorrhea (b/c ducts just Rokitansky-Kuster- ovaries; normal no uterus) regress (it just Hauser Syndrome) pubic hair and happens) breasts
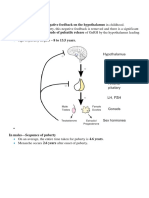
Normale MALE T range; LH = elevated; E = elevated
T & E = normal LH = normal or increased
looks same as androgen insensitivity but at puberty, you override the hormones and get a penis;
webbed neck, cubitus valgus, short 4th metacarpal, shield phenotypic female chest, wide with normal spaced nipple; vagina, normal other anomalies: uterus, streak renal anomalies, ovaries, scant hearing loss, Turner Syndrome pubic hair, small 45, X autoimmune dx breasts, primary (10% amenorrhea, hypothyroid), CV delayed puberty anomalies (33%) or primary like bicuspid amenorrhea aortic valve, coarctation of aorta, aortic aneurysms SRY deletion or Dax O/E 46 XY Females (b/c Dax-9 inhibits Sox9) SRY translocation to X chromosome
high FSH, high LH, low estradiol, normal androgens; ultrasound/MRI : prepubertal uterus (normal), no gonads seen
provide estrogen/progestin (BC pills) for breast dev, bone density, increase uterine size, stimulates endometrium; FERTILITY: egg donation, adoption
early oocyte death => streak ovaries; LH induces enzymes that convert cholesterol into androstendione and testosterone => SCANT PUBIC HAIR (from low T)
46 XX Males
Menstrual Cycle Disorders Kallman's Syndrome (extreme ex of hypothalamic amenorrhea)
Oligomenorrhea: menstrual interval greater than 35 days (i.e. infrequent menstrual cycles); Polymenorrhea: menstrual interval less than 21 days; Menorrhagia: normal interval, excessive flow (>80mL, >7 days); Metrorrhagia: irregular intervals, bleeding btwn menses; Menometrorrhagia: heavy & irregular bleeding congenital - x linked form anosmia/ (Xp22.3 = Kal1 => anosmin 1) hyposmia; absent and anosmin 1 is required for olfactory sulci in migration of GnRH and rhinencephalon olfactory neurons
no GnRH neurons in hypothalamus
phenotypic female with normal Hypergonadotropi vagina, normal c Hypogonadism uterus, streak (aka ovarian ovaries possible, failure - ex. scant pubic hair, Turner's small breasts, Syndrome) primary or secondary amenorrhea
summary: high gonadotropins (FSH, LH), low estrogen, often abnormal karyotype (ex. 45,X), ovarian failure (no more eggs, no more hormone production)
high FSH, high LH, low estradiol, normal androgens; ultrasound/MRI : prepubertal uterus (normal)
as T increases => increased body hair > facial hair > menstrual disturbances > clitorimegaly > muscle mass abnormal female and/or balding; Hyperandrogenic hair pattern (more see acanthosis Anovulation AKA male pattern), nigracans and PCOS (Polycystic usually skin tags from Ovarian overweight/obese hyperinsulinemic Syndrome) , normal uterus, state; KEY normal ovaries POINTS: anovulation, hyperandrogenis m, hyperinsulinemia, risk obesity, HTN, DM, endometrial Pediatric / Adolescent GYN Issues Neonatal Findings
androgens (DHEA, DHEAS, androstenedione , T) increased, LH/FSH ratio > 3:1; progesterone is low; basal body temp is monophasic/an ovulatory; ultrasound multiple peripheral cysts < 10mm ("string of pearls")
etiology: idiopathic, autoimmune, provide estrogen/progestin (BC gonadal pills) for breast dev, bone density, agenesis/dysgenesis, increase uterine size, stimulates genetic, "resistant endometrium; FERTILITY: egg ovaries", donation, adoption, 10% POI chemo/radiation, ovulation (sporadic, unpredictable infection (mumps), NOT genetic causes surgery, SCD, trauma, StAR defect provide hormonal factors estrogen/progestin affecting HPO axis: (BC pills) to cycle androgens converted endometrium to estrogens in fat (prevent (by aromatase); more hyperplasia), lower fat => more FSH and LH, conversion; prevent ovarian estrogens => ~ cysts, decrease increase in LH and androgens, and estrogens (low increase SHBG; levels) => decrease FERTILITY: in FSH; LH => ovulation induction increase in ovarian with clomiphene androgens - cycle citrate (estrogen just continues; antagonist --> insulin => increase increase FSH), in LH, decrease in gonadotropins SHBG => more (FSH), insulin free androgens (why sensitizers tx with metformin); (METFORMIN) i.e. too many GnRH
Normal Findings: breast buds, nipple discharge, white vaginal discharge (from mom's estrogen in utero) - resolve in 6-8 weeks; vaginal bleeding (from maternal estrogen withdrawal) - resolve in 10 days adjacent edges labia minora adhere (commonly posteriorly) no impact on puberty, growth, sex, fertility; due to low estrogen +/inflammation
Labial Agglutination
Congenital Adrenal Hyperplasia (CAH) Neonatal Vulvar Disorders
ambiguous genitalia at birth (ex. clitoral enlargement) Candidiasis: diaper rash (candida albicans); Molluscum Contagiosum: smooth papules with central cheesy plug, "umbilicated"; condyloma acuminata: genital warts (HPV, perinatal transmission)
need to determine the source (vaginal, vulvar, endometrial); etiology: inflammatory (most common): vulvovaginitis (atrophy, inflammation, pruritis); Premenarchal STD (condyloma acuminata), parasite (shigella); hormonal: precocious puberty, exogenous hormones, sex hormone tumors; trauma: exclude sexual Disorders: Vaginal abuse, physical activities (straddle injuries), foreign body (toilet paper); urologic: urethral prolapse ("angry red donut"), UTI, hematuria, neoplasms; Bleeding vulvar lesions: Lichen sclerosis (flat ivory colored papules, may coalesce into plaques; tumors: sarcoma botryoides, ovarian germ cell tumor etiology: most common is idiopathic; McCune Albright Syndrome: polyostotic fibrous dysplasia, caf au lait skin pigment lesions, activating mutations of G-protein coupled receptors; exogenous hormones; sex hormone tumors Etiology: 1) non-specific (70%) secondary to urinary/fecal hygiene (mixed bacterial / e. coli) 2) specific vulvovaginitis: enteric = shigella; respiratory = H. influenzae; skin = staph, strep; STD = sexual abuse (G & C); candida; pinworm = enterobius vermicularis (scotch tape test) risk factors: anatomic (no pubic hair, no labial fat pads, close to rectum); poor hygiene (wipe front to back, diapers); hormonal (thin atrophic vagina pH 6.5 7.5); others: obesity, DM, other vulvar dermatoses, immune status; most common prepubertal GYN complaint (50% outpt visits)
Precocious Puberty
secondary sex characteristics (<7 y/o W, <6 y/o AA)
Vulvovaginitis
discharge (50%), presents in dysuria, pruritis, pediatrics; pain, genital inflammation of irritation, vulvar and vaginal erythema, tissues excoriation, vaginal bleeding inability to conceive after 1 year of trying
Infertility / Pregnancy
Infertility
primary: never been pregnant before; secondary: has been pregnant in the past
Testing Female for Ovulation: BBT, luteal progesterone (3 weeks after last period); test for sub-clinical endocrinopathies (hyper/hypo thyroid, hyperprolactin, hypergonadotropic; Test Female anatomy: hystersalpingogram (HSG), laparoscopy, hysteroscopy; Test Male via Semen Analysis (volume, count, motility, morphology) and for anti-sperm antibodies; Female-Male Evaluation: sexual intercourse (timing, frequency), postcoital test, female anti-sperm antibodies
Ovulation Induction: clomiphene citrate, gonadotropins, dopamine agonists, side effects of many steroids; IVF infertility tx = (harvest eggs after multiple gestations induction by gonadotropins and mix egg & sperm in dish then reimplant in uterus)
Placentation
placenta previa: placenta implants too low in the uterine cavity (covers cervical os); placenta accreta: placenta invades uterine wall too deeply; ** placenta previa and placenta accreta tend to occur together! abruptio placentae: early separation of placenta from uterus => hemorrhage (etiology of abruptio = 1) trauma 2) some sort of pathophysiologic injury to spiral arteries e.g. infection => inflammation => rupture of spiral artery) CMV: immunocompromise d pts; hematogenous spread; Acute chorioamnionitis: ascending bacterial infection in placenta (group B strep, e coli) from bacteria that colonize vaginal/cervical tract Single Umbilical Artery; Velamentous Insertion (cord inserts into fetal membranes then travels w/in membranes to placenta => exposed vessels vulnerable to rupture); True Knot; Pseudoknot; Helical Coiling; Nuchal Cord placenta = thin; chronic infarct = tan/yellow appearance & acute = hemorrhagic i.e. red and congested; infarct has well defined borders ghost villi; chorionic villi that are no longer identifiable; coagulative necrosis or sometimes liquefactive necrosis (large cysts)
2 types of infection in placenta: 1) organisms cross over placental barrier Placental Infection (transplacental infection) or 2) infections arise by hematogenous spread (mostly viral like
Acute chorioamnionitis: preterm = serious problem (infection = most common cause of spontaneous preterm birth)
TORCH infections
CMV: see CMV inclusions in nucleus and cytoplasm; Acute Chorioamnionit is: PNMs present
Umbilical Cord Pathology
see single umbilical artery think fetal CV abnormalities
Placental Infarct
chronic placental malperfusion => placentas that are small for gestational age (IUGR)
Benign Breast Pathology
Disorder of Development: Persistent Milk Line
heterotopic breast tissue; supernumerary nipples or breasts
bilaterally from the mid-axillae through normal breasts then inferior to medial groins; embryological milk line atrophies except for short segment at pectoral region to make breasts needs to be considered when considering cancer uncommon; need to include "inflammatory breast cancer" (peau d'orange) in the differential dx localized necrosis of fat tissues; related to trauma (32%), surgery and radiation can cause it
Disorder of palpable mass Development: with lactational Accessory Axillary change Breast Inflammatory Disorders erythematous swollen painful breast Etiologies: fat necrosis, duct ectasia, acute mastitis, recurrent subareolar abscess, granulomatous mastitis, lymphocytic mastopathy mammogram can reveal spiculated poorly defined mass w/ calcifications (i.e. mimic cancer) features depend on stage: necrotic fat surrounded by foamy macrophages, giant cells, fibroblasts and fibrosis; +/Ca++ dilated ducts with insipissated secretions; epithelial degeneration and periductal chronic lymphocytic reaction antibiotics SMOLD (squamous metaplasia of lactiferous ducts - Zuska dx); keratinizing squamous metaplasia surgery of nipple duct; intense Cl and granulomatous inflammation collagenized stroma surround atrophic lobule with Cl and circumferential inflammation
Fat Necrosis
painless mass, palpable and firm mimics cancer (usually present clinically w/o any significant hx)
Duct Ectasia
intermittent clear nipple discharge acute infection (with staph aureus), abscess secondary infection
Acute Mastitis
from breast feeding
Periductal Mastitis some periductal (aka recurrent chronic subareolar inflammation abscess) Lymphocytic Mastopathy (aka Diabetic Mastopathy)
palpable mass
type 1 DM, autoimmune dx
Nonproliferative Breast Changes (fibrocystic change)
palpable lump, some pts have nipple discharge
cysts (lined by thin wall), fibrosis, mammographic adenosis (increased # lobular density or units); calcifications (blue), calcifications apocrine change (pink) **increased risk of invasive breast cancer 1.5 to 2.0 more than 2 cell layers (usually inner cell layer that proliferates); architecture: irregular space, peripheral fenestration, cells look streaming or whirling; cytological: heterogenous variations in size and shape of cells and nuclei
epithelial hyperplasia usual type, Proliferative Breast sclerosing adenosis, complex dx w/o Atypia sclerosing lesion, papillomas
Epithelial Hyperplasia Usual Type
usually incidental
Papilloma
nipple discharge (can even be bloody) imaging density, calcification or mass that mimics invasive carcinoma radiographic images show central mass with long projections
fibrovascular cores extend into duct lumens covered by epithelium; epithelium can have epithelial hyperplasia, apocrine metaplasia, sometimes atypia or DCIS increased number of acinar (distorted and compressed), may be present with other FCC or form mass itself sclerosing adenosis, papilloma, epithelial hyperplasia, stroma is distorted with entrapped benign cords and ducts/lobules
Sclerosing Adenosis
palpable mass
Radial Sclerosing Lesion / Complex Sclerosing Lesion Atypical Ductal Proliferative Breast Hyperplasia & Dx w/ Atypia Atypical Lobular Hyperplasia
benign mimicker of invasive cancer
** 4-5x risk of developing invasive breast cancer
Atypical Ductal Hyperplasia
harbor acquired genetic gain or loss present in DCIS
Atypical Lobular Hyperplasia
most common benign tumor of female breast; young Fibroadenoma women (20-30 yo), hormonal responsive (increase during pregnancy) localized or most common diffuse, tender, male breast only has ducts (no clinical and assymmetric area lobules), increased dense associated with pathological of firmness and collagenous CT; in the ducts, see Gynecomastia hyperestrinism abnormality of male enlargement some epithelial hyperplasia with and medications breast; etiology: (unilateral or micropapillary type hormonal imbalance bilateral) ; pt (ex. Klinefelter's) presents with Risk Factors: first degree relative w/ breast ca; genetic predisposition (BRCA1&2, Li-Fraumeni syndrome (germ line mutation of p53), Cowden dx (multiple hamartoma syndrome)); increasing age; age at first full term pregnancy over 30 or nulliparous; early onset menstruation/late menopause; previous bx dx of proliferative breast dx; race (caucasian: highest rates; AA: present at higher stage, higher grade, younger age, receptor neg; hispanic: fastest growing rate of ca); obesity (decrease in young women, increase in older women); hormonal imbalance (estrogen excess); radiation Breast Carcinoma exposure; dietary fat (increases risk); smoking NOT associated; Prognostic Factors: Tumor size (<1 cm 98% 10 year survival); Lymph Node Status (MOST IMPT): neg LN - 70-80% 10 year survival; Distant Metastasis; Other Prognostic Factors: histologic subtypes that aren't NOS, ER/PR + is better; lymphovascular invasion is worse; oncogene expression (Her-2/Neu) is worse; loss of tumor suppressor gene fxn (p53 mutation) is worse; Tx: pts undergo surgery +/- adjuvant therapy (hormonal manipulation - PR/ER+; external radiation, brachytherapy; chemotherapy; antibody therapy (Herceptin against Her2)) early onset (15-20 years earlier); occurrence in several close relatives; more than 1 type of ca; bilateral in Familial Cancer 80% of families with Syndrome (BRCA paired organs; occurrence in less cancers are from 1&2, Li-Fraumeni) affected sex (males w/ breast ca); sporadic mutations mult ca across generations; rare ca clusters
monotonous (diff from hetero in typical) cells forming complex architecture (cribiform, micropapillary etc); forms arcades (diff from whirling in typical); nuclei are evenly spaced with distinct cell borders cytologically like LCIS (lobular carcinoma in situ) but only partially involving a lobular unit; punched out holes (cookie cutter appearance), cells look monotonous w/ uniform evenly spaced nuclei hyalinized or oval, white, myxoid stroma rubbery, mobile compress mass, well epithelium (i.e. circumscribed prolif of stroma)
seen in 5-17% bx calcifications; cellular proliferation resembling DCIS but quantitative and qualitative insufficiency
mostly incidental
BRCA 1 & 2
no palpable lesion (in-situ carcionmas, sm invasive lesions) OR breast mass, skin retraction/dimpling, peau d'orange / changes of inflammatory carcinoma, nipple retraction, discharge (invasive carcinoma, palpable tumors 2cm) ductal carcinoma in situ (DCIS) / intraductal carcinoma (80%) ; lobular carcinoma in situ (LCIS) (20%)
hereditary AD breast ca susceptibility genes
detection: incidental finding on palpation (women find themselves), yearly clinical breast exams and monthly self; screening: mammography starts at 40, screening MRI for high risk pts
<5 % of breast ca but 30% of breast ca in pts <30 yo; risk of other tumors; Ashkenazi Jewish ancestry
In situ/ noninvasive carcinoma (1530%)
proliferation of epithelial cells that have undergone malignant transformation but remain at site of origin (i.e. w/in duct or lobule) confined by a BM; metastatic spread CANNOT occur by definition calcifications on mammogram (screening begins at 40 yo look for changes over time); pathologic evaluation: ?invasion, grade, size, margins incidence = 30% (was rare 20 years ago b/c of not screening); grade based on nuclear features & necrosis; considered precursor lesion to invasive carcinoma
rarely forms a mass, rarely bilateraly (10Ductal Carcinoma 20%) - as In Situ (DCIS) opposed to lobular carcinoma which tends to be bilateral contiguous intraductal spread from nipple ducts ulcerating oozing to nipple skin and nipple areola => scale crust on skin surface
4 types: solid, cribiform, comedocarinoma, micropapillary; low grade = uniform, smaller nuclei w/ infrequent necrosis; high grade = large bizarre nuclei w/ central necrosis little balloons w/ dots in the middle = cancer cells
Paget's Dx of the Nipple
underlying DCIS, invasion present in ~60% of cases
bilateral (in Lobular approx 40%) => Carcinoma In Situ no palpable mass more risk of (LCIS) invasive tumor in both breasts
proliferation of small uniform cells w/in terminal ducts and lobules (acini); no necrosis or microcalcifications
close observation
incidental finding; significance: considered a marker of increased risk for developing invasive carcinoma (8-10x)
Invasive carcinoma (70%) aka infiltrating carcinoma; adenocarcinoma
neglected tumors present needle loc usually presents mets first to as large invasive ductal mammogram as a (palpable) axillary nodes ulcerating carcinoma (90%); (use needle to mass; can cause then to liver, lesions; invasive lobular locate lesion for skin dimpling and lung, bones, scirrhous (= carcinoma (10%) excision during nipple retraction CNS stony hard), surgery) gritty consistency w/
surgery, tamoxifen, lymph node evaluation, ER/PR receptor antagonist
proliferation of epithelial cells that have undergone malignant transformation and have grown beyond the confines of the BM (of the duct / lobule) into breast parenchyma; metastatic spread CAN occur
Invasive Ductal Carcinoma
mets to bone, liver, lungs
colloid/mucinous: nest of ca cells floating in mucinous tissue; tubular: ca cells in nests in tubules; medullary: looks like high grade ca, lot's of inflammatory rxn (esp lymphocytes) pleomorphic cells; tubules, nests, sheets
NOS = 90%; colloid (mucinous) = 1-6%; tubular = 2%; medullary = 1-5%; all look diff under microscope and behave better than NOS (less aggressive and invasive); grading: modified bloom richardson score - nuclear pleomorphism (1-3); tubule formation (1-3); mitotic activity (1-3) best differentiated => total = 3
Invasive Lobular Carcinoma
bilateral and multicentric
mets to abdomen and retroperitoneum
ill defined firm areas
single file pattern and targetoid patterns present NOT graded -uniform cells; single cells, multicentric
invade in a diffuse pattern
Problems in Puberty Kallman's Syndrome (see above) Hypothalamic Hamartoma form of central precocious puberty if happens in fetal life => micropenis in boys (b/c no GH, FSH, LH, ACTH, TSH in fetal life) document with MRI (but usually leave alone) congenital (structural or midline abnormalities; mutations in pituitary TFs) or acquired (CNS insult, trauma, basal skull fracture, radiation, chemo) medical therapy heterotopic mass of GnRH secreting neurons; present from birth may involve isolated or multiple hormones
hypopituitarism
delayed puberty
idiopathic central precocious precocious puberty puberty Turner's Syndrome (see above)
early fusion of epiphysial growth plates (severe short stature)
GnRH stimulation test to dx (do FSH measurement after GnRH stim => normal levels when should be prepubertal); then can do head MRI
premature activation of HPG axis; more common in girls
delayed puberty
classic triad: precocious puberty, caf au McCune-Albright lait skin Syndrome pigmentation & fibrous bone dysplasia Cervical Pathology Non Neoplastic Lesions usually Polyps (Nonasymptomatic but neoplastic Lesion) can cause vaginal spotting Follicular Cervicitis (Nonneoplastic lesion) Infections infections and inflammation of cervix
activating mutation in Gs alpha lg ovarian cysts in => hyper fxn of endocrine tissues; somatic mutation in girls mosaic distribution
looks red when glandular and white when squamous often caused by chlamydia
fibromuscular stroma lined by benign epithelium form a lymphoid follicle (not specific pattern)
impt in differential dx of vaginal bleeding (b/c also in differential is endometrial ca)
Other infections not shown: gonococcus, chlamydia, syphilis, CMV, schistomiasis, TB
Candida
STD
pap smear
see squamous cells but also see lymphocytes; see branching tree looking thing (that's the fungus)
be weary of STDs increasing in senior citizens
Trichomonas
STD
pap smear
see bluish glandular thing with flagellum which is the parasite
Herpes Simplex Virus
STD
pap smear
cell crowded with ground glass nuclei (multinucleated cell)
NO CURE just there are vaccines in antivirals to control trials breakouts
HPV Cervical Premalignancy and Malignancy
HPV causes cervical cancer but NOT all HPV infections cause cancer; HPV is often species and site specific; causes many types of warts; has many subtypes; genital tract subtypes related to cervical cancer; epidemiology of genital HPV infecton: not usually seen prior to sexual activity; likelihood of infection directly related to number of sexual partners, young age at first intercourse, and male's sexual partners; same risk factors for cervical precancer and cancer; Majority of cervical cancers occur in the transitional zone; pts with lesions get colposcopy (use dissecting microscope and put vinegar on the cervix => lesions turn white and then biopsy the lesions) pap smear with viral subtyping little if any undifferentiated cells, limited to bottom layers of epithelium (action is near surface); hallmark: koilocyte (enlarged, dark irregular nucleus with perinuclear cytoplasmic clearing (halo) with "hard" edge
CIN1 / LGSIL (low grade squamous intraepthelial lesion)
low overall risk of progression; most regress
can have any low risk HPV (6, 11, 42, 44) OR high risk HPV (16, 18, 31, 33)
includes flat condyloma and low grade dysplasia; this is a productive HPV lesion (i.e. cell is a factory making more HPV virus => halo)
CIN2/ HGSIL (high grade squamous intraepthelial lesion)
high risk HPV subtypes ONLY (16, 18, 31, 33) much higher risk high risk HPV of persistence subtypes and progression ONLY (16, 18, 31, 33)
undifferentiated cells and mitoses up to middle layers of epithelium
CIN3/ HGSIL
full thickness undifferentiated cells and mitoses, qualitatively worse cellular features, look for mitoses (in all layers) - bottom image = squamous lining transformed into high grade lesion and is tracking backwards to replace endometrial gland; cytology: higher nuclear: cytoplasmic ratio than LGSIL (dark angry looking nuclei in sm cells)
includes high grade dysplasia and carcinoma in situ
Squamous Carcinoma
ulcerated or fungating mass originating in transformation zone; often large since pt not screened; can present with vaginal discharge / + LN; high risk subtypes
fungating mass (looks like mushroom); red = glandular tissue; white = squamous tissue
invasive histologic pattern (local tissue destruction)
surgery and radiation
stage most impt for prognosis - ca much worse when reaches pelvic wall (stage III, 35% 5 yr survival vs 75% for stage II); morbidity greater from direct extension rather than mets incidence appears to be rising
endocervical adenocarcinoma
goes through in situ phase but progression not as well understood
associated with HPV 18 (18 can also cause squamous cancers)
pap smears (not as effective at detecting as they are at detecting squamous lesions)
glandular cells become dysplastic => ca
Uterine Pathology
Ovulatory Causes of Infertility not common limited to bacterial infections that arise after delivery or miscarriage
acute endometritis
bleeding, discomfort, pain
PNMs and eosinophils invade glands and stroma
antibiotics
chronic endometritis
abnormal bleeding, pain, discharge, infertility
PLASMA CELLS in stroma, some bleeding
etiology: retained products of conception, IUD associated infections, TB; most common in setting of PID dx: culture, detect gonoccocal DNA or RNA, endocervical contents; Tx: ascending infection that begins in vulva or vagina; common; with ectopic pregnancy - can get
PID: Gonorrhea
pelvic pain, adnexal tenderness, fever, vaginal discharge complications
cervical envolvement common (asymptomatic), preferentially
abscess formation in fallopian tube
islands of inflammatory cells (PNMs, lymphos, macros) in
PID: Chlamydia
infects same sites as gonorrhea but recurrent with milder infections = symptoms and common less acuity
chlamydia = obligate intracellular pathogen; exists in 2 forms: 1) elementary body (infectious but NEVER divides) & 2) reticulate body (divides but not infectious); often recog by persistence of symptoms after tx gonorrhea - ** other PID pathogen = enteric bacteria composed of mature smooth muscle (looks ressection of like myometrium) fibroid, cauterization, hysterectomy
leiomyomas
endometrial bleeding, infertility, heaviness, pain
4 locations: intramural, subendometrial, subserosal, pedunculated
dx: need to see gross masses then look at histo
very well demarcated, located in myometrium
most common tumors in women; estrogen responsive
adenomyosis
menometrorrhagia (irregular and heavy menses), colicky dysmenorrhea, dyspareunia, pelvic pain (esp during menstrual period)
spongy looking myometrium from endometrial glands/stroma
endometrial glands/stroma in myometrium (surrounded by smooth muscle)
presence of endometrial glands and stroma w/in myometrium; 20% of women
endometriosis
depends on location involved; common complaints: dysmenorrhea, dyspareunia, pelvic pain, menstrual irregularities, infertility (30-40% women)
rarely shows features (mets and invasion) similar to malignant tumors => serious complications; distribution: ovaries, uterine ligaments, rectovaginal septum, pelvic peritoneum, laparatomy scars; associated with ovarian malignancies (clear cell carcinoma & endometrioid carcinoma)
dx: cytology needs pigemented macrophages and blood, stroma, and glands (need 2 out of 3) AND clinical hx
chocolate cysts when ovaries
have to see endometrial glands AND stroma mixed with diff tissue type; hemosiderin laden macrophages (from blood)
DUB (dysfunctional uterine bleeding)
clinical term for uterine bleeding not caused by any underlying organic (structural) abnormality; common at menarche and perimenopausal
presence of endometrial glands and stroma outside uterus; dx of women in reproductive life; 10% women; theories: 1) regurgitation theory 2) metaplastic theory 3) vascular or lymphatic dissemination theory functional disturbance; most cases have no cause but are from anovulatory cycle; when cause: from 1) endocrine disorder 2) primary lesion of ovary (PCOS) 3) generalized metabolic disorder (eg DM)
clinically infertile low progesterone; endometrial bx: Inadequate Luteal inadequate corpus luteum formation => (irregular secretory endometrium lags in Phase (ovulatory low progesterone => can't maintain ovulatory cycles), histological charac (eg. Day 26 has dysfunctional endometrium increased bleeding subnuclear vacuoles) bleeding) or amenorrhea Uterine most common female genital cancer; post-menopausal women (median age 58); cause unknown but E plays a role; tamoxifen => 3x risk increase Malignancies: for uterine ca (in uterus, tamoxifen is agonist) Endometrial Carcinoma
Endometriod Carcinoma
phenotype: nulliparous, obese, younger, DM, HTN, late onset menopause, irregular or post- 5x risk to get well- pre and post menopausal differentiated menopausal bleeding endometrial ca women at risk arising in background of complex hyperplasia with atypa, low grade
bx and endometrial curettage (to r/o polyps, hyperplasia, leoimyomas), frequently ER/PR+
pink, polyploid looking mass kind of looks like curdled milk
well diff = glands w/ no intervening stroma, complex glands with infoldings (cribiform); poorly diff = solid mass of tumor w/ some good outcome glands; nuclei &long term jumbled, round, survivial prominent nucleoli, clearing of chromatin (atypia features); squamous differentiation: pink-keratin part of tumor background appears atrophic; complex branching papillae (w/ pink fibrovascular core) lined by pleomorphic cells with high nuclear grade; tumor cell tufts; psammoma bodies (calcifications); histo identical to ovarian serous ca, no hyperplasia
Classical Pathway (type 1) endometroid is most common, others: adenocarcinoma with squamous differentiation, mucinous, squamous; estrogen related prototype; almost always p53 NEGATIVE
phenotype: multiparous, thin, older, poorly irregular or postpost differentiated Serous Carcinoma menopausal menopausal deeply invasive bleeding women lesions (myometrium), high grade
bx and endometrial curettage; POSITIVE for p53 & ER/PR -
poor prognosis (widespread lymphovascular permeation)
alternate pathway (type II) - type of alt is clear cell; nonhormonal prototype; precursor = EIC (endometrial intraepithelial carcinoma) - in situ replacement of benign endometrial glandular epithelium by malignant cells;
Clear Cell Carcinoma
aggressive, high stage, poor prognosis
architectural pattern: solid, papillary, tubular, cystic; atypical nuclei see clear cell w/ clearing of cytoplasm (might be eosinophilic)
4% of endometrial ca
Uterine Sarcomas Stromal Nodule (BENIGN)
3-5% of uterine malignancies (w/ mixed tumors)
premenopausal women Low Grade Endometrial Stromal Sarcoma
worms distinctive gross appearance / may extend beyond uterus
malignant endometrial stroma; clusters of sm uniform cells infiltrate myometrium & fill lymphatics; minimal atypia & mitoses; cells look bland & haphazardly arranged, sm & hyperchromatic nuclei significant atypia with mitoses, hemorrhage & necrosis common; nuclei = pleomorphic, prominent nucleoli,
Undifferentiated Endometrial Sarcoma (formerly high grade endometrial stromal sarcoma)
post menopausal
aggressive - most have spread beyond uterus at dx dev indep from leiomyomas ; incidental dx = presumed leiomyoma
Leiomyosarcoma
post-menopausal bleeding, uterine enlargement, average age = 53 abdominal pain, constipation, urinary frequency
malignant smooth muscle; firm and white hypercellular tumor that's w/ spindle cells hemorrhagic w/ in fasicular some necrotic pattern; areas pleomorphic cells, chromatic nuclei, mitoses
Mixed Tumors Adenofibroma (BENIGN) Adenomyoma (BENIGN)
malignant epithelium and stroma
Adenosarcoma
recurrence and distant mets after many years
polyploid mass
epithelial component resembles proliferative endometrium is BENIGN; stromal w/ slit-like glands; mitoses component is MALIGNANT
Carcinosarcoma (MMMT = malignant mixed mullerian tumor)
elderly pts with pelvic pain and bleeding
glandular component usually endometrioid ca; stroma can be homologous (differentiation same as uterus bulky mass w/ endometrial hemorrhage and smooth muscle necrosis or stroma) or heterogenous (diff from uterus mesenchymal from elsewhere like fat, cartilage, skeletal muscle large polyploid lesions in endometrial cavity
BOTH epithelial and non-epithelial poor prognosis (5 components = year survival = 18MALIGNANT; 39%); uniformly mets to LN = aggressive epithelial (carcinoma mets not sarcoma)
Endometrial Polyps (BENIGN)
localized proliferation of glands and stroma; see thick walled BVs and fibrotic stroma (pink); cystic dilated glands w/ normal sized glands scattered btwn; fibrotic background + stroma
Endometrial Hyperplasia
diffuse proliferation of glands and stroma; result of prolonged estrogen stimulation via anovulatory cycles, obesity, hormone-producing ovarian tumors (ex. granulosa cell tumor); progression of "untreated" atypical hyperplasia to endometrioid adenocarcinoma numerous glands that look crowded but simple and tubular; increased abundance of glands compared to background stroma; nuclei are still well oriented, they're elongated, no prominent nucleoli
Simple Endometrial Hyperplasia w/o Atypia
Simple Endometrial Hyperplasia w/ Atypia
simple b/c glands are still round and tubular (NOT branching); ATYPIA: nuclei are rounding up, chromatin is clearing out in some places, nuclei look crowded / jumbled, prominent nucleoli
Complex Endometrial Hyperplasia w/ Atypia
glands look complex - not round and tubular; ATYPIA: nuclei jumbled, rounding up in some places, chromatin clearing, lose orientation to BM
most likely to be associated with or progress to endometrial cancer
Ovarian Pathology Non-Neoplastic Cystic Changes / Lesiosn Fallopian Tube Disorders Primary Ovarian Tumors Follicle Derived: cystic follicle (< 3cm) & follicular cyst ( > 3cm); corpora-lutea derived: cystic corpus luteum (< 3cm) & corpus luteum cyst (> 3cm); epithelial inclusion cyst Infections (PID); Endometriosis; Tubal Pregnancy (common site = ampulla); Tumors (adenocarcinoma - BRCA mutation; TIC (tubal intraepithelial carcinoma) 3 categories: 1) surface epithelial tumors (65-70%) 2) germ cell tumors (15-20%) 3) sex cord-stromal tumors (5-10%)
Surface Epithelial Benign: cystadenoma, cystadenofibroma; Borderline: serous borderline tumor, mucinous borderline tumor; Malignant: serous carcinoma, mucinous carcinoma, endometrioid carcinoma, clear cell carcinoma Tumors Serous: cyst collapses (looks like a tent); when full, it's full of clear fluid; cauliflower like growth can be seen; Mucinous: find many cysts inside (daughter cysts), looks like jelly -> mucin cystic (unilocular or multilocular); monolayer lining on BM; serous: cuboidal, ciliated cells; mucinous: columnar cells w/ copious apical mucin
Cystadenoma
cytsic + adenoma; serous or mucinous; papillary or not; neoplastic part = epithelium
Borderline Tumors : Serous Type
cystic with nodular buds, excrescences can grow on ovarian surface; very solid looking (b/c of more overgrowth and proliferation)
epithelial proliferation -> stratification; hierarchy papillary architecture (looks like tree trunk w/ branches); mild to moderate cellular atypia
have mixed features: not quite benign or malignant (clinically and hist), more proliferative (m); NO INVASION (b) (but may have local spread to peritoneal surface); can met to LN, can recur, rarely => death (b)
Ovarian Carcinoma
screening: pelvic exam, ultrasound, CA-125 (more sensitive than tend to be specific; marker relatively most cases = in blood); genetic asymptomatic; cause testing for affect women >40 unknown BRCA1/2 yo carriers => prophylactic bilateral salpingooophorectomy
Hereditary Ovarian Carcinoma
most cases associated w/ family hx, BRCA1/2: younger age at dx AD, high penetrance
ugly cells prognosis not so 8th most common (obvious good b/c it's hard cancer but 5th killer malignant to detect early (in US), more than features), 80% of malignant stromal ovarian tumors = invasion; varied epithelial tumors; growth patterns protective factors: (serous: oral contraceptives, solid with or papillary, gland multiparity, tubal w/o cysts, bulky forming, solid; ligation; risk factors: (usually) - looks mucinous/endo nulliparity, hereditary fleshy metrioid/ clear predisposition cell: gland forming or solid; psammoma bodies (concentric calcifications) approx 10% of ovarian cancers; BRCA = tumor suppressor gene; "two hit" theory -> 1) inherited germline mutation of one copy of gene, 2) somatic mutation of backup copy of gene; loss of suppressor fxn => dev of tumor
Germ Cell Tumors
derived from transformed germ cells (follicles / oocytes); most common tumors in young women (<20 yo); ovarian teratoma: aberrant differentiation of totipotent cells to any/all germ layers (ecto/endo/mesoderm) hasn't been fertilized; analogous to seminoma starts to look like embryo
dysgerminoma embryonal carcinoma
choriocarcinoma yolk sac tumor (endodermal sinus tumor)
increased bHCG increase serum AFP schiller-duval body (looks glomeruloid) composed of mature elements histo evidence from all germ of all 3 germ layers (teeth, layers hair, etc) see thyroid tissue like colloid composed of immature (embryonic or fetal-like) elements from any/all germ layers; blue surgery, adjuvent staining; see immature chemotherapy neuroepithelium (left pic) and immature mesenchyme (right)
similar to placenta
Teratoma: Mature Cystic Teratoma (dermoid cyst)
peak incidence 2040
most common benign ovarian germ cell tumor but may have malignant transformation to squamous cell carcinoma struma ovarii: mature thyroid tissue predominantly; carcinoid tumor
Teratoma: Monodermal (or highly specialized)
Teratoma: Immature
very rare in women >30
malignant; graded by amt of immature neuroepithelium
Sex-Cord Stromal Tumors
tumors derived from ovarian stroma (developed from sex cords of the embryo); may be hormonally active; Classification: fibroma, thecoma (fibrothecoma), granulosa cell tumor, sertoli-leydig cell tumor classic looking "coffee bean fleshy and cystic nuclei" nuclei looking; with cleared out hemorrhagic; center and line yellowish in down middle; color Call-Exner Bodies cellular, bland spindled solid, firm, tanfibroblasts; yellow mass collagen bundles
can present with hyperestrinism Ovarian Granulosa (tumor Cell Tumor hormonally active)
low grade malignancy; occurs at any age (2/3 post menopausal)
tumors recaptiulating granulosa cells of follicle; at risk for endometrial carcinoma
Fibroma
most common sex cord tumor; no hormonal activity; can be associated with Meigs syndrome & Gorlin Syndrome
Thecoma
may present with hyperestrinism
uniform, solid consistency; yellowish
spindle looking for fibroma part and epithelioid for thecoma part
tumors recapitulating theca cells of follicles; rare to exist as pure form (commonly with fibroma - fibrothecoma; may produce estrogen => hyperestrinism => risk of endometrial hyperplasia or carcinoma
Ovarian Metastatic most common origin = non-mullerian primary - colon > breast > stomach; endometrial carcinoma = most common mullerian origin Tumors Krukenberg Tumor Pseudomyxoma Peritonei Prostate Benign Prostate Pathology bilateral ovarian mets abdominal cavity full of mucin regulation of prostate growth mainly by T (and DHT by conversion) elevated PSA levels possible with hyperplasia and inflammation can present with urinary tract obstruction due to compression and increased muscle extremely tone => common in men hesitency, >50 dribbling, frequency, nocturia, urgency, hydroureter, hydronephrosis fever, tenderness of prostate (b/c it's infectious in etiology) transurethral resection of prostate (TURP) glandular: stroma is reduced and and suprapubic glands are enlarged, have prostatectomy; 5projections (papillae) protruding alpha reductase into lumen due to hyperplasia; inhibitor stromal: rarely find glands, (finasteride => spindle nuclei and abundant inhibits action of eosinophilic cytoplasma (look T); alpha 1 receptor similar to smooth muscle cells) antagonist to relax the smooth muscle (stromal cells) PNMs fill prostatic glands antibiotics => microabcesses not many PNMs, lot's of lymphocytes and plasma cells signet-ring carcinoma commonly from GI primary appendical origin
Benign Prostatic Hyperplasia (BPH)
can have elevated PSA
enlargement of prostate gland by stromal or glandular proliferation and nodular formation; etiology: androgens & estrogens
Prostatitis: Acute
etiology: typically bacterial infection can be due to inability to cure acute prostatitis (can flip back to acute => alterations back and forth); many cases it's noninfectious
Prostatis: Chronic
Prostatis: Granulomatous
aggregate of histiocytes and lymphocytes form a nodule
idiopathic
etiology: hereditary factors (~9% of prostate ca); racial difference (genetic and environmental probs); sex hormones (T and androgen receptors, Malignnat Prostate which are very sensitive to T so can also activate w/ lower amts of T); dietary fat (free radicals, sex hormone production); PSA Screening: low sensitivity & specificity - high preoperative PSA =. poor prognosis; use Gleason Grading (predominant grade + second most predominant grade) Pathology higher score => worse prognosis pathogenesis poorly understood but recurrent spreads fusions of lymphovascularly; TMPRSS2 to very slow growing can go to bones ERG or cancer => many (relatively ETV1 => don't present common => brings clinically increase alkaline androgenic phosphatase) ERG under control of T => more androgens (in ~50% of ca) low power (architecture): tumor glands = sm or intermediate (opposite of BPH where glands or stroma are BIGGER); decrease in stroma; infiltrative; cancerous glands most commonly infiltrate btwn benign glands; diagnosed ca in high power: loss of basal cells; enlarged nuclei w/ clumping men; higher incidence in AA; chromatin; prominent nucleoli; helpful features: perineural invasion, crystalloids (pink rectangular material in lumen), acidic mucin (looks blue b/c very acidic so picks up basic dye which is blue) high grade PIN => cytological features similar to carcinoma but no invasion; still have stroma; nuclei with very big prominent nucleoli detection: DRE, trans-rectal ultrasound (TRUS), PSA (4-10ng/mL - 25-35% w/ ca; >10ng/mL - 50% w/ ca), decrease in free PSA (<10% total PSA) increases chance for detection of ca (specificity); PSA velocity (measure PSA over time to see trend in changes); PSA density (PSA value versus volume of prostate); management: observation, surgery (radical prostatectomy), radiation (external beam, brachytherapy), hormonl therapy (blocking T via orchiectomy, inhib GnRH, inhib androgen synthesis, inhib androgen binding to receptor, inhib 5 alpha reductase - finasteride), chemotherapy (for hormone-resistant ca) precancerous cellular proliferation of prostatic ducts and acini
Adenocarcinoma
Prostatic Intraepithelial Neoplasia (PIN) Testicular Pathology Abnormal Gonadal Fxn not gone through puberty, no secondary sexual hair, genitalia anosmia almost prepubescent, sm testicular volume
Kallman's Syndrome
FSH = LOW; LH = LOW; T = LOW
give GnRH replacement OR give gonadotropins (LH first to get T up b/c need intratesticular T to make sperm, then give FSH => spermatogenesis)
hypogonadotropic hypogonadism from missing GnRH neurons
gynecomastia from T being low but potentially T tx options: 1) injectible very small firm estrogen still being higher fasting testosterone enanthate every 2-4 testes, very tall, made; prolactin is glucose; FSH = weeks; 2) transdermal testosterone high in labs from normal male hair Klinefelter's karyotype = HIGH; LH = patches, change daily; 3) transdermal high LH => high E pattern, normal Syndrome 47, XXY HIGH; T= T cream, apply daily; 4) oral penis, female => high prolactin; LOW; substituted T, methyl T (not as obesity pattern, elevated prolactin = good, hepatotoxic) gynecomastia gonadotropins b/c HIGH trying to stimulate testes to make sperm elevated temperature => lowered sperm Mediterranean counts Fever source can be exogenous (i.e. steroids) or endogenous (i.e. CAH) but => Excess Androgens suppression of gonadotropins and aspermia causes of testicular atrophy: cryptorchidism, progressive atherosclerosis, inflammatory orchitis, hypopituitarism (low FSH and LH), generalized Benign Conditions malnutrition, irradiation, prolonged administration of anti-androgens (prostate ca), exhaustion atrophy (high levels of FSH), primary failure of of Testes genetic origin (eg. Klinefelter) progressive Testicular descent most descend atrophy and occurs in 2 phases: spontaneously into incomplete fibrosis of 1) abdomen to pelvic scrotum w/in first descent of testes testicle => brim (MIF) 2) pelvic tends to be year; orchioplexy by major sites = inferitility; brim to scrotom unilateral; rarely 2 years old: surgical inguinal canal or cause not well changes start to (androgens); Cryptorchidism associated with placement of testis higher up in understood take place at 2 relatively common: defined hormonal into scrotal sac => scrotum yo; BM looks 1% of 1 yo; risk of disorder improved fertility (intraabdominal = thick, infertility; 5-10x (not guaranteed) rare) interstitium increased risk of and may decrease looks testicular ca (in both risk of testicular ca prominent testes) children (congenital urinary associated w/ tract anomalies) - gram neg UTIs (urethritis, rods; young adults: G and C; acute inflammation => mixed cystitis, prostatits) adults: e coli and pseudomonas; inflammatory infiltration (PNMS, Inflammation of => secondary mumps (viral): >20% of adults lymphocytes, eosinophils); w/ TB Testis involvement of => orchitis; syphilis => orchitis see caseating granulomas (necrosis, testis / w/o epididymitis; TB: begins in giant cells, hyaline) epidydimu;s; can epididymis and may spread to form abscess tesis
granulomatous orchitis
unilateral enlargement (+/probably middle aged men pain) - may mimic autoimmune tumor may be associated w/: incomplete descent, atrophy, defective scrotal ligaments venous occulsion +/arterial compromise
granulomas present diffusely throughout testis, confined to seminiferous tubules; macrophages, plasma cells, lymphocytes congestion, enlargement, hemorrhage, infarction/necr osis surgical emergency: orchioplexy; need can be in association to manually untwist w/ strenuous w/in 6 hours or physical activity lose testis
Vascular Disturbances (Testicular Torsion) Tumors Germ Cell Tumors
pain, enlarged testis
most common tumor in men 15-35; incidence is increasing (maybe environmental); most common in white males (5:1 compared to blacks); 95% are germ cell tumors; lymphoma is common in older men; testicular cancer associated with testicular dysgensis syndrome seminomatous includes: seminoma & "spermatocytic seminoma" ; non-seminomatous includes: embryonical carcinoma, yolk sac tumor, choriocarcinoma, teratoma; LDH (lactate dehydrogenase) - can be used to assess tumor burden Placental Alkaline Phosphatase (PLAP) peak incidence: 30s sheets of uniform cells ("fried eggs"); bulky, lobulated large masses; round/ovoid homogenous; nuclei; surgery (radical tan, fleshy; prominent orchiectomy) + usually devoid of nucleoli; clear radiation hemorrhage/nec cytoplasm; rosis - "potato lymphocytes; neoplasm" fibrous separations (+/) crowded sheets of cells; architectural formations (papillary, glandular, bulky or small chemotherapy + tubular); ugly tumors, orchioectomy + cells (lg hemorrhage, retroperitoneal LN pleomorphic necrosis dissection cells, hyperchromatic nuclei, prominent often mult nucleoli) most common germ cell tumor (~50%); frequently occurs pure; variants: typical (~85%), anaplastic (~5-10%); spermatocytic: << 5%; usually low stage at dx; spread initially to LN
Seminoma
Embryonal Carcinoma
peak incidence: 20-30s
PLAP
more aggressive than seminoma (VERY impt to distinguish); rare in pure form
Yolk Sac Tumor
well circumscribed tumor w/ pale alpha feto yellow mucoid protein in serum cut surface; can have hemorrhagic and cystic foci looks kind of like placenta; elevated bHCG areas of hemorrhage and necrosis
"lace-like" reticular architecture; "SchillerDuval" bodies; stains for AFP synctiotrophobl ast and cytotrophoblast ; nuclei with prominent nucleoli and pink cytoplasm
most common in children <3 yo; very rare in adults
Choriocarcinoma
can present with gynecomastia
most aggressive; rare in pure form; early mets
painless/ painful enlargement; chemotherapy + metastases with clinically occult or tumor derived from see evidence of orchioectomy + unapparent tumors; extragonadal Teratoma one or more germ diff tissue types retroperitoneal LN signs/symptoms; lung or layer dissection retroperitoneal LNs physiologic conditions: medical: DM, CAD, neurologic disorder (ex. MS), renal failure, vulvovaginal atrophy, infections, alcoholism, drug abuse, heavy smoking, incontinence; hormonal: iatrogenic (OCPs, Depo Provera, etc), cancer tx, hair loss tx; drugs: antilipids, antipsychotics, benzos, bariburates, anti-HTN, anti-reflux, phenytoin, TCAs, SSRIs, alcohol, antihistamines, anticholinergics, amphetamines, narcotics; psychologic Sexual Disfunction causes: predisposing factors: family attitudes to sexuality, inadequate sex education, traumatic sexual experiences; precipitants: psychiatric illness, childbirth, infidelity, dysfxn in partner / prob in relationship; maintaining factors: anxiety, poor communication, lack of foreplay, depression, dysfxn in partner, poor sexual info, prob in general relationship Female Disorders persistently or recurrently deficient Hypoactive Sexual (or absent) sexual fantasies and desire for sexual activity, which Desire Disorder causes personal distress persistent or recurrent inability to attain, or to maintain until completion of the sexual activity, an Female Sexual Arousal Disorder adequate lubrication/swelling response of sexual excitement, which causes personal distress persistent or recurrent delay in, or Female Orgasmic absence of, orgasm following normal excitement phase, which causes Disorder distress don't need to distinguish btwn women who are aroused mentally by non-genital stimuli from those who are not aroused by any stimuli limitations of this def: women with female orgasmic disorder often have sexual arousal disorder; often it's intensity of orgasm that has diminished => distress
Dyspareunia
recurrent or persistent genital pain associated with sexual intercourse, which causes distress recurrent or involuntary spasm of musculature of outer 1/3 of vagina that interferes with sexual intercourse recurrent or persistent genital pain associated with noncoital sexual stimulation
penile-vaginal movement of intercourse may be impossible b/c of pain from partial or complete penile entry
Vaginismus
"muscular spasm" has never been documented; reflexive muscle tightening, fear of vaginal entry, and pain w/ its attempt are characteristic
Noncoital Sexual Pain Disorder
Male Disorders deficient or absent sexual fantasies judgement must and desire for be made by sexual activity, Hypoactive Sexual clinician taking which causes Desire Disorder into account pts distress and can't age and life be accounted for circumstances by other physical dx recurring inability to achieve or maintain an erection until Male Erectile completion of sexual activity, which Disorder causes distress; can't be accounted (impotence) for by other medical condition delay or absence of orgasm must be judged following normal by clinician Male Orgasmic excitement or taking into acct Disorder sexual activity; pts age and life must be persistent circumstances or frequent & => distress
evidence suggests that relationship issues and/or sexual trauma in childhood may play role; also life stressors and other interpersonal difficulties
tx includes discovering and resolving underlying conflict or life difficulties
prognosis: course can be consistent or periodic; i.e. can get remission if life stressors reemerge
short of other physiologic cause, usually a result of 'performance anxiety' or fears of not being able to achieve or maintain an erection often thought of as beginning in adolescence or early adulthood b/c sexual intimacy becomes related with a negative life event or aspect
tx for non-medical management = 'Sensate Focus' (involves progression of sexual intimacy typically over several weeks ultimately leading to penetration and orgasm) tx includes working through the underlying issues; therapists also use behavioral techniques like sensate focus
medical causes must be ruled out first; prognosis: very good with high success rates medical cause must be r/o first (including using substances); prognosis is very good
premature ejaculation
Sexual Aversion Disorder
ejaculation with minimal sexual stimulation before or shortly after penetration and before person wishes it; must be persistent or frequent & => distress when presented persistent or w/ sexual recurring aversion opportunity, pt to or avoidance of may get panic sexual activity that attacks or => distress extreme anxiety recurrent or persistent genital pain ass. w/ sex
relationship stress, novelty of new relationship, anxiety, issues related to control and intimacy can all play role in its dev
tx involves relaxation training, education, and working through underlying issues; if its due to new relationship, difficulty will resolve as relationship matures tx involves discovering and resolving underlying conflict or life difficulties tx: resolve underlying sexual and relationship issues
medical causes must be r/o first (including substance use); prognosis is good prognosis: varies but increases w/ ability to gain insight and work through issues that => disorder need to r/o other medical conditions first (including substance use); prognosis: varies
Dyspareunia
relationship issues and/or sexual trauma in childhood may play role relationship of this can be dx in disorder w/ males or females victims of rape and sex abuse
You might also like
- Rimary Menorrhea: By: Zaineb Talib Nimaa Zahra Ali Hasoon Zina Safaa AldienDocument28 pagesRimary Menorrhea: By: Zaineb Talib Nimaa Zahra Ali Hasoon Zina Safaa AldienZaineb TalibNo ratings yet
- Pemicu 4 "Air Seni Tidak Memancar Lurus": Adrian Pratama - 405100018Document34 pagesPemicu 4 "Air Seni Tidak Memancar Lurus": Adrian Pratama - 405100018Charles FerdinandNo ratings yet
- Obesteric Gynacology Short Notes For Usmle Step 2Document32 pagesObesteric Gynacology Short Notes For Usmle Step 2Rodaba Ahmadi Meherzad100% (1)
- Early Pregnancy Complications: by Harvir Singh Supervised by DR Ranjit and DR SyafiqahDocument33 pagesEarly Pregnancy Complications: by Harvir Singh Supervised by DR Ranjit and DR SyafiqahShre RanjithamNo ratings yet
- High Risk PregnancyDocument10 pagesHigh Risk PregnancyRoy Mujeres CabueñasNo ratings yet
- Identification and Management of Ambiguous GenitaliaDocument31 pagesIdentification and Management of Ambiguous Genitaliateslimolakunleraji100% (1)
- First Trimester BleedingDocument12 pagesFirst Trimester BleedingKevin de SilvaNo ratings yet
- MEETING 6 AmenorrheaDocument41 pagesMEETING 6 AmenorrheaNader KhouryNo ratings yet
- Sexuality and IntersexualityDocument23 pagesSexuality and IntersexualityManu BharadwazNo ratings yet
- GynecologyDocument18 pagesGynecologyLuai Tuma KhouryNo ratings yet
- GynecologyDocument27 pagesGynecologyDaniel Dwi NugrohoNo ratings yet
- Undescended TestisDocument66 pagesUndescended TestisalaaNo ratings yet
- Ultrasound Showing Uterus and Tubal PregnancyDocument58 pagesUltrasound Showing Uterus and Tubal PregnancyassssadfNo ratings yet
- Bleeding in First Trimester - Miscarriage, Non-Obstetrical ReasonsDocument23 pagesBleeding in First Trimester - Miscarriage, Non-Obstetrical ReasonsDiana TiganucNo ratings yet
- Disoreder Sex of DevelopmentDocument37 pagesDisoreder Sex of DevelopmentBesth To Frynce HutabaratNo ratings yet
- Obs WrittenDocument21 pagesObs WrittenHeno FayizNo ratings yet
- Abnormalities Of The Testis And Scrotum: A Concise GuideDocument34 pagesAbnormalities Of The Testis And Scrotum: A Concise GuideIma MoriNo ratings yet
- Amenorrhea SGDDocument44 pagesAmenorrhea SGDMichelle Lariosa ChangNo ratings yet
- Ectopic Pregnancy - Prof Zakaria SanadDocument76 pagesEctopic Pregnancy - Prof Zakaria SanadHerman FiraNo ratings yet
- Clinical Guidelines ForDocument54 pagesClinical Guidelines ForHenry SudharsonoNo ratings yet
- Menses: Dr. Said SarahnehDocument41 pagesMenses: Dr. Said Sarahnehpal_pal_palNo ratings yet
- Abnormal OBDocument34 pagesAbnormal OBLawrence NemirNo ratings yet
- Undescended Testes (CryptorchidismDocument6 pagesUndescended Testes (CryptorchidismJayson Almario AranasNo ratings yet
- Assesment of Amen or RheaDocument49 pagesAssesment of Amen or Rheakhadzx100% (2)
- Obstetrics & Gynecology NotesDocument7 pagesObstetrics & Gynecology NotesMary Ella WoodNo ratings yet
- AMENORRHOEADocument13 pagesAMENORRHOEAsuleiman AbdullahiNo ratings yet
- Amenorrhea, Hirsutism, VirilismDocument12 pagesAmenorrhea, Hirsutism, VirilismAly MorganNo ratings yet
- AmenorrhoeaDocument38 pagesAmenorrhoeaheydydNo ratings yet
- Dr. Shehla Jamal Assistant Professor: OBG S M S & RDocument69 pagesDr. Shehla Jamal Assistant Professor: OBG S M S & RAppy LoveNo ratings yet
- 2 Nursing Care of the Pregnant Client Gestational ConditionDocument120 pages2 Nursing Care of the Pregnant Client Gestational ConditionjustinjareddNo ratings yet
- Student'S Gynaecology Notes: For Students, by StudentsDocument45 pagesStudent'S Gynaecology Notes: For Students, by StudentsAdli IsmailNo ratings yet
- OB Intern's Review - Dra LeeDocument214 pagesOB Intern's Review - Dra LeeKathleenZunigaNo ratings yet
- 2.Amenorrhea.Document41 pages2.Amenorrhea.Kilp MosesNo ratings yet
- InfertilityDocument29 pagesInfertilityCristina StanleeNo ratings yet
- AmenorrheaDocument41 pagesAmenorrheaBonitavanyNo ratings yet
- Rps138 Slide AmenorrheaDocument41 pagesRps138 Slide Amenorrheasyahidah nadiahNo ratings yet
- OB/GYNDocument58 pagesOB/GYNpaulineNo ratings yet
- Gonadal Failure: Pure Gonadal Dysgenesis (46, XX and 46, XY With Gonadal Streaks) As Noted, This Abnormality May Have ADocument2 pagesGonadal Failure: Pure Gonadal Dysgenesis (46, XX and 46, XY With Gonadal Streaks) As Noted, This Abnormality May Have ALuis PadillaNo ratings yet
- Nursing Care in Clients With General Disturbance inDocument48 pagesNursing Care in Clients With General Disturbance inJADE MAIKHA A. MIERGASNo ratings yet
- SEO-Optimized Title for Document on Various Gynecological and Obstetric TopicsDocument140 pagesSEO-Optimized Title for Document on Various Gynecological and Obstetric TopicsjNo ratings yet
- Primary Amenorrhea: Rabika Almina RabiaDocument30 pagesPrimary Amenorrhea: Rabika Almina RabiaAlmina RehmanNo ratings yet
- DR Atin Singhai, Pathology LectureDocument41 pagesDR Atin Singhai, Pathology LectureHubdar Ali SahitoNo ratings yet
- Gyn and Ob notesDocument6 pagesGyn and Ob notesDr-Hashem Al-ShareefNo ratings yet
- Anomalies of The Female Genital TractDocument2 pagesAnomalies of The Female Genital TractAnastasia KasapiNo ratings yet
- Development of Female Genital Tract and Its AnomaliesDocument44 pagesDevelopment of Female Genital Tract and Its AnomaliesSuresh KatakamNo ratings yet
- Amenorrhea PDFDocument5 pagesAmenorrhea PDFHeden ColladoNo ratings yet
- Amen or RheaDocument41 pagesAmen or Rheakhadzx100% (2)
- Testiculartorsion Alias OrkitisDocument18 pagesTesticulartorsion Alias OrkitisNabilaNo ratings yet
- AmennorheaDocument14 pagesAmennorheakutra3000No ratings yet
- Gynecological Disorders in The Adolescent Gynecological Disorders in The AdolescentDocument31 pagesGynecological Disorders in The Adolescent Gynecological Disorders in The AdolescentarrypotterNo ratings yet
- Amenorrheamadeeasyslideshare 2015 150423165553 Conversion Gate01 PDFDocument51 pagesAmenorrheamadeeasyslideshare 2015 150423165553 Conversion Gate01 PDFHerman FiraNo ratings yet
- Sub-fertility Management GuideDocument41 pagesSub-fertility Management GuideBukola AjokeNo ratings yet
- WH Buzzwords Compilation 2020Document3 pagesWH Buzzwords Compilation 2020hexagridledsNo ratings yet
- Ch22 Female 1Document136 pagesCh22 Female 1Louis FortunatoNo ratings yet
- OSCE Gynae-OSCE-MMSSDocument24 pagesOSCE Gynae-OSCE-MMSSMohammad Saifullah100% (1)
- Evaluation of Testicular DisordersDocument56 pagesEvaluation of Testicular Disordersamal.fathullahNo ratings yet
- AmenorrheaDocument51 pagesAmenorrheabisharatNo ratings yet
- Guide to Pediatric Urology and Surgery in Clinical PracticeFrom EverandGuide to Pediatric Urology and Surgery in Clinical PracticeNo ratings yet
- Approach To The Patient With An Adnexal Mass - UpToDateDocument31 pagesApproach To The Patient With An Adnexal Mass - UpToDateRamackNo ratings yet
- GYNECOLOGY - 2.8 B&M Lesions of The OvariesDocument6 pagesGYNECOLOGY - 2.8 B&M Lesions of The OvariesAngela CaguitlaNo ratings yet
- Pathology ofDocument114 pagesPathology ofRahul Audenesen BratNo ratings yet
- FINAL Case Pres Ovarian CancerDocument31 pagesFINAL Case Pres Ovarian CancerXan LopezNo ratings yet
- 10 3322@caac 21559Document25 pages10 3322@caac 21559DIANA ALEXANDRA RAMIREZ MONTAÑONo ratings yet
- Intl J Gynecology Obste - 2021 - BerekDocument25 pagesIntl J Gynecology Obste - 2021 - BerekKalaivathanan VathananNo ratings yet
- Malignant Ovarian TumourDocument42 pagesMalignant Ovarian TumourJones MarinaNo ratings yet
- Ovarian CADocument62 pagesOvarian CAeza floresNo ratings yet
- Updates in Molecular Testing and Front-Line Treatment in Ovarian Cancer - Bradley MonkDocument30 pagesUpdates in Molecular Testing and Front-Line Treatment in Ovarian Cancer - Bradley Monkinfo7615No ratings yet
- Characterization of A Preclinical Model of Simultaneous Breast and Ovarian Cancer ProgressionDocument6 pagesCharacterization of A Preclinical Model of Simultaneous Breast and Ovarian Cancer ProgressionYuda AlhabsyNo ratings yet
- Fallopian Tube CancerDocument17 pagesFallopian Tube CancerDiana ApriliyanaNo ratings yet
- Tumor Marker DENDocument12 pagesTumor Marker DENArtheswara SidhajatiNo ratings yet
- Stewart 2019Document6 pagesStewart 2019tika apriliaNo ratings yet
- Reproduction Pathology ReviewDocument9 pagesReproduction Pathology ReviewkishorechandraNo ratings yet
- Textbook of Gynaecological OncologyDocument58 pagesTextbook of Gynaecological OncologyMarco Vinicio Benavides Osorto100% (2)
- Passmrcog Patho, Embryo 2016Document171 pagesPassmrcog Patho, Embryo 2016Mona HelouNo ratings yet
- Benign and Malignant Ovarian Tumors (Gynaecology)Document70 pagesBenign and Malignant Ovarian Tumors (Gynaecology)Dr Ali MehdiNo ratings yet
- Noor PDFDocument826 pagesNoor PDFAnonymous d1CGjMTi100% (1)
- Special Pathology Viva Questions by AMS 46Document32 pagesSpecial Pathology Viva Questions by AMS 46Mohan Dass100% (1)
- Management of Borderline Ovarian Tumors - RCOGDocument6 pagesManagement of Borderline Ovarian Tumors - RCOGYossi Agung AriosenoNo ratings yet
- GYNE 5.01 - Neoplastic Disease of The Ovary - Dr. TinioDocument7 pagesGYNE 5.01 - Neoplastic Disease of The Ovary - Dr. TinioMonique BorresNo ratings yet
- Benign Ovarian MassDocument39 pagesBenign Ovarian MassVidhi Chaudhary100% (1)
- Mucinous Tumors of The Ovary: Current Thoughts On Diagnosis and ManagementDocument9 pagesMucinous Tumors of The Ovary: Current Thoughts On Diagnosis and ManagementKenneth DomasianNo ratings yet
- Ovarian CancerDocument71 pagesOvarian CancerAyuni SallehNo ratings yet
- Precancerous Lesions of The Gynecologyc Tract - 2016 PDFDocument324 pagesPrecancerous Lesions of The Gynecologyc Tract - 2016 PDFCésar Garavito Quijaite50% (2)
- Philippine Journal of Gynecologic Oncology Volume 9 Number 1 2012Document48 pagesPhilippine Journal of Gynecologic Oncology Volume 9 Number 1 2012Dasha VeeNo ratings yet
- Pathology of The Female Genital Tract Course OutlineDocument2 pagesPathology of The Female Genital Tract Course OutlineDanica BiteraNo ratings yet
- Cancer of The Ovary PDFDocument12 pagesCancer of The Ovary PDFDiego Fernando Alzate GomezNo ratings yet
- Detecting Cancer Spread Through Peritoneal Fluid AnalysisDocument16 pagesDetecting Cancer Spread Through Peritoneal Fluid AnalysisnanxtoyahNo ratings yet
- Female Reproductive System Tumors and PathologyDocument115 pagesFemale Reproductive System Tumors and PathologyKhafidz Asy'ariNo ratings yet