Professional Documents
Culture Documents
HW 9 2011 Solutions
Uploaded by
Paramesh AnuguOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HW 9 2011 Solutions
Uploaded by
Paramesh AnuguCopyright:
Available Formats
Home-work #9_solutions
9.2 At 500C (930F), what is the maximum solubility (a) of Cu in Ag? (b) Of Ag in Cu?
Solution
(a) From Figure 9.7, the maximum solubility of Cu in Ag at 500C corresponds to the position of
the |(o + |) phase boundary at this temperature, or to about 2 wt% Cu.
(b) From this same figure, the maximum solubility of Ag in Cu corresponds to the position of the
o(o + |) phase boundary at this temperature, or about 1.5 wt% Ag.
9.5 Consider a specimen of ice that is at 210C and 1 atm pressure. Using Figure 9.2, the pressure
temperature phase diagram for H
2
O, determine the pressure to which the specimen must be raised or
lowered to cause it (a) to melt, and (b) to sublime.
Solution
The figure below shows the pressure-temperature phase diagram for H
2
O, Figure 10.2; a vertical
line has been constructed at -10C, and the location on this line at 1 atm pressure (point B) is also noted.
(a) Melting occurs, (by changing pressure) as, moving vertically (upward) at this temperature, we
cross the Ice-Liquid phase boundary. This occurs at approximately 570 atm; thus, the pressure of the
specimen must be raised from 1 to 570 atm.
(b) In order to determine the pressure at which sublimation occurs at this temperature, we move
vertically downward from 1 atm until we cross the Ice-Vapor phase boundary. This intersection occurs at
approximately 0.0023 atm.
9.8 Cite the phases that are present and the phase compositions for the following alloys:
(a) 90 wt% Zn-10 wt% Cu at 400C (750F)
(b) 75 wt% Sn-25 wt% Pb at 175C (345F)
(c) 55 wt% Ag-45 wt% Cu at 900C (1650F)
(d) 30 wt% Pb-70 wt% Mg at 425C (795F)
(e) 2.12 kg Zn and 1.88 kg Cu at 500C (930F)
(f) 37 lb
m
Pb and 6.5 lb
m
Mg at 400C (750F)
(g) 8.2 mol Ni and 4.3 mol Cu at 1250C (2280F)
(h) 4.5 mol Sn and 0.45 mol Pb at 200C (390F)
Solution
This problem asks that we cite the phase or phases present for several alloys at specified
temperatures.
(a) That portion of the Cu-Zn phase diagram (Figure 9.19) that pertains to this problem is shown
below; the point labeled A represents the 90 wt% Zn-10 wt% Cu composition at 400C.
As may be noted, point A lies within the c and q phase field. A tie line has been constructed at 400C; its
intersection with the cc + q phase boundary is at 87 wt% Zn, which corresponds to the composition of the
c phase. Similarly, the tie-line intersection with the c + qq phase boundary occurs at 97 wt% Zn, which is
the composition of the q phase. Thus, the phase compositions are as follows:
C
c
= 87 wt% Zn-13 wt% Cu
C
q
= 97 wt% Zn-3 wt% Cu
(b) That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown
below; the point labeled B represents the 75 wt% Sn-25 wt% Pb composition at 175C.
As may be noted, point B lies within the o + | phase field. A tie line has been constructed at 175C; its
intersection with the oo + | phase boundary is at 16 wt% Sn, which corresponds to the composition of the
o phase. Similarly, the tie-line intersection with the o + || phase boundary occurs at 97 wt% Sn, which
is the composition of the | phase. Thus, the phase compositions are as follows:
C
o
= 16 wt% Sn-84 wt% Pb
C
|
= 97 wt% Sn-3 wt% Pb
(c) The Ag-Cu phase diagram (Figure 9.7) is shown below; the point labeled C represents the
55 wt% Ag-45 wt% Cu composition at 900C.
As may be noted, point C lies within the Liquid phase field. Therefore, only the liquid phase is present; its
composition is 55 wt% Ag-45 wt% Cu.
(d) The Mg-Pb phase diagram (Figure 9.20) is shown below; the point labeled D represents the
30 wt% Pb-70 wt% Mg composition at 425C.
As may be noted, point D lies within the o phase field. Therefore, only the o phase is present; its
composition is 30 wt% Pb-70 wt% Mg.
(e) For an alloy composed of 2.12 kg Zn and 1.88 kg Cu and at 500C, we must first determine
the Zn and Cu concentrations, as
C
Zn
=
2.12 kg
2.12 kg + 1.88 kg
100 = 53 wt%
C
Cu
=
1.88 kg
2.12 kg + 1.88 kg
100 = 47 wt%
That portion of the Cu-Zn phase diagram (Figure 9.19) that pertains to this problem is shown below; the
point labeled E represents the 53 wt% Zn-47 wt% Cu composition at 500C.
As may be noted, point E lies within the | + phase field. A tie line has been constructed at 500C; its
intersection with the || + phase boundary is at 49 wt% Zn, which corresponds to the composition of the
| phase. Similarly, the tie-line intersection with the | + phase boundary occurs at 58 wt% Zn, which is
the composition of the phase. Thus, the phase compositions are as follows:
C
|
= 49 wt% Zn-51 wt% Cu
C
= 58 wt% Zn-42 wt% Cu
(f) For an alloy composed of 37 lb
m
Pb and 6.5 lb
m
Mg and at 400C, we must first determine the
Pb and Mg concentrations, as
C
Pb
=
37 lb
m
37 lb
m
+ 6.5 lb
m
100 = 85 wt%
C
Mg
=
6.5 lb
m
37 lb
m
+ 6.5 lb
m
100 = 15 wt%
That portion of the Mg-Pb phase diagram (Figure 9.20) that pertains to this problem is shown below; the
point labeled F represents the 85 wt% Pb-15 wt% Mg composition at 400C.
As may be noted, point F lies within the L + Mg
2
Pb phase field. A tie line has been constructed at 400C;
it intersects the vertical line at 81 wt% Pb, which corresponds to the composition of Mg
2
Pb. Furthermore,
the tie line intersection with the L + Mg
2
Pb-L phase boundary is at 93 wt% Pb, which is the composition of
the liquid phase. Thus, the phase compositions are as follows:
C
Mg
2
Pb
= 81 wt% Pb-19 wt% Mg
C
L
= 93 wt% Pb-7 wt% Mg
(g) For an alloy composed of 8.2 mol Ni and 4.3 mol Cu and at 1250C, it is first necessary to
determine the Ni and Cu concentrations, which we will do in wt% as follows:
n
Ni
'
= n
m
Ni
A
Ni
= (8.2 mol)(58.69 g/mol)= 481.3 g
n
Cu
'
= n
m
Cu
A
Cu
= (4.3 mol)(63.55 g/mol)= 273.3 g
C
Ni
=
481.3 g
481.3 g + 273.3 g
100= 63.8 wt%
C
Cu
=
273.3 g
481.3 g + 273.3 g
100= 36.2 wt%
The Cu-Ni phase diagram (Figure 9.3a) is shown below; the point labeled G represents the 63.8 wt% Ni-
36.2 wt% Cu composition at 1250C.
As may be noted, point G lies within the o phase field. Therefore, only the o phase is present; its
composition is 63.8 wt% Ni-36.2 wt% Cu.
(h) For an alloy composed of 4.5 mol Sn and 0.45 mol Pb and at 200C, it is first necessary to
determine the Sn and Pb concentrations, which we will do in weight percent as follows:
n
Sn
= n
m
Sn
A
Sn
= (4.5 mol)(118.71 g/mol)= 534.2 g
n
Pb
'
= n
m
Pb
A
Pb
= (0.45 mol)(207.2 g/mol)= 93.2 g
C
Sn
=
534.2 g
534.2 g + 93.2 g
100= 85.1 wt%
C
Pb
=
93.2 g
534.2 g + 93.2 g
100=14.9 wt%
That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the
point labeled H represents the 85.1 wt% Sn-14.9 wt% Pb composition at 200C.
As may be noted, point H lies within the | + L phase field. A tie line has been constructed at 200C; its
intersection with the L| + L phase boundary is at 74 wt% Sn, which corresponds to the composition of
the L phase. Similarly, the tie-line intersection with the | + L| phase boundary occurs at 97.5 wt% Sn,
which is the composition of the | phase. Thus, the phase compositions are as follows:
C
|
= 97.5 wt% Sn-2.5 wt% Pb
C
L
= 74 wt% Sn-26 wt% Pb
9.13 For an alloy of composition 74 wt% Zn-26 wt% Cu, cite the phases present and their compositions at
the following temperatures: 850C, 750C, 680C, 600C, and 500C.
Solution
This problem asks us to determine the phases present and their concentrations at several
temperatures, for an alloy of composition 74 wt% Zn-26 wt% Cu. From Figure 9.19 (the Cu-Zn phase
diagram), which is shown below with a vertical line constructed at the specified composition:
At 850C, a liquid phase is present; C
L
= 74 wt% Zn-26 wt% Cu
At 750C, and liquid phases are present; C
= 67 wt% Zn-33 wt% Cu; C
L
= 77 wt% Zn-23 wt%
Cu
At 680C, o and liquid phases are present; C
o
= 73 wt% Zn-27 wt% Cu; C
L
= 82 wt% Zn-18 wt%
Cu
At 600C, the o phase is present; C
o
= 74 wt% Zn-26 wt% Cu
At 500C, and c phases are present; C
= 69 wt% Zn-31 wt% Cu; C
c
= 78 wt% Zn-22 wt% Cu
9.17 A 90 wt% Ag-10 wt% Cu alloy is heated to a temperature within the | + liquid phase region. If the
composition of the liquid phase is 85 wt% Ag, determine:
(a) The temperature of the alloy
(b) The composition of the | phase
(c) The mass fractions of both phases
Solution
(a) In order to determine the temperature of a 90 wt% Ag-10 wt% Cu alloy for which | and liquid
phases are present with the liquid phase of composition 85 wt% Ag, we need to construct a tie line across
the | + L phase region of Figure 9.7 that intersects the liquidus line at 85 wt% Ag; this is possible at about
850C.
(b) The composition of the | phase at this temperature is determined from the intersection of this
same tie line with solidus line, which corresponds to about 95 wt% Ag.
(c) The mass fractions of the two phases are determined using the lever rule, Equations 9.1 and
9.2 with C
0
= 90 wt% Ag, C
L
= 85 wt% Ag, and C
|
= 95 wt% Ag, as
W
|
=
C
0
C
L
C
|
C
L
=
90 85
95 85
= 0.50
W
L
=
C
|
C
0
C
|
C
L
=
95 90
95 85
= 0.50
9.27 A 45 wt% Pb55 wt% Mg alloy is rapidly quenched to room temperature from an elevated
temperature in such a way that the high-temperature microstructure is preserved. This microstructure is
found to consist of the phase and Mg
2
Pb, having respective mass fractions of 0.65 and 0.35. Determine
the approximate temperature from which the alloy was quenched.
Solution
We are asked to determine the approximate temperature from which a 45 wt% Pb-55 wt% Mg
alloy was quenched, given the mass fractions of o and Mg
2
Pb phases. We can write a lever-rule expression
for the mass fraction of the o phase as
W
o
= 0.65 =
C
Mg
2
Pb
C
0
C
Mg
2
Pb
C
o
The value of C
0
is stated as 45 wt% Pb-55 wt% Mg, and C
Mg
2
Pb
is 81 wt% Pb-19 wt% Mg, which is
independent of temperature (Figure 9.20); thus,
0.65 =
81 45
81 C
o
which yields
C
o
= 25.6 wt% Pb
The temperature at which the o(o + Mg
2
Pb) phase boundary (Figure 9.20) has a value of 25.6 wt% Pb is
about 360C (680F).
9.33 The microstructure of a lead-tin alloy at 180C (355F) consists of primary and eutectic structures.
If the mass fractions of these two microconstituents are 0.57 and 0.43, respectively, determine the
composition of the alloy.
Solution
Since there is a primary | microconstituent present, then we know that the alloy composition, C
0
is between 61.9 and 97.8 wt% Sn (Figure 9.8). Furthermore, this figure also indicates that C
|
= 97.8 wt%
Sn and C
eutectic
= 61.9 wt% Sn. Applying the appropriate lever rule expression for W
|'
W
|'
=
C
0
C
eutectic
C
|
C
eutectic
=
C
0
61.9
97.8 61.9
= 0.57
and solving for C
0
yields C
0
= 82.4 wt% Sn.
9.37 For a 30 wt% Zn-70 wt% Cu alloy, make schematic sketches of the microstructure that would be
observed for conditions of very slow cooling at the following temperatures: 1100C (2010F), 950C
(1740F), 900C (1650F), and 700C (1290F). Label all phases and indicate their approximate
compositions.
Solution
The illustration below is the Cu-Zn phase diagram (Figure 9.19). A vertical line at a composition
of 30 wt% Zn-70 wt% Cu has been drawn, and, in addition, horizontal arrows at the four temperatures
called for in the problem statement (i.e., 1100C, 950C, 900C, and 700C).
On the basis of the locations of the four temperature-composition points, schematic sketches of the four
respective microstructures along with phase compositions are represented as follows:
9.39 The room-temperature tensile strengths of pure lead and pure tin are 16.8 MPa and 14.5 MPa,
respectively.
(a) Make a schematic graph of the room-temperature tensile strength versus composition for all
compositions between pure lead and pure tin. (Hint: you may want to consult Sections 9.10 and 9.11, as
well as Equation 9.24 in Problem 9.64.)
(b) On this same graph schematically plot tensile strength versus composition at 150C.
(c) Explain the shapes of these two curves, as well as any differences between them.
Solution
The (a) and (b) portions of the problem ask that we make schematic plots on the same graph for
the tensile strength versus composition for lead-tin alloys at both room temperature and 150C; such a
graph is shown below.
(c) Upon consultation of the Pb-Sn phase diagram (Figure 9.8) we note that, at room temperature
(20C), about 1.5 wt% of Sn is soluble in Pb (within the o-phase region at the left extremity of the phase
diagram). Similarly, only about 1 wt% of Pb is soluble in Sn (within the |-phase region at the left
extremity). Thus, there will a solid-solution strengthening effect on both ends of the phase diagram
strength increases slightly with additions of Sn to Pb [in the o phase region (left-hand side)] and with
additions of Pb to Sn [in the | phase region (right-hand side)]; these effects are noted in the above figure.
This figure also shows that the tensile strength of pure lead is greater than pure tin, which is in agreement
with tensile strength values provided in the problem statement.
In addition, at room temperature, for compositions between about 1.5 wt% Sn and 99 wt% Sn,
both o and | phase will coexist, (Figure 9.8), Furthermore, for compositions within this range, tensile
strength will depend (approximately) on the tensile strengths of each of the o and | phases as well as their
phase fractions in a manner described by Equation 9.24 for the elastic modulus (Problem 9.64). That is, for
this problem
(TS)
alloy
~ (TS)
o
V
o
+ (TS)
|
V
|
in which TS and V denote tensile strength and volume fraction, respectively, and the subscripts represent
the alloy/phases. Also, mass fractions of the o and | phases change linearly with changing composition
(according to the lever rule). Furthermore, although there is some disparity between the densities of Pb and
Sn (11.35 versus 7.27 g/cm
3
), weight and volume fractions of the o and | phases will also be similar (see
Equation 9.6).
At 150C, the curve will be shifted to significantly lower tensile strengths inasmuch as tensile
strength diminishes with increasing temperature (Section 6.6, Figure 6.14). In addition, according to Figure
9.8, solubility limits for both o and | phases increasefor the o phase from 1.5 to 10 wt% Sn, and for the
| phase from 1 to about 2 wt% Pb. Thus, the compositional ranges over which solid-solution strengthening
occurs increase somewhat from the room-temperature ranges; these effects are also noted on the 150C
curve above. Furthermore, at 150C, it would be expected that the tensile strength of lead will be greater
than that of tin; and for compositions over which both o and | phases coexist, strength will decrease
approximately linearly with increasing Sn content.
9.42 Figure 9.36 is the aluminum-neodymium phase diagram, for which only single-phase regions are
labeled. Specify temperature-composition points at which all eutectics, eutectoids, peritectics, and
congruent phase transformations occur. Also, for each, write the reaction upon cooling.
Solution
Below is shown the aluminum-neodymium phase diagram (Figure 9.36).
There are two eutectics on this phase diagram. One exists at 12 wt% Nd-88 wt% Al and 632C.
The reaction upon cooling is
L Al + Al
11
Nd
3
The other eutectic exists at about 97 wt% Nd-3 wt% Al and 635C. This reaction upon cooling is
L AlNd
3
+ Nd
There are four peritectics. One exists at 59 wt% Nd-41 wt% Al and 1235C. Its reaction upon
cooling is as follows:
L + Al
2
Nd Al
11
Nd
3
The second peritectic exists at 84 wt% Nd-16 wt% Al and 940C. This reaction upon cooling is
L + Al
2
Nd AlNd
The third peritectic exists at 91 wt% Nd-9 wt% Al and 795C. This reaction upon cooling is
L + AlNd AlNd
2
The fourth peritectic exists at 94 wt% Nd-6 wt% Al and 675C. This reaction upon cooling is
L + AlNd
2
AlNd
3
There is one congruent melting point at about 73 wt% Nd-27 wt% Al and 1460C. Its reaction
upon cooling is
L Al
2
Nd
No eutectoids are present.
9.43 Figure 9.37 is a portion of the titanium-copper phase diagram for which only single-phase
regions are labeled. Specify all temperature-composition points at which eutectics, eutectoids, peritectics,
and congruent phase transformations occur. Also, for each, write the reaction upon cooling.
Solution
Below is shown the titanium-copper phase diagram (Figure 9.37).
There is one eutectic on this phase diagram, which exists at about 51 wt% Cu-49 wt% Ti and
960C. Its reaction upon cooling is
L Ti
2
Cu + TiCu
There is one eutectoid for this system. It exists at about 7.5 wt% Cu-92.5 wt% Ti and 790C.
This reaction upon cooling is
| o + Ti
2
Cu
There is one peritectic on this phase diagram. It exists at about 40 wt% Cu-60 wt% Ti and
1005C. The reaction upon cooling is
| + L Ti
2
Cu
There is a single congruent melting point that exists at about 57.5 wt% Cu-42.5 wt% Ti and
982C. The reaction upon cooling is
L TiCu
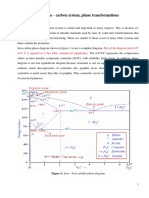
.50 Consider 1.0 kg of austenite containing 1.15 wt% C, cooled to below 727C (1341F).
(a) What is the proeutectoid phase?
(b) How many kilograms each of total ferrite and cementite form?
(c) How many kilograms each of pearlite and the proeutectoid phase form?
(d) Schematically sketch and label the resulting microstructure.
Solution
(a) The proeutectoid phase will be Fe
3
C since 1.15 wt% C is greater than the eutectoid
composition (0.76 wt% C).
(b) For this portion of the problem, we are asked to determine how much total ferrite and
cementite form. Application of the appropriate lever rule expression yields
W
o
=
C
Fe
3
C
C
0
C
Fe
3
C
C
o
=
6.70 1.15
6.70 0.022
= 0.83
which, when multiplied by the total mass of the alloy (1.0 kg), gives 0.83 kg of total ferrite.
Similarly, for total cementite,
W
Fe
3
C
=
C
0
C
o
C
Fe
3
C
C
o
=
1.15 0.022
6.70 0.022
= 0.17
And the mass of total cementite that forms is (0.17)(1.0 kg) = 0.17 kg.
(c) Now we are asked to calculate how much pearlite and the proeutectoid phase (cementite)
form. Applying Equation 9.22, in which
C
1
'
= 1.15 wt% C
W
p
=
6.70 C
1
'
6.70 0.76
=
6.70 1.15
6.70 0.76
= 0.93
which corresponds to a mass of 0.93 kg. Likewise, from Equation 9.23
W
Fe
3
C'
=
C
1
'
0.76
5.94
=
1.15 0.76
5.94
= 0.07
which is equivalent to 0.07 kg of the total 1.0 kg mass.
(d) Schematically, the microstructure would appear as:
You might also like
- Week 11Document12 pagesWeek 11lduran_63No ratings yet
- Chap 07 PDFDocument26 pagesChap 07 PDFdada jamdarNo ratings yet
- Engineering Materials 2 - Chapter 18Document4 pagesEngineering Materials 2 - Chapter 18Andres PerezNo ratings yet
- Cast IronDocument15 pagesCast IronJohnNo ratings yet
- Report Heat Treatment Eng Lab 3Document7 pagesReport Heat Treatment Eng Lab 3khalifawhan43% (7)
- Reeds-8 General Engineering Knowledge For Marine Engineers.Document265 pagesReeds-8 General Engineering Knowledge For Marine Engineers.habesha0288% (16)
- Module 06 Part 7 Control CableDocument17 pagesModule 06 Part 7 Control CableAviation WorldNo ratings yet
- Design and MFG of Hydraulic PressesDocument54 pagesDesign and MFG of Hydraulic Pressesraghumn100% (3)
- HCP A C Ratio PDFDocument16 pagesHCP A C Ratio PDFInderpal SinghNo ratings yet
- ch16 Advance CompositesDocument52 pagesch16 Advance CompositesAnonymous 3TpOzKPj5100% (1)
- Solutions ManualDocument17 pagesSolutions ManualTzu-li LiuNo ratings yet
- HW 5 2010 SolutionsDocument7 pagesHW 5 2010 SolutionsArlindo Lopes Faria100% (1)
- Nihonto Summary PDFDocument54 pagesNihonto Summary PDFtogakure0880No ratings yet
- Assignment 7 SolutionsDocument11 pagesAssignment 7 SolutionsIsaiah Qe LiewNo ratings yet
- Problems Phase FeDocument9 pagesProblems Phase FeAshutoshKumarNo ratings yet
- Phase Diagrams: Solubility LimitDocument163 pagesPhase Diagrams: Solubility LimitseaNo ratings yet
- Fall2010 Ch4&5 Sug HW KeyDocument20 pagesFall2010 Ch4&5 Sug HW KeyjacobtianNo ratings yet
- CH 09Document97 pagesCH 09BeAu ZorAko50% (2)
- Closed-Book Practice-Ch 09 (2017!08!07)Document9 pagesClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- CH 21Document32 pagesCH 21Indro ParmaNo ratings yet
- ch11 AllProblem KeyDocument56 pagesch11 AllProblem Keyladyinred90No ratings yet
- CH 13Document30 pagesCH 13Laurertan TavaresNo ratings yet
- Construction Materials Density and Dimensional ChangesDocument4 pagesConstruction Materials Density and Dimensional ChangesIngrid Zetina Tejero100% (1)
- Material Science and Engineering Ch. 10 SolDocument72 pagesMaterial Science and Engineering Ch. 10 SolPatrick GibsonNo ratings yet
- Atomic and Ionic Arrangements CalculationsDocument19 pagesAtomic and Ionic Arrangements CalculationsRafael AraújoNo ratings yet
- PMMA Stress-Strain AnalysisDocument5 pagesPMMA Stress-Strain AnalysisAnam PirachaNo ratings yet
- ECEN 248 Digital Systems Design Chapter 6.1 Multiplexers and DecodersDocument28 pagesECEN 248 Digital Systems Design Chapter 6.1 Multiplexers and DecodersnicksoldenNo ratings yet
- CH 10Document71 pagesCH 10Ipshita Ranjana100% (1)
- Calculate Vacancy Formation Energies and Concentrations in SolidsDocument3 pagesCalculate Vacancy Formation Energies and Concentrations in SolidsDavid Sahry Mark100% (1)
- CH 07Document60 pagesCH 07IlhamBintang100% (1)
- 5.111 Practice 1 Solutions PDFDocument8 pages5.111 Practice 1 Solutions PDF15klaNo ratings yet
- 21 Askeland ChapDocument8 pages21 Askeland Chapel_dualcNo ratings yet
- MIT2 080JF13 Lecture11Document21 pagesMIT2 080JF13 Lecture11combatps1No ratings yet
- Physics of VLSI Devices (ECE-5018) Digital Assignment - IDocument5 pagesPhysics of VLSI Devices (ECE-5018) Digital Assignment - IShreyas RaoNo ratings yet
- 20 Askeland ChapDocument10 pages20 Askeland Chapel_dualcNo ratings yet
- Homework and Solutions - ch5 Ch6.IMSDocument18 pagesHomework and Solutions - ch5 Ch6.IMSHery RobiyantoroNo ratings yet
- Sheet (4) SolDocument6 pagesSheet (4) Solsamadony100% (1)
- Combustion Processes ExplainedDocument18 pagesCombustion Processes ExplainedMd. Ahsanur RahmanNo ratings yet
- WDocument11 pagesWShrey PandeyNo ratings yet
- Second Edition (: 2001 Mcgraw-Hill)Document11 pagesSecond Edition (: 2001 Mcgraw-Hill)AbdullahNo ratings yet
- Chapter 5 Practice Problems SolutionsDocument8 pagesChapter 5 Practice Problems SolutionsShafiq HafizullahNo ratings yet
- Homework 2Document8 pagesHomework 2jinx50100% (1)
- PDFDocument29 pagesPDFSaurabh PatelNo ratings yet
- CH 06Document85 pagesCH 06Gerardo VieyraNo ratings yet
- Question & Answer Set-5Document8 pagesQuestion & Answer Set-5rahul kumar100% (1)
- Ch11 AllProblem KeyDocument55 pagesCh11 AllProblem KeyHarnani Sharuddin100% (3)
- HW#5 SolutionDocument5 pagesHW#5 Solutionmtydaics100% (2)
- Assignment 2 - 2023 - SolutionsDocument23 pagesAssignment 2 - 2023 - SolutionsLinhan ChuNo ratings yet
- ch12 PDFDocument66 pagesch12 PDFSurekha Pravin AlshiNo ratings yet
- MIT5 111F14 Lec04SolnDocument2 pagesMIT5 111F14 Lec04SolnFaiza Jan IftikharNo ratings yet
- Thesis MosfetDocument84 pagesThesis Mosfetawanath100% (1)
- ch29 PDFDocument29 pagesch29 PDFRodrigo S QuirinoNo ratings yet
- Tutorials - 1 To 12Document19 pagesTutorials - 1 To 12Subhash ChandraNo ratings yet
- Problem Set 3Document3 pagesProblem Set 3AshutoshKumarNo ratings yet
- 9 SolutionsDocument31 pages9 SolutionsLaurertan TavaresNo ratings yet
- Tutorial 2 HelperDocument3 pagesTutorial 2 HelperDedy SaputraNo ratings yet
- Assignment 8 SolutionDocument6 pagesAssignment 8 SolutionBrishen Hawkins100% (1)
- HW SolutionsDocument16 pagesHW SolutionsoerbilNo ratings yet
- Mse230 E2 Practice SolDocument20 pagesMse230 E2 Practice SolAkshit PandeyNo ratings yet
- Composition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Document7 pagesComposition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Muhammad Ibkar YusranNo ratings yet
- CH 09Document8 pagesCH 09Antoinette ChuaNo ratings yet
- Solving Phase Equilibrium ProblemsDocument5 pagesSolving Phase Equilibrium ProblemsJulian GarcíaNo ratings yet
- HW8 Solution KeyDocument3 pagesHW8 Solution KeyHerlina PebrianiNo ratings yet
- 9과 상태도 답지Document23 pages9과 상태도 답지이수연No ratings yet
- CH 10Document9 pagesCH 1000gp1200r100% (1)
- Engineering Materials: Tutorial / Chapter 9Document11 pagesEngineering Materials: Tutorial / Chapter 9Alim SultangazinNo ratings yet
- Chapter 11: Transformation in AlloysDocument48 pagesChapter 11: Transformation in AlloysbadaboyNo ratings yet
- Ferrous Metals Production ProcessesDocument31 pagesFerrous Metals Production Processessabasaktir4142No ratings yet
- FeC and TTT DiagramsDocument12 pagesFeC and TTT DiagramsMohamed El-WakilNo ratings yet
- Micro Constituents of Iron and SteelDocument1 pageMicro Constituents of Iron and SteelAshwani KansaraNo ratings yet
- FL&O Section 10Document24 pagesFL&O Section 10Muhammad FaizNo ratings yet
- Lec 8 (Iron-Iron Carbide Diagram)Document20 pagesLec 8 (Iron-Iron Carbide Diagram)HdjdNo ratings yet
- Properties of Engineering Materials Lesson 3 (I)Document11 pagesProperties of Engineering Materials Lesson 3 (I)Douglas Kufre-Abasi GilbertNo ratings yet
- Problem Solving 3Document4 pagesProblem Solving 3Raphael Pizarro ArceoNo ratings yet
- Effect of Chill On Ni Cast Iron PDFDocument18 pagesEffect of Chill On Ni Cast Iron PDFsachinguptachdNo ratings yet
- Physical Metallurgy Laboratory Manual PDFDocument83 pagesPhysical Metallurgy Laboratory Manual PDFafnene1100% (1)
- Vol4 MICRESS ExamplesDocument83 pagesVol4 MICRESS ExamplesVm GobinathNo ratings yet
- Fe-Fe3C Phase Diagram ExplainedDocument6 pagesFe-Fe3C Phase Diagram Explainedolid_zoneNo ratings yet
- 2019 03 28 Phase DiagramsDocument72 pages2019 03 28 Phase DiagramsHarish Subramanian100% (1)
- Congruent Transformation: No Change in Composition Upon PhaseDocument21 pagesCongruent Transformation: No Change in Composition Upon PhaseTuna ÇelikNo ratings yet
- Iron - Carbon SystemDocument21 pagesIron - Carbon SystemYavana KeerthiNo ratings yet
- Mechanical Properties and Fatigue Behavior of Railway Wheel Steels As Influenced by Mechanical and Thermal LoadingsDocument8 pagesMechanical Properties and Fatigue Behavior of Railway Wheel Steels As Influenced by Mechanical and Thermal LoadingsSTBz09No ratings yet
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanNo ratings yet
- Iron Carbon Phase DiagramDocument3 pagesIron Carbon Phase DiagramrabikmNo ratings yet
- High Carbon Hot-Rolled Steel Sheet With Excellent Formability "SUPERHOT - F"Document3 pagesHigh Carbon Hot-Rolled Steel Sheet With Excellent Formability "SUPERHOT - F"dhafi keceNo ratings yet
- Homework 14 Solutions Spring 2001Document2 pagesHomework 14 Solutions Spring 2001Ikhwan Wf Miscellaneous AveroesNo ratings yet
- KMTL Cast Iron Turning Guide 32 65Document34 pagesKMTL Cast Iron Turning Guide 32 65cristian111111No ratings yet
- Assignment 3 PMTDocument2 pagesAssignment 3 PMTDewi Lestari Natalia MarpaungNo ratings yet
- Application of Phase DiagramDocument66 pagesApplication of Phase Diagrammm11_nedNo ratings yet