Professional Documents
Culture Documents
Assignment 23
Uploaded by
Jenny GoOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Assignment 23
Uploaded by
Jenny GoCopyright:
Available Formats
Engineering Materials BMM 1523
Assignment 2: submit this assignment within 2 weeks once it has been given to you (Your lecturer will let you know the exact date for this to be submitted: 1 group = 3 students). Important!! (On the front page of your assignment, you must state your section OR name of the lecturer who teaches you in the class). This is compulsory!! -----------------------------------------------------------------------------------------------------------------------------------------Question 1 In the pure water pressure-temperature equilibrium phase diagram in Fig 8.1 in Smiths book, page 312, what phases are in equilibrium for the following conditions: i. Along the freezing line ii. Aling the vaporization line iii. At the triple point Question 2 What is a binary isomorphous alloy system?
Question 3 For the following 4 equations, determine the name of each reaction.
Question 4 How are eutectic and eutectoid reactions similar? What is the significance of the oid suffix?
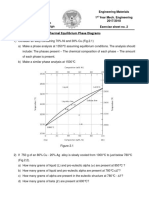
Question 5 Consider an alloy consisting 70 wt% Ni and 30 wt% Cu (see Fig 1 below). i. At 1350C make a phase analysis assuming equilibrium conditions. In the phase analysis include the following: a) What phases are present? b) What is the chemical composition of each phase c) What amount of each phase is present? ii. Make a similar phase analysis at 1500C. iii. Sketch the microstructure of the alloy at each of these temperatures by using circular microscopic fields.
Figure 1
Question 6 Consider the binary eutectic copper-silver phase diagram in Figure 2 below. Make phase analysis of an 88 wt% Ag 12 wt% Cu alloy at the temperatures: a) 1000C b) 800C c) 780C + T d) 780C T
Also, in the phase analysis, include: i. ii. iii. iv. The phases present The chemical compositions of the phases The amounts of each phase Sketch the microstructure by using 2 cm diameter circular fields.
Figure 2
Question 7 An alloy of 30 wt% Pb and 70 wt% Sn is slowly cooled from 250C to 27C (see Figure 3 below). i. ii. iii. iv. v. Is this alloy hypoeutectic or hypereutectic? What is the composition of the solid to form? What are the amounts and compositions of each phase that is present at 183C + T? What is the amount and composition of each phase that is present at 183C T? What are the amounts of each phase present at room temperature?
Figure 3 Question 8 A copper-nickel alloy contains 47 wt% Cu and 53 wt% Ni and is at 1300C. Use Fig 8.5 in Smiths book page 317 and answer the following question: i. What is the weight percent of copper in the liquid and solid phases at this temperature? ii. What weight percent of this alloy is liquid and what weight percent is solid?
Question 9 Make phase analysis of the equilibrium (ideal) solidification of lead-tin alloys at the following points in the leadtin phase diagram of Fig. 8.13 Smiths book page 326. State phases present, compositions of phases and amount of phases for below questions: i. ii. iii. iv. At the eutectic composition of the equilibrium just below 183C (eutectic temperature). The point C at 40% Sn and 230C. The point d at 40% Sn and 183C + T. The point e at 40% Sn and 183C T.
Answers: 5. iii 6. ii iii. iv. iii. iv.
amount of phases 33.3% and 66.67% amount of phases 33.3% and 66.6% amount of phases 16.6% and 83.4% amount of phases 3.84% and 96.16% 77.2% and 22.8% = 35.1% and = 64.8% eutectic = 77.2% and = 22.8% = 45.5% and = 54.5% = 30.6% and = 69.4% 38% and 62% 45.5% and 54.5% 76% and 24% 49% and 51% 73% and 27%
7.
v. 8. 9. ii. i. ii. iii. iv.
You might also like
- 2002UNIT2PAPER2Document16 pages2002UNIT2PAPER2petey78No ratings yet
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- Friction Stir Welding of High Strength 7XXX Aluminum AlloysFrom EverandFriction Stir Welding of High Strength 7XXX Aluminum AlloysNo ratings yet
- 2phase Flow and Boiling Heat TransferDocument218 pages2phase Flow and Boiling Heat TransfercmegmhiNo ratings yet
- Heat and Mass Transfer: "Solved Problems"Document16 pagesHeat and Mass Transfer: "Solved Problems"qiritical99No ratings yet
- Specification and Schedule of Quantities BrickworkDocument11 pagesSpecification and Schedule of Quantities BrickworkSyed AbthahirNo ratings yet
- Aws Cwi QuestionDocument8 pagesAws Cwi Questionfrenskiran75% (4)
- Tablet Coating PDFDocument6 pagesTablet Coating PDFAsif Hasan Niloy100% (1)
- Pile Jacking FullTextDocument223 pagesPile Jacking FullTextGeorge Ardianda CrNo ratings yet
- Metal Casting Processes Chapter ExplainedDocument88 pagesMetal Casting Processes Chapter ExplainedTham Wai Hung89% (9)
- Metal Casting Processes Chapter ExplainedDocument88 pagesMetal Casting Processes Chapter ExplainedTham Wai Hung89% (9)
- Infinity For Cement Equipment: Quality & Composition of Cement ClinkerDocument48 pagesInfinity For Cement Equipment: Quality & Composition of Cement ClinkerYhaneNo ratings yet
- Ashok Leyland Placement PaperDocument57 pagesAshok Leyland Placement PaperPriyanka NegiNo ratings yet
- PSet 8 FL13Document5 pagesPSet 8 FL13cacer009No ratings yet
- EG 244 Assignment 3 Phase DiagramDocument3 pagesEG 244 Assignment 3 Phase DiagramGoodson KolalaNo ratings yet
- Tutorial 7 - Phase DiagramsDocument4 pagesTutorial 7 - Phase DiagramsSYAFIQAH ISMAILNo ratings yet
- Engineering Materials Phase DiagramsDocument6 pagesEngineering Materials Phase DiagramsOmar AssalNo ratings yet
- MLE1101 Tutorial 4 - Suggested Solutions AnalysisDocument7 pagesMLE1101 Tutorial 4 - Suggested Solutions AnalysisYin HauNo ratings yet
- Sample Paper - 2010 Class - X Subject - Physics: General InstructionsDocument6 pagesSample Paper - 2010 Class - X Subject - Physics: General InstructionsReeshabh KaranNo ratings yet
- Che 3330 - Spring 2012 HW 5Document5 pagesChe 3330 - Spring 2012 HW 5Brett CasserlyNo ratings yet
- MECHANICAL SCIENCE (SEMESTER 2Document9 pagesMECHANICAL SCIENCE (SEMESTER 2DebojyotiMukherjeeNo ratings yet
- PM PaperDocument4 pagesPM PaperSomya KabraNo ratings yet
- Chapter 4-Phase DiagramDocument16 pagesChapter 4-Phase Diagramtky96No ratings yet
- 9A03703 Finite Element MethodsDocument8 pages9A03703 Finite Element MethodssivabharathamurthyNo ratings yet
- Cge 566 - Reservoir Engineering Assignment 1 - Phase Behavior of FluidsDocument2 pagesCge 566 - Reservoir Engineering Assignment 1 - Phase Behavior of FluidsMohamad AslamNo ratings yet
- INGE 5005 - 2 Semester 2014-2015 Name: Student NumberDocument2 pagesINGE 5005 - 2 Semester 2014-2015 Name: Student Numberluis riveraNo ratings yet
- F2020 HW5 SolutionsDocument7 pagesF2020 HW5 SolutionsWilliam Carl KistlerNo ratings yet
- WWW - Manaresults.Co - In: II B. Tech I Semester Regular/Supplementary Examinations, October/November - 2018 ThermodynamicsDocument8 pagesWWW - Manaresults.Co - In: II B. Tech I Semester Regular/Supplementary Examinations, October/November - 2018 Thermodynamicsashoku24007No ratings yet
- Failure Analysis of SA-213TP347H High-TemperatureDocument6 pagesFailure Analysis of SA-213TP347H High-TemperatureLakshminarayanNo ratings yet
- HT andPI2009-2010Document6 pagesHT andPI2009-2010sajni123No ratings yet
- Piazzoni AntonioSebastianoDocument87 pagesPiazzoni AntonioSebastianoAntonio PiazzoniNo ratings yet
- Thermodynamics Exp 2 ThermocoupleDocument15 pagesThermodynamics Exp 2 Thermocouplehayder alaliNo ratings yet
- Tutorial Questions For Part 1Document5 pagesTutorial Questions For Part 1Ng Yan XiongNo ratings yet
- AttachmentDocument7 pagesAttachmentgodspower odiorNo ratings yet
- THT ExamDocument7 pagesTHT Examabdilrhman sulimanNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced LevelDocument16 pagesCambridge International Advanced Subsidiary and Advanced LevelCallie Jia LiNo ratings yet
- Radioactivity Decay, Nuclear Energy SafetyDocument7 pagesRadioactivity Decay, Nuclear Energy Safetyzuliana1No ratings yet
- Atomic Structure and Bonding in MaterialsDocument16 pagesAtomic Structure and Bonding in MaterialsRasyidi AhmadNo ratings yet
- SPM 2009 Physics Paper2 MelakaDocument21 pagesSPM 2009 Physics Paper2 Melakawass2012No ratings yet
- Solve Only For 25 PointsDocument6 pagesSolve Only For 25 PointsOscar HechtNo ratings yet
- TYPD ExercisesDocument10 pagesTYPD ExercisesConstance Lynn'da GNo ratings yet
- Class 11C - Physics Paper 2Document6 pagesClass 11C - Physics Paper 2St. Andrew's High School KarachiNo ratings yet
- HYD ME 2 1 Thermo Set 1Document10 pagesHYD ME 2 1 Thermo Set 1manikantar15No ratings yet
- II B.tech I Sem Question Bank For r18Document30 pagesII B.tech I Sem Question Bank For r18saiharish634No ratings yet
- IES OBJ Civil Engineering 2004 Paper IDocument17 pagesIES OBJ Civil Engineering 2004 Paper Iravi maharajNo ratings yet
- ZEBAR SCHOOL PERIODIC TEST-3 SCIENCEDocument4 pagesZEBAR SCHOOL PERIODIC TEST-3 SCIENCERohan VayaNo ratings yet
- 3 Phase Induction Motor Types Connection Types 3 Phase Squirrel - Cage MotorDocument4 pages3 Phase Induction Motor Types Connection Types 3 Phase Squirrel - Cage MotorMikail AslanNo ratings yet
- SPM Tips Light Paper 2Document11 pagesSPM Tips Light Paper 2Salmizam IzamNo ratings yet
- Mechanics II Independent Revision ResourceDocument16 pagesMechanics II Independent Revision ResourcefejhvdhdwvwoyqiNo ratings yet
- A6DEC19BMEADocument4 pagesA6DEC19BMEA2K19/EC/101 LOKESHNo ratings yet
- Materials 1 Exam: Phase Diagrams, Microstructure and PropertiesDocument4 pagesMaterials 1 Exam: Phase Diagrams, Microstructure and Propertiesshauno9997No ratings yet
- Phase DiagramsDocument48 pagesPhase DiagramszanretNo ratings yet
- 2011-12-07 APSC278 Final ExamDocument7 pages2011-12-07 APSC278 Final ExamNik AgarwalNo ratings yet
- Semester-1 - Chemistry Stream - Mid+end PaperDocument15 pagesSemester-1 - Chemistry Stream - Mid+end PaperGopiNo ratings yet
- Salford University Engineering Materials and Electrical Systems ExamDocument7 pagesSalford University Engineering Materials and Electrical Systems ExamBrody CrossNo ratings yet
- ASSIGNMENT # 3 W KEYDocument16 pagesASSIGNMENT # 3 W KEYRashedul IslamNo ratings yet
- Problem Set 2 - Determining Maximum Cs-137 Production and Energy Deposition from Broken SourceDocument3 pagesProblem Set 2 - Determining Maximum Cs-137 Production and Energy Deposition from Broken SourcepstgouveiaNo ratings yet
- Jntuworld: R07 Set No. 2Document6 pagesJntuworld: R07 Set No. 2Dolly PriyaNo ratings yet
- 雙金屬料管感應加熱製程模擬Document88 pages雙金屬料管感應加熱製程模擬CCNo ratings yet
- 17.3 Oscillations - Cie Ial Physics - QP TheoryDocument11 pages17.3 Oscillations - Cie Ial Physics - QP TheoryRhibhav PalNo ratings yet
- Please See Your Exam Timetable or Check On FASER For The Deadline To Upload Your AnswersDocument11 pagesPlease See Your Exam Timetable or Check On FASER For The Deadline To Upload Your AnswersVlad SimizeanuNo ratings yet
- Elements of Mechanical EngineeringDocument2 pagesElements of Mechanical Engineeringrahul106No ratings yet
- Dynamic Damage and FragmentationFrom EverandDynamic Damage and FragmentationDavid Edward LambertNo ratings yet
- Resistivity Modeling: Propagation, Laterolog and Micro-Pad AnalysisFrom EverandResistivity Modeling: Propagation, Laterolog and Micro-Pad AnalysisNo ratings yet
- Week 1-1 IntroductionDocument22 pagesWeek 1-1 IntroductionJenny GoNo ratings yet
- (Week 8) Pneumatics Components and Circuit DesignDocument53 pages(Week 8) Pneumatics Components and Circuit DesignJenny Go100% (1)
- Fourier's Law and The Heat Equation: Chapter TwoDocument16 pagesFourier's Law and The Heat Equation: Chapter TwoSky OnnNo ratings yet
- (Week 8) Pneumatics Components and Circuit DesignDocument53 pages(Week 8) Pneumatics Components and Circuit DesignJenny Go100% (1)
- Week 1-3 Pascal, BernoulliDocument19 pagesWeek 1-3 Pascal, BernoulliJenny GoNo ratings yet
- Compilation of MUET Speaking QuestionsDocument35 pagesCompilation of MUET Speaking QuestionsJenny GoNo ratings yet
- Observation For Orifice at Speed Number 2Document4 pagesObservation For Orifice at Speed Number 2Jenny GoNo ratings yet
- Flow patterns and wall shear stress in arteriovenous fistula modelDocument11 pagesFlow patterns and wall shear stress in arteriovenous fistula modelJenny GoNo ratings yet
- F212 Module 1 Enzymes File NotesDocument7 pagesF212 Module 1 Enzymes File NotesJenny GoNo ratings yet
- Lesson 6 PID Control of Heat Exchanger Temp PDFDocument2 pagesLesson 6 PID Control of Heat Exchanger Temp PDFJenny GoNo ratings yet
- Objective: - To Design Complete Measurement Technique For Fluid Flow and Verify Bernoulli'sDocument2 pagesObjective: - To Design Complete Measurement Technique For Fluid Flow and Verify Bernoulli'sJenny GoNo ratings yet
- F212 Module 1 Enzymes File NotesDocument7 pagesF212 Module 1 Enzymes File NotesJenny GoNo ratings yet
- Manu FactDocument2 pagesManu FactJenny GoNo ratings yet
- General Plane MotionDocument14 pagesGeneral Plane MotionJenny GoNo ratings yet
- Module 1 - Enzymes PDFDocument5 pagesModule 1 - Enzymes PDFJenny GoNo ratings yet
- LogDocument4 pagesLogJenny GoNo ratings yet
- F212 Module 1 Enzymes File NotesDocument7 pagesF212 Module 1 Enzymes File NotesJenny GoNo ratings yet
- Business Letter EpcDocument1 pageBusiness Letter EpcJenny GoNo ratings yet
- Module 1 - Enzymes PDFDocument5 pagesModule 1 - Enzymes PDFJenny GoNo ratings yet
- Chemistry STPM Chapter 1 by STUDY SMART WWW - Studysmart.page - TLDocument8 pagesChemistry STPM Chapter 1 by STUDY SMART WWW - Studysmart.page - TLAcyl Chloride Hariprem95% (40)
- Question Bank (PG 1-58)Document58 pagesQuestion Bank (PG 1-58)Jm'' GarrickNo ratings yet
- Business Letter EpcDocument1 pageBusiness Letter EpcJenny GoNo ratings yet
- Project Statement 1Document2 pagesProject Statement 1Jenny GoNo ratings yet
- Engineering Materials Assignment 1 Due Nov 1stDocument1 pageEngineering Materials Assignment 1 Due Nov 1stJenny GoNo ratings yet
- Assignment 22Document2 pagesAssignment 22Jenny GoNo ratings yet
- Concrete Slipforming: A Cost-Effective Formwork TechniqueDocument117 pagesConcrete Slipforming: A Cost-Effective Formwork Techniqueparamarthasom1974No ratings yet
- V4500 Bypass Mulitslide Installation Instructions BypassDocument17 pagesV4500 Bypass Mulitslide Installation Instructions BypassJames ReiterNo ratings yet
- Smaw 12 Module 1Document7 pagesSmaw 12 Module 1Francis Rico Mutia RufonNo ratings yet
- Dosage FormulationDocument5 pagesDosage FormulationArham AhmedNo ratings yet
- Cmos Process FlowDocument25 pagesCmos Process FlowSHAIK MUSTHAFANo ratings yet
- S. I. 8 National Environmental Protection (Effluent Limitation) Regulations, 1991Document21 pagesS. I. 8 National Environmental Protection (Effluent Limitation) Regulations, 1991Ajus WaziriNo ratings yet
- Product Data Sheet: Sikacor® Eg-1Document4 pagesProduct Data Sheet: Sikacor® Eg-1Thompson LaiNo ratings yet
- CS2 - Carbon Steel Bars For The Reinforcement of Concrete (1995)Document36 pagesCS2 - Carbon Steel Bars For The Reinforcement of Concrete (1995)don2hmrNo ratings yet
- Product Data Sheet SKD-S2: Spotcheck Non Aqueous DeveloperDocument3 pagesProduct Data Sheet SKD-S2: Spotcheck Non Aqueous DeveloperMai Anh TaiNo ratings yet
- Soil test kit instructions and reagent listDocument8 pagesSoil test kit instructions and reagent listhromeroeNo ratings yet
- Abstracts Book EWWM2014Document276 pagesAbstracts Book EWWM2014azilleNo ratings yet
- Gove Operations Process Flow Single PageDocument4 pagesGove Operations Process Flow Single PageMayke Cezar WippelNo ratings yet
- Elevator Buckets CatalogueDocument30 pagesElevator Buckets CatalogueYeffreyn EscalonaNo ratings yet
- 15E282 18lab1Document10 pages15E282 18lab1Yadhuvanth kumarNo ratings yet
- Carriers of The Protective Effectiveness of Used Motor OilsDocument4 pagesCarriers of The Protective Effectiveness of Used Motor OilsChristian D JiménezNo ratings yet
- Chondroitin sulfate sodium analytical methods guideDocument3 pagesChondroitin sulfate sodium analytical methods guideAchmad LatiefNo ratings yet
- Functional Gage DesignDocument32 pagesFunctional Gage DesignnaveedsidhuNo ratings yet
- MQ SP M 4002 PDFDocument28 pagesMQ SP M 4002 PDFjaseelNo ratings yet
- OPGWDocument74 pagesOPGWAnonymous 3y4Z5cUNo ratings yet
- Technical Catalogue PP v1Document84 pagesTechnical Catalogue PP v1Fluidra Group0% (1)
- Performance Chemicals for the Polyurethane IndustryDocument8 pagesPerformance Chemicals for the Polyurethane IndustrySiriluck NevestNo ratings yet
- Complaint AnalysisDocument8 pagesComplaint AnalysisJKP OperationNo ratings yet
- Antacid Lab Effectiveness AnalysisDocument42 pagesAntacid Lab Effectiveness Analysisumesh123patilNo ratings yet
- Plant Design Solutions Master Contents and Updates: Pds Master Catalog For Cadworx 2015Document2 pagesPlant Design Solutions Master Contents and Updates: Pds Master Catalog For Cadworx 2015calebneltonNo ratings yet
- Spectre M 1 Ds EnglishDocument2 pagesSpectre M 1 Ds EnglishOgbedande Awo OrunmilaNo ratings yet
- Adsorption of Flouride Using Nanoparticles of Aluminium OxideDocument15 pagesAdsorption of Flouride Using Nanoparticles of Aluminium OxideIsa musaNo ratings yet