Professional Documents
Culture Documents
Klinkenberg Effect
Uploaded by
Qaiser HafeezOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Klinkenberg Effect
Uploaded by
Qaiser HafeezCopyright:
Available Formats
KLINKENBERG EFFECT:
BACKGROUND:
There is a difference between the flow of a gas and a liquid through a reservoir. This defiantly affecting the measurement of absolute permeability. As gas flow is less impeded by grain surfaces than liquid flow. Thus, this effect of gas flow being less impeded than liquid flow is considered to be a KLINKENBERG EFFECT.
INTRODUCTION:
The difference between gas and water permeabilities is significant not only for solving gas-water two-phase flow problems, but also for quick measurements of permeability using gas as pore fluid. The observed difference in gas and water permeabilities has been analyzed in view of the KLINKENBERG EFFECT. The flow of gas through porous media was investigated by Klinkenberg (1941).
EXPLANATION:
When the mean free path of the measuring gas is greater than the diameter of the capillary (pores) through which it is traveling, the random kinetic energy of the gas is transferred to movement of the gas molecule through the capillary or slippage of the molecules occur at the pore walls. This slippage causes the molecules of the gas to travel at a higher velocity in the direction of transfer. This phenomenon, known as the KLINKENBERG EFFECT, causes the measured permeability of a gas to be greater than the absolute permeability of the sample. This effect is due to slip flow of gas at pore walls which enhances gas flow when pore sizes are very small. Experimental results show (1) that gas permeability is larger than water permeability, (2) that gas permeability increases with increasing pore pressure, and (3) that water permeability slightly increases with increasing pore-pressure gradient across the specimen. The results (1) and (2) can be explained by Klinkenberg effect quantitatively with an empirical power law for Klinkenberg constant. Thus water permeability can be estimated from gas permeability. The Klinkenberg effect is important when permeability is lower than 10-18 m2 and at low differential pore pressures, and its correction is essential for estimating water permeability from the measurement of gas permeability.
Factors Affecting Measured Permeability Values:
Air permeabilities measured in a routine core analysis laboratory on rock samples from nonfractured reservoirs will give higher values than the actual reservoir permeability. This difference is dependent upon the magnitude of permeability as well as the pore geometry. The higher laboratory values are thought to be caused by gas slippage (the Klinkenberg effect), relative permeability, reactive fluids, and overburden pressure effects.
THE KLlNKENBERG GAS SLIPPAGE EFFECT:
The flow of gas through porous media was investigated by Klinkenberg (1941). He found that the permeability of a core sample was not constant, but varied with the gas used to make the measurement, as well as the mean (average) pressure existing in the core at the time of measurement. His investigations indicated that at low mean pressures-for example, at atmospheric pressure-the gas molecules are so far apart that they slip through the pore spaces with little friction loss, and yield a higher value of permeability. At higher mean pressures for example, 1000 psi (6895 kPa) or greater the gas molecules are closer together and experience a friction drag at the side of the pore walls. This increases as higher mean pressure increases, with the gas acting more and more like a liquid. This means that the measured value of permeability decreases as reservoir or laboratory mean pressure increases.
EXPERIMENTAL VERIFICATION:
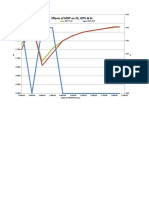
Experiments show that a plot of gas permeability versus the reciprocal mean pressure existing at the time of the gas permeability measurement forms a straight line that can be extrapolated to infinite mean pressure (Figure 5.7). This extrapolated value of permeability, referred to as the Klinkenberg permeability or equivalent liquid permeability, is lower than the measured gas permeabilities and is comparable to the permeability that would be obtained if the core were saturated with a nonreactive liquid such as oil. Figure 5.10 (Klinkenberg permeability determination) shows an example of this relationship for low permeability reservoir.
The Klinkenberg value (kL) can be correlated with the value of permeability determined with air at the mean pressure normally used in the laboratory measurements. Table 1, below, offers examples of the relationship between the air permeability and Klinkenberg-corrected values for sandstones. The correction, on a percentage basis, is greater in low permeability sand and becomes progressively smaller as permeability value increases.
Noncorrected permeability (md) 1.0 10.0 100.0 1000.0
Klinkenberg corrected permeability* (md) 0.7 7.8 88.0 950.0
*Air permeability that has been corrected for gas slippage. The Klinkenberg value is the equivalent liquid permeability assuming no reaction between the rock and the fluid.
Table 1. A comparison of noncorrected and Klinkenberg-corrected air permeability for some sandstones.
The table represents the results of laboratory measurements on a suite of core samples that covered a wide range of air permeabilities. In early core analysis reports, measured air permeability was corrected to the Klinkenberg permeability value, using correlations such as those presented in Table 1. above. In early core analysis the Klinkenberg permeability was estimated by using the following correlations;
Where km and kL are the measured- and the absolute (liquid) permeability, respectively. The parameter b depends on the type of gas used and reflects, to some extent, properties of the rock. NOTE: In most laboratory measurements of gas permeability, it is safe to neglect the Klinkenberg effect if the gas pressure is higher than 10 bar. In reservoirs, the pressure will be much higher and consequently the significance of the Klinkenberg effect of no importance.
You might also like
- Pressure Transient Formation and Well Testing: Convolution, Deconvolution and Nonlinear EstimationFrom EverandPressure Transient Formation and Well Testing: Convolution, Deconvolution and Nonlinear EstimationRating: 2 out of 5 stars2/5 (1)
- Rock Permeability: Reservoir Rock Properties LabDocument12 pagesRock Permeability: Reservoir Rock Properties LabhashoNo ratings yet
- Advanced Water Injection for Low Permeability Reservoirs: Theory and PracticeFrom EverandAdvanced Water Injection for Low Permeability Reservoirs: Theory and PracticeRating: 4 out of 5 stars4/5 (2)
- Klinkenberg Effect For Gas PermeabilityDocument12 pagesKlinkenberg Effect For Gas PermeabilityOmid MohamadiNo ratings yet
- Working Guide to Reservoir Rock Properties and Fluid FlowFrom EverandWorking Guide to Reservoir Rock Properties and Fluid FlowRating: 3 out of 5 stars3/5 (1)
- Water Coning in Vertical Wells 2Document26 pagesWater Coning in Vertical Wells 2Suleiman BaruniNo ratings yet
- Thermal Properties and Temperature-Related Behavior of Rock/Fluid SystemsFrom EverandThermal Properties and Temperature-Related Behavior of Rock/Fluid SystemsNo ratings yet
- 4 Frontal DisplacementDocument89 pages4 Frontal DisplacementHemant SrivastavaNo ratings yet
- Reservoir Engineering Exercise Set 1Document9 pagesReservoir Engineering Exercise Set 1Mohammad HaqNo ratings yet
- Well Test Analysis for Fractured Reservoir EvaluationFrom EverandWell Test Analysis for Fractured Reservoir EvaluationRating: 5 out of 5 stars5/5 (1)
- Material Balance CalculationDocument11 pagesMaterial Balance Calculationginozky100% (1)
- Practical Petroleum Geochemistry for Exploration and ProductionFrom EverandPractical Petroleum Geochemistry for Exploration and ProductionNo ratings yet
- Water and Gas Coning 1 PDFDocument15 pagesWater and Gas Coning 1 PDFBunga Arafah100% (2)
- The Practice of Reservoir Engineering (Revised Edition)From EverandThe Practice of Reservoir Engineering (Revised Edition)Rating: 5 out of 5 stars5/5 (3)
- Reservoir Engineering Quiz Questions AnswerDocument2 pagesReservoir Engineering Quiz Questions AnswerHikmatullah khan100% (1)
- Seminar On Water Influx and Well TestingDocument46 pagesSeminar On Water Influx and Well TestingJujupNo ratings yet
- Injection Well Transient Testing and AnalysisDocument82 pagesInjection Well Transient Testing and AnalysisBarbara_LFC100% (1)
- Water Influx Models ExplainedDocument43 pagesWater Influx Models Explainedlee100% (1)
- Vertical Lift & Flowline Pressure CurvesDocument25 pagesVertical Lift & Flowline Pressure CurvesSayaf Salman100% (2)
- Wellbore Storage EffectDocument6 pagesWellbore Storage EffectitshimelNo ratings yet
- Wellbore Storage Effects on Pressure Transient AnalysisDocument6 pagesWellbore Storage Effects on Pressure Transient AnalysisVictor Fernandez0% (1)
- Gas Material BalanceDocument29 pagesGas Material BalanceHadi HendizadehNo ratings yet
- Van Everdingen Hurst AquifersDocument21 pagesVan Everdingen Hurst Aquifers13670319100% (1)
- Reservoir SimulationDocument487 pagesReservoir SimulationAbhinav Shahi100% (2)
- Reservoir Engineering I: Barham S. Mahmood E-Mail: Petroleum Engineering DepartmentDocument61 pagesReservoir Engineering I: Barham S. Mahmood E-Mail: Petroleum Engineering DepartmentHadi Bapir SlemanNo ratings yet
- Hydraulic Fracturing Design Optimization-Bakken Case StudyDocument19 pagesHydraulic Fracturing Design Optimization-Bakken Case Studyjafar100% (1)
- Reservoir Rock Properties Lab.: Capillary PressureDocument6 pagesReservoir Rock Properties Lab.: Capillary PressureHeven HassanNo ratings yet
- Reservoir engineering water drive analysisDocument16 pagesReservoir engineering water drive analysisLauraIsabelVargas100% (1)
- Thermal Recovery Monograph Volume 7 by Michael PratsDocument5 pagesThermal Recovery Monograph Volume 7 by Michael Pratsserag SalamNo ratings yet
- GMBE Derivation and ApplicationDocument25 pagesGMBE Derivation and ApplicationHomam MohammadNo ratings yet
- 5 - Gas Well Testing and AnalysisDocument50 pages5 - Gas Well Testing and AnalysisHashem HashemNo ratings yet
- 04 - CompressibilityDocument15 pages04 - CompressibilityNhi VoNo ratings yet
- Assignment 2 MBEDocument3 pagesAssignment 2 MBEBadut SarkasNo ratings yet
- HW3 SolutionDocument5 pagesHW3 SolutionMohammad Iqbal Mahamad Amir100% (4)
- Reservoir Engineering CourseDocument129 pagesReservoir Engineering CourseLeonardo Barrios CarreraNo ratings yet
- Calculation of BG Gas Formation Volume FDocument7 pagesCalculation of BG Gas Formation Volume FMohammad Iqbal Mahamad Amir100% (2)
- Well test analysis provides reservoir insightsDocument20 pagesWell test analysis provides reservoir insightsSaa D ShamimNo ratings yet
- Introduction To Reservoir EngineeringDocument28 pagesIntroduction To Reservoir EngineeringChijioke Zion OkabieNo ratings yet
- Natural Gas Engineering Material Balance EquationsDocument47 pagesNatural Gas Engineering Material Balance EquationsIbrahim_Kocabas100% (1)
- Principles of Transient Testing: 1.1.1 Description of A Well TestDocument23 pagesPrinciples of Transient Testing: 1.1.1 Description of A Well TestEdgar Chiquito100% (3)
- Wellbore Heat Losses and Casing Temperatures During Steam Injection - API-66-025Document8 pagesWellbore Heat Losses and Casing Temperatures During Steam Injection - API-66-025nicessg@gmail.comNo ratings yet
- Diffusivity Equation For Spherical FlowDocument2 pagesDiffusivity Equation For Spherical FlowMuhammad Shahrukh50% (2)
- Pressure Buildup and Horner PlotDocument46 pagesPressure Buildup and Horner PlotKAORU AmaneNo ratings yet
- PVT AnalysisDocument40 pagesPVT AnalysisBrian CbtngnNo ratings yet
- Chap 17 - Water InfluxDocument57 pagesChap 17 - Water InfluxSlim.BNo ratings yet
- Chapter 10 Relative PermeabilityDocument27 pagesChapter 10 Relative PermeabilityAndrew Guo100% (1)
- Differential Liberation PVT Fluid TestDocument11 pagesDifferential Liberation PVT Fluid TestAhmed M. Saad0% (1)
- Pressure Traverse CurvesDocument29 pagesPressure Traverse CurvesYusrohDarmantoroNo ratings yet
- CCE Experiment for Determining Bubble Point PressureDocument6 pagesCCE Experiment for Determining Bubble Point PressureNasih NooriNo ratings yet
- TutorialDocument1 pageTutorialabdounouNo ratings yet
- Liquid Hold Up in Gas WellsDocument6 pagesLiquid Hold Up in Gas WellsEngr Sadiq WazirNo ratings yet
- Immiscible and Miscible Gases Injection To Improve Oil Recovery in Oil ReservoirsDocument12 pagesImmiscible and Miscible Gases Injection To Improve Oil Recovery in Oil ReservoirsAli MahmoudNo ratings yet
- Volve Field 1Document20 pagesVolve Field 1Siddharth VermaNo ratings yet
- Lab Measurement of Rock PermeabilityDocument14 pagesLab Measurement of Rock PermeabilityPugalNo ratings yet
- Waterflooding Using EclipseDocument18 pagesWaterflooding Using EclipseAakriti BhandariNo ratings yet
- Chapter 01 IntroductionDocument30 pagesChapter 01 IntroductionSurya Adhie100% (1)
- 3.3 Capillary PressureDocument78 pages3.3 Capillary PressureHind Lionne100% (1)
- Chapter 3 Gas Well TestingDocument93 pagesChapter 3 Gas Well TestingIrawan RianNo ratings yet
- Reservoir DeliverabilityDocument305 pagesReservoir DeliverabilityAjay Suri100% (3)
- SPE-192878-MS Maximizing Pipeline Flexibility With Drag Reducing AgentsDocument9 pagesSPE-192878-MS Maximizing Pipeline Flexibility With Drag Reducing AgentsQaiser HafeezNo ratings yet
- Capturing The Next Frontier of Value Operating Models For Oil and Gas Fields of The FutuDocument15 pagesCapturing The Next Frontier of Value Operating Models For Oil and Gas Fields of The FutuQaiser HafeezNo ratings yet
- Gas Well Deliquification-Comparison BW Foam Sequeeze and Foam Batch ApproachDocument3 pagesGas Well Deliquification-Comparison BW Foam Sequeeze and Foam Batch ApproachQaiser HafeezNo ratings yet
- Candidate Well SelectionDocument7 pagesCandidate Well SelectionQaiser HafeezNo ratings yet
- Devex-2018-Base-Management-workshopDocument27 pagesDevex-2018-Base-Management-workshopQaiser HafeezNo ratings yet
- 18 Best Practice For Stand Alone Screens Ian WattieDocument30 pages18 Best Practice For Stand Alone Screens Ian WattieQaiser HafeezNo ratings yet
- For Crude Oil: BackgroundDocument2 pagesFor Crude Oil: BackgroundQaiser HafeezNo ratings yet
- SPE 58976 Effect of Drag-Reducing Agents in Multiphase, Oil/Gas Horizontal FlowDocument7 pagesSPE 58976 Effect of Drag-Reducing Agents in Multiphase, Oil/Gas Horizontal FlowQaiser HafeezNo ratings yet
- Experiments With Various Drag Reducing Additives in Turbulent Flow in Dense Phase Gas PipelinesDocument12 pagesExperiments With Various Drag Reducing Additives in Turbulent Flow in Dense Phase Gas PipelinesQaiser HafeezNo ratings yet
- SPE 82284 Assessing Profitability of Well Interventions On Mature Fields: Well Intervention Profitability Evaluation Method, Case Study From CameroonDocument10 pagesSPE 82284 Assessing Profitability of Well Interventions On Mature Fields: Well Intervention Profitability Evaluation Method, Case Study From CameroonQaiser HafeezNo ratings yet
- Psig 1901Document29 pagesPsig 1901Qaiser HafeezNo ratings yet
- 06 PTL 02 LimitDiagramsDocument9 pages06 PTL 02 LimitDiagramsQaiser HafeezNo ratings yet
- A. LSPI Website PublicationsDocument3 pagesA. LSPI Website PublicationsQaiser HafeezNo ratings yet
- SPE 58976 Effect of Drag-Reducing Agents in Multiphase, Oil/Gas Horizontal FlowDocument7 pagesSPE 58976 Effect of Drag-Reducing Agents in Multiphase, Oil/Gas Horizontal FlowQaiser HafeezNo ratings yet
- IADC/SPE-180689-MS Samarang Well Intervention Performance Evaluation For Production Enhancement PortfolioDocument11 pagesIADC/SPE-180689-MS Samarang Well Intervention Performance Evaluation For Production Enhancement PortfolioQaiser HafeezNo ratings yet
- SPE-185428-MS Optimizing Cost and Effectiveness of Well Interventions: An Holistic ApproachDocument17 pagesSPE-185428-MS Optimizing Cost and Effectiveness of Well Interventions: An Holistic ApproachQaiser HafeezNo ratings yet
- WFT177034Document1 pageWFT177034Qaiser HafeezNo ratings yet
- SPE-185428-MS Optimizing Cost and Effectiveness of Well Interventions: An Holistic ApproachDocument17 pagesSPE-185428-MS Optimizing Cost and Effectiveness of Well Interventions: An Holistic ApproachQaiser HafeezNo ratings yet
- Sand Control Screen Plugging & ControlDocument14 pagesSand Control Screen Plugging & ControlQaiser HafeezNo ratings yet
- Pressure Drop and Closure Forces in Velocity Type SSSV API-77-B001 PDFDocument64 pagesPressure Drop and Closure Forces in Velocity Type SSSV API-77-B001 PDFQaiser Hafeez100% (1)
- Fault Seal AnalysisDocument8 pagesFault Seal AnalysisQaiser HafeezNo ratings yet
- Why Oil and Gas Companies Must Act On AnalyticsDocument5 pagesWhy Oil and Gas Companies Must Act On AnalyticsQaiser HafeezNo ratings yet
- SPE 82284 Assessing Profitability of Well Interventions On Mature Fields: Well Intervention Profitability Evaluation Method, Case Study From CameroonDocument10 pagesSPE 82284 Assessing Profitability of Well Interventions On Mature Fields: Well Intervention Profitability Evaluation Method, Case Study From CameroonQaiser HafeezNo ratings yet
- An introduction to multiphase flow measurementDocument14 pagesAn introduction to multiphase flow measurementHeverth OsorioNo ratings yet
- StimTube GunDocument1 pageStimTube GunQaiser HafeezNo ratings yet
- Wet Gas Metering by Isokinetic Sampling TechnicalDocument11 pagesWet Gas Metering by Isokinetic Sampling TechnicalQaiser HafeezNo ratings yet
- Tubing Swage Tool Opens Collapsed PipeDocument1 pageTubing Swage Tool Opens Collapsed PipeQaiser HafeezNo ratings yet
- Log Parameters FormulaeDocument1 pageLog Parameters FormulaeQaiser HafeezNo ratings yet
- Prosper Model UnderstandingDocument6 pagesProsper Model UnderstandingQaiser HafeezNo ratings yet
- Fracture Design Spresdsheet KF 6KDocument23 pagesFracture Design Spresdsheet KF 6KQaiser HafeezNo ratings yet
- Krystaline 2 (English)Document4 pagesKrystaline 2 (English)José Luis CampelloNo ratings yet
- Aqua SilencerDocument18 pagesAqua SilencerManideep AlluNo ratings yet
- 5d Ultra FG Product Specification ShhetDocument3 pages5d Ultra FG Product Specification Shhetfmk342112No ratings yet
- Experimental Study of Humidification AndDehumidifiDocument19 pagesExperimental Study of Humidification AndDehumidifiHanz GianNo ratings yet
- Structure Determination:: Proton 1.00728 U Neutron 1.00866 U Electron 0.00055 UDocument12 pagesStructure Determination:: Proton 1.00728 U Neutron 1.00866 U Electron 0.00055 Ufouad elferdiNo ratings yet
- Analytical Method Development and Validation For The Test Related Substances of Pomalidomide in Pomalidomide CapsulesDocument8 pagesAnalytical Method Development and Validation For The Test Related Substances of Pomalidomide in Pomalidomide CapsulesInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Lab 6 (Soaps & Detergents)Document21 pagesLab 6 (Soaps & Detergents)AmeerRashidNo ratings yet
- Glydant Plus Liquid (I) 2006Document2 pagesGlydant Plus Liquid (I) 2006Enrique Alberto Valenzuela Hernandez0% (1)
- Study of Process Parameters in High Pressure Die CastingDocument9 pagesStudy of Process Parameters in High Pressure Die Castingruben6286No ratings yet
- Synthesis of 2-NitropropeneDocument3 pagesSynthesis of 2-NitropropeneVictor VikeneNo ratings yet
- 2018 Gen Ed Reviewer: Chemistry: Welcome To LET Exam Questions and Answers!!!Document28 pages2018 Gen Ed Reviewer: Chemistry: Welcome To LET Exam Questions and Answers!!!Christine Grace IlanNo ratings yet
- Srivastava DissertationDocument144 pagesSrivastava DissertationleNo ratings yet
- Lecture BIOCHEMISTRY of CYTOSOL AlfiDocument56 pagesLecture BIOCHEMISTRY of CYTOSOL Alfisennaavia12No ratings yet
- SSP 665 Audi A8 Type 4N New Air Conditioning Features and Introduction of Refrigerant R744Document36 pagesSSP 665 Audi A8 Type 4N New Air Conditioning Features and Introduction of Refrigerant R744ylk1No ratings yet
- MBH Metals CatalogueDocument56 pagesMBH Metals CataloguetaichiNo ratings yet
- Mop Strip 1217 MSDSDocument2 pagesMop Strip 1217 MSDSSage Chemical InternationalNo ratings yet
- Rosemarinus Officinalis Mediated Synthesis of Silver Nanoparticles and Its ActivityDocument6 pagesRosemarinus Officinalis Mediated Synthesis of Silver Nanoparticles and Its ActivityHafsaAliNo ratings yet
- Development of Yondelis (Trabectedin, ET-743) - A Semisynthetic Process Solves The Supply ProblemDocument16 pagesDevelopment of Yondelis (Trabectedin, ET-743) - A Semisynthetic Process Solves The Supply ProbleminsaNo ratings yet
- For Gas Testing Examination. Unit 1 Air CompositionDocument3 pagesFor Gas Testing Examination. Unit 1 Air CompositionAman KumarNo ratings yet
- PV ParaffinDocument40 pagesPV ParaffinAbi DesonNo ratings yet
- Assignment 1Document3 pagesAssignment 1Yeleti FamilyNo ratings yet
- Sop For GcmsDocument3 pagesSop For GcmsDrMd IdrisNo ratings yet
- Fuji Dri-Chem Slide Ca-Piii: Date of Issue: 1/may/2012Document1 pageFuji Dri-Chem Slide Ca-Piii: Date of Issue: 1/may/2012susey tepaNo ratings yet
- Asme Section Viii Div.1Document87 pagesAsme Section Viii Div.1balu100% (7)
- Progress in Starch Modification in The Last Decade PDFDocument7 pagesProgress in Starch Modification in The Last Decade PDFLau MaRtiinezNo ratings yet
- Solution stoichiometry calculations tutorialDocument6 pagesSolution stoichiometry calculations tutorialJayakumar SankaranNo ratings yet
- Product Data Sheet TIC 9061 Heat Transfer Cement: DescriptionDocument2 pagesProduct Data Sheet TIC 9061 Heat Transfer Cement: DescriptionsusantaNo ratings yet
- Proposal ChlorobenzeneDocument12 pagesProposal ChlorobenzeneDavid Akomolafe100% (1)
- Metallic EjDocument88 pagesMetallic EjaayopercivalNo ratings yet
- Bender Elements ManualDocument14 pagesBender Elements ManualOrlando FernandezNo ratings yet
- Rolling Bearing Tribology: Tribology and Failure Modes of Rolling Element BearingsFrom EverandRolling Bearing Tribology: Tribology and Failure Modes of Rolling Element BearingsNo ratings yet
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseFrom EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseRating: 4.5 out of 5 stars4.5/5 (50)
- Design of Foundations for Offshore Wind TurbinesFrom EverandDesign of Foundations for Offshore Wind TurbinesRating: 5 out of 5 stars5/5 (3)
- Introduction to Applied Thermodynamics: The Commonwealth and International Library: Mechanical Engineering DivisionFrom EverandIntroduction to Applied Thermodynamics: The Commonwealth and International Library: Mechanical Engineering DivisionRating: 2.5 out of 5 stars2.5/5 (3)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsFrom EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo ratings yet
- Hyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionFrom EverandHyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionRating: 4.5 out of 5 stars4.5/5 (3)
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationFrom EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationNo ratings yet
- The Laws of Thermodynamics: A Very Short IntroductionFrom EverandThe Laws of Thermodynamics: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (10)
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationFrom EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationRating: 4 out of 5 stars4/5 (18)
- Pressure Vessels: Design, Formulas, Codes, and Interview Questions & Answers ExplainedFrom EverandPressure Vessels: Design, Formulas, Codes, and Interview Questions & Answers ExplainedRating: 5 out of 5 stars5/5 (1)
- Control of Power Electronic Converters and Systems: Volume 1From EverandControl of Power Electronic Converters and Systems: Volume 1Rating: 5 out of 5 stars5/5 (1)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Quantum Mechanics 4: Spin, Lasers, Pauli Exclusion & Barrier PenetrationFrom EverandQuantum Mechanics 4: Spin, Lasers, Pauli Exclusion & Barrier PenetrationRating: 1 out of 5 stars1/5 (1)
- Practical Guides to Testing and Commissioning of Mechanical, Electrical and Plumbing (Mep) InstallationsFrom EverandPractical Guides to Testing and Commissioning of Mechanical, Electrical and Plumbing (Mep) InstallationsRating: 3.5 out of 5 stars3.5/5 (3)
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)
- Chemical Process Safety: Learning from Case HistoriesFrom EverandChemical Process Safety: Learning from Case HistoriesRating: 4 out of 5 stars4/5 (14)
- Process Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentFrom EverandProcess Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentNo ratings yet
- Guidelines for Siting and Layout of FacilitiesFrom EverandGuidelines for Siting and Layout of FacilitiesNo ratings yet
- Machinery Failure Analysis Handbook: Sustain Your Operations and Maximize UptimeFrom EverandMachinery Failure Analysis Handbook: Sustain Your Operations and Maximize UptimeRating: 3.5 out of 5 stars3.5/5 (4)