Professional Documents
Culture Documents
Teks 11c Notes
Uploaded by
api-236826747Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Teks 11c Notes
Uploaded by
api-236826747Copyright:
Available Formats
Name ___________________________ Class ________ Date ___________
TEKS 11C Use thermochemical equations to calculate energy changes that occur in
chemical reactions and classify reactions as exothermic or endothermic.
TEKS Lesson 11C:
Thermochemical Equations
How can you express the enthalpy change for a reaction in
a chemical equation?
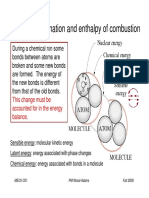
As you will learn in TEKS Lesson 11E, the enthalpy (H) o a system accounts or the heat low o the
system at constant pressure! The heat a"sor"ed or released "y a reaction at constant pressure is the same
as the change in enthalpy, sym"oli#ed as $H!
% you mi& calcium o&ide with water, the water in the mi&ture "ecomes warm! This e&othermic reaction
occurs when cement, which contains calcium o&ide, is mi&ed with water to ma'e concrete! (hen 1 mol
o calcium o&ide reacts with 1 mol o water, 1 mol o calcium hydro&ide orms and )*!+ ', o heat is
released! %n a chemical equation, the enthalpy change or the reaction can "e written as either a reactant or
a product! %n the equation descri"ing the e&othermic reaction o calcium o&ide and water, the enthalpy
change can "e considered a product!
-a.(s) / 0+.(l) -a(.0)+(s) / )*!+ ',
This equation is presented 1isually "elow! A chemical equation that includes the enthalpy change is called
a thermochemical equation.
The heat of reaction is the enthalpy change or the chemical equation e&actly as it is written! 2ou will
usually see heats o reaction reported as 3H, which is equal to the heat low at constant pressure! The
physical state o the reactants and products must also "e gi1en! The standard conditions are that the
reaction is carried out at 141!5 '6a (1 atm) and that the reactants and products are in their usual physical
states at +*7-! The heat o reaction, or 3H, in the a"o1e e&le is 8)*!+ ',! Each mole o calcium o&ide
and water that reacts to orm calcium hydro&ide produces )*!+ ', o heat!
-a.(s) / 0+.(l) -a(.0)+(s) 3 H 9 8)*!+ ',
%n this and other e&othermic processes, the chemical potential energy o the reactants is higher than the
chemical potential energy o the products!
1
TEKS
Chemistry
Lesson 11C
Name ___________________________ Class ________ Date ___________
.ther reactions a"sor" heat rom the surroundings! :or e&le, "a'ing soda (sodium
"icar"onate) decomposes when it is heated! The car"on dio&ide released in the
reaction causes muins to rise while "a'ing! This process is endothermic!
+;a0-.5(s) / <* ', ;a+-.5(s) / 0+.(l) / -.+(g)
=emem"er that 3H is positi1e or endothermic reactions! Thereore, you can write the reaction as
ollows>
+;a0-.5(s) ;a+-.5(s) / 0+.(l) / -.+(g) 3 H 9 <* ',
The igure "elow shows the enthalpy diagram or this reaction! %n this and other endothermic processes,
the chemical potential energy o the products is higher than the chemical potential energy o the reactants!
How can you calculate the enthalpy change for a reaction
using a chemical equation?
-hemistry pro"lems in1ol1ing enthalpy changes are similar to stoichiometry pro"lems! The amount o
heat released or a"sor"ed during a reaction depends on the num"er o moles o the reactants in1ol1ed!
The decomposition o + mol o sodium "icar"onate, or e&le, requires <* ', o heat! Thereore, the
decomposition o ? mol o the same su"stance would require twice as much heat, or 1@4 ',!
To see why the physical state o the reactants and products in a thermochemical reaction must "e stated,
compare the ollowing two equations or the decomposition o 1 mol 0+.>
0+.(l) 0+(g) /
1
+
.+(g) 3 H 9 +<*!< ',
0+.(g) 0+(g) /
1
+
.+(g) 3 H 9 +?1!< ',
dierence 9 ??!4 ',
Although the two equations are 1ery similar, the dierent physical states o 0+. result in dierent 3H
1alues! %n one case, the reactant is a liquidA in the other case, the reactant is a gas! The 1apori#ation o
1 mol o liquid water to water 1apor at +*7- requires ??!4 ', o heat!
0+.(l) 0+.(g) 3 H 9 ??!4 ',
Sample ro!lem: "sing the Heat of #eaction to Calculate
Enthalpy Change Use the ollowing thermochemical equation to calculate the energy change (in ',)
that occurs when +!+? mol o ;a0-.5(s) decomposes!
$
TEKS
Chemistry
Lesson 11C
Name ___________________________ Class ________ Date ___________
+;a0-.5(s) / <* ', ;a+-.5(s) / 0+.(l) / -.+(g)
1. Analyze List the knowns and the unknown. Use the thermochemical equation a"o1e
to write a con1ersion actor relating 'iloBoules o heat and moles o ;a0-.5(s)! Then
use the con1ersion actor to determine $H or +!+? mol ;a0-.5(s)!
Knowns
C amount o ;a0-.5(s) that decomposes 9 +!+? mol
C $H 9 <* ', or + mol ;a0-.5(s)
Unknown
C $H 9 D ', or +!+? mol ;a0-.5(s)
2. Calculate Solve for the unknown.
(rite the con1ersion actor relating ', o heat and moles o ;a0-.5(s)!
<* ',
+ mol ;a0-.
5
(s)
Using dimensional analysis, sol1e or $H.
H =+!+? mol ;a0-.
5
(s)
<* ',
+ mol ;a0-.
5
(s)
=E* ',
3. Evaluate Does the result make sense? The <* ', in the thermochemical equation reers to the
decomposition o + mol ;a0-.5(s)! Thereore, the decomposition o +!+? mol should a"sor" more heat
than <* ',! The answer o E* ', is consistent with this estimate!
%
TEKS
Chemistry
Lesson 11C
Name ___________________________ Class ________ Date ___________
Lesson Chec&
1' #e(iew How are enthalpy changes expressed in chemical equations?
________________________________________________________________________________
________________________________________________________________________________
________________________________________________________________________________
$' )escri!e When 2 mol of solid magnesium (g! com"ine with # mol of oxygen gas ($2!% 2 mol of
solid magnesium oxide (g$! are formed and #2&' () of heat is released* Write the thermochemical
equation for this com"ustion reaction*
________________________________________________________________________________
%' Classify #eactions +he following thermochemical equation descri"es the com"ustion of methane%
which is found in natural gas*
CH'(g! , 2$2(g! C$2(g! , 2H2$(l! , -.& ()
Classify the a"o/e reaction as exothermic or endothermic*
________________________________________________________________________________
********************************************************************************
+' Calculate Energy Changes 0asohol contains ethanol% C2H1$(l!* When ethanol "urns% it reacts with
$2(g! to produce C$2(g! and H2$(l!* 2se the thermochemical equation to calculate the energy
change that occurs when #2*3 g of ethanol "urns*
C2H1$(l! , 4$2(g! 2C$2(g! , 4H2$(l! 5 H 6 7#41- ()
+
TEKS
Chemistry
Lesson 11C
You might also like
- Chemistry Notes 2.1 NotesDocument10 pagesChemistry Notes 2.1 NotesOsama Bin AmerNo ratings yet
- Chem Project DharanishDocument9 pagesChem Project Dharanishnathinsp8mvmNo ratings yet
- OWL Tutorial 2ADocument16 pagesOWL Tutorial 2ANatNo ratings yet
- Entropy NotesDocument9 pagesEntropy NotescusgakungaNo ratings yet
- AP Chemistry 2013-2014 Lab #13 - Hot Pack/Cold Pack Design ChallengeDocument4 pagesAP Chemistry 2013-2014 Lab #13 - Hot Pack/Cold Pack Design ChallengeAman GuptaNo ratings yet
- Endothermic and Exothermic Reaction: ThermochemistryDocument5 pagesEndothermic and Exothermic Reaction: Thermochemistryelizz_zabeth_3960598No ratings yet
- 161 Lab Experiment 7 CalorimetryDocument14 pages161 Lab Experiment 7 CalorimetryInês LapaNo ratings yet
- EquillibruimDocument46 pagesEquillibruimMeenakshi SoodNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Ap Chemistry: Designing A Hand WarmerDocument7 pagesAp Chemistry: Designing A Hand WarmerRishabh KotturgowdraNo ratings yet
- Energy ChangesDocument40 pagesEnergy ChangesKissiedu YirenkyiNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersNo ratings yet
- Enthalpy of Formation PDFDocument10 pagesEnthalpy of Formation PDFatulsemiloNo ratings yet
- H2 Chemistry DefinitionsDocument2 pagesH2 Chemistry DefinitionsEugene TayNo ratings yet
- T8 - Energetics IDocument28 pagesT8 - Energetics II Kadek Irvan Adistha PutraNo ratings yet
- The Kinetics and Thermodynamics of The Phenol From Cumene Process: A Physical Chemistry ExperimentDocument5 pagesThe Kinetics and Thermodynamics of The Phenol From Cumene Process: A Physical Chemistry Experimentkanokwan jaruekNo ratings yet
- First Law of ThermodynamicsDocument67 pagesFirst Law of ThermodynamicsRhodelyn TolentinoNo ratings yet
- Discussion Lab Report 1.3Document6 pagesDiscussion Lab Report 1.3Aishah FatimahNo ratings yet
- H2SO4Document12 pagesH2SO4pedroNo ratings yet
- Ib Enthalpy KHDocument39 pagesIb Enthalpy KHSamer EhabNo ratings yet
- LAS General Chemistry 2 Q4W12Document16 pagesLAS General Chemistry 2 Q4W12Marlon C. CambayNo ratings yet
- 1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 AppendicesDocument16 pages1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 Appendicesmonkeystar12100% (3)
- 1 Lab Handout PDFDocument5 pages1 Lab Handout PDFKhud SarNo ratings yet
- Guía TermodinámicaDocument17 pagesGuía TermodinámicamiayalacNo ratings yet
- Homework - 8chemical Equilibrium FINALDocument20 pagesHomework - 8chemical Equilibrium FINALNwachukwu ObiNo ratings yet
- Vapor-Liquid Equilibria Multicomponent Aqueous Solutions Volatile Weak ElectrolytesDocument11 pagesVapor-Liquid Equilibria Multicomponent Aqueous Solutions Volatile Weak ElectrolytesJoão Pedro GomesNo ratings yet
- 0 - Acid Base Equilibrium Notes PDFDocument48 pages0 - Acid Base Equilibrium Notes PDFGary VeeNo ratings yet
- IChO-2013 Theoretical Official English VersionDocument38 pagesIChO-2013 Theoretical Official English VersionTôn Thất HuyNo ratings yet
- 141 The Adiabatic State EquationsDocument1 page141 The Adiabatic State EquationspartoNo ratings yet
- Enthalpy PogilDocument4 pagesEnthalpy Pogilapi-213793181No ratings yet
- Experiment 4 Charles LawDocument4 pagesExperiment 4 Charles LawNurasyilah YakubNo ratings yet
- POSTLAB 9 - Heat of Formation of NaClDocument7 pagesPOSTLAB 9 - Heat of Formation of NaClRaniel Miranda100% (1)
- General Chemistry Ch. 11 TBDocument35 pagesGeneral Chemistry Ch. 11 TBZara V. FeldmanNo ratings yet
- Solubilidad Explicada RSCDocument8 pagesSolubilidad Explicada RSCSebastian Finger CaraccioliNo ratings yet
- Activity No.4 CalorimetryDocument5 pagesActivity No.4 CalorimetryRexzon DumanguitNo ratings yet
- THERMOCHEMISTRY Hand Outs 2023Document6 pagesTHERMOCHEMISTRY Hand Outs 2023Paul Willard GumapacNo ratings yet
- Thermo ChemistryDocument11 pagesThermo ChemistryEugenTutunaruNo ratings yet
- BT - Chương 3-4 - LTCB pha-CB Pha He 1 Cau Tu - SVDocument2 pagesBT - Chương 3-4 - LTCB pha-CB Pha He 1 Cau Tu - SVminhsiucute3112No ratings yet
- Equilibrium: Three Stooges in Chemical ReactionsDocument5 pagesEquilibrium: Three Stooges in Chemical ReactionsKhud SarNo ratings yet
- Heat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherDocument12 pagesHeat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherAeyyjayyNo ratings yet
- 11 Chemistry Notes Ch07 EquilibriumDocument8 pages11 Chemistry Notes Ch07 EquilibriumShishirRanjanNo ratings yet
- Module 2Document23 pagesModule 2Suzanne GuzmanNo ratings yet
- Making Hot and Cold Packs C11!4!04Document5 pagesMaking Hot and Cold Packs C11!4!04Resta THawNo ratings yet
- Heat of Neutralization f10Document9 pagesHeat of Neutralization f10Nishat AhmedNo ratings yet
- Equi Lib RumDocument95 pagesEqui Lib RumRichard MitchellNo ratings yet
- Amali Kimia 1Document5 pagesAmali Kimia 1Syahmi RifqiNo ratings yet
- AED Design Requirements - Hydropneumatic Tanks - Sep-09Document16 pagesAED Design Requirements - Hydropneumatic Tanks - Sep-09skywalker_handsomeNo ratings yet
- Experiment #6: ThermochemistryDocument11 pagesExperiment #6: Thermochemistryhera_sulistiawatiNo ratings yet
- Johnmar S. Deligero: Chemistry/Biology 12 (Nova Scotia Curriculum)Document36 pagesJohnmar S. Deligero: Chemistry/Biology 12 (Nova Scotia Curriculum)Sahid SantosNo ratings yet
- Class05 ChemistryG12 Notes and HomeworkDocument51 pagesClass05 ChemistryG12 Notes and HomeworkAndy Rei KouNo ratings yet
- Chemical Reaction222Document8 pagesChemical Reaction222Anonymous W6zPy3fImvNo ratings yet
- AP Chem Unit 4 Thermochem Student PacketDocument28 pagesAP Chem Unit 4 Thermochem Student PacketMiron WolfNo ratings yet
- Chapter 05 Energetics TextbookDocument26 pagesChapter 05 Energetics TextbookMirei IidaNo ratings yet
- 5.1 EnergeticsDocument8 pages5.1 EnergeticsEldin EnggNo ratings yet
- Lesson 1 4th GP Gen Chem 2Document12 pagesLesson 1 4th GP Gen Chem 2Alex Jethro TigoyNo ratings yet
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationFrom EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo ratings yet
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringFrom EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringNo ratings yet
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4From Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4No ratings yet
- Properties of Matter KeyDocument4 pagesProperties of Matter Keyapi-236826747No ratings yet
- Teks 11b Heat Transfer Lesson NotesDocument4 pagesTeks 11b Heat Transfer Lesson Notesapi-236826747No ratings yet
- Teks 12a Nuclear Radiation Lesson NotesDocument5 pagesTeks 12a Nuclear Radiation Lesson Notesapi-236826747No ratings yet
- Equipment List - KeyDocument4 pagesEquipment List - Keyapi-236826747No ratings yet
- Test 1 Review KeyDocument4 pagesTest 1 Review Keyapi-236826747No ratings yet
- Teks 11a Forms of Energy Lesson NotesDocument4 pagesTeks 11a Forms of Energy Lesson Notesapi-236826747No ratings yet
- C 11de 2 0 Explain Stemscopedia StudentDocument7 pagesC 11de 2 0 Explain Stemscopedia Studentapi-236826747No ratings yet
- Stem C 10cd Explain StemscopediaDocument6 pagesStem C 10cd Explain Stemscopediaapi-236826747No ratings yet
- Rules For Assigning Oxidation NumbersDocument1 pageRules For Assigning Oxidation Numbersapi-236826747No ratings yet
- Teks 10i PH Concept Lesson NotesDocument8 pagesTeks 10i PH Concept Lesson Notesapi-236826747No ratings yet
- Review KeyDocument2 pagesReview Keyapi-236826747No ratings yet
- HW Solubility Curve 3 30Document5 pagesHW Solubility Curve 3 30api-236826747No ratings yet
- Teks 10g Defining Acids Bases Lesson NotesDocument9 pagesTeks 10g Defining Acids Bases Lesson Notesapi-236826747No ratings yet
- Question Prompts For Aqueous Solutions - StudentDocument3 pagesQuestion Prompts For Aqueous Solutions - Studentapi-236826747No ratings yet
- C 10ef 2 0 Explain StemscopediaDocument7 pagesC 10ef 2 0 Explain Stemscopediaapi-2368267470% (1)
- نام گذاری اسیدها و بازهاDocument2 pagesنام گذاری اسیدها و بازهاapi-3706290100% (1)
- C 10ab 2 0 Explain Stemscopedia StudentDocument8 pagesC 10ab 2 0 Explain Stemscopedia Studentapi-2368267470% (1)
- Moles Notes Student VersionDocument4 pagesMoles Notes Student Versionapi-236826747No ratings yet
- VocabDocument2 pagesVocabapi-236826747No ratings yet
- Unit 6 Work To UploadDocument18 pagesUnit 6 Work To Uploadapi-236826747No ratings yet
- Unit 7 Notes For WebDocument13 pagesUnit 7 Notes For Webapi-236826747No ratings yet
- Ionic Naming and Formula NotesDocument1 pageIonic Naming and Formula Notesapi-236826747No ratings yet
- Unit 4 Test Review KeyDocument3 pagesUnit 4 Test Review Keyapi-236826747No ratings yet
- Naming Acids and BasesDocument1 pageNaming Acids and Basesapi-236826747No ratings yet
- Covalent Rules For Naming and FormulasDocument2 pagesCovalent Rules For Naming and Formulasapi-236826747No ratings yet
- Covalent Formulas and Naming PracticeDocument2 pagesCovalent Formulas and Naming Practiceapi-236826747No ratings yet
- Molecular Geometry ModelsDocument1 pageMolecular Geometry Modelsapi-236826747No ratings yet
- Teks 7d Metallic Bonding Lesson NotesDocument4 pagesTeks 7d Metallic Bonding Lesson Notesapi-236826747No ratings yet
- VseprDocument1 pageVseprapi-236826747No ratings yet
- Lecture Slides: Fatigue Failure Resulting From Variable LoadingDocument43 pagesLecture Slides: Fatigue Failure Resulting From Variable LoadingAbdulNo ratings yet
- Chem 162 Formal Lab ReportDocument5 pagesChem 162 Formal Lab ReportLiem LimantoNo ratings yet
- BPCL Report MechanicalDocument44 pagesBPCL Report MechanicalritikaNo ratings yet
- Topical, Gastrointestinal, NI, Radio-, MiscDocument113 pagesTopical, Gastrointestinal, NI, Radio-, MiscArk Olfato ParojinogNo ratings yet
- Environmental Studies: by Prof. Sanjukta MistriDocument10 pagesEnvironmental Studies: by Prof. Sanjukta MistriramNo ratings yet
- Usp 788Document3 pagesUsp 788Wesley OliveiraNo ratings yet
- 2013 Synthesis and Cationic Photopolymerization of A Difunctional Episulfide Monomer PDFDocument6 pages2013 Synthesis and Cationic Photopolymerization of A Difunctional Episulfide Monomer PDFMarion ChenalNo ratings yet
- BASF Patent On Double Contact Double AbsorptionDocument4 pagesBASF Patent On Double Contact Double AbsorptionANo ratings yet
- Specifications: Specifications & Material Safety Data Sheet Xyz General Cleaner LemonDocument3 pagesSpecifications: Specifications & Material Safety Data Sheet Xyz General Cleaner LemonnicholasyudhistiraNo ratings yet
- Separacion Fusel OilDocument10 pagesSeparacion Fusel OilKarenLissNo ratings yet
- Solar Refrigeration ReportDocument21 pagesSolar Refrigeration ReportCj MoLanoNo ratings yet
- Proteins and Peptides 20msc01029Document10 pagesProteins and Peptides 20msc01029Ritu ParmarNo ratings yet
- Polywithe® - 8000 CLDocument1 pagePolywithe® - 8000 CLsébastien cardinaleNo ratings yet
- Manufacture of RadiopharmaceuticalsDocument9 pagesManufacture of RadiopharmaceuticalsRainMan75No ratings yet
- Lec 22Document20 pagesLec 22Simanchal KarNo ratings yet
- Physics Chapter 19 Class ProblemsDocument3 pagesPhysics Chapter 19 Class ProblemsRuba AlNo ratings yet
- Phenates (Alkyl Phenate Sulfides) : Product Stewardship SummaryDocument4 pagesPhenates (Alkyl Phenate Sulfides) : Product Stewardship SummaryPayal MinochaNo ratings yet
- McCabe-Thiele Method 1Document37 pagesMcCabe-Thiele Method 1HariKrishnaBushi100% (2)
- Well Testing PresentationDocument24 pagesWell Testing PresentationSaid71% (7)
- Cryogenics IntroductionDocument26 pagesCryogenics Introductionsenior high100% (1)
- ISC 2013 Chemistry Theory Paper 1 Solved PaperDocument20 pagesISC 2013 Chemistry Theory Paper 1 Solved PaperAakash Singh100% (1)
- Syll-2 MSC Organic Chemistry 2019Document22 pagesSyll-2 MSC Organic Chemistry 2019Saqib Faheem KachrooNo ratings yet
- Thermit ReactionDocument10 pagesThermit ReactionAndrei PetreNo ratings yet
- Hydrogen Embrittlemnt - Gas PipelinesDocument36 pagesHydrogen Embrittlemnt - Gas PipelinesCesar MoreNo ratings yet
- 2 Tutorial Pressure Sept19Document7 pages2 Tutorial Pressure Sept19hairinnisaNo ratings yet
- FY 2018 Self Identified Generic Drug Facilities Sites and OrganizationsDocument175 pagesFY 2018 Self Identified Generic Drug Facilities Sites and OrganizationsDavid Paul HensonNo ratings yet
- SSI AFD270 9in DiscDiff 012210Document2 pagesSSI AFD270 9in DiscDiff 012210Chris HdezNo ratings yet
- Comparison Chart: (828 Ratings) (877 Ratings)Document11 pagesComparison Chart: (828 Ratings) (877 Ratings)RoseNo ratings yet
- Kitel Et Al 2023 Discovery of Inhibitory Fragments That Selectively Target Spire2 Fmn2 InteractionDocument13 pagesKitel Et Al 2023 Discovery of Inhibitory Fragments That Selectively Target Spire2 Fmn2 InteractionNgô HuyNo ratings yet
- Redox ReactionsDocument15 pagesRedox ReactionsAdarsh YadavNo ratings yet