Professional Documents
Culture Documents
Jurnal Sitologi PDF
Uploaded by
Ibnu Satria Apriansa0 ratings0% found this document useful (0 votes)
2K views5 pagesSolid pseudopapillary tumor of pancreas (SPTP) is a rare pancreatic tumor of uncertain histogenesis usually affecting young women. This study was designed to demonstrate the utility of CD 99 immunostaining along with cytological features for making a pre-operative diagnosis.
Original Description:
Original Title
jurnal sitologi.pdf
Copyright
© Attribution Non-Commercial (BY-NC)
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentSolid pseudopapillary tumor of pancreas (SPTP) is a rare pancreatic tumor of uncertain histogenesis usually affecting young women. This study was designed to demonstrate the utility of CD 99 immunostaining along with cytological features for making a pre-operative diagnosis.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
2K views5 pagesJurnal Sitologi PDF
Uploaded by
Ibnu Satria ApriansaSolid pseudopapillary tumor of pancreas (SPTP) is a rare pancreatic tumor of uncertain histogenesis usually affecting young women. This study was designed to demonstrate the utility of CD 99 immunostaining along with cytological features for making a pre-operative diagnosis.
Copyright:
Attribution Non-Commercial (BY-NC)
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 5
151 Journal of Cytology / July 2013 / Volume 30 / Issue 3
RANAJOY GHOSH, SAUMYA R. MALLIK, SANDEEP R. MATHUR, VENKATESWARAN K. IYER
Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Address for correspondence: Dr. Sandeep R Mathur, Department of Pathology, 1
st
Floor Teaching Block, All India Institute of Medical Sciences,
New Delhi, India. E-mail: mathuraiims@gmail.com
ABSTRACT
Background: Solid pseudopapillary tumor of pancreas (SPTP) is a rare pancreatic tumor of uncertain histogenesis usually
affecting young women. Though these tumors have characteristic cytomorphology, it is sometimes diffcult to differentiate
them from neuroendocrine tumors of the pancreas. We reviewed cases of SPTP to delineate the diagnostic cytological
features and also observed utility of CD 99 (MIC 2) immunostaining to aid in the diagnosis of this tumor.
Aims: This study was designed to demonstrate the utility of CD 99 immunostaining along with cytological features for making
a pre-operative diagnosis and delineating it from the neuroendocrine tumor of pancreas which is a close mimic.
Materials and Methods: Cytomorphological features of 11 cases of solid pseudopapillary neoplasm diagnosed by
pre-operative fne-needle aspiration cytology (FNAC) at our institute were reviewed. Immunocytochemistry for CD 99 was
also performed on the smears.
Results: All the cases had cellular smears with monomorphic cells lying singly, as loosely cohesive clusters as well as
forming delicate pseudopapillae. Presence of intra and extra-cellular basement membrane material, background foamy
macrophages and nuclear grooves were the other salient features. Immunocytochemistry for CD 99 could be performed on
eight cases and demonstrated typical paranuclear dot-like positivity.
Conclusions: Pre-operative early diagnosis of SPTP can be made by FNAC which can further be aided by CD 99
immunocytochemistry.
Key words: CD 99; immunohistochemistry; solid pseudopapillary tumor of pancreas.
Introduction
Solid pseudopapillary tumor of the pancreas (SPTP) is a
rare neoplasm of unknown histogenesis and low malignant
potential first reported by Frantz in 1959.
[1]
The tumor was
known by various names like solid and cystic tumor, solid
cystic and papillary epithelial neoplasm, and solid and
papillary tumor before the present consensus name solid
pseudopapillary tumor of pancreas (SPTP).
[2]
SPTP is more
common in young females although cases in males are also
reported in the literature.
[3]
Early pre-operative diagnosis is
of paramount importance as adequate resection is usually
curative.
[4]
SPT constitutes approximately 3% of the cystic
lesions of pancreas
[5]
and about 60 cases diagnosed by
fine-needle aspiration cytology (FNAC) are reported in the
literature.
[6]
The cytomorphology of this tumor is highly
characteristic, with features that are distinctive from those
of other cystic and solid tumors of the pancreas. However,
monomorphic population of discohesive cells and eccentric
nuclei sometimes makes it difficult to differentiate from
some other pancreatic tumors like the neuroendocrine
tumors. It is very important to distinguish this tumor
from other pancreatic tumors as these may have similar
clinical presentation and radiologic appearance but with
different prognosis and treatment. Immunohistochemically
these tumors are usually positive for vimentin and -1
antitrypsins
[7]
but no specific immunocytochemical markers
CD99immunocytochemistryinsolidpseudopapillarytumorof
pancreas:Astudyonfne-needleaspirationcytologysmears
Access this article online
Website:
www.jcytol.org
Quick Response Code
DOI:
10.4103/0970-9371.117645
Ori gi nal Arti cl e
[Downloadedfreefromhttp://www.jcytol.orgonWednesday,October16,2013,IP:36.74.61.1]||ClickheretodownloadfreeAndroidapplicationforthisjournal
152
Ghosh, et al.: CD 99 immunocytochemistry in solid pseudopapillary tumor of pancreas
Journal of Cytology / July 2013 / Volume 30 / Issue 3
are present which could be used to distinguish it from
other pancreatic tumors. Some other markers like CD56,
neurone-specific enolase, progesterone receptor and CD10
may be immunopositive in SPTP
[8]
but may also be positive
in various other tumors.
[9]
Here we have studied detailed cytomorphological features
of 11 cases of SPTP for accurate pre-operative diagnosis along
with use of immunocytochemical marker CD 99 as a specific
marker for SPTP with a unique staining pattern.
Materials and Methods
Eleven cases of SPTPs with pre-operative cytological
diagnosis were retrieved from the archives of the
cytopathology laboratory of our institute. FNAC was done
with 23G needle under ultrasound guidance and in one case
EUS-guided aspirate was done. Toluidine blue stain was
done for specimen adequacy assessment and preliminary
diagnostic interpretation on site. Smears were fixed in
95% alcohol for Papanicolaou stain and air dried for May
GrnwaldGiemsa staining. Detailed cytomorphological
evaluation was performed in each case. Alcohol-fixed
slides were also used for immunocytochemistry. In five
cases, spare alcohol-stained slides were available and
immunocytochemistry was done on them. In three cases,
a Papanicolaou-stained slide was destained by dipping
in xylene for 2-3 h followed by immersing in methanol
for 15 minutes. Immunohistochemical staining was
done with monoclonal antibody against CD 99 (Dako,
Mouse antihuman antibody clone 12E7) using a routine
streptavidin-biotin horseradish peroxidase detection system
with diaminobenzidine (DAB) as chromogen.
Results
During the period of 2005-2012, 11 patients of SPTP
presented to our institute, with age ranging from 13 to
40 years. Only one of the patients was male and rest were
all females. The clinical and radiological features of these
patients are summarized in Table 1.
Cytomorphology
The smears ranged from being moderately cellular to richly
cellular. A pseudopapillary pattern with a fibrovascular core
surrounded by two or more layers of cells was conspicuous
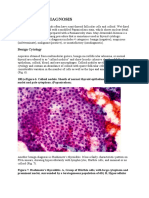
in all the cases [Figure 1a and d]. Pseudorosettes were
observed in four cases [Figure 1c]. There were also many
loosely cohesive cells and bare nuclei in the background.
Extra-cellular deposition eosinophilic material was another
finding observed in all the cases while intracellular
eosinophilic material was seen in two cases [Figure 1e and f].
Five of the cases had foamy macrophages in the background
[Figure 1c]. The cells were monomorphic with round to oval
nuclei and moderate to abundant amount of cytoplasm with
five of the cases showing fine sago grain like vacuoles. Mild
anisonucleosis was observed in the cases. Nuclear grooves
were seen in many of the cells. Most of the cells had one
to two inconspicuous nucleoli [Figure 1b]. Mitosis was not
observed in any of the case, while one of the cases had
necrosis.
Immunocytochemistry
Immunocytochemistry for CD 99 was performed in eight of
the cases where alcohol-fixed smears were available. All the
cases demonstrated dot-like paranuclear positivity [Figure 2].
Immunocytochemistry for CD 99 was also performed in two
cases of pancreatic neuroendocrine tumor which were negative.
Table 1: Clinicopathological features of patients
Case no. Age/Sex Presentation Site Radiology
1 35/F Epigastric lump Head Cystic SOL in head of pancreas on USG
2 40/F Epigastric pain and vomiting, weight loss Tail and body Cystic mass near tail of pancreas not encasing vessels
on CT scan and USG
3 20/F Pain in left flank lump in epigastrium Body CT scan showed solid mass in body of pancreas
suggestive of adencoarcinoma
4 32/F Pain, intestinal obstruction due to adhesion Head Multiple mixed echogenic intra-hepatic area
5 20/F Pain abdomen and vomiting Body SOL in head of pancreas suggestive of tuberculosis/
carcinoma
6 22/F Lump in left upper abdomen with restricted mobility Head Solid cystic mass in head
7 13/F Pain abdomen Neck Mass in neck of pancreas and multiple mesenteric
lymphadenopathy
8 20/F Pain abdomen, weight loss hard lump in left upper abdomen Head CT scan suggestive of solid and cystic neoplasm
9 31/M Lump abdomen Body Solid heteroechoic mass in pancreas on USG MRI/CT
Scan shows cyst in body of pancreas suggestive of
Serous cystadenoma
10 27/F Pain abdomen Body Cystic mass in CT scan
11 26/F Lump and pain abdomen Body Solid cystic mass
[Downloadedfreefromhttp://www.jcytol.orgonWednesday,October16,2013,IP:36.74.61.1]||ClickheretodownloadfreeAndroidapplicationforthisjournal
153
Ghosh, et al.: CD 99 immunocytochemistry in solid pseudopapillary tumor of pancreas
Journal of Cytology / July 2013 / Volume 30 / Issue 3
Discussion
Solid pseudopapillary tumors account for 1% of all
pancreatic neoplasms.
[10]
Solid pseudopapillary neoplasm
mostly occurs in adolescent girls and young women
(mean age 25-35 years).
[11]
SPTP in men is very rare, and
accounts for about 7% of cases. In our case series, 1 of 11
patients was male and 1 case was a middle aged female.
All other cases occurred in young females ranging from
13 to 35 years. Histogenesis of this tumor is uncertain;
however, it is believed to arise from uncommitted
epithelial cells that can show both endocrine and
exocrine differentiation.
[12]
Clinically, SPTP usually presents with abdominal mass and
discomfort or pain, or it may be an incidental finding in
work-up for unrelated conditions.
[13]
In our series, all patients
had pain abdomen and four patients presented with lump in
upper abdomen. One of the patients presented with intestinal
obstruction due to adhesions.
Approximately 70% of SPTPs originate in the body and tail of
the pancreas and less frequently in the head.
[11]
In our series,
4 out of 11 cases had tumor in head of pancreas and 6 had
tumor in body and tail of pancreas, while 1 had in neck of
pancreas.
Correct diagnosis of SPTP is very important for proper
management of this tumor . Resection of tumor is curative
and no chemotherapy and radiotherapy is required unlike
many other malignant pancreatic tumors. Moreover, as
these tumors are very vascular, pre-operative correct
diagnosis helps in anticipating complications like intra-
operative hemorrhage, or hemoperitonium. Imaging-
guided FNA biopsy is the cardinal choice for pre-operative
pathological diagnosis as the tumor is of low malignant
potential. The cytomorphology of SPTP is classical and
usually different from other cystic lesions of pancreas.
[14]
The cytological findings in our case was similar to those
of earlier reports in the literature.
[4,6,15]
Numerous cellular
papillary fragments as previously described were seen
in our cases some having an irregular Chinese letter-like
pattern. Pseudorosettes described in some of the studies
were also seen in four of our cases.
[16]
The papillary
fragments composed of monomorphic bland round to oval
cells were seen in all our cases confirming with the earlier
studies.
[4]
Many cells in the tumor had fine vacuolation in
the cells as described by Pettinato et al.
[14]
Intra-cytoplasmic
inclusions were seen in our case similar to that by Jayram
et al.
[17]
Cappelari et al
[18]
described nuclear grooves as
one of the typical findings of this tumor which was seen
in our cases.
Although SPTP have these classical morphological
features, there is considerable overlap in cytomorphology
and immunocytochemistry with some other pancreatic
tumors specially the pancreatic endocrine tumors.
[19]
Many of the immunocytochemical markers of endocrine
tumors are also expressed in SPTP.
[20]
Moreover, some
other markers of SPTP like CD10 and CD56 are also
positive in tumors like pancreatoblastoma and acinic
cell carcinoma.
[20]
Staining of CD56 in SPTP is also
inconsistent. Beta Catenin and E-cadherin were previously
considered good markers for SPTP,
[21]
and Holly Burford
et al.
[22]
had proposed a short panel comprising of
CD10, E-cadherin and -catenin for diagnosis of SPTP;
however, these markers are also found to be positive in
tumors like intra-ductal papillary mucinous neoplasm,
Figure 1: (a and d) Pseudopapillae with a delicate fibrovascular core
(Pap, x100 and MGG x100 respectvely). (b) Round to oval cells with fne
chromatn and inconspicuous nucleoli (Pap, x200). (c) Pseudorosete with
foamy macrophages in the background (Pap, x400). (e) Nuclear overlapping
in pseudopapillae (MGG, x400). (f) Intra-cellular basement membrane-like
material (MGG, x400)
a b c
f e d
Figure 2: (a and b) Paranuclear dot-like positvity of CD 99 (ICC, x200 and
x100, respectvely)
a b
[Downloadedfreefromhttp://www.jcytol.orgonWednesday,October16,2013,IP:36.74.61.1]||ClickheretodownloadfreeAndroidapplicationforthisjournal
154
Ghosh, et al.: CD 99 immunocytochemistry in solid pseudopapillary tumor of pancreas
Journal of Cytology / July 2013 / Volume 30 / Issue 3
pancreatoblatoma and acinic cell carcinoma.
[23-25]
To rule
out the other morphologic mimics of SPTPs, a relatively
specific and consistent marker which is expressed on FNA
smears should be used.
CD 99 immunoreactivity in SPTP has been reported
recently in two studies on histopathology sections. CD
99 was demonstrated to have a characteristic paranuclear
dot-like positivity in these studies.
[26,27]
They reported
that this positivity pattern was quite classical of SPTP
among the different pancreatic tumors as it was negative
in pancreatoblastoma, adenocarcinoma and acinic cell
carcinoma. In pancreatic endocrine tumors, it was either
negative or had a membranous pattern of positivity.
[28]
Similar to our study, the studies on tissue sections had
100% of the SPTP positive for CD 99 [Table 2]. There has
been no published study which has evaluated utility
of CD 99 on FNA smears. In our series, CD 99 staining
in cytology smears also demonstrated a similar result
with paranuclear dot-like positivity in the eight SPTP
cases while negative in two endocrine tumors. Though,
in this study we performed immunocytochemistry in
Papanicolaou-stained slide by de-staining. The cell
block is ideal as it gives better cellularity and allows
performance of more tests in the same sample. A major
limitation of this study is the number of cases especially
only two neuroendocrine tumors were included in the
study for comparison. Immunocytochemistry on larger
number of cases may have been better and helped to
establish predictive values, our study is thus mainly a
descriptive study of this unique immunocytochemical
property.
SPTP is a tumor of low malignant potential where early
diagnosis and resection is curative. This is the first study
to demonstrate the paranuclear dot-like expression of CD
99 on FNA smears. This may help especially to differentiate
from the other close cytological differential diagnosis like
pancreatic endocrine tumors. CD 99 immunocytochemistry
thus may be used as a good adjunct to cytomorphology
for diagnosis of SPTP where there is an overlap with other
entities on imaging and morphology. The pathophysiology
for this unique staining pattern however is still to be
established.
References
1. Frantz VK. Tumors of the pancreas. In: Atlas of tumor pathology, 1st
series. Washington, DC, USA: US Armed Forces Institute of Pathology;
1959. p. 32-3.
2. Adamthwaite JA, Verbeke CS, Stringer MD, Guillou PJ, Menon KV.
Solid pseudopapillary tumor of the pancreas: Diverse presentation,
outcome and histology. J Pancreas 2006;7:635-42.
3. Mohan H, Bal A, Punia RP, Attri AK. Solid and cystic papillary epithelial
neoplasm of the pancreas. J Postgrad Med 2006;52:141-2.
4. Naresh KN, Borges AM, Chinoy RF, Soman CS, Krishnamurthy SC.
Solid and Papillary epithelial neoplasm of the pancreas. Diagnosis by
fne needle aspiration cytology in four cases. Acta Cytol 1995;39:489-93.
5. Solcia E, Capella C, Kloppel G. Tumors of the pancreas. Atlas of Tumor
Pathology, third series, Fascicle 20, Washington D.C. Armed Forces
Institute of Pathology, 1995.Rosai J, Sobin LH, editors.
6. Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, Guiter G,
et al. Endoscopic ultrasound-guided fne-needle aspiration cytology
diagnosis of solid-pseudopapillary tumor of pancreas : A rare neoplasm
of elusive origin but characteristic cytomorphology features. Am J Clin
Pathol 2004;121:654-62.
7. Pelosi G, Iannucci A, Zamboni G, Bresaola E, Iacono C, Serio G. Solid
and cystic papillary neoplasm of the pancreas: A clinico-cytopathologic
and immunocytochemical study of five new cases diagnosed by
fne-needle aspiration cytology and a review of the literature. Diagn
Cytopathol 1995;13:233-46.
8. Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, et al. Solid
pseudopapillary tumor of the pancreas: A review of 553 cases in Chinese
literature. World J Gastroenterol 2010;14:1209-14.
9. Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol
2007;20:S94-112.
10. Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I,
Pantazopoulou A, Dappola V. Endoscopic ultrasound-guided fne-
needle aspiration cytology diagnosis of solid pseudopapillary tumor of
the pancreas: A case report and literature review. World J Gastroenterol
2007;13:5158-63.
11. Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, et al.
Cystic neoplasms of the pancreas and tumor-like lesions with cystic
features: A review of 418 cases and a classifcation proposal. Virchows
Arch 2004;445:168-78.
12. Mendelson G. Papillary cystic tumor of pancreas: An enigma. Am J Clin
Pathol 1992;98:476-7.
13. Hamilton SR, Aaltonen LA, Editors. World Health Organization
Classifcation of Tumors. Pathology and Genetics of Tumors of the
Digestive System. Lyon: IARC Press; 2000. p. 246-8.
14. Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma
P, Insabatao L. Solid-pseudopapillary tumor of the pancreas: A neoplasm
with highly distinct and characteristic cytological features. Diagn
Cytopathol 2002;27:325-34.
15. Al-Kaisi N, Sieger EE. Fine Needle aspiration cytology of the pancreas.
Acta Cytol 1989;33:145-52.
16. Mehta N, Modi L, Patel T, Shah M. Study of cytomorphology of solid
pseudopapillary tumor of pancreas and its differential diagnosis. J Cytol
2010;27:118-22.
17. Jayram G, Chaturvedi KU, Jindal RK, Venugopal S, Kapoor R. Papillary
cystic neoplasm of pancreas Report of a case diagnosed by fne needle
aspiration cytology. Acta Cytol 1990;34:429-33.
18. Cappellari JO, Geisinger KR, Albertson DA, Wolfman NT, Kute TE.
Malignant papillary cystic tumor of pancreas. Cancer 1990;66:193-8.
19. Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of
solid-pseudopapillary neoplasm and endocrine tumors of the pancreas.
World J Gastroenterol 2010;16:1025-30.
20. Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K,
Mizobuchi K, et al. Solid-pseudopapillary tumor of the pancreas:
Table 2: Studies on CD 99 staining in SPTP
Number of
SPTPs
Number of cases positive
for CD 99
Guo et al.
[27]
62 62
Li et al.
[26]
37 37
Present study 8 8
[Downloadedfreefromhttp://www.jcytol.orgonWednesday,October16,2013,IP:36.74.61.1]||ClickheretodownloadfreeAndroidapplicationforthisjournal
155
Ghosh, et al.: CD 99 immunocytochemistry in solid pseudopapillary tumor of pancreas
Journal of Cytology / July 2013 / Volume 30 / Issue 3
immunohistochemical localization of neuroendocrine markers and
CD10. Am J Surg Pathol 2000;24:1361-71.
21. Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan
M, et al. Solid-pseudopapillary tumors of the pancreas are genetically
distinct from pancreatic ductal adenocarcinomas and almost always
harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9.
22. Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/
beta-catenin and CD10: A limited immunohistochemical panel to
distinguish pancreatic endocrine neoplasm from solid pseudopapillary
neoplasm of the pancreas on endoscopic ultrasound-guided fne-needle
aspirates of the pancreas. Am J Clin Pathol 2009;132:831-9.
23. Chetty R, Serra S, Salahshor S, Alsaad k, Shih W, Blaszyk H, et al.
Expression of Wnt-signaling pathway proteins in intraductal papillary
mucinous neoplasms of the pancreas: A tissue microarray analysis. Hum
Pathol 2006;37:212-7.
24. Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, Yeo CJ, et al.
Distinctive molecular genetic alterations in sporadic and familial
adenomatouss polyposisassociated pancreatoblastomas: Frequent
alterations in the APC/ beta-catenin pathway and chromosome 11p. Am
J Pathol 2001;159:1619-27.
25. Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, et al.
Genetic and immunohistochemical analysis of pancreatic acinar cell
carcinoma: frequent allelic loss on chromosome 11p and alterations in
the APC/beta-catenin pathway. Am J Pathol 2002;160:953-62.
26. Li L, Li J, Hao C, Zhang C, Mu K, Wang Y, et al. Immunohistochemical
evaluation of solid pseudopapillary tumors of the pancreas: The
expression pattern of CD99 is highly unique. Cancer Lett 2011;310:9-14.
27. Guo Y, Yuan F, Deng H, Wang HF, Jin XL, Xiao JC. Paranuclear dot-
like immunostaining for CD99: A unique staining pattern for diagnosing
solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol
2011;35:799-806.
28. Goto A, Niki T, Terado Y, Fukushima J, Fukayama M Prevalence
of CD99 protein expression in pancreatic endocrine tumors (PETs).
Histopathology 2004;45:384-92.
How to cite this article: Ghosh R, Mallik SR, Mathur SR, Iyer VK. CD
99 immunocytochemistry in solid pseudopapillary tumor of pancreas: A
study on fne-needle aspiration cytology smears. J Cytol 2013;30:151-5.
Source of Support: Nil, Confict of Interest: None declared.
Author Help: Online submission of the manuscripts
Articles can be submitted online from http://www.journalonweb.com. For online submission, the articles should be prepared in two files (first
page file and article file). Images should be submitted separately.
1) First Page File:
Prepare the title page, covering letter, acknowledgement etc. using a word processor program. All information related to your identity
should be included here. Use text/rtf/doc/pdf files. Do not zip the files.
2) Article File:
The main text of the article, beginning with the Abstract to References (including tables) should be in this file. Do not include any information
(such as acknowledgement, your names in page headers etc.) in this file. Use text/rtf/doc/pdf files. Do not zip the files. Limit the file size
to 1 MB. Do not incorporate images in the file. If file size is large, graphs can be submitted separately as images, without their being
incorporated in the article file. This will reduce the size of the file.
3) Images:
Submit good quality color images. Each image should be less than 4 MB in size. The size of the image can be reduced by decreasing the
actual height and width of the images (keep up to about 6 inches and up to about 1800 x 1200 pixels). JPEG is the most suitable file format.
The image quality should be good enough to judge the scientific value of the image. For the purpose of printing, always retain a good quality,
high resolution image. This high resolution image should be sent to the editorial office at the time of sending a revised article.
4) Legends:
Legends for the figures/images should be included at the end of the article file.
[Downloadedfreefromhttp://www.jcytol.orgonWednesday,October16,2013,IP:36.74.61.1]||ClickheretodownloadfreeAndroidapplicationforthisjournal
You might also like
- Fine Needle Aspiration Cytology (FNAC) of GISTDocument7 pagesFine Needle Aspiration Cytology (FNAC) of GISTurfriendanshul100% (1)
- Neuroendocrine Tumors: Surgical Evaluation and ManagementFrom EverandNeuroendocrine Tumors: Surgical Evaluation and ManagementJordan M. CloydNo ratings yet
- The Histomorphological Study of Prostate LesionsDocument5 pagesThe Histomorphological Study of Prostate LesionsIOSRjournalNo ratings yet
- Diagnostic and Prognostic Markers For Gastrointestinal Stromal Tumors in NorwayDocument8 pagesDiagnostic and Prognostic Markers For Gastrointestinal Stromal Tumors in NorwayNengLukmanNo ratings yet
- ECP 2017 Asbtracts Supplement (12 Lucrari)Document14 pagesECP 2017 Asbtracts Supplement (12 Lucrari)Cristiana PoppNo ratings yet
- YagciDocument7 pagesYagciPatricia BezneaNo ratings yet
- Neuroendocrine Tumors Dr. WarsinggihDocument20 pagesNeuroendocrine Tumors Dr. WarsinggihAndi Eka Putra PerdanaNo ratings yet
- Malignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesDocument4 pagesMalignant Mesothelioma Presenting With Unexplained Recurrent Pleurisy EpisodesNurul NingrumNo ratings yet
- Clinical and Cytopathological Aspects in Phyllodes Tumors of The BreastDocument7 pagesClinical and Cytopathological Aspects in Phyllodes Tumors of The Breastreeves_coolNo ratings yet
- Title: Latent Primary Papillary Micro Tumor in Thyroglossal Duct Cyst Wall - A Rare Case Report With Review of LiteratureDocument9 pagesTitle: Latent Primary Papillary Micro Tumor in Thyroglossal Duct Cyst Wall - A Rare Case Report With Review of LiteratureRathinaKumarNo ratings yet
- Nature0538420160724 27817 2z23gn With Cover Page v2Document6 pagesNature0538420160724 27817 2z23gn With Cover Page v2Sherlyy Kristiani.SNo ratings yet
- Tumorpediatricoembrionalcerebelo PDFDocument8 pagesTumorpediatricoembrionalcerebelo PDFRafael Mujica OreNo ratings yet
- Citologie-Fna Tiroida DocumentDocument10 pagesCitologie-Fna Tiroida DocumentMocanu RalucaNo ratings yet
- Solitary TumorDocument7 pagesSolitary TumorOla AzzahraNo ratings yet
- His 12031Document4 pagesHis 12031JeevikaGoyalNo ratings yet
- Plasmacytoma of The Thyroid Gland: Case Report and Review of The LiteratureDocument10 pagesPlasmacytoma of The Thyroid Gland: Case Report and Review of The Literaturedede wawaNo ratings yet
- Pediatric Surgery Update Volume 33, 2009Document12 pagesPediatric Surgery Update Volume 33, 2009rajarshikNo ratings yet
- Philippine Journal of Gynecologic Oncology Volume 9 Number 1 2012Document48 pagesPhilippine Journal of Gynecologic Oncology Volume 9 Number 1 2012Dasha VeeNo ratings yet
- Mucin1 Expression in Relation To Histomorphological Type of Colorectal Carcinoma: A Cross Sectional StudyDocument6 pagesMucin1 Expression in Relation To Histomorphological Type of Colorectal Carcinoma: A Cross Sectional StudyIJAR JOURNALNo ratings yet
- Abdominal Wall Extraskeletal Ewing Sarcoma - Case ReportDocument3 pagesAbdominal Wall Extraskeletal Ewing Sarcoma - Case ReportInternational Organization of Scientific Research (IOSR)No ratings yet
- Articulo Citologia Carcinoma EndometrialDocument11 pagesArticulo Citologia Carcinoma Endometrialangelo marinoNo ratings yet
- Pancreatic NETsDocument37 pagesPancreatic NETseztouch12No ratings yet
- NeuroblastomaDocument19 pagesNeuroblastomaTri Andhika Dessy WahyuniNo ratings yet
- 9032 FTPDocument6 pages9032 FTPSelapoom PairorNo ratings yet
- Diagnostic Accuracy Fine Needle Aspiration Cytology of Thyroid Gland LesionsDocument6 pagesDiagnostic Accuracy Fine Needle Aspiration Cytology of Thyroid Gland LesionsinventionjournalsNo ratings yet
- RPB14150079015Document5 pagesRPB14150079015Ijupbs IjupbsNo ratings yet
- Kim 2017Document14 pagesKim 2017Cota AncutaNo ratings yet
- Cancer GuidelinesDocument17 pagesCancer GuidelinesJoni WitziNo ratings yet
- Yolk Sac Tumor Testis Suatu Laporan Kasus: Meta Zulyati OktoraDocument8 pagesYolk Sac Tumor Testis Suatu Laporan Kasus: Meta Zulyati OktorafreakNo ratings yet
- Tonsil TumorDocument4 pagesTonsil TumorafrisiammyNo ratings yet
- Expression of CD133 in Endometrial Cancer Cells and Its ImplicationsDocument16 pagesExpression of CD133 in Endometrial Cancer Cells and Its Implicationsbee yournitaNo ratings yet
- SCLC LancetDocument15 pagesSCLC LancetLeeTomNo ratings yet
- Expression of CD133 in Endometrial Cancer Cells and Its ImplicationsDocument16 pagesExpression of CD133 in Endometrial Cancer Cells and Its Implicationsbee yournitaNo ratings yet
- Neoplasia ExamDocument9 pagesNeoplasia ExamYheng Gaosaii100% (1)
- Adult NeuroblastomaDocument3 pagesAdult NeuroblastomaJoanne HoNo ratings yet
- Transplant International Special Issue: Abstracts of The 14th Congress of The European Society For Organ Transplantation Volume 22, Issue Supplement s2, Pages 95-222, August 2009Document128 pagesTransplant International Special Issue: Abstracts of The 14th Congress of The European Society For Organ Transplantation Volume 22, Issue Supplement s2, Pages 95-222, August 2009Enrique Moreno GonzálezNo ratings yet
- Inflammatory Myofibroblastic TumourDocument4 pagesInflammatory Myofibroblastic TumourThiruNo ratings yet
- A Diagnostic Flow Chart For Non-Immune HydropsDocument2 pagesA Diagnostic Flow Chart For Non-Immune HydropsIvan BejarNo ratings yet
- File 1Document4 pagesFile 1Marsya Yulinesia LoppiesNo ratings yet
- Follicular Proliferative Lesion Arising in Struma Ovarii: Min Jee Park Min A Kim Mi Kyung Shin Hye Sook MinDocument5 pagesFollicular Proliferative Lesion Arising in Struma Ovarii: Min Jee Park Min A Kim Mi Kyung Shin Hye Sook MinRebecca Agustine KristianNo ratings yet
- OvaryDocument4 pagesOvaryCarolina MartinezNo ratings yet
- Thyroid Nodule: Statpearls (Internet) - Treasure Island (FL) : Statpearls Publishing 2019 JanDocument8 pagesThyroid Nodule: Statpearls (Internet) - Treasure Island (FL) : Statpearls Publishing 2019 JanRara SafikaNo ratings yet
- Malignant Pleural MesotheliomaDocument14 pagesMalignant Pleural MesotheliomaIulyaNo ratings yet
- Frantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case ReportDocument3 pagesFrantz's Tumor (Solid Pseudopapillary Tumor) of The Pancreas. A Case ReportcostachemfNo ratings yet
- NotesDocument4 pagesNotesRaza HodaNo ratings yet
- OncologyDocument15 pagesOncologyapi-3840195100% (5)
- Retrospective Diagnosis of Malignant Struma Ovarii After Discovery of PulmonaryDocument17 pagesRetrospective Diagnosis of Malignant Struma Ovarii After Discovery of PulmonaryGeorge LazarNo ratings yet
- CD133: A Potential Indicator For Differentiation and Prognosis of Human CholangiocarcinomaDocument8 pagesCD133: A Potential Indicator For Differentiation and Prognosis of Human CholangiocarcinomaNovita ApramadhaNo ratings yet
- Morphologic Criteria of Invasive Colonic Adenocarcinoma On Biopsy SpecimensDocument8 pagesMorphologic Criteria of Invasive Colonic Adenocarcinoma On Biopsy SpecimensFadilatul HalimahNo ratings yet
- 42 Amita EtalDocument4 pages42 Amita EtaleditorijmrhsNo ratings yet
- RG 256055137Document20 pagesRG 256055137Adel SalehNo ratings yet
- Jurnal MelenaDocument4 pagesJurnal MelenaMadetolis PhotoworksNo ratings yet
- Flucke 2013Document8 pagesFlucke 2013FANIA PARRANo ratings yet
- Argani 2000Document15 pagesArgani 2000karen.cobenaNo ratings yet
- Klein2005Document7 pagesKlein2005Nurjannah MuhdiNo ratings yet
- Ann Oncol-2014-Dreyling-iii83-92 PDFDocument10 pagesAnn Oncol-2014-Dreyling-iii83-92 PDFHessler Hannsen Zambrano CondoriNo ratings yet
- Retroperitoneal Non-Functioning Paraganglioma: A Case ReportDocument4 pagesRetroperitoneal Non-Functioning Paraganglioma: A Case ReportDebo AdeosoNo ratings yet
- Inmunoterapia Basada en ODN CPG Es Efectiva en El Control Del Crecimiento de Celulas Metastasicas HAN-A KIM 2009Document5 pagesInmunoterapia Basada en ODN CPG Es Efectiva en El Control Del Crecimiento de Celulas Metastasicas HAN-A KIM 2009Ramiro J. Rodriguez GarciaNo ratings yet
- Semb 57 287Document18 pagesSemb 57 287samruizayalaNo ratings yet
- ACLS Megacode Testing ScenariosDocument12 pagesACLS Megacode Testing Scenariosealm10100% (2)
- Sample Questions For HAAD Prometric and DHA For NursesDocument46 pagesSample Questions For HAAD Prometric and DHA For NursesJaezelle Ella Sabale100% (4)
- TRS 987Document286 pagesTRS 987NGOTTANo ratings yet
- Prostate Cancer Between Prognosis and Adequate/proper TherapyDocument8 pagesProstate Cancer Between Prognosis and Adequate/proper TherapyGabriel NguyenNo ratings yet
- Basic Anaestetic DrugsDocument1 pageBasic Anaestetic DrugsLuthfi AfiatNo ratings yet
- Pulp Exposure in Permanent Teeth CG-A012-04 UpdatedDocument7 pagesPulp Exposure in Permanent Teeth CG-A012-04 UpdatedmahmoudNo ratings yet
- Psychiatric Mental Health Nursing Concepts of Care in Evidence Based Practice Townsend 7th Edition Test BankDocument24 pagesPsychiatric Mental Health Nursing Concepts of Care in Evidence Based Practice Townsend 7th Edition Test BankAnitaCareyfemy100% (35)
- Primitive ReflexesDocument10 pagesPrimitive Reflexesbun_yulianaNo ratings yet
- Ministry Health Medicines Formulary 1 2015Document371 pagesMinistry Health Medicines Formulary 1 2015David Lim Su ShenNo ratings yet
- Emergence of Multidrug Resistant Acinetobacter Baumannii As Nosocomial Pathogen: Clinical Significance and Antimicrobial SensitivityDocument5 pagesEmergence of Multidrug Resistant Acinetobacter Baumannii As Nosocomial Pathogen: Clinical Significance and Antimicrobial SensitivityIOSRjournalNo ratings yet
- Textbook ReadingDocument275 pagesTextbook ReadingNovitasari EkaNo ratings yet
- 2018 AHA Guidelines For Management of Blood CholesterolDocument66 pages2018 AHA Guidelines For Management of Blood CholesterolsarumaxNo ratings yet
- HiperkolesterolDocument60 pagesHiperkolesterolAnonymous 1jCVqQuNo ratings yet
- Ecg For The Exam-Score 2020Document17 pagesEcg For The Exam-Score 2020prasannasimhaNo ratings yet
- Prevalence of Abnormal Pap Smear During Pregnancy in A Teaching Hospital in South IndiaDocument4 pagesPrevalence of Abnormal Pap Smear During Pregnancy in A Teaching Hospital in South IndiapebripulunganNo ratings yet
- The Impact of Menstrual Periods On Performance of Female WorkersDocument5 pagesThe Impact of Menstrual Periods On Performance of Female WorkersEiman Khowaja (22GJKB-CLSTEC)No ratings yet
- NehaDocument91 pagesNehaPawan MeenaNo ratings yet
- Focus On Adult Health Medical Surgical Nursing Pellico Edition Test BankDocument8 pagesFocus On Adult Health Medical Surgical Nursing Pellico Edition Test BankCarolineAndersoneacmg100% (33)
- Special Needs in The General Education ClassroomDocument15 pagesSpecial Needs in The General Education Classroomapi-267526572No ratings yet
- Sim LabDocument4 pagesSim Laballycat2390% (10)
- Guardian - Dental PPO Plan Summary 2022Document4 pagesGuardian - Dental PPO Plan Summary 2022Jessi ChallagullaNo ratings yet
- 7325-300 C-Peptide & Insulin AccuBind VAST ELISA Rev 6Document2 pages7325-300 C-Peptide & Insulin AccuBind VAST ELISA Rev 6Luisa MaríaNo ratings yet
- Lecture Notes Malarial and Non Malarial Drugs For Blues 1Document5 pagesLecture Notes Malarial and Non Malarial Drugs For Blues 1chn pastranaNo ratings yet
- Reaction Paper On The IntegumentaryDocument2 pagesReaction Paper On The IntegumentaryCamile Elaine Borromeo NocheNo ratings yet
- Medicine 018 Final PDFDocument22 pagesMedicine 018 Final PDFdrkefyalewtayeNo ratings yet
- Gait AnalysisDocument2 pagesGait AnalysisIvin KuriakoseNo ratings yet
- Specialize Immunity at Epithelial Barriers and in Immune Privilege TissuesDocument27 pagesSpecialize Immunity at Epithelial Barriers and in Immune Privilege TissuesUmar UsmanNo ratings yet
- K1 Hubungan Biomedik Dasar Dengan Ilmu KedokteranDocument21 pagesK1 Hubungan Biomedik Dasar Dengan Ilmu Kedokteranhartinissa vaniaNo ratings yet
- Amacon2022-Accepted Paper Poster ListDocument35 pagesAmacon2022-Accepted Paper Poster ListViraj ShahNo ratings yet
- Pinhole NDocument4 pagesPinhole NNizam MohamedNo ratings yet
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 4 out of 5 stars4/5 (6)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (28)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeRating: 2 out of 5 stars2/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDFrom EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDRating: 5 out of 5 stars5/5 (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (404)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (42)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (81)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- Sleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningFrom EverandSleep Stories for Adults: Overcome Insomnia and Find a Peaceful AwakeningRating: 4 out of 5 stars4/5 (3)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)From EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)No ratings yet
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- The Marshmallow Test: Mastering Self-ControlFrom EverandThe Marshmallow Test: Mastering Self-ControlRating: 4.5 out of 5 stars4.5/5 (58)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessFrom EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessRating: 4.5 out of 5 stars4.5/5 (328)
- Dark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingFrom EverandDark Psychology: Learn To Influence Anyone Using Mind Control, Manipulation And Deception With Secret Techniques Of Dark Persuasion, Undetected Mind Control, Mind Games, Hypnotism And BrainwashingRating: 4 out of 5 stars4/5 (1138)
- Troubled: A Memoir of Foster Care, Family, and Social ClassFrom EverandTroubled: A Memoir of Foster Care, Family, and Social ClassRating: 4.5 out of 5 stars4.5/5 (26)