Professional Documents
Culture Documents
Hets Project
Uploaded by
api-212894050Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hets Project
Uploaded by
api-212894050Copyright:
Available Formats
1
Megan Whitley Heterogeneity Project March 5, 2013 Heterogeneity Project When radiation was first used for cancer treatment, the research had not yet been done to determine tissue densities impact on treatment. Once computed tomography (CT) became standard of care the influences of these densities became clear. Nevertheless, the field of radiation oncology developed without the data provided by CTs. Within this timeframe, many charts, concepts, protocols and standards were devised, that are still in place today. Many of our algorithms and treatment planning software (TPS) still utilize these older data sets, and they are also used for performing hand calculations. The problem with the data collected before advent of CT simulation is that it assumes that all tissues within the patient have a uniform density, that they are homogenous.1 Thus, the data on which most treatment decisions are based, depends on dose calculations in which inhomogeneity corrections were not made.2 As we now know, this is inaccurate, and many protocols today are fighting to remedy this by collecting new information and reconstructing these data sets for use in calculations. But, until this time, extra strides are required to ensure that the most accurate treatment regimen is planned. To compensate for the different tissue densities within the body, a correction factor was developed and implemented. This factor, as mentioned earlier from Bentel, is known as an inhomogeneity correction. The reason this correction is vital is because radiation travels through tissues of different densities in different ways. In lung tissue, theres less tissue to attenuate the radiation, therefore less energy is required for penetration. The opposite is true in bone, with the increased density of bone requiring more energy for adequate penetration. And, at the interfaces between lesser and greater densities, for example the interface of the soft tissue of the breast, abutting more dense rib, coupled with the less dense lung, the necessity for a correction factor truly becomes apparent. So, from the CT scan, to compensate for these differences, a number is assigned to the different densities, a Hounsfield Unit (HU). Hounsfield Units assigned to tissue are ranked from +1000, which would represent dense bone, to -1000, for air, while water is 0.3 If there are varying degrees of differentiation within the tissue densities, the area is known as
heterogeneous. If the densities are similar, the tissues are homogenous. So, now knowing what defines these tissue differences, we need to establish what defines their impact. To compensate for the influence of heterogeneity on treatment, the correction factor must be obtained. The correction equates to the dose calculated in a heterogeneous medium being divided by the dose calculated at the same point in a homogeneous medium. Again, the reason that compensating for these differences in densities is due to their influence on radiation. When radiation enters the body, tissues impede its travel. When compensation is not made, the planning process not only becomes easier, but less accurate. If a correction was not used in planning, the beams would simply create boxes of dose, centered within the transection of the beams. As in, a 3 field plan would have a 3 field box, and a 4 field plan, a 4 field box.
Figure 1. Volumetric comparison between two plans, the one on the left has the heterogeneity corrections on, and the one on the right is planned without the corrections. Take Figure 1 for example; in the plan on the right, the dose simply distributes into a shape of relatively uniform dose. The only things shaping the dose are the multileaf collimators (MLCs), the wedges, the weighting, and the sloping of the skin. But, in the plan on the left, the
corrections have been turned on, compensating for the different densities of soft tissue and lung, thus impacting the fluence of the dose, and making the impact of these tissues visible.
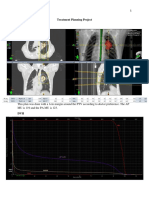
Figure 2. Single slice comparison of the isodose lines through the same isocenter within the same plan, except the left plan has the heterogeneity corrections on. As in Figure 2, the plan on the right side depicts a treatment regimen that would be expected from a plan generated for a homogenous attenuation region, and it represents a plan that is not struggling to accomplish goals. On the other hand, the plan on the left accurately portrays
the typical difficulty met when planning a lung. It is very difficult to accurately get radiation to a tumor within the lung, and oftentimes, the anterior and posterior chest walls, and the scapula, receive very high doses. This region of high dose is known as a hot spot, and it represents the buildup region of dose brought on by the lack of attenuation from lung that increases dose within and beyond the lung. As is shown in the upper right hand of the screen shots, the plan without the corrections used is cooler, at 110% hot, and the plan that includes the corrections has a hot area of 114%. Unfortunately, this is both accurate and unavoidable. If these accuracies arent obtained, the true dose distributions are unchecked and unaccounted for, causing great potential for areas of overdose and underdose. The differences are clear as we can see from Figure 2.
The inhomogeneity correction factor was introduced at a time when many radiation oncologists had a great deal of practice, and they were not used to seeing these new areas of concern and the increased complications within the plans. As is logical, this new correction was not received well. So, for a while, doctors chose not to use this factor. In the lung for example, areas can be seen where the isodose lines fall apart and regions of both under and overdose occur. Also, to obtain proper penetration, a decrease in monitor units (MU) can be seen, and optimal outcomes in treatment planning are much more difficult to achieve. For this, they blamed the factor. Although some physicians still make the choice not to use it today, now physicians are taught to use the inhomogeneity correction while in school, and come into the field with experience viewing plans with these characteristics. Such differences in MU can be seen in Figures 3 and 4.
Figure 3. This is the beam data report generated from the plan with the inhomogeneity correction factor.
Figure 4. This is the beam data report generated from the plan without the inhomogeneity correction factor. Heterogeneity correction is evident when viewing differences in the monitor units required in each plan. The MLCs for both plans were designed from a margin of 1.5 centimeters around the tumor volume. There are two fields, both an anterior-posterior (AP) and a posterior-
anterior (PA). The weighting was placed as 60% on the AP field and 40% on the PA field. The same wedge was used on each field, a 15 wedge with the heel placed superiorly. The beam data in Figure 3, for the plan with corrections on, demonstrates the MU for field A at 186, and for field B at 146. The beam data in Figure 4, for the plan with corrections off, demonstrates the MU for field A at 200, and for field B at 174. These differences reflect that lung tissue provides less attenuation than bone or soft tissue. Without the corrections, the algorithm assumed the lung was actually soft tissue. This difference in tissue requires less MU for accurate penetration, rendering the homogenous plan with concerns for overdose. Herein resides the need for both remedying the outdated data sets and tables, and furthering implementation of the inhomogeneity correction factor. This figure comparison demonstrates the differences in the plans with and without the corrections, in regards to their impacts on the target and organs at risk. A dose volume histogram, or DVH, is the ideal mechanism in which to illustrate these differences. This DVH has two lines. The one with squares illustrates the plan that utilizes the inhomogeneity correction factor, and the line with triangles illustrates the plan without the correction. If the line on the DVH illustrated with squares is analyzed, it shows that the target is not covered as well as the triangle line. The healthy structures are also receiving more dose with the corrections on. As stated before, corrections may not make a task easier, but, the plan is more accurate. And, after all, accuracy is the back bone and mainstay of cancer treatment.
Figure 5. This is a comparative DVH that represents the dosages to both the target and the organs at risk in both plans, and allows for a simple, visual comparison. The last 2 figures show the more complete overviews of the difference in the final products of the plans. Figure 6 demonstrates the dose at isocenter in a coronal, sagittal, and axial view. It also includes a DVH with only the plan with corrections. Then, for comparison, the same illustration is used for the plan without corrections in Figure 7.
Figure 6. Multi plane views of a plan created with the inhomogeneity corrections.
10
Figure 7. Multi plane views of a plan created without the inhomogeneity corrections. These treatment plans were generated using Varians Eclipse TPS with the convolution/superposition algorithm. Convolution/superposition is one of the semiempirical algorithms used today, collectively named correction-based algorithms.4 The name correctionbased algorithms reflects the impact that the inhomogeneity correction factor has had on
11
treatment planning and radiation oncology. In fact, within Eclipse there are means to turn the correction factor off, but no one in the department knew how, exemplifying how stringently the factor is used in modern day treatment planning. Without this factor, both under and over dosing may occur, leaving some areas too damaged, and some with a higher incidence of recurrence. With continued use of the factor, and the research to recreate widely used data sets, the future of both dosimetry and cancer treatment look to be more accurate, beneficial, and complete.
12
References 1. Hendee WR, Ibbott GS, Hendee EG. Radiation Therapy Physics. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2005:250. 2. Bentel GC. Radiation therapy planning. 2nd ed. Colombia: Macmillan Publishing Company; 1992:99. 3. Washington CM, Leaver D. Principles and Practice of Radiation Therapy. 3rd ed. St. Louis, MO: Mosby-Elsevier; 2010: 435. 4. Khan F, Gibbons J, Mihailidis D, Alkhatib H. Khans Lectures: Handbook of the Physics of Radiation Therapy. Baltimore, MD: Lippincott Williams & Wilkins; 2011: 184.
You might also like
- Treatment Planning Project Guide Heterogeneity Correction 1 2Document9 pagesTreatment Planning Project Guide Heterogeneity Correction 1 2api-645453685No ratings yet
- Solie 523paper FinalDocument11 pagesSolie 523paper Finalapi-571726550No ratings yet
- TxplanningprojectDocument9 pagesTxplanningprojectapi-432489466No ratings yet
- TPC Paper AllisonwrightDocument9 pagesTPC Paper Allisonwrightapi-632741700No ratings yet
- Treatment Planning PaperDocument13 pagesTreatment Planning Paperapi-569210408No ratings yet
- Heterogeneity ProjectDocument10 pagesHeterogeneity Projectapi-302831047No ratings yet
- Treatment Planning PaperDocument10 pagesTreatment Planning Paperapi-633057533No ratings yet
- Treatment Planning Paper Raver 4 1Document8 pagesTreatment Planning Paper Raver 4 1api-635923017No ratings yet
- TX Planning PaperDocument8 pagesTX Planning Paperapi-633434674No ratings yet
- Treatment Planning ProjectDocument11 pagesTreatment Planning Projectapi-484763634No ratings yet
- Treatment Planning Project PDFDocument10 pagesTreatment Planning Project PDFapi-692385376No ratings yet
- Treatment Planning Paper 3Document12 pagesTreatment Planning Paper 3api-642376263No ratings yet
- Tissue Heterogeneity PaperDocument8 pagesTissue Heterogeneity Paperapi-213038536No ratings yet
- Heterogeneity Corrections For Lung Treatment PlanningDocument11 pagesHeterogeneity Corrections For Lung Treatment Planningapi-543077883No ratings yet
- Heterogeneity Correction Paper PDF WeeblyDocument16 pagesHeterogeneity Correction Paper PDF Weeblyapi-630263039No ratings yet
- TX Planning PaperDocument15 pagesTX Planning Paperapi-633087057No ratings yet
- Treatment Planning Heterogeneity Vs Homogeneity Lung ProjectDocument13 pagesTreatment Planning Heterogeneity Vs Homogeneity Lung Projectapi-299138743No ratings yet
- TP HeterogeneityDocument10 pagesTP Heterogeneityapi-529438966No ratings yet
- Treatment Planning PaperDocument15 pagesTreatment Planning Paperapi-632827798No ratings yet
- TxplanningpaperDocument10 pagesTxplanningpaperapi-530717893No ratings yet
- Lung Cancer Plan With and Without Heterogeneity CorrectionDocument11 pagesLung Cancer Plan With and Without Heterogeneity Correctionapi-264047496No ratings yet
- HeterogeneityDocument8 pagesHeterogeneityapi-635186395No ratings yet
- Lung Treatment Planning PaperDocument10 pagesLung Treatment Planning Paperapi-527782385No ratings yet
- TP PaperDocument10 pagesTP Paperapi-312374730No ratings yet
- Heterogeneity Corrections - Treatment PlanningDocument10 pagesHeterogeneity Corrections - Treatment Planningapi-395602816100% (1)
- Treatment Planning Project 4Document11 pagesTreatment Planning Project 4api-282901112No ratings yet
- Treatment Planning Project PaperDocument12 pagesTreatment Planning Project Paperapi-332574751No ratings yet
- Treatment Planning PaperDocument14 pagesTreatment Planning Paperapi-631272802No ratings yet
- Treatment Planning PaperDocument8 pagesTreatment Planning Paperapi-598487146No ratings yet
- Treatment Planning PaperDocument10 pagesTreatment Planning Paperapi-632529930No ratings yet
- Treatment Planning ProjectDocument12 pagesTreatment Planning Projectapi-523912641No ratings yet
- Heterogeneity and Lung TreatmentsDocument8 pagesHeterogeneity and Lung Treatmentsapi-691277740No ratings yet
- Treatment Planning Final Copy 1Document13 pagesTreatment Planning Final Copy 1api-373572658No ratings yet
- Heterogenity Paper 1Document7 pagesHeterogenity Paper 1api-309296181No ratings yet
- Heterogeneity Versus Homogeneity in Treatment PlanningDocument8 pagesHeterogeneity Versus Homogeneity in Treatment Planningapi-632493717No ratings yet
- Staceysong Treatmentplanninglab 2023spring 1Document8 pagesStaceysong Treatmentplanninglab 2023spring 1api-634067897No ratings yet
- Lung Lab Dos 771Document18 pagesLung Lab Dos 771api-632146706No ratings yet
- Michals Treatment Planning ProjectDocument10 pagesMichals Treatment Planning Projectapi-480222490No ratings yet
- Dos 523 ProjectDocument13 pagesDos 523 Projectapi-375157618No ratings yet
- TreatmentplanninglabDocument11 pagesTreatmentplanninglabapi-644802109No ratings yet
- Hieu Tran Lung Treatment Planning LabDocument14 pagesHieu Tran Lung Treatment Planning Labapi-642692363No ratings yet
- Treatment Planning Project BmeyerDocument7 pagesTreatment Planning Project Bmeyerapi-350437453No ratings yet
- E Winn LungDocument12 pagesE Winn Lungapi-574567198No ratings yet
- TP PaperDocument11 pagesTP Paperapi-243468464No ratings yet
- Shahad Alward Treatment Planning PaperDocument9 pagesShahad Alward Treatment Planning Paperapi-691667702No ratings yet
- Paper UpdateDocument8 pagesPaper Updateapi-630484455No ratings yet
- Iacobo TxplanningpaperDocument11 pagesIacobo Txplanningpaperapi-524151719No ratings yet
- Treatment Planning ProjectDocument7 pagesTreatment Planning Projectapi-631250296No ratings yet
- Treatment Planning PaperDocument6 pagesTreatment Planning Paperapi-632526087No ratings yet
- Wael TP ProjectDocument15 pagesWael TP Projectapi-267335639No ratings yet
- PelvislabpdfDocument17 pagesPelvislabpdfapi-632827798No ratings yet
- Final DraftDocument15 pagesFinal Draftapi-334402872No ratings yet
- Treatment Planning ProjectDocument12 pagesTreatment Planning Projectapi-302696314No ratings yet
- Group 1-Vijay Brittany Pat Seth Veronica Stephanie Sadie Ashley Draft 1Document15 pagesGroup 1-Vijay Brittany Pat Seth Veronica Stephanie Sadie Ashley Draft 1api-334644774No ratings yet
- Dos 523 - Treatment Planning LabDocument10 pagesDos 523 - Treatment Planning Labapi-645531105No ratings yet
- Csi Write UpDocument9 pagesCsi Write Upapi-631736561No ratings yet
- Csi PaperDocument9 pagesCsi Paperapi-602488644No ratings yet
- Draft 1Document15 pagesDraft 1api-334402872No ratings yet
- Treatment PlanningDocument9 pagesTreatment Planningapi-426094285No ratings yet
- New Patient Flow ChartDocument1 pageNew Patient Flow Chartapi-212894050No ratings yet
- March Case StudyDocument13 pagesMarch Case Studyapi-212894050No ratings yet
- Extra Credit Problems Submit Upto 20 QuesDocument7 pagesExtra Credit Problems Submit Upto 20 Quesapi-174496267No ratings yet
- Attenuation ProjectDocument10 pagesAttenuation Projectapi-212894050No ratings yet
- Guidelines For SKPMG2 TSSP - Draft For Consultation 10.10.17Document5 pagesGuidelines For SKPMG2 TSSP - Draft For Consultation 10.10.17zqhnazNo ratings yet
- Unit 7: Anthropology: Q2e Listening & Speaking 4: Audio ScriptDocument6 pagesUnit 7: Anthropology: Q2e Listening & Speaking 4: Audio ScriptĐại học Bạc Liêu Truyền thông100% (1)
- Work ProblemsDocument19 pagesWork ProblemsOfelia DavidNo ratings yet
- Howard R700X - SPL - INTDocument44 pagesHoward R700X - SPL - INTJozsefNo ratings yet
- 8.ZXSDR B8200 (L200) Principle and Hardware Structure Training Manual-45Document45 pages8.ZXSDR B8200 (L200) Principle and Hardware Structure Training Manual-45mehdi_mehdiNo ratings yet
- Chemistry: Crash Course For JEE Main 2020Document18 pagesChemistry: Crash Course For JEE Main 2020Sanjeeb KumarNo ratings yet
- Vendor Information Sheet - LFPR-F-002b Rev. 04Document6 pagesVendor Information Sheet - LFPR-F-002b Rev. 04Chelsea EsparagozaNo ratings yet
- Quantitative Methods For Economics and Business Lecture N. 5Document20 pagesQuantitative Methods For Economics and Business Lecture N. 5ghassen msakenNo ratings yet
- Oracle Forms & Reports 12.2.1.2.0 - Create and Configure On The OEL 7Document50 pagesOracle Forms & Reports 12.2.1.2.0 - Create and Configure On The OEL 7Mario Vilchis Esquivel100% (1)
- Roleplayer: The Accused Enchanted ItemsDocument68 pagesRoleplayer: The Accused Enchanted ItemsBarbie Turic100% (1)
- Time-Sensitive Networking - An IntroductionDocument5 pagesTime-Sensitive Networking - An Introductionsmyethdrath24No ratings yet
- Global Geo Reviewer MidtermDocument29 pagesGlobal Geo Reviewer Midtermbusinesslangto5No ratings yet
- Report On GDP of Top 6 Countries.: Submitted To: Prof. Sunil MadanDocument5 pagesReport On GDP of Top 6 Countries.: Submitted To: Prof. Sunil MadanAbdullah JamalNo ratings yet
- Appendix - Pcmc2Document8 pagesAppendix - Pcmc2Siva PNo ratings yet
- Plaza 66 Tower 2 Structural Design ChallengesDocument13 pagesPlaza 66 Tower 2 Structural Design ChallengessrvshNo ratings yet
- PM CH 14Document24 pagesPM CH 14phani chowdaryNo ratings yet
- Decision Making and The Role of Manageme PDFDocument20 pagesDecision Making and The Role of Manageme PDFRaadmaan RadNo ratings yet
- Technical Specification For 33KV VCB BoardDocument7 pagesTechnical Specification For 33KV VCB BoardDipankar ChatterjeeNo ratings yet
- Electives - ArchitDocument36 pagesElectives - Architkshitiz singhNo ratings yet
- AnticyclonesDocument5 pagesAnticyclonescicileanaNo ratings yet
- E Flight Journal Aero Special 2018 Small PDFDocument44 pagesE Flight Journal Aero Special 2018 Small PDFMalburg100% (1)
- Monkey Says, Monkey Does Security andDocument11 pagesMonkey Says, Monkey Does Security andNudeNo ratings yet
- WL-80 FTCDocument5 pagesWL-80 FTCMr.Thawatchai hansuwanNo ratings yet
- ReadmeDocument3 pagesReadmedhgdhdjhsNo ratings yet
- IEC ShipsDocument6 pagesIEC ShipsdimitaringNo ratings yet
- Hdfs Default XML ParametersDocument14 pagesHdfs Default XML ParametersVinod BihalNo ratings yet
- Application of Graph Theory in Operations ResearchDocument3 pagesApplication of Graph Theory in Operations ResearchInternational Journal of Innovative Science and Research Technology100% (2)
- 04 - Fetch Decode Execute Cycle PDFDocument3 pages04 - Fetch Decode Execute Cycle PDFShaun HaxaelNo ratings yet
- 15.053/8 February 7, 2013: More Linear and Non-Linear Programming ModelsDocument42 pages15.053/8 February 7, 2013: More Linear and Non-Linear Programming ModelsShashank SinglaNo ratings yet
- Excon2019 ShowPreview02122019 PDFDocument492 pagesExcon2019 ShowPreview02122019 PDFSanjay KherNo ratings yet